Abstract
Background
Current treatment options for Malassezia folliculitis (MF) are limited. Recent research has demonstrated the inhibitory effect of cold atmospheric plasma (CAP) on the growth of Malassezia pachydermatis in vitro, suggesting CAP as a potential therapeutic approach for managing MF.
Objectives
The objective of our study is to assess the in vitro antifungal susceptibility of Malassezia yeasts to CAP. Additionally, we aim to evaluate the efficacy and tolerability of CAP in treating patients with MF.
Methods
We initially studied the antifungal effect of CAP on planktonic and biofilm forms of Malassezia yeasts, using well‐established techniques such as zone of inhibition, transmission electron microscopy, colony count assay and 2,3‐bis(2‐methoxy‐4‐nitro‐5‐sulfophenyl)‐2H‐tetrazolium‐5‐carboxanilide salt assay. Subsequently, a randomized (1:1 ratio), active comparator‐controlled, observer‐blind study was conducted comparing daily CAP therapy versus itraconazole 200 mg/day for 2 weeks in 50 patients with MF. Efficacy outcomes were measured by success rate, negative microscopy rate and changes in Dermatology Life Quality Index (DLQI) and Global Aesthetic Improvement Scale (GAIS) scores. Safety was assessed by monitoring adverse events (AEs) and local tolerability.
Results
In laboratory investigations, CAP time‐dependently inhibited the growth of Malassezia yeasts in both planktonic and biofilm forms. Forty‐nine patients completed the clinical study. At week 2, success was achieved by 40.0% of subjects in the CAP group versus 58.3% in the itraconazole group (p = 0.199). The negative direct microscopy rates of follicular samples were 56.0% in the CAP group versus 66.7% in the itraconazole group (p = 0.444). No significant differences were found in the proportion of subjects achieving DLQI scores of 0/1 (p = 0.456) or in the GAIS responder rates (p = 0.588) between the two groups. Three patients in the CAP group and one patient in the itraconazole group reported mild AEs.
Conclusion
CAP demonstrated significant antifungal activity against Malassezia yeasts in vitro and exhibited comparable efficacy to itraconazole in treating MF patients. Without the associated adverse effects of oral antifungal drugs, CAP can be considered a promising and safe treatment modality for MF.
Keywords: antifungal agents, cold atmospheric plasma, itraconazole, Malassezia, Malassezia folliculitis
1. INTRODUCTION
The Malassezia genus is a common resident of the skin microbiota, constituting approximately 50%–80% of the total skin mycobiome. 1 However, under specific conditions, Malassezia species can contribute to the pathogenesis of various common skin disorders, including folliculitis, tinea versicolor, seborrheic dermatitis, and atopic dermatitis. In extreme cases, they may result in potentially fatal bloodstream and visceral infections especially in patients undergoing parenteral hyperalimentation. 2 , 3
Malassezia folliculitis (MF) arises from the infection of pilosebaceous units in sebaceous skin regions by Malassezia, often triggered by factors such as sweating, hot climates, exercise, high sebum production and recent corticosteroid use. M. furfur, M. sympodialis, M. pachydermatis and M. globosa are commonly isolated species from MF lesions. Current treatment options for MF are limited, with suggested therapies including topical azoles, topical non‐azoles, and oral azoles. 4 While oral antifungals such as fluconazole and itraconazole are more effective than topical ones, they may not always be appropriate due to gastrointestinal discomfort, potential hepatotoxicity and QT prolongation. 5 Additionally, up to 100% of individuals with MF experience relapse after treatment, 4 highlighting the need for expanded medical options.
Plasma is the fourth state of matter, besides solid, liquid and gas. Cold atmospheric plasma (CAP), currently generated at atmospheric pressure and room temperature, is well‐tolerated by human tissues. 6 As an innovative non‐intrusive application, CAP has been applied in various medical specialties, including infection control, dentistry, oncology and neurology. In dermatology, long‐term safety analyses have indicated that CAP treatment was safe for both normal and diseased skin. Beyond its established role in wound healing, CAP has shown promising results in treating skin infections, inflammatory skin disorders and skin malignancies. 7 , 8 CAP effectively inhibits various skin microorganisms, including viruses, bacteria and fungi, through the generation of reactive oxygen and nitrogen species. 8 Studies on dermatophyte fungal infections demonstrated that CAP suppresses the growth of Trichophyton rubrum, Epidermophyton floccosum, Microsporum canis, Microsporum gypseum, and Candida albicans by disrupting fungal spores and cell membrane integrity. 9 , 10 , 11 As a physical therapy modality without promoting fungal resistance or triggering allergic or toxic reactions, plasma treatment combined with nail plate abrasion and refreshment achieved clinical and mycological cure rates of 70.0% and 85.7%, respectively, in patients with onychomycosis. 12 A controlled split‐face study on acne patients demonstrated improved aesthetics on the treated side, with 79% of patients reporting enhancement compared to 45% on the untreated side. 13 Notably, studies by Wiegand et al., and Tae‐Hyun Lee et al., indicated that plasma reduced the growth of M. pachydermatis in vitro. 14 , 15 In this study, we initially confirmed the inhibitory effect of CAP on Malassezia yeasts in vitro and subsequently designed a prospective study to provide insights into the efficacy and safety of CAP compared to itraconazole in patients with MF.
2. MATERIALS AND METHODS
2.1. Plasma source
A detailed description of the CAP device, the so‐called ST‐P101 plasma skin therapy instrument (Hefei CAS Ion Medical and Technical Devices Co., Ltd, Hefei, China), can be found in a paper by Miaomiao Li et al. 16 Briefly, the hand‐held therapeutic electrode head consisted of 1‐mm‐diameter copper needles connected to the reactor, where the high‐voltage electrode was located. The low‐voltage electrode was connected to the skin. Air was introduced into the reactor, and the plasma stream was generated at the copper needles. The plasma parameters employed in this study were as follows: an AC voltage signal ranging from 9–15 kV, and a ballast resistance of 10 MΩ. The treated surface‐to‐needle tip distance was maintained at 10 mm.
2.2. Strain and culture medium
M. furfur (2.181) from the Guangdong Microbial Culture Collection Center (GDMCC, Guangdong, China), M. sympodialis (Y14b), M. pachydermatis (Y15b1) and M. globosa (MC14) from the Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College (ID‐CAMS & PUMC, Najing, China) were cultured in modified Dixon agar (MDA, Hopebio, Qingdao, China) at 37°C under aerobiosis. A 4‐day‐old colony was suspended in 20 mL of modified Dixon broth (MD, Hopebio, Qingdao, China) and cultured for 2 days at 32°C and 150 rpm. Afterward, cells were washed twice with 20 mL PBS and resuspended in MD to obtain standardized suspensions containing 5 × 106 colony forming units (CFUs)/mL.
2.3. Effect of CAP on planktonic cells
2.3.1. The zone of inhibition
The antifungal activity of CAP against planktonic cells of different Malassezia species was evaluated based on the zone of inhibition (ZOI). Standardized suspensions (100 µL) were plated onto MDA plates. Each plate was placed below the CAP electrode and treated for 0, 20, 40, 60, 120 and 180s, respectively. Following 72 h of incubation the area of the ZOI was measured using ImageJ (https://imagej.nih.gov/ij/). The ZOI was normalized by calculating the percentage of ZOI area to plate area.
2.3.2. Morphology
Transmission electron microscopy (TEM) was used to assess the effect of CAP on the structures of M. furfur planktonic cells. Standardized suspensions (100 µL) were deposited in each well of a 96‐well microplate and treated with CAP for 180s. Subsequently, after incubation for 2, 4 and 6 h, the suspensions were transferred to Eppendorf tubes and centrifuged. The cells were prefixed with 2.5% glutaraldehyde and postfixed with 1% osmium tetroxide, and then dehydrated in series acetones. The samples were embedded in epoxy resin and sliced into ultrathin section. Afterward, the sections were stained with uranyl acetate and lead citrate. The images were taken by TEM (JEOL JEM‐1400 FLASH, Tokyo, Japan).
2.4. Effect of CAP on biofilms
2.4.1. Formation of biofilms and CAP exposure
To form Malassezia biofilms, standardized suspensions (150 µL) of different Malassezia species were inoculated into selected wells of a 96‐well microplate. After incubation at 32°C, 150 rpm for 5 days, the supernatants were gently aspirated and the formed biofilms washed three times with PBS (200 µL) to remove the planktonic or loosely attached pathogens. Subsequently, each biofilm was positioned below the nozzle of CAP for a specific duration.
2.4.2. Quantification of viable cells
After CAP exposure, PBS (100 µL) were transferred to selected wells and biofilms were mechanically broken to get a homogenous suspension. The suspensions were proper decimal diluted and then plated in MDA incubated at 37°C, 5% CO2 for 48 h to process for colony count assay. A sample not exposed to CAP was used as a positive control.
2.4.3. Semiquantification of viable cells
The antibiofilm efficacy of the CAP at different time points was evaluated semiquantitatively using the 2,3‐bis‐(2‐methoxy‐4‐ nitro‐5‐sulfophenyl)−2H‐tetrazolium‐5‐carboxanilide (XTT) assay. The XTT assay relies on the metabolic reduction of XTT into a water‐soluble formazan product in viable cells, which can be directly quantified by spectrophotometric measurements. In brief, PBS (100 µL) were added to selected wells of a 96‐well microplate containing a plasma‐exposed or unexposed biofilm. After incubation for 6 h, 20 µL of the XTT labelling solution (activation reagent and XTT reagent) (Solarbio, Beijing, China), prepared according to manufacturer's recommendations, were added. The absorbance of the solution in each well was measured in a microtiter plate reader (Thermo) at 450 nm after incubated at 37°C, 5% CO2 for 6 h in the dark.
2.5. Clinical study
2.5.1. Subjects
Fifty subjects aged 18–65 years with MF were enrolled in this 2‐week, exploratory, active comparator‐controlled, randomized, observer‐blind clinical trial. The study was performed between August 2022 and December 2023 at the Department of Dermatology and Venereology, the Second Affiliated Hospital of Anhui Medical University, Anhui, China.
The main inclusion criteria for participants are the occurrence of pruritic papules and pustules in sebaceous skin regions. Diagnosis is confirmed by examining pus from these lesions with a 10%–20% potassium hydroxide solution and Gram staining on a microscope slide. Typically, this examination reveals unipolar budding yeasts, conidia and occasionally hyphae. The distinct absence of comedones is crucial for differentiating these cases from acne vulgaris. 4
The main exclusion criteria included pregnancy and lactation; subjects undergoing treatment with topical corticosteroids or antifungals within the previous 2 weeks; systemic corticosteroids or immunosuppressants within the previous 1 month; and systemic antifungals within the previous 3 months; as well as subjects with diabetes, liver disease, cardiac or renal failure, and a known malignancy.
All patients were evaluated and enrolled in the study after signing informed consent. This study was reviewed and approved by the Ethics Committee at the Second Affiliated Hospital of Anhui Medical University, Anhui, China, and followed the ethical standards of the Declaration of Helsinki of 1964.
2.5.2. Study design
Fifty patients were allocated into two groups by a computer‐generated sequence of random numbers. Twenty‐five subjects were randomly assigned to the itraconazole group, treated with oral itraconazole capsules (Sporanox; Johnson & Johnson) at a dosage of 200 mg per day for 2 weeks, and another 25 subjects were randomly assigned to the CAP group to receive CAP treatment over the same period. For each treatment session in the CAP group, the plasma was delivered through a hand‐held therapeutic electrode head, which was consistently maintained at a 10 mm distance from the skin surface. The irradiation time for targeted lesions was set at 3 min. Following this, the electrode head was gradually moved to the next untreated area, ensuring that all lesions received uniform CAP irradiation during each session. A baseline visit, an intermediate visit after 1 week and a final visit after 2 weeks were planned. The Dermatology Life Quality Index (DLQI) was utilized to measure the impact on patients' quality of life. Additionally, the Global Aesthetic Improvement Scale (GAIS), a 5‐point relative scale, was appraised by two experienced dermatologists who were blinded to the patients' treatment allocations. The GAIS is delineated as follows: +3 = “very much improved”, +2 = “much improved”, +1 = “improved”, 0 = “no change”, and −1 = “worse”. Similarly, treatment efficacy was evaluated using the 4‐point Physician's Global Assessment (PGA) scale by the same blinded dermatologists: 0 = “healed” (no visible disease signs), 1 = “markedly improved” (minimal residual lesions, no active disease), 2 = “moderately improved” (decreased severity, active disease remains), 3 = “unchanged” or “worsened”. Participants from the CAP group who did not achieve healing were eligible to enroll in an additional 2‐week extension of the treatment. Post‐treatment, healed subjects were observed for a recurrence period of 2 weeks.
2.5.3. Efficacy and safety assessments
The two coprimary efficacy endpoints included the success rate, assessed via the PGA scales (defined as achieving a PGA score of 0 or 1), and the proportion of subjects exhibiting negative results in direct microscopic examinations of follicular samples. The two secondary endpoints were the proportion of subjects achieving a score of 0 or 1 on the DLQI, indicative of minimal to no impact on life quality, and the GAIS responder rate, with responses categorized as “improved”, “much improved” or “very much improved”. Adverse events (AEs) were recorded, and physical examinations were conducted at each visit to evaluate dermal tolerability and safety. This trial was registered with ClinicalTrials.gov, number NCT 04886323.
2.6. Statistical analysis
For in vitro studies, statistical significance of variations among experimental groups was assessed via the ANOVA test. For the clinical study, given the exploratory study design, 25 patients in each group were considered adequate. Categorical variables were captured by absolute numbers and percentages. Continuous variables were presented as means with standard deviations (SD) or median values with interquartile ranges (IQR). The rates of success, negative direct microscopy, DLQI 0/1 and GAIS responder were analyzed for significance using either the Chi‐square test or Fisher's exact test, depending on the data. Changes in DLQI scores from baseline to the end of treatment were analyzed using a t‐test. Statistical analysis was performed using SPSS (version 22.0, IBM) and statistical significance was set at p < 0.05.
3. RESULTS
3.1. Laboratory investigations
3.1.1. CAP inhibits the planktonic cells
The normalized ZOI of different Malassezia species on plates are represented in Figure 1A for each tested exposure time. Samples treated with CAP at all exposure durations exhibited a significant ZOI. Consequently, an increase in CAP exposure time led to a significant augmentation of the ZOI. Figure 2 shows the impact of CAP treatment on the cell morphology of M. furfur through TEM. After 2 h of 180s CAP treatment, the cell wall of M. furfur noticeably expanded with a significant reduction in electron density compared to untreated cells. After 4 h, necrosis was observed with granular cytoplasm. After 6 h, the cells were completely necrotic with subcellular organelles broken down. These observations suggest that CAP induces direct damage to the cell wall of planktonic M. furfur cells, leading to the loss of barrier function and ultimately cell death.
FIGURE 1.

The antifungal effect of CAP at different time points on planktonic and biofilm forms of different Malassezia species in vitro. (A) The normalized zone of inhibition (Norm. ZOI) and photographs of ZOI on plates. (B) Percentage of biofilms surviving cells normalized to the control group. (C) The XTT biofilm viability normalized to the control group. CAP, cold atmospheric plasma; ZOI, zone of inhibition.
FIGURE 2.
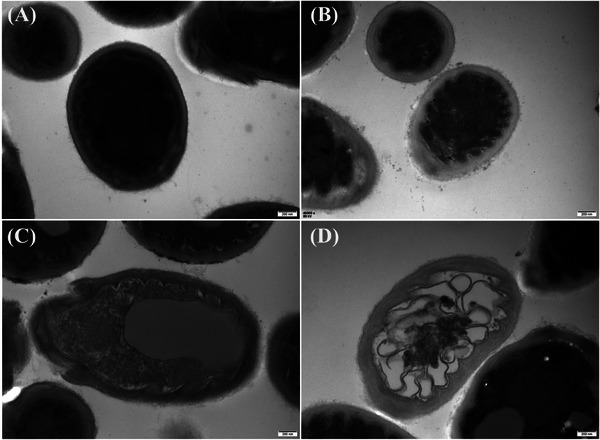
Transmission electron microscopic findings of non‐treated Malassezia furfur cells (A) and 180s cold atmospheric plasma treated cells after 2 h (B) and 4 h (C) and 6 h (D). (A) Normal cells with a healthy subcellular structure. (B) Affected cells with noticeably expanded and transparent cell walls. (C) Affected cells with proliferation buds showing granular cytoplasm. (D) Affected cells showing destruction of subcellular organelles.
3.1.2. CAP inhibits the biofilms
Colony counting, a commonly used quantification assay for investigating pathogen growth inactivation, revealed a significant reduction in CAP treated Malassezia biofilm samples. With prolonged plasma exposure time, the colony count substantially decreased (Figure 1B). The antifungal efficacy of CAP on Malassezia biofilms at different time points was further evaluated using the XTT assay. As expected, the longest CAP exposure time showed the highest inhibition rate (Figure 1C). When treated with CAP for 180s, the metabolic activity of Malassezia biofilms decreased to 15.1%–22.4%.
3.2. Clinical study
3.2.1. Patient disposition, demographic and clinical characteristics
Forty‐nine of the enrolled subjects (96.0%) completed the study. One patient in the itraconazole group withdrew before week 1 for reasons unrelated to the study and was excluded from the research (Figure 3). The demographic and baseline clinical characteristics for the two groups were comparable as presented in Table 1.
FIGURE 3.
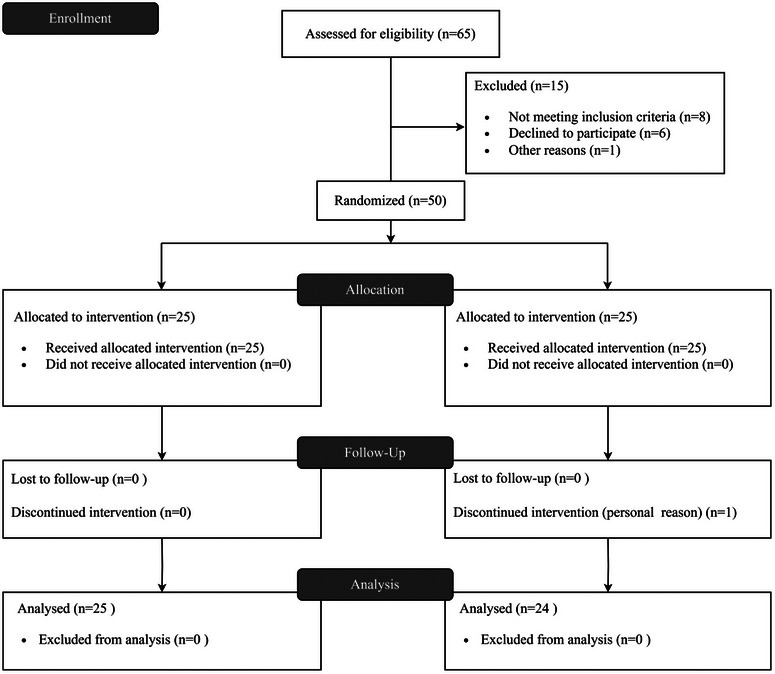
Clinical study flowchart.
TABLE 1.
Demographic and baseline clinical characteristics.
| CAP (n = 25) | Itraconazole (n = 24) | p | |
|---|---|---|---|
| Age, years (median, IQR) | 28.0 (22.0, 34.5) | 24.5 (21.0, 32.8) | 0.548 |
| Female, n (%) | 10 (40.0) | 9 (37.5) | 0.858 |
| Fitzpatrick skin type, n (%) | 0.633 | ||
| II | 3 (12.0) | 2 (8.3) | |
| III | 13 (52.0) | 16 (66.7) | |
| IV | 9 (36.0) | 6 (25.0) | |
| Duration, days (median, IQR) | 17.0 (7.5, 36) | 15.5 (8.3, 27.8) | 0.528 |
| Distribution, n (%) | 0.907 | ||
| Face | 2 (8.0) | 1 (4.2) | |
| Neck | 3 (12.0) | 2 (8.3) | |
| Chest | 12 (48.0) | 14 (58.3) | |
| Abdomen | 0 (0.0) | 1 (4.2) | |
| Back | 7 (28.0) | 5 (20.8) | |
| Upper limbs | 1 (4.0) | 1 (4.2) | |
| Prior antifungal therapy, n (%) | 5 (20.0) | 3 (12.5) | 0.702 |
| Baseline DLQI (mean, SD) | 9.7 (5.5) | 8.9 (5.8) | 0.638 |
Abbreviations: CAP, cold atmospheric plasma; DLQI, Dermatology Life Quality Index.; IQR, interquartile range.
3.2.2. Efficacy
The results of all coprimary and secondary efficacy endpoints at week 2 were comparable for the two treatment groups (p > 0.05) (Table 2). The success rates, defined as either 0 (healed) or 1 (markedly improved) based on the PGA scales at week 2, were 40.0% in the CAP group and 58.3% in the itraconazole group, which was statistically insignificant (p = 0.199). At week 2, 56.0% and 66.7% of the subjects exhibited negative results in direct microscopic examinations of follicular samples in the CAP group and itraconazole group, respectively, without a significant difference between the two sides (p = 0.444). In the CAP group, the mean (SD) DLQI score significantly decreased from an initial value of 9.7 (5.5) to 6.2 (5.1) following treatment completion (p = 0.026). Similarly, in the itraconazole group, the mean (SD) DLQI score significantly declined from a baseline of 8.9 (5.8) to 5.6 (5.1) (p = 0.039). There was no significant difference in the proportion of subjects achieving DLQI 0/1 in week 2 between the CAP group and the itraconazole group (p = 0.456). The GAIS responder rate also indicated no significant difference between the two sides (p = 0.588). The PGA scores and GAIS scores from baseline to week 1 and week 2 are shown in Figure 4. Figure 5 shows representative photographic images of subjects treated with CAP, categorized by different PGA scores.
TABLE 2.
Summary efficacy result.
| CAP (n = 25) | Itraconazole (n = 24) | p | |
|---|---|---|---|
| Coprimary efficacy endpoints | |||
| PGA success, n (%) | |||
| Week 1 | 5 (20.0) | 8 (33.3) | 0.291 |
| Week 2 | 10 (40.0) | 14 (58.3) | 0.199 |
| Negative direct microscopy, n (%) | |||
| Week 1 | 8 (32.0) | 11 (45.8) | 0.320 |
| Week 2 | 14 (56.0) | 16 (66.7) | 0.444 |
| Secondary efficacy endpoints | |||
| DLQI 0/1, n (%) | |||
| Week 1 | 2 (8.0) | 5 (20.8) | 0.247 |
| Week 2 | 5 (20.0) | 7 (29.2) | 0.456 |
| GAIS responder, n (%) | |||
| Week 1 | 10 (40.0) | 13 (54.2) | 0.321 |
| Week 2 | 17 (68.0) | 18 (75.0) | 0.588 |
Abbreviations: CAP, cold atmospheric plasma; DLQI, Dermatology Life Quality Index; GAIS, Global Aesthetic Improvement Scale; PGA, Physician's Global Assessment.
FIGURE 4.
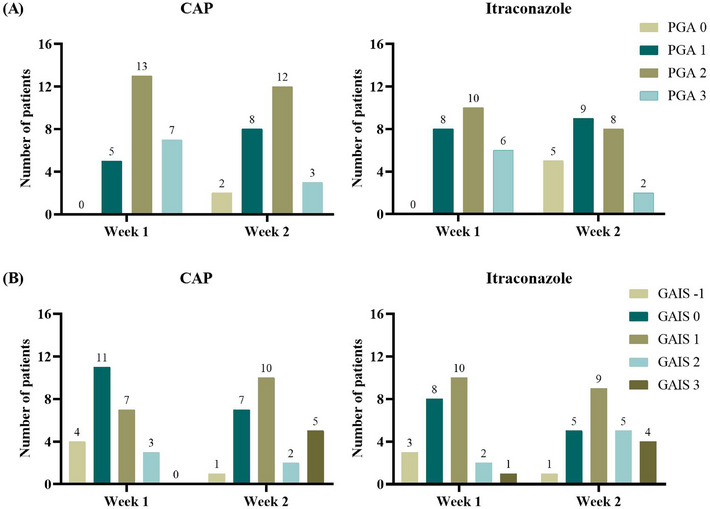
PGA scores and GAIS scores at week 1 and week 2. (A) PGA scores. (B) GAIS scores. GAIS, Global Aesthetic Improvement Scale; PGA, Physician's Global Assessment.
FIGURE 5.
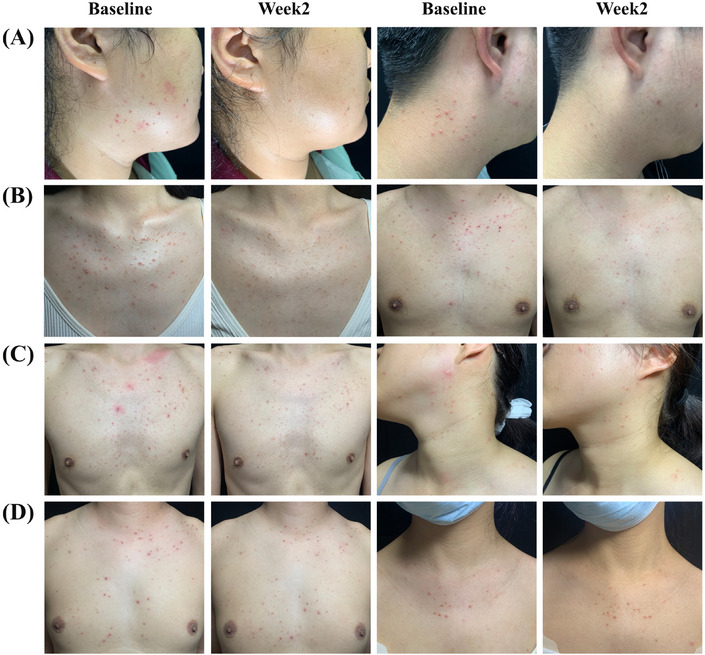
Photographs of subjects treated with cold atmospheric plasma at baseline and 2 weeks after treatment. (A) Patients achieved a PGA score of 0. (B) Patients achieved a PGA score of 1. (C) Patients achieved a PGA score of 2. (D) Patients achieved a PGA score of 3. PGA, Physician's Global Assessment.
Two weeks after the end of treatment, no recurrence was observed in the two healed patients with CAP treatment, while 1 of 5 (20.0%) healed patients with itraconazole treatment experienced recurrence. Five patients in the CAP group who were not cured volunteered for an extended 2‐week treatment. Following this extension, three patients achieved a PGA score of 0, whereas one patient experienced recurrence in the 2 weeks after the cessation of treatment.
3.2.3. Safety and tolerability
It was observed that 3 of 25 (12.0%) patients treated with CAP and 1 of 24 (4.2%) patients treated with itraconazole reported product‐related AEs. Three patients in the CAP group experienced local skin dryness, itching and erythema, respectively, while one patient in the itraconazole group experienced nausea during the course of treatment. The severity of AEs in four patients was mild and resolved over time. No patient withdrew due to study‐related intolerance reactions.
4. DISCUSSION
To the best of our knowledge, this is one of the few reports that evaluate the effect of CAP on Malassezia genus in vitro. Furthermore, we present here the first exploration of the efficacy and safety of CAP in a proof‐of‐concept clinical trial on patients with MF.
In this study, CAP demonstrated a significant antifungal effect against planktonic cells of Malassezia yeast. The observed increase in ZOI with longer CAP treatment durations suggested a time‐dependent inhibition of planktonic cell growth, consistent with previous studies on Malassezia. 15 Moreover, microscopic examination of the M. furfur planktonic cell wall revealed substantial morphological alterations compared to non‐exposed samples. Progressive damage of organelles may be sequelae to the loss of barrier function. In line with our results, Safi‐Samghabadi et al. reported similar CAP‐induced cell wall damage in T. rubrum under scanning electron microscopy. 17 Traditionally, studies evaluating the effect of CAP in Malassezia genus have focused on planktonic cells in pure culture despite the fact that most microbes exist as organized biofilm communities of cells in natural ecosystems. The biofilm structure enhances the inherent resistance of microbes to both physical and chemical stressors and mediates the regulation of community‐driven gene expression. 18 Luciana et al. found that the formation of biofilm is associated with increased antifungal resistance in M. pachydermatis. 19 In this study, we presented the initial exploration of the impact of CAP on Malassezia biofilms. The combination of colony assay and XTT assay was employed to detect and quantify viable and metabolically active cells. The results indicated that CAP exhibits effective antifungal activity against Malassezia biofilms.
The safety of the CAP device used in this study has been previously established in both rats and humans. 16 Having demonstrated its ability to inhibit Malassezia yeasts in vitro, we conducted a prospective randomized controlled trial, demonstrating that CAP is a secure and efficient approach for adult MF management. With the prolongation of treatment duration, the therapeutic effect improved. By the end of the treatment, the PGA scales indicated success rates of 40.0% in the CAP group and 58.3% in the itraconazole group (p = 0.199), while direct microscopic examinations revealed negative results in 56.0% and 66.7% of the subjects, respectively (p = 0.444). Despite these results not reaching statistical significance, the apparent numerical discrepancy warrants further discussion. The lack of statistical significance could be attributed to several factors, including sample size, variability in patient response, or the presence of confounding factors that were not controlled for in the study. Notably, the 40.0% success rate in the CAP group, coupled with a 56.0% rate of negative results in direct microscopic examinations indicates that it remains a valuable treatment option. Moreover, both treatments significantly improved quality of life as evidenced by reductions in DLQI scores from baseline to study completion, with no significant difference in the proportion of subjects achieving DLQI 0/1 between the groups (p = 0.456). The GAIS responder rate further supports this, showing no significant difference between the two groups (p = 0.588), indicating comparable aesthetic improvements. The availability of CAP as an alternative treatment enhances personalized medicine by offering options where standard treatments are unsuitable. In our randomized controlled trial, we ethically prioritized patient welfare by not restricting treatment options for those who remained uncured, ensuring that all participants could pursue the best possible care. Among the three patients treated with CAP who achieved remission and were observed post‐treatment cessation, one experienced a recurrence of symptoms within 2 weeks, indicating that this therapeutic approach may face challenges similar to other standard treatments in managing post‐treatment recurrence.
MF involves a substantial invasion of yeasts into sebaceous follicles, leading to follicular dilatation. 20 CAP, acting against certain pathogenic factors, demonstrated efficacy in preclinical studies against Malassezia planktonic cells and biofilms. Moreover, research has shown that hair can serve as a conduit, facilitating the delivery of plasma into the deeper regions of the hair follicles. The reactive oxygen and nitrogen species generated by plasma effectively penetrate into the hair follicles, leading to local antisepsis within the follicular reservoir. This contrasts with traditional topical antibacterial agents, which fail to reach deeper follicular areas. 21 Malassezia species, excluding M. pachydermatis, lack the fatty acid synthase gene and rely on exogenous lipids. The Malassezia that exist in the cornified layer and hair papilla follicles use their own lipase and phospholipase to hydrolyze triglycerides in sebum into free fatty acids, which serve as their own lipid source. This explains why the skin diseases caused by Malassezia are mainly distributed in sebaceous areas of the skin. 20 Heesu Kim et al. found that the sebaceous glands can be specifically damaged through selecting gaseous agents and appropriate energy setting plasma without causing thermal necrosis in the dermis of animal models. 22 Cho et al. discovered that CAP effectively inhibits lipogenesis and proliferation of human sebocytes, leading to a reduction in sebum production in the facial skin of humans. 23 Therefore, it can be hypothesized that CAP treats MF by inactivating Malassezia in deeper follicular areas and reducing sebaceous gland secretion.
Our study has limitations that require further investigation. In vitro studies were confined to standard Malassezia strains; additional clinically isolated strains should be explored. Clinically, the investigation was limited to a single center and a small‐scale cohort over a 2‐week period, without restricting treatment options for uncured patients. This underscores the necessity for larger‐scale studies with extended treatment durations and follow‐ups to better determine therapeutic effects and understand the dynamics of recurrence post‐treatment. Additionally, the impact of different treatment durations and frequencies requires further determination.
5. CONCLUSION
In conclusion, CAP demonstrated significant antifungal activity against Malassezia in vitro and comparable efficacy in treating MF patients with itraconazole. Without the potential adverse effects associated with oral antifungal drugs, such as hepatotoxicity and gastrointestinal discomfort, it can be considered a promising and safe treatment modality for MF.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICAL STATEMENT
All patients were evaluated and enrolled in the study after signing informed consent. This study was reviewed and approved by the Ethics Committee at the Second Affiliated Hospital of Anhui Medical University, Anhui, China (2021‐028) and followed the ethical standards of the Declaration of Helsinki of 1964.
PATIENT CONSENT STATEMENT FOR USE OF PHOTOGRAPHS
Written informed consent was obtained from the patient for the use of their photographs in this publication.
ACKNOWLEDGMENTS
We thank the study participants for their valuable contributions to the research. The work was supported by the Research Fund of Anhui Medical University, Hefei, China. Grant/Award Number: 2019xkj033. We are acknowledged to Professor Zhou Liu from the Department of Laboratory Medicine, the Second Affiliated Hospital of Anhui Medical University for providing the necessary facilities of this study.
Wang N, Yan T, Mei X, Liu J, Lei Y, Yang C. Cold atmospheric plasma therapy for Malassezia folliculitis: Laboratory investigations and a randomized clinical trial. Skin Res Technol. 2024;30:e13850. 10.1111/srt.13850
DATA AVAILABILITY STATEMENT
The data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Oh J, Byrd AL, Deming C, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514(7520):59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tragiannidis A, Bisping G, Koehler G, Groll AH. Minireview: Malassezia infections in immunocompromised patients. Mycoses. 2010;53(3):187‐195. [DOI] [PubMed] [Google Scholar]
- 3. Aguirre C, Euliarte C, Finquelievich J, Sosa Mde L, Giusiano G. Fungemia and interstitial lung compromise caused by Malassezia sympodialis in a pediatric patient. Rev Iberoam Micol. 2015;32(2):118‐121. [DOI] [PubMed] [Google Scholar]
- 4. Henning MAS, Hay R, Rodriguez‐Cerdeira C, et al. Position statement: Recommendations on the diagnosis and treatment of Malassezia folliculitis. J Eur Acad Dermatol Venereol. 2023;37(7):1268‐1275. [DOI] [PubMed] [Google Scholar]
- 5. Tsai YC, Wang JY, Wu YH, Wang YJ. Clinical differences in pediatric and adult Malassezia folliculitis: Retrospective analysis of 321 cases over 9 years. J Am Acad Dermatol. 2019;81(1):278‐280. [DOI] [PubMed] [Google Scholar]
- 6. Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species‐mediated functions. Front Immunol. 2022;13:868386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazdani Z, Pasandi MS, Golpour M, Eslami M, Rafiei A. Effect of cold atmospheric plasma on changing of biomolecular structures involved in apoptosis pathways of melanoma cancer. Skin Res Technol. 2024;30(1):e13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan F, Wang Y, Zhang S, Shui R, Chen J. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shemer A, Daniel R, Kassem R, Geffen Y, Galili E. Cold sub‐atmospheric and atmospheric pressure plasma for the treatment of Trichophyton rubrum onychomycosis: An in‐vitro study. Dermatol Ther. 2020;33(6):e14084. [DOI] [PubMed] [Google Scholar]
- 10. Ouf SA, El‐Adly AA, Mohamed AH. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J Med Microbiol. 2015;64(10):1151‐1161. [DOI] [PubMed] [Google Scholar]
- 11. Lin L, Zhuo Y, Dong Q, Yang C, Cheng C, Liu T. Plasma activated Ezhangfeng Cuji as innovative antifungal agent and its inactivation mechanism. AMB Express. 2023;13(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lux J, Dobias R, Kuklova I, et al. Inactivation of dermatophytes causing onychomycosis and its therapy using non‐thermal plasma. J Fungi (Basel). 2020;6(4):214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karrer S, Berneburg M, Zeman F, Koller M, Müller K. A prospective, randomised, controlled, split‐face clinical trial to assess the safety and the efficacy of cold atmospheric plasma in the treatment of acne vulgaris. Applied Sciences. 2021;11(23):11181. [Google Scholar]
- 14. Wiegand C, Fink S, Hipler UC, et al. Cold atmospheric pressure plasmas exhibit antimicrobial properties against critical bacteria and yeast species. J Wound Care. 2017;26(8):462‐468. [DOI] [PubMed] [Google Scholar]
- 15. Lee TH, Hyun JE, Kang YH, Baek SJ, Hwang CY. In vitro antifungal activity of cold atmospheric microwave plasma and synergistic activity against Malassezia pachydermatis when combined with chlorhexidine gluconate. Vet Med Sci. 2022;8(2):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M, Gao J, Wang L, et al. Basic research and clinical exploration of cold atmospheric plasma for skin wounds. Bioeng Transl Med. 2023;8(5):e10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Safi‐Samghabadi A, Atyabi SM, Razzaghi‐Abyaneh M. Anti‐dermatophytic activity of cold atmospheric plasma against Trichophyton rubrum via affecting fungal growth, morphology, drug susceptibility and HSP90 gene expression. Sci Rep. 2022;12(1):9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int J Microbiol. 2012;2012:528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Figueredo LA, Cafarchia C, Otranto D. Antifungal susceptibility of Malassezia pachydermatis biofilm. Med Mycol. 2013;51(8):863‐867. [DOI] [PubMed] [Google Scholar]
- 20. Saunte DML, Gaitanis G, Hay RJ. Malassezia‐associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol. 2020;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lademann O, Kramer A, Richter H, et al. Antisepsis of the follicular reservoir by treatment with tissue‐tolerable plasma (TTP). Laser Physics Letters. 2011;8(4):313‐317. [Google Scholar]
- 22. Kim H, Kim HJ, Kim HK, Hong JY, Cho SB. Effects of argon and nitrogen plasma pulses on the skin and skin appendages in an in vivo animal model. Skin Res Technol. 2020;26(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 23. Cho SB, Lee S, Yoo DS, et al. Cold atmospheric plasma inhibits lipogenesis and proliferation of human sebocytes and decreases sebum production in human facial skin. Dermatologic Therapy. 2023;2023:1‐9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.


