Abstract
The continued presence of virus-specific CD8+ T cells within the central nervous system (CNS) following resolution of acute viral encephalomyelitis implicates organ-specific retention. The role of viral persistence in locally maintaining T cells was investigated by infecting mice with either a demyelinating, paralytic (V-1) or nonpathogenic (V-2) variant of a neurotropic mouse hepatitis virus, which differ in the ability to persist within the CNS. Class I tetramer technology revealed more infiltrating virus-specific CD8+ T cells during acute V-1 compared to V-2 infection. However, both total and virus-specific CD8+ T cells accumulated at similar peak levels in spinal cords by day 10 postinfection (p.i.). Decreasing viral RNA levels in both brains and spinal cords following initial virus clearance coincided with an overall progressive loss of both total and virus-specific CD8+ T cells. By 9 weeks p.i., T cells had largely disappeared from brains of both infected groups, consistent with the decline of viral RNA. T cells also completely disappeared from V-2-infected spinal cords coincident with the absence of viral RNA. By contrast, a significant number of CD8+ T cells which contained detectable viral RNA were recovered from spinal cords of V-1-infected mice. The data indicate that residual virus from a primary CNS infection is a vital component in mediating local retention of both CD8+ and CD4+ T cells and that once minimal thresholds of stimuli are lost, T cells within the CNS cannot survive in an autonomous fashion.
CD8+ T cells are primary effector cells capable of controlling many intracellular pathogens. Acute infections can trigger potent antigen (Ag)-driven expansion and differentiation of CD8+ T cells into cytotoxic T cells (CTL), resulting in substantial populations of virus-specific CD8+ T cells (1, 13, 23). During infections localized to specific tissues, Ag-specific T cells predominate at the site of virus replication to exert effector function (5, 13). Although the majority of activated, Ag-experienced T cells undergo apoptosis concomitant with clearance of the infectious agent (6, 27), a variable portion survive to become long-lived memory T cells (1). These regulatory mechanisms serve to minimize tissue damage and maintain homeostasis. Following viral clearance, memory cells are generally not retained at the original site of infection but redistribute into secondary lymphoid organs and blood (8, 13). However, accumulation of Ag-experienced CD8+ T cells at a preferred site has been observed following mucosal immunization and subsequent challenge (14) and following infection of the central nervous system (CNS) (5, 16, 20, 21). These data suggest that CD8+ T-cell homing and retention may be dependent on the anatomical site of initial virus entry and replication (14). It is not clear whether accumulation or retention of CD8+ T cells at the site of previous infection is dependent on residual Ag, expression of adhesion molecules, soluble mediators specific for the local microenvironment, and/or the inability to recirculate due to local barriers. In particular, little is known about the fate of CD8+ T cells following resolution of acute infections associated with restricted access to immune cells, such as the CNS parenchyma.
Retention of reactivated CD8+ memory T cells within the CNS has been demonstrated in immune mice challenged with a neurotropic influenza virus (16). The inability to detect persisting Ag or viral genome and lack of T-cell proliferation suggested that the CNS can harbor functional CD8+ T cells for a prolonged period in the absence of cognate Ag (16). Similarly, CD8+ T cells were also recovered from the CNS following clearance of the neurotropic JHM strain of mouse hepatitis virus (JHMV) (5, 20, 21). In contrast to influenza virus-specific T cells, JHMV-specific CD8+ T cells were associated with persisting virus, as demonstrated by the presence of viral RNA (vRNA) and rapidly lost cytolytic activity. These observations suggested that the CNS may provide a unique environment for the maintenance of CD8+ T cells irrespective of persisting Ag. However, both of these infectious models differ not only in the association with persisting vRNA but also in the CNS cell types infected and the activation of naive versus memory CD8+ T-cell subsets. Therefore, the mechanisms involved in the continued presence of CD8+ T cells within the CNS, as well as regulation of activational status following infection and their role in CNS pathology, require further elucidation.
This study takes advantage of two monoclonal antibody (MAb)-selected JHMV variants to investigate the role of persisting Ag in maintaining the presence of CD8+ T cells within the CNS following clearance of infectious virus. JHMV variants 2.2-V-1 and 2.2/7.2-V-2 (9, 10), designated V-1 and V-2, have similar growth characteristics in infected mice but vastly different disease outcomes (10, 21). Mice infected with V-1 develop a nonfatal encephalomyelitis associated with transient paralysis and extensive demyelination. Both the pathology and cellular immune components of V-1 infection have been characterized extensively (9, 21). By contrast, encephalomyelitis induced by infection with the closely related V-2 variant is essentially asymptomatic and is characterized by little or no demyelination (10, 21). Despite these vastly different disease outcomes, infectious virus is cleared from the CNS with similar kinetics (11, 21). During acute JHMV infection, CD8+ T cells are the primary immune effectors responsible for eliminating infectious virus (29). Following infection with V-1 or V-2, virus-specific CD8+ T cells constitute up to 50% of the infiltrating CD8+ mononuclear cell population (5, 21). Furthermore, large numbers of virus-specific CD8+ T cells are found within the CNS for several weeks following clearance of both infectious viruses (5, 21). Nevertheless, V-1-infected mice fail to achieve sterile immunity, resulting in a persistent infection as evidenced by the detection of life-long persisting vRNA (4, 12). Persistence is primarily localized to the spinal cord white matter and is associated with ongoing focal demyelination and chronic mononuclear cell infiltration, hallmarks reminiscent of the human disease multiple sclerosis. By contrast, histological evidence suggests that V-2 only transiently infects cells of the spinal cord (21). These distinct infections were therefore used as experimental models to examine the correlation between persisting virus and maintenance of virus-specific CD8+ T cells within the CNS.
Analysis of brains and spinal cords revealed that T-cell infiltration closely reflected the distribution and level of vRNA characteristic for either infection. During both infections, T-cell recruitment was maximal between days 7 and 10 postinfection (p.i.), with delayed infiltration of virus-specific CD8+ T cells into the spinal cord compared to the brain. However, recovery of T cells 2 months after clearance of infectious virus was clearly dependent on the presence of detectable vRNA, independent of T-cell specificity. The data suggest that survival and/or continued recruitment of both virus-specific and heterologous CD8+ T cells as well as CD4+ T cells in CNS tissue following control of a primary viral infection is mediated by the presence of persisting virus.
MATERIALS AND METHODS
Mice and viruses.
Male BALB/c (H-2d) mice were purchased from the National Cancer Institute (Frederick, Md.) at 6 weeks of age and certified naive to prior mouse hepatitis virus exposure. Mice were housed in an accredited animal facility at the University of Southern California and infected within 1 week of arrival. CNS infections were induced by intracranial injection of 30 μl containing 1,000 PFU of JHMV MAb-selected variants V-1 and V-2 (9, 10). Viruses were propagated and quantitated by plaque assay using the murine DBT astrocytoma cell line as described elsewhere (28).
Determination of vRNA by RT-PCR.
Mice were sacrificed by CO2 asphyxiation, and individual brains and spinal cords were taken separately after severing the junction between the brain stem and spinal cord. RNA was extracted from one-quarter brain or one-half spinal cord as described previously (4). Briefly, samples were lysed in guanidinium thiocyanate using a Ten Broeck homogenizer, passed through a 25-gauge needle, and then centrifuged over 5.4 M CsCl at 105 × g for 16 h at ambient temperature. cDNA was synthesized by mixing 2 μg of RNA with random hexanucleotide primers and avian myeloblastosis reverse transcriptase (RT; Promega, Madison, Wis.) and incubated for 1 h at 42°C. Nested PCR primers for amplification of viral nucleocapsid (N) gene nucleotides 534 to 898 (GTCGCAAGCCAACAGGCCG and GGAGTCCTCTTTTGACGAGGC) and 548 to 858 (CCATGGCCGAGACTAGGACCTCT and CTGCCTGACTTCTTTGGCACT) as well as for host hypoxanthine phosphoriboxyltransferase (HPRT) cDNA (25) were purchased from Genosys Biotechnologies (The Woodlands, Tex.). PCR amplification conditions consisted of 5 min at 95°C followed by 30 cycles for HPRT, 35 cycles for the external N primer set, and 20 cycles for the internal N primer set at 95°C for 1 min, 58°C for 1 min, and 72°C for 2 min.
Isolation of CNS-derived CD8+ T cells and IFN-γ ELISPOT assays.
Mononuclear cells were derived from the CNS of mice as described previously (5, 21). Briefly, brains or spinal cords were pooled from groups of six to eight mice and homogenized in RPMI 1640 supplemented with 25 mM HEPES and 1% fetal bovine serum using Ten Broeck tissue homogenizers. Cells were suspended in 30% Percoll, concentrated onto a 1-ml cushion of 70% Percoll by centrifugation at 800 × g for 20 min at 4°C, and collected from the interphase. Typical yields of mononuclear cells ranged from ∼5 × 105 to ∼1.5 × 106 per brain and ∼8 × 104 to ∼2 × 105 per spinal cord, with the lower range of cells being recovered during latter stages of infection. To control for contamination with blood-borne mononuclear cells, mice were perfused with 30 ml of phosphate-buffered saline prior to tissue isolation where indicated. Enzyme-linked immunospot (ELISPOT) assays to measure the frequency of Ag-specific gamma interferon (IFN-γ)-secreting cells among splenocytes were carried out as described previously (20). Spots from two mononuclear cell dilutions (n = 6) were counted.
Fluorescence-activated cell sorting analysis.
Expression of cell surface markers was determined by staining cells with MAbs specific for CD8 (53.67) and CD4 (GK1.5) as described elsewhere (5). All MAbs were purchased from PharMingen (San Diego, Calif.). The Ld major histocompatibility complex (MHC) class I tetramer associated with pN318-326 peptide (Ld-pN) has been described elsewhere (5).
RESULTS
JHMV variant V-1 persistently infects the spinal cord, whereas V-2 is cleared.
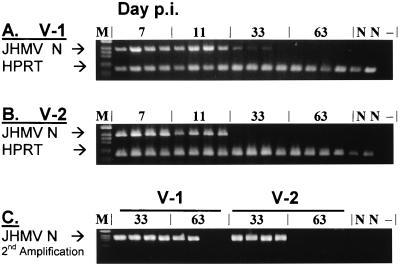
Chronic demyelination in V-1-infected mice is associated with persisting vRNA, which can be detected for up to 2 years p.i. (4, 12). Although V-2 infection is clinically asymptomatic, the ability of V-2 to persist is unknown. To assess the relative abilities of V-1 and V-2 to persist in infected BALB/c mice (H-2d), CNS tissues were examined for the presence of viral N RNA as well as the housekeeping gene HPRT by RT-PCR at multiple intervals p.i. Brain RNA and spinal cord RNA were analyzed separately due to the more rapid and extensive virus spread to the spinal cord during V-1 compared to V-2 infection (21). Similar levels of V-1 and V-2 RNA were observed in brains during acute infection at days 7 and 11 p.i. (Fig. 1A and B). vRNA in brains declined sharply by day 33 p.i. and was undetectable by day 63 p.i. following infection with both viruses. Relative to naive mice, HPRT products exhibited similar levels of intensity at all time points, confirming the specific decline in vRNA after day 11 p.i. (Fig. 1A and B), coincident with clearance of infectious virus (21). To detect low levels of persisting vRNA, RNA isolated on days 33 and 63 p.i. was analyzed following two rounds of amplification using a nested set of viral N-gene-specific primers. Following the second amplification, vRNA was readily detected in brain samples from all infected mice at day 33 p.i. (Fig. 1C). At day 63 p.i. vRNA was still detected in two of four V-1-infected mice but was below detection levels in all V-2-infected mice. These data indicate that vRNA was cleared to a variable extent from the brains of V-1-infected mice. By contrast, V-2 RNA was completely cleared from the brains of V-2-infected mice within 8 to 9 weeks p.i.
FIG. 1.
Presence of vRNA in brains of V-1- and V-2-infected mice. Total RNA was prepared from brains of four individual mice infected with either V-1 (A) or V-2 (B) at days 7, 11, 33, and 63 p.i. as indicated. Sequences from the viral N RNA and host HPRT RNA (A and B) were amplified and analyzed by gel electrophoresis. PCR products from days 33 and 63 p.i. were further amplified using a nested set of primers for the viral N gene (C). M, marker; N, naive control mouse; −, no RNA used during the cDNA synthesis.
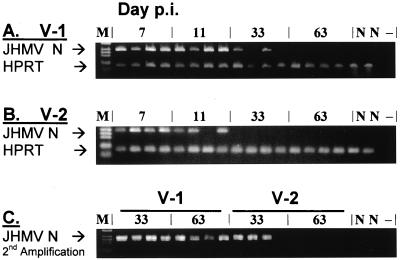
Although V-1 and V-2 initially replicate in the brain, V-1 Ag and RNA are most abundant in the spinal cords in persistently infected mice (4, 12, 21). To verify complete clearance of V-2 RNA from the CNS while confirming the ability of V-1 to establish persistence within the spinal cord, the presence of vRNA in spinal cords from infected and naive mice was tested (Fig. 2). Following a single round of amplification, V-1 RNA was readily detectable in all mice to day 11 p.i. and in two of four mice at day 33 p.i. (Fig. 2A). By contrast, V-2 RNA levels were lower and no longer detected at day 33 p.i. (Fig. 2B). Following reamplification using the nested N primer set, vRNA was detected in spinal cords from all V-1-infected mice at days 33 and 63 p.i. (Fig. 2C). By contrast, vRNA was detected in only three out of four V-2-infected mice at day 33 p.i. and was undetectable by day 63 p.i. (Fig. 2C). These data provided crucial information for subsequent analysis of T-cell retention within the CNS: (i) V-1 established a chronic CNS infection in BALB/c mice, whereas V-2 was effectively cleared; (ii) the spinal cord was confirmed as the preferential site of chronic V-1 infection; and (iii) vRNA was transiently found in the spinal cords of V-2 infected mice, albeit at lower levels than in V-1 infected mice.
FIG. 2.
Presence of vRNA in spinal cords of V-1- and V-2-infected mice. Total RNA was prepared from spinal cords of four individual mice infected with either V-1 (A) or V-2 (B) at each of the indicated time points. Sequences from the viral N RNA and host HPRT RNA (A and B) were amplified and analyzed by gel electrophoresis. PCR products from days 33 and 63 p.i. were further amplified using a nested set of primers for the viral N gene (C). M, marker; N, naive control mouse; −, no RNA used during the cDNA synthesis.
Clearance of vRNA coincides with disappearance of CD8+ T cells from the CNS.
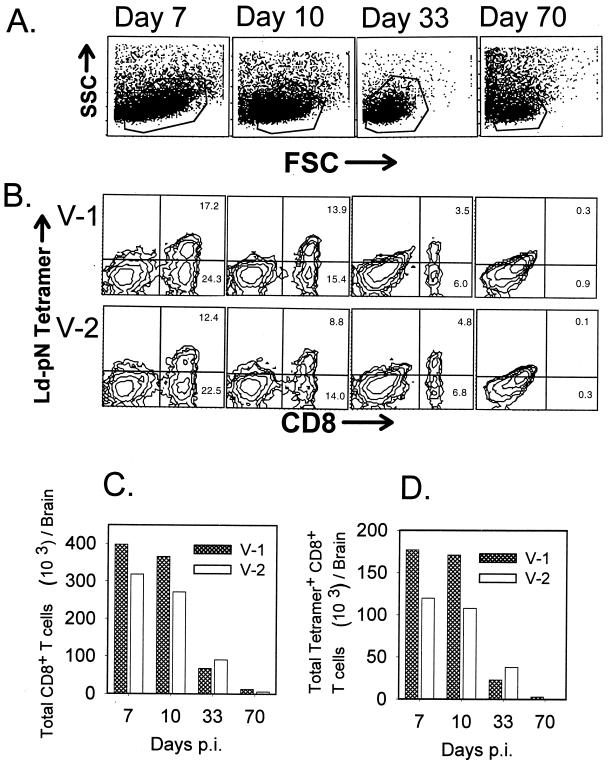
Infection of the CNS by both V-1 and V-2 in BALB/c mice induces vigorous local CD8+ T-cell responses specific for the dominant N protein epitope (20, 21). Furthermore, these CD8+ T cells are present in the CNS for at least 1 month following clearance of infectious virus (5, 21). To determine whether persisting virus plays a role in maintaining T cells within the CNS, mice infected with either V-1 or V-2 were analyzed for CNS mononuclear cell infiltration during and after acute infection. As comparisons of CNS tissue from perfused and nonperfused mice revealed no differences in either the relative percentage or absolute numbers of CNS infiltrating T-cell subsets (data not shown), all data were derived from nonperfused mice. Brains and spinal cords were examined separately, as vRNA is sequestered differentially between these tissues at different times p.i. Due to the low number of cells recovered during the latter stages of infection, especially within the spinal cord (∼8 × 104 cells per mouse), CNS mononuclear cells pooled from six to eight mice were analyzed per time point. Brain-derived mononuclear cells were stained with anti-CD8 MAb and the Ld-pN tetramer specific for the immunodominant N epitope recognized in H-2d mice and analyzed by flow cytometry. Representative gates set for live mononuclear cells from each time point are shown in Fig. 3A. During the peak of T-cell infiltration (day 7 p.i.), the frequency of total CD8+ T cells (41.5% versus 34.9%) as well as of tetramer+ CD8+ T cells (17.2% versus 12.4%) was higher in brains of V-1-infected mice compared to V-2-infected mice (Fig. 3B), confirming a superior response induced by V-1 infection (21). By day 33 p.i., CD8+ T-cell percentages had decreased; however, the proportion of tetramer+ cells within the brain remained fairly constant, although total CD8+ T cells and tetramer+ CD8+ T cells were slightly higher in V-2-infected mice (Fig. 3B). By day 70 p.i., when vRNA was undetectable in the brains of all V-2-infected and 50% of V-1-infected mice (Fig. 1C), levels of CD8+ T cells induced by both infections were significantly reduced (Fig. 3B). A slightly higher percentage of brain CD8+ T cells in V-1-infected mice compared to V-2-infected mice (1.2% versus 0.4%) may result from low levels of vRNA in some animals. However, the frequency of CD8+ T cells isolated from brains of infected mice at day 70 p.i. approximate those found within the brains of naive animals (data not shown).
FIG. 3.
Presence of virus-specific CD8+ T cells in the brain. Brain mononuclear cells were isolated from six to eight mice sacrificed at days 7, 10, 33, and 70 p.i. as indicated and pooled prior to analysis. Live mononuclear cells were gated based on forward and side scatter (FSC and SSC) analysis, and representative gates are shown for each time point (A). Brain mononuclear cells were stained directly ex vivo with MAb to CD8 and the Ld-pN tetramer reagent (B). Numbers in the right quadrants represent percentages of tetramer+ CD8+ T cells (upper number) and tetramer− CD8+ T cells (lower number). The data presented in panel B were used to calculate the total number of infiltrating CD8+ T cells (C) and total number of tetramer+ CD8+ T cells (D) per brain.
To compensate for higher yields of mononuclear cells during acute infection, absolute numbers of total CD8+ T cells and tetramer+ CD8+ T cells recovered from the brain were determined (Fig. 3C and D, respectively). Infiltration by total CD8+ T cells and tetramer+ CD8+ T cells peaked at day 7 p.i., with slightly greater numbers in V-1-infected animals. By day 33 p.i., both total CD8+ and tetramer+ CD8+ T-cell numbers declined more than fivefold in V-1-infected animals, but only about threefold in V-2-infected animals. By day 70 p.i., however, CD8+ T cells were reduced approximately 30-fold in V-1-infected animals and approximately 55-fold in V-2-infected animals compared to the respective peak levels of infiltration at day 7 p.i. These data suggest that the vast majority of CD8+ T cells are ultimately cleared from the brains of JHMV-infected animals when vRNA levels are near or below detection thresholds.
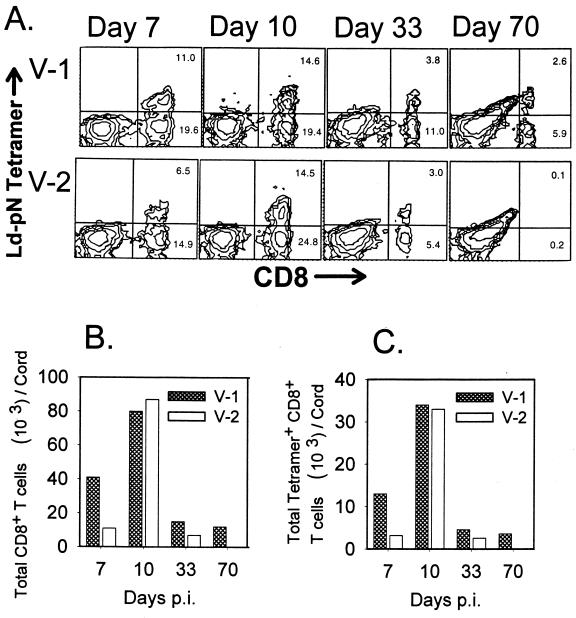
All infections were initiated by intraparenchymal injection; however, V-1 spreads down the ependymal cells lining the central canal of the spinal cord and migrates centripetally through the surrounding gray matter of the cord, where it establishes persistent infection of the spinal cord white matter (32). By contrast, V-2 is more limited in its ability to infect the spinal cord (21). Consistent with the distribution of Ag, flow cytometric analysis of mononuclear cells from spinal cords revealed a delay in peak CD8+ T-cell infiltration compared to the brain during both infections (Fig. 4A). CD8+ T-cell infiltration in the spinal cord was maximal at day 10 p.i., comprising 34% for V-1-infected mice, compared to 39% of the mononuclear cell population for V-2 infection (Fig. 4A). Although frequencies of tetramer+ CD8+ T cells were initially lower in spinal cords of V-2-infected mice, levels approximated those in spinal cords of V-1-infected mice by day 10 p.i. As found for the brain, total CD8+ T-cell percentages dropped significantly by day 33 p.i. Most importantly, by day 70 p.i. CD8+ T cells, including tetramer+ T cells, comprised distinct populations within mononuclear cells from spinal cords of mice persistently infected with V-1 but were virtually absent in mice that had cleared V-2, as demonstrated by the absence of detectable vRNA (Fig. 1C).
FIG. 4.
Presence of virus-specific CD8+ T cells in the spinal cord. Mononuclear cells were isolated from the spinal cords of V-1- and V-2-infected mice (n = 6 to 8) at the indicated time points and pooled prior to ex vivo staining with anti-CD8 MAb and the Ld-pN tetramer reagent (A). Numbers shown in the right quadrants represent percentages of tetramer+ CD8+ T cells (upper number) and tetramer− CD8+ T cells (lower number). The data presented in panel A were used to calculate the total number of infiltrating CD8+ T cells (B) and total number of tetramer+ CD8+ T cells (C) per spinal cord.
Numbers of total CD8+ T cells and tetramer+ CD8+ T cells recovered are presented on a per-cord basis in Fig. 4B and C, respectively. Although V-1 infection recruited over four times as many total CD8+ T cells and tetramer+ CD8+ T cells as V-2 infection on day 7 p.i., populations peaked at similar numbers at day 10 p.i. Consistent with the severely reduced vRNA levels, CD8+ T-cell numbers declined by day 33 p.i. By day 70 p.i., spinal cords of V-1-infected mice contained approximately 11,000 CD8+ T cells per spinal cord, compared to fewer than 200 CD8+ T cells per spinal cord of mice that had cleared V-2 infection. Thus, two lines of evidence indicate that persistent virus is required to locally maintain CD8+ T cells recruited to a primary infection within the CNS: CD8+ T cells disappear from both the brain and spinal cord concomitant with clearance of vRNA from V-2-infected mice; and CD8+ T cells localize preferentially to regions with more abundant vRNA, i.e., within the spinal cord rather than the brain during persistent V-1 infection.
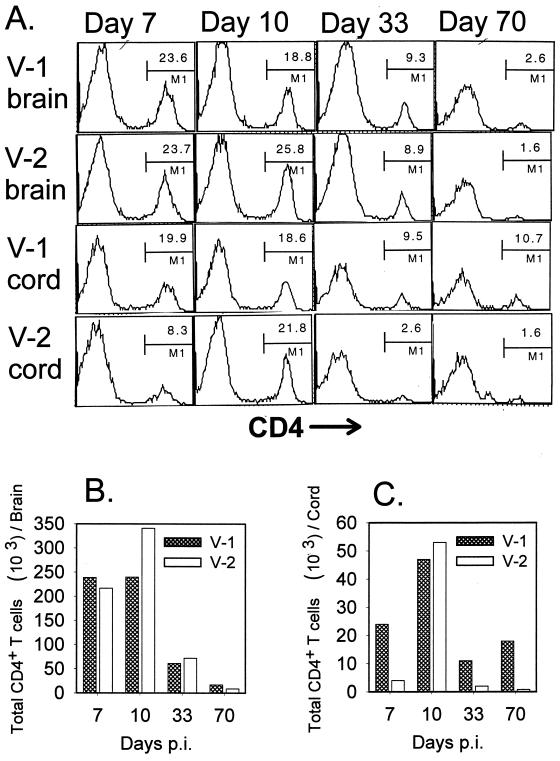
CD4+ T cells follow similar patterns of CNS retention as CD8+ T cells.
As survival and function of CD8+ T cells in the CNS are dependent on the presence of CD4+ T cells (30), mice infected with either V-1 or V-2 were analyzed for the retention of CD4+ T cells within the brain and spinal cord. Analysis of brain-derived mononuclear cells revealed that infiltration by CD4+ T cells was maximal at days 7 and 10 p.i., ranging from 19 to 26% of the total mononuclear cell population (Fig. 5A). As found for CD8+ T cells, these frequencies dropped to half (8 to 9%) by day 33 p.i. and declined to approximately 2 to 3% by day 70 p.i. (Fig. 5A). Furthermore, brain-derived mononuclear cells contained similar total numbers of CD4+ T cells throughout infection with both viruses, with the exception of day 10 p.i., when the brains of V-2-infected mice contained a slightly increased number of CD4+ T cells (Fig. 5B). These data demonstrate similar kinetics of CD4+ T-cell recruitment during both infections, followed by a decline subsequent to virus reduction and leading to ultimate clearance in the absence of detectable vRNA.
FIG. 5.
Presence of CD4+ T cells in the brain and spinal cord. Mononuclear cells were isolated from brains and spinal cords of V-1- and V-2-infected mice at the indicated time points (n = 6 to 8). For each time point, mononuclear cells from brains were pooled as one set and mononuclear cells from spinal cords were pooled as a separate set prior to ex vivo staining with anti-CD4 MAb (A). The data presented in panel A were used to calculate the average number of infiltrating CD4+ T cells on a per-brain basis (B) as well as the average number of CD4+ T cells per spinal cord (C).
In contrast to the brain, the frequency of CD4+ T cells within the spinal cord was two to six times higher in V-1-infected mice than in V-2-infected mice at all time points except day 10 p.i. (Fig. 5A). These differences were more evident when total infiltrating CD4+ T cells were compared on a per-cord basis (Fig. 5C). Overall CD4+ T cells were maintained at a high level within the spinal cord throughout the course of V-1 infection. By contrast, CD4+ T cells infiltrated the spinal cord only transiently during acute V-2 infection and declined rapidly by day 33 p.i. Thus, viral persistence appears to mediate a prolonged presence not only of CD8+ T cells but also of CD4+ T cells within the CNS.
Continued presence of T cells in the CNS does not alter maintenance or function in the periphery.
Retention of virus-specific CD8+ T cells within the CNS during V-1 persistence may be associated with rapid turnover of newly recruited cells, ultimately leading to a decline of responsive memory T cells in peripheral lymphoid tissue. To compare relative frequencies of virus-specific CD8+ T cells in the periphery of V-1- and V-2-infected mice, N-epitope-responsive T cells from the spleen were enumerated by IFN-γ ELISPOT (Table 1), as the frequencies are too low for accurate determination by tetramer staining. In contrast to observations within the CNS, acute V-2 infection was associated with higher frequencies of Ag-specific T cells in the periphery (days 7 and 14 p.i.) compared to V-1 infection. At later time points when T cells were no longer prevalent within the CNS of V-2-infected animals, the frequencies of N-specific splenocytes were comparable between V-1- and V-2-infected animals. Specifically, V-1-infected mice contained 31 Ag-specific T cells/106 splenocytes and V-2-infected mice contained 47 Ag-specific T cells/106 splenocytes at day 63 p.i. Thus, persistent V-1 infection did not appear to result in a significant depletion of memory CD8+ T cells compared to transient V-2 infection.
TABLE 1.
Frequencies of splenocytes secreting IFN-γ in response to pN peptide
| Virus infection | Response at indicateda days p.i.
|
|||
|---|---|---|---|---|
| 7 | 14 | 35 | 63 | |
| V-1 | 231 ± 119 | 333 ± 35 | 54 ± 13 | 31 ± 9 |
| V-2 | 1,635 ± 553 | 1,380 ± 419 | 165 ± 24 | 47 ± 4 |
Number of virus-specific CD8+ T cells per 106 splenocytes responding to pN peptide stimulation as determined by IFN-γ ELISPOT. The average number of nonspecific IFN-γ-secreting cells from control wells which lacked pN peptide was subtracted from the number of cells counted in each well cultured in the presence of pN peptide.
DISCUSSION
Recent studies of T-cell regulation following acute encephalitis induced by either neurotropic influenza virus (16) or JHMV (5, 21) demonstrate that the CNS is unique in harboring significant numbers of Ag-specific CD8+ T cells after clearance of infectious virus. However, the factors that promote their survival are not well understood. Whereas the continued presence of reactivated influenza virus-specific memory CD8+ T cells appears to be independent of virus retention (16), JHMV-specific CD8+ T cells may be maintained by persisting virus (4, 5). This dichotomy was addressed by concomitantly examining the fate of vRNA as a marker of viral persistence and CD8+ T cells in the CNS following primary infections with two closely related neurotropic viruses. RT-PCR analysis to detect vRNA confirmed that injection of the nondemyelinating V-2 variant results in a transient infection of the brain and spinal cord (21), which is ultimately cleared from both tissues by day 63 p.i. By contrast, mice infected with the demyelinating V-1 variant all had detectable levels of vRNA in spinal cords, with variable detection in the brain, confirming predominant persistence within the spinal cord. Enumeration of virus-specific CD8+ T cells in the CNS by flow cytometry using class I tetramers demonstrated that tetramer+ CD8+ T cells constituted prominent populations in both the brains and spinal cords to day 33 p.i. in both infected groups. Even at day 70 p.i., spinal cords from persistently V-1-infected mice still contained a considerable frequency of CD8+ T cells comprised of 30% N-specific cells. In striking contrast, CD8+ T cells had dropped to background levels in spinal cords of mice that had completely cleared V-2. Similarly, CD8+ and CD4+ T cells had disappeared from the brains of mice once vRNA levels dropped to or below RT-PCR detection thresholds. These data indicated that vRNA expression is a key factor in promoting the presence of CD8+ T cells within the CNS following clearance of infectious virus. Whether these cells survive locally or are continually recruited remains to be elucidated.
Ultimate loss of CD8+ T cells from the V-2-infected CNS could not be attributed to overall quantitative or qualitative differences in T-cell infiltrates during acute replication. Infiltration by both tetramer+ and total CD8+ populations was highest between days 7 and 10 p.i. and declined abruptly by day 33 p.i. in both brains and spinal cords, coincident with significantly reduced vRNA levels. Peak T-cell infiltration into the spinal cord was delayed compared to the brain during both infections, reaching remarkably similar maximal levels in both V-1- and V-2-infected mice at day 10 p.i. Furthermore, lower numbers of CD8+ T cells in spinal cords of V-2-infected mice reflected diminished detection of V-2 compared to V-1 RNA in the cord tissue. Despite the divergent clinical symptoms and propensities to persist, the JHMV variants induced CD8+ T-cell responses comparable in not only magnitude but also function (21). An underestimate of Ag-specific cells due to down-regulation of T-cell receptor expression is unlikely, as the percentage of IFN-γ-producing CD8+ T cells determined by intracellular staining never exceeded that of tetramer+ CD8+ T cells throughout the course of infection (data not shown). Thus, clearance of CD8+ T cells cannot be attributed to differential expression of effector function or regulation of CD8+ T cells induced to V-1 versus V-2. This was supported by continued activation of CD8+ T cells throughout the infection, as evidenced by peak expression of the early activation marker CD69 on 90% of CD8+ T cells at day 10 p.i., and a decline only to 80% positive cells by day 33 p.i., despite the drop in vRNA throughout this period (data not shown). CD69 expression is a common hallmark of CD8+ T cells retained in the CNS following viral infection (5, 16, 21).
Two major differences between the influenza virus and the JHMV models may account for disparate roles of persisting Ag in CD8+ T-cell survival within the CNS. Whereas CD8+ T cells found in the CNS of mice infected with JHMV arise from a primary CNS infection, T cells recruited during influenza virus CNS infection are of memory origin (16). Ag-specific memory T cells are present at a higher frequency and are less dependent on costimulation than naive CTL precursor cells; furthermore, memory cells demonstrate an increased ability to infiltrate extravascular tissues (8). Reactivation of influenza virus-specific memory CD8+ T cells may thus result in substantially greater virus-specific CD8+ T-cell infiltration during secondary viral challenge compared to the primary JHMV response (13, 31). In addition, memory T cells infiltrating the CNS may be maintained in a different state of activation or autonomous proliferation compared to CD8+ T cells, which never acquire the classical memory phenotype due to persistent infection. The second difference is the neuronal tropism of the influenza virus versus the predominant glial tropism of the two JHMV variants. Neurons have a highly limited capacity for class I presentation (17, 22). By contrast, glial cells, primarily microglia and to a lesser degree astrocytes, present both class I- and class II-restricted Ag, thus potentially providing a superior activation stimulus (2, 3, 15, 17, 22, 26, 33). Furthermore, expression of costimulatory molecules expressed by activated microglia may contribute to enhanced T-cell receptor activation when MHC presentation is low (2, 3, 26).
Disappearance of T cells from both brains and spinal cords following the decline of vRNA supports the notion that persisting virus provides a signal for maintaining the presence of T cell within the CNS. However, it is unclear whether chronic activation occurs directly via T-cell receptor recognition or indirectly by ongoing pathogenic processes. Plausible mechanisms for continued MHC presentation may reside in low-level viral Ag expression and class I processing, low class I turnover on resident CNS cells, and/or cross-priming of exogenous viral protein products. The inability to detect intact viral Ag by histochemistry after 35 days p.i. (reference 21 and data not shown) suggests that proof of direct class I presentation will be experimentally challenging. Furthermore, the tight link between persisting vRNA and chronic demyelination in V-1-infected mice makes it difficult to assess relative cause and effects of persisting T cells. In contrast to V-2 infection, V-1 induces expansive demyelination by day 14 p.i.; as demyelination is associated with both astrocyte and macrophage/microglia activation, this process may recruit or maintain CD8+ T cells via cytokine or chemokine secretion (2, 18). If demyelination, rather than Ag presentation, were a major factor in maintaining the presence of CD8+ T cells, spinal cords from V-1-infected mice would be expected to contain considerably more CD8+ T cells at day 33 p.i., when RNA levels are already low. However, despite clearance of infectious virus by day 15 p.i. (21), both infections are associated not only with a persisting presence of CD8+ T cells in the brain and spinal cords to day 33 p.i. but also with similar absolute levels. These data do not support the notion that the demyelinating process itself enhances the presence of CD8+ T cells within the CNS. The CNS thus appears to differ from other nonlymphoid tissue in delaying the exit or death of T cells after the vast majority of Ag is cleared.
The specificities of tetramer− CD8+ T cells found in the CNS throughout both infections are still elusive. The ratio of tetramer+ to tetramer− populations remained fairly similar throughout the courses of both infections, suggesting early recruitment of bystander cells or cells responding to some as yet undefined viral epitope(s). Interestingly, after vRNA levels dropped below the level of detection, disappearance of T cells was not limited to the tetramer+ CD8+ T cells but was found for all CD8+ and CD4+ T cells. These data indicate that the tetramer− CD8+ T cells are not specific for a CNS-derived Ag. Although it cannot be excluded that the tetramer− CD8+ T cells during V-1 and V-2 infections comprise distinct populations with heterologous specificities, disappearance of these cells, concomitant with vRNA clearance, supports the suggestion that they are not potential autoimmune cells.
In summary, these data demonstrate that viral persistence, as measured by vRNA, is necessary to provide sufficient stimulus to maintain the presence of both CD8+ and CD4+ T cells in the CNS. The continued presence of T cells within the CNS is independent of the magnitude of initial infiltration or propensity of the infection to induce either acute or chronic demyelination. Thus, unlike Ag-independent turnover of memory T cells in the periphery (24), T cells recruited to the CNS from a naive precursor pool are incapable of autonomous proliferation within the CNS. Furthermore, the absence of CNS pathology following V-2 infection, despite extensive local CD8+ T-cell infiltration and effector function (21), followed by ultimate disappearance of all T cells from the CNS suggests that the initial presence of CD8+ T cells within the CNS is not sufficient to break tolerance to host epitopes. Thus, the similarities between the immune responses, despite vastly differing pathologies, argue against either molecular mimicry or epitope spreading as a means of chronic CD8+ T-cell stimulation and CNS pathology.
ACKNOWLEDGMENTS
This work was supported by NIH grants NS 18146, AI 33314, and NS 07149.
We thank Wenqiang Wei and Margaret Kornacki for exceptional technical assistance.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–59. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi F, Rea F, Adorini L. Regulation of T cell response by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann C C, Dimacali E, Stohl S, Wei W, Lai M M C, Tahara S, Marten N. Variability of persisting MHV RNA sequences comprising immune and replication relevant domains. Virology. 1998;244:563–572. doi: 10.1006/viro.1998.9147. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann C C, Altman J D, Hinton D, Stohlman S A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the CNS. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 6.Combadiere B, Reis e Sousa C, Trageser C, Zheng L X, Kim C R, Lenardo M J. Differential TCR signaling regulates apoptosis and immunopathology during antigen responses in vivo. Immunity. 1998;9:305–313. doi: 10.1016/s1074-7613(00)80613-5. [DOI] [PubMed] [Google Scholar]
- 7.Dhib-Jalbut S S, Xia Q, Drew P D, Swoveland P T. Differential up-regulation of HLA class I molecules on neuronal and glial cell lines by virus infection correlates with differential induction of IFN-beta. J Immunol. 1995;155:2096–2108. [PubMed] [Google Scholar]
- 8.Doherty P C, Hou S, Tripp R A. CD8+ T-cell memory to virus. Curr Opin Immunol. 1994;6:545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 9.Fleming J O, Trousdale M, Zactarim F E, Stohlman S A, Weiner L P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibody. J Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming J O, Trousdale M, Stohlman S A, Weiner L P. Pathogenic characteristics of neutralization-resistant variants of JHM coronavirus (MHV-4) Adv Exp Med Biol. 1987;218:333–342. doi: 10.1007/978-1-4684-1280-2_42. [DOI] [PubMed] [Google Scholar]
- 11.Fleming J O, Trousdale M D, Bradbury J, Stohlman S A, Weiner L P. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb Pathog. 1987;3:9–20. doi: 10.1016/0882-4010(87)90033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming J O, Houtman J J, Alaca H, Hinze H C, McKenzie D, Aiken J, Bleasdale T, Baker S. Persistence of viral RNA in the central nervous system of mice inoculated with MHV-4. In: Laude H, Vautherot J F, editors. Coronaviruses—1994. New York, N.Y: Plenum Press; 1994. pp. 327–332. [DOI] [PubMed] [Google Scholar]
- 13.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 14.Gallichan W S, Rosenthal K L. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic infections. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart M N, Fabry Z. CNS antigen presentation. Trends Neurosci. 1994;18:475–481. doi: 10.1016/0166-2236(95)92767-k. [DOI] [PubMed] [Google Scholar]
- 16.Hawke S, Stevenson P G, Freeman S, Bangham C R M. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz M S, Evans C F, Klier F G, Oldstone M B. Detailed in vivo analysis of interferon-gamma induced major histocompatibility complex expression in the central nervous system: astrocytes fail to express major histocompatibility complex class I and II molecules. Lab Investig. 1999;79:235–242. [PubMed] [Google Scholar]
- 18.Lane T E, Asensio V C, Yu N, Paoletti A D, Campbell I L, Buchmeier M J. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- 19.Li X Y, Matsuzaki G, Yoshikai Y, Muramori K, Nomoto K. T cells expressing both L-selectin and CD44 molecules increase in number in peritoneal exudates cells and in vitro-stimulated spleen cells from mice immunized intraperitoneally with Listeria monocytogenes. Immunology. 1993;78:28–34. [PMC free article] [PubMed] [Google Scholar]
- 20.Marten N W, Stohlman S A, Smith-Begolka W, Miller S D, Dimacali E, Yao Q, Stohl S, Goverman J, Bergmann C C. Selection of CD8+ T cells with a highly focused specificity during viral persistence in the central nervous system. J Immunol. 1999;162:3905–3914. [PubMed] [Google Scholar]
- 21.Marten N W, Stohlman S A, Atkinson R D, Hinton D R, Fleming J O, Bergmann C C. Contributions of CD8+ T cells and viral spread to demyelinating disease. J Immunol. 2000;164:4080–4088. doi: 10.4049/jimmunol.164.8.4080. [DOI] [PubMed] [Google Scholar]
- 22.Massa P T, Ozato K, McFarlin D E. Cell type-specific regulation of major histocompatibility complex (MHC) class I gene expression in astrocytes, oligodendrocytes, and neurons. Glia. 1993;8:201–217. doi: 10.1002/glia.440080307. [DOI] [PubMed] [Google Scholar]
- 23.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J S, Slansky J, Ahmed R. Counting antigen-specific CD8+ T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8+ T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 25.Murphy E, Hieny S, Sher A, O'Garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993;162:211–223. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- 26.Pope J G, Vanderlugt C L, Rahbe S M, Lipton H L, Miller S D. Characterization of and functional antigen presentation by central nervous system mononuclear cells from mice infected with Theiler's murine encephalomyelitis virus. J Virol. 1998;72:7762–7771. doi: 10.1128/jvi.72.10.7762-7771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon M, Pilling D, Borthwick N J, Viner N, Janossy G, Bacon P A, Akbar A N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 28.Stohlman S A, Brayton P R, Fleming J O, Weiner L P, Lai M C. Murine coronaviruses: isolation and characterization of two plaque morphology variants of the JHM neurotropic strain. J Gen Virol. 1982;63:265–275. doi: 10.1099/0022-1317-63-2-265. [DOI] [PubMed] [Google Scholar]
- 29.Stohlman S A, Bergmann C C, van der Veen R, Hinton D. Mouse hepatitis virus-specific cytotoxic T lymphocytes protect from lethal infection without eliminating virus from the central nervous system. J Virol. 1995;69:684–694. doi: 10.1128/jvi.69.2.684-694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 31.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 32.Wang F I, Hinton D R, Gilmore W, Trousdale M D, Fleming J O. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab Investig. 1992;66:744–753. [PubMed] [Google Scholar]
- 33.Xu J, Ling E A. Upregulation and induction of major histocompatibility complex class I and II antigens on microglial cells in early postnatal rat brain following intraperitoneal injections of recombinant interferon-gamma. Neuroscience. 1994;60:959–967. doi: 10.1016/0306-4522(94)90275-5. [DOI] [PubMed] [Google Scholar]