Abstract
Phosphorylation is the most studied post-translational modification, and has multiple biological functions. In this study, we have reanalyzed publicly available mass spectrometry proteomics data sets enriched for phosphopeptides from Asian rice (Oryza sativa). In total we identified 15,565 phosphosites on serine, threonine, and tyrosine residues on rice proteins. We identified sequence motifs for phosphosites, and link motifs to enrichment of different biological processes, indicating different downstream regulation likely caused by different kinase groups. We cross-referenced phosphosites against the rice 3,000 genomes, to identify single amino acid variations (SAAVs) within or proximal to phosphosites that could cause loss of a site in a given rice variety and clustered the data to identify groups of sites with similar patterns across rice family groups. The data has been loaded into UniProt Knowledge-Base—enabling researchers to visualize sites alongside other data on rice proteins, e.g., structural models from AlphaFold2, PeptideAtlas, and the PRIDE database—enabling visualization of source evidence, including scores and supporting mass spectra.
Keywords: phosphoproteomics, Oryza sativa, false localization rate, database searching, software, statistics
Introduction
Rice is one of the most important crops for human nutrition, acting as a staple food for around a third of the global human population.1 Asian domesticated rice, Oryza sativa, has historically been subcategorized into two major varietal groups: Japonica and Indica, although further subdivisions have also been proposed, including the Aus and Admixed families. There is great genetic diversity both within and between varietal groups. Major efforts are underway to understand that diversity through genomic techniques, and to exploit diversity to find alleles conferring desirable traits (such as resistance to biotic and abiotic stresses), which could be bred into high yielding varieties. The genome sequence of a reference Japonica variety, Nipponbare, was sequenced by the International Rice Genome Sequencing Project (IRGSP), with a first release of gene models in 2005.2 A group led from China also sequenced a reference Indica variety (IR-93), and independently annotated gene models.3 Despite Indica rice varieties accounting for around six times the size of international market as Japonica rice varieties,4 the Nipponbare assembly is generally considered the “canonical reference” genome for research and breeding efforts.
There are two current, nonsynchronized annotations of the Oryza sativa Japonica (Nipponbare variety) genome assembly: the Rice Genome Annotation Project at Michigan State University (MSU)5 and the Rice Annotation Project Database (RAP-DB).6 MSU gene models are no longer updated but still used frequently in research projects and cited in publications. RAP-DB is regularly updated, and serves as the source for gene models loaded into other databases such as Ensembl Plants and Gramene7,8 (the two databases being mostly synchronized and using the same underlying technologies), and the source protein sequences for UniProtKB (UniProt Knowledge-base),9 the most popular protein knowledge-base. Over several years UniProtKB has performed some manual curation, where improvements can be identified in protein sequences, meaning that UniProtKB protein sequences are not identical to Ensembl Plants/Gramene. Other key initiatives and data sets include the rice 3,000 genomes project,10 which provides a resource for understanding genetic variants within Oryza sativa. More recently, new rice “platinum standard” genomes are being sequenced,11,12 with new predicted gene models for these varieties now available in Ensembl Plants and Gramene.
Genetic variation data and GWAS analyses can be key for identifying candidate genes or chromosomal regions associated with traits of interest. However, discovery of a SNP (Single Nucleotide Polymorphism) significantly associated with a trait can give only limited information about associated biological function or mechanism. For example, to understand why a given trait confers stress resistance involves understanding the function of proteins, and the pathways and networks they are involved with. A key component relates to understanding cell signaling, such as fast responses to the detection of stress, via reversible post-translational modifications (PTMs) of proteins. The most widely studied reversible modifications include phosphorylation (by far the most studied one, and our primary focus here), acetylation, methylation, and attachment of small proteins, such as ubiquitin and SUMO. There is increasing evidence that sites of PTMs can be important alleles for breeding efforts, examples including the “green revolution” DELLA genes that have an altered response to the gibberellin hormone, via loss of PTM sites13 and root branching toward water controlled by SUMOylation.14
In this work, we aim to provide a high-quality resource providing phosphorylation sites in rice. Phosphosites on proteins are detected and localized on a large scale using tandem mass spectrometry (MS), via “phosphoproteomics” methods. These methods generally involve the proteins extracted from samples being digested by enzymes such as trypsin and phosphorylated peptides being enriched in these samples using reagents such as TiO2, or other metal ions, attached to a column (affinity chromatography), to which phosphate binds preferentially. These bound peptides are then eluted and analyzed using liquid chromatography-mass spectrometry (LC-MS/MS).15 The tandem MS data is then usually queried against a protein sequence database, via a search algorithm. Scores or statistics are calculated for the confidence that the correct peptide sequence has been identified (including the mass of any PTMs), and then for PTM-enriched data, a second step is usually performed to assess the confidence that the site of modification has been correctly identified, if there are multiple alternative potential residues in the peptide. We have recently published an approach to assess the global false localization rate (FLR) of PTMs using searches for PTMs on decoy amino acids, and demonstrated its importance for controlling multiple sources of error in the analytical pipeline.16 We have also extended the model to demonstrate how to combine evidence coming from multiple spectra, and to combine evidence and control FLR across multiple studies.17
Many papers that report phospho-proteomes do not adequately control for the site false discovery rate (FDR), and just use ad hoc score thresholds for peptide identification or site localization scores. For example, in the popular PhosphoSitePlus resource we estimated that around ∼67% of the phosphosites reported in the database are likely to be false positives, and those with only an observation from one single study are very unlikely to be true.18 To provide FDR-controlled data on phosphosites to the research community requires reprocessing MS data, using well controlled statistical procedures, and applying post hoc approaches to control the FDR when aggregating data across multiple studies.
There are several online databases for gaining information about PTMs in plants, sourced from published studies. The Plant PTM Viewer19 aggregates results from published studies that used PTM enrichment and MS, and has good coverage of studies for a range of PTM types, including phosphorylation, acetylation, ubiquitination, and several others. Over 13,000 rice proteins are reported to be modified, and >27,000 Arabidopsis thaliana proteins, mapped to ∼15,000 genes (translated from 54,000 transcripts of the 27,600 coding genes). For detailed study of the A. thaliana phosphoproteome, there also exists the PhosPhAT database,20 which similarly loads phosphoproteomics data from published studies on Arabidopsis, containing evidence for 55,000 phosphorylation sites on ∼9,000 A. thaliana proteins. Plant PTM Viewer and PhosPhAT are useful for community resources, although by loading data from published studies (rather than reprocessing data), are likely to contain variable data quality and cannot control for FLR across multiple data sets.
UniProtKB is a leading cross-species resource for studying protein function, including extensive expert manual curation. For PTM-related data, UniProtKB mostly loads data by curating individual studies, and has not previously loaded large-scale MS data reporting on plant PTMs. PTMs are reported on just 320 rice proteins in the UniProtKB and on 2,763 Arabidopsis proteins (October 2023, Release 2023_04).
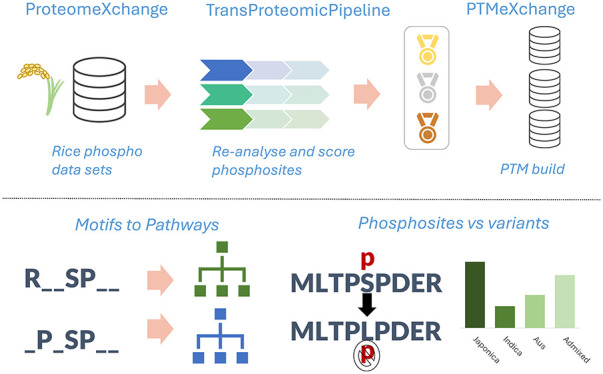
The PRIDE database at the European Bioinformatics Institute (EMBL-EBI) is the largest MS-based proteomics data repository.21 PRIDE is leading the ProteomeXchange (PX) consortium of proteomics resources, whose mission is to standardize open data practices in proteomics worldwide.22 PeptideAtlas is also a PX member, focused on the consistent reanalysis of data sets23 for a variety of species, including recent builds for Arabidopsis.24 Widespread public deposition of proteomics data in PX resource now enables meta-analysis studies to be performed, by reanalyzing groups of related data sets. As part of the “PTMeXchange” project (www.proteomexchange.org/ptmexchange), our consortium aims to complete a large-scale reanalysis of public PTM enriched data sets, using robust analysis pipelines incorporating strict FDR control, and correction for FDR inflation in meta-analyses.
In this work, we have reanalyzed phospho-enriched rice data sets and integrated results into PeptideAtlas, PRIDE, and UniProtKB, for visualization of the confident phosphosites alongside other data on rice proteins. Downstream analysis is also performed on the confident sites to identify PTMs that may be of biological interest. These analyses include investigations on common motifs seen around the phosphosites, pathway enrichment analysis for these motifs and analysis of single amino acid variations (SAAVs) identified close by to the confident phosphosites. From these analyses, we aim to identify rice phosphoproteins that may be of biological interest.
Methods
Phosphosite Identification
The ProteomeXchange Consortium25 was used to identify suitable rice phosphoproteomics data sets, via the PRIDE repository.26 From this, 111 proteomics data sets were identified for the Oryza sativa species. Of these, 13 were identified as being enriched in phosphopeptides and then potentially suitable for reanalysis: PXD000923,27 PXD001168,28 PXD000857,28 PXD002222,29 PXD001774,30 PXD004939,31 PXD005241,32 PXD004705,33 PXD002756,34 PXD012764,35 PXD007979,36 PXD010565,37 and PXD01929138 (Supplementary Table 1). These 13 data sets were investigated further to evaluate their quality for use within the phosphoproteomics reanalysis. It was found that PXD001168, PXD001774, and PXD010565 contained very few phosphopeptides for the size of the data set, these data sets were therefore excluded from the analysis. PXD007979 was identified to be a meta-analysis of the PXD002222 and PXD000923 data sets and was therefore also excluded. Finally, PXD000857 was excluded as this is a relatively old data set containing only one raw file. This resulted in 8 high quality data sets being carried forward for the phosphopeptide reanalysis. Sample and experimental metadata were manually curated and adhering to the Sample-Data Relationship Format (SDRF)-Proteomics file format.39
The search database was created consisting of protein sequences derived from the MSU Rice Genome Annotation Project, the Rice Annotation Project Database (RAP-DB), including both translated CDS and predicted sequences, and UniProtKB, including both reviewed and unreviewed sequences. A fasta file was generated from the combination of these databases. If a protein sequence occurs in more than one resource, RAP-DB was used as the primary identifier; this was then followed by any other IDs for that protein. cRAP contaminant sequences were also added to the database (https://www.thegpm.org/crap/, accessed April 2022), and decoys across all protein and contaminant sequences were generated for each entry using the de Brujin method (with k = 2).40 The database was deposited in PRIDE along with the reprocessed data files (PRIDE ID: PXD046188).
The analysis was conducted using the pipeline as previously described.16 Using the Trans-Proteomic Pipeline (TPP),41,42 the data set files were first searched using Comet.43 The resulting files were then combined and processed using PeptideProphet,44 iProphet,45 and PTMProphet,46 for each data set. The files were searched with the variable modifications: Oxidation (MW), N-terminal acetylation, ammonia loss (QC), pyro-glu (E), deamination (NQ), and phosphorylation (STYA). Phosphorylation on alanine was included as a decoy to estimate the false localization rate (FLR), using the count of pAla identified, following the methods previously described by our group.16 Carbamidomethylation (C) was used as a fixed modification and the iTRAX8plex label was included for the search on the PXD012764 data set. Maximum missed cleavage used was 2, with a maximum number of modifications per peptide of 5. Table 1 outlines the data sets used and the tolerance parameters used for each data set.
Table 1. Tolerance Parameters Used for Comet Search for Each Data Set.
| Original data set identifier | Tissue | Instrument | Count of. RAW files | Count MS2 spectra | Peptide mass tolerance (ppm) | Fragment tolerance (Da) | Count of observed sites reported | Statistical control method used (Peptides) | Statistical control method used (Sites) |
|---|---|---|---|---|---|---|---|---|---|
| PXD00092327 | Flower | TripleTOF 5600 | 10 | 97388 | 20 | 0.02 | 2347 | ion score >34 (p < 0.05) | Ascore ≥ 19, p ≤ 0.01 |
| PXD00222229 | Leaf | Q Exactive | 6 | 121117 | 20 | 0.02 | 2367 | filtered for peptide rank 1 and high identification confidence, corresponding to 1% FDR | PhosphoRS probability >90%, in at least 2 of 3 biological replicates |
| Mascot score >20 | |||||||||
| PXD00275634 | Anther | LTQ Orbitrap Elite | 5 | 225674 | 20 | 1.0005 | 8973 | Expectation value p < 0.05 | phosphoRS (no threshold given) |
| PXD00470533 | Leaf | Q Exactive | 9 | 208050 | 20 | 0.02 | 3412 | Protein Discoverer <1% FDR | PhosphoRS probability >90% in at least 2 of 3 biological replicates |
| PXD00493931 | Leaf | Q Exactive | 9 | 212875 | 20 | 0.02 | 2271 | Protein Discoverer <1% FDR | PhosphoRS probability >90% |
| Mascot score >20 | |||||||||
| PXD00524132 | Shoot, Leaf, Panicles (Young and Mature) | Q Exactive | 18 | 1040757 | 20 | 1.0005 | 5523 | Protein Discoverer <1% FDR | PhosphoRS probability >90% in at least 2 of 3 biological replicates |
| PXD01276435 | Root | Q Exactive | 6 | 260560 | 10 | 0.02 | 2674 | Protein Discoverer <1% FDR | PhosphoRS score ≥50; PhosphoRS site probability ≥75% |
| PXD01929138 | Pollen | Q Exactive | 4 | 110117 | 10 | 0.02 | 2246 | Not reported | Not reported |
The data files obtained from searching with TPP were processed by custom Python scripts (https://github.com/PGB-LIV/mzidFLR). The data was analyzed in the same way as in a previous study.16,17 The global FDR was calculated from the decoy counts and the peptide-spectrum matches (PSMs) were filtered for 1% PSM FDR. From these filtered PSMs, a site-based file was generated giving individual localization scores for each phosphosite found on each PSM, removing PSMs not containing a phosphate, decoy PSMs, and contaminant hits. These site-based PSMs were ordered by a combined probability, calculated by multiplying the PSM probability by the localization probability.
It is common to observe many PSMs giving evidence for sites on the same peptidoform, where a peptidoform is a peptide sequence with a specific set of modified residues. In previous work,17 we have shown that collapsing results to the peptidoform-site level simply by taking the maximum final probability was suboptimal as many of the high scoring decoy (and thus false) hits are supported by only a single PSM. We therefore applied a statistical model for multiple observations of a PTM site, using a binomial adjustment of the PTM probabilities to collapse these results by protein position.17 This adjustment considered the number of times a specific site has been seen and the number of times this same site has been seen as a phosphosite, allowing us to give weight to those sites that are supported by multiple PSMs.
The global FLRs for all the data sets were estimated using the identification of phosphorylated Alanine (pAla) as a decoy. These are known to be false localizations and can therefore be used to estimate the FLR, following the method previously established,16 alongside the binomial adjustment. The global FLR was estimated for every ranked site, across all PSMs and in the collapsed protein position site-based format, from which we can later apply a threshold at the lowest scoring site that delivers a desired global FLR (e.g., 1%, 5%, or 10%), similar to the q-value approach for standard proteomic database searching.
When aggregating data across multiple studies, we must control the inflation of the FLR. FLR inflation is observed when the same correct sites are seen identified across multiple studies and tend to accumulate slowly, whereas each study reports different and random false positives that then accumulate rapidly as more data sets are added. PTM localization has been shown to be incomparable between independent studies.17 As a result, we developed an empirical approach to categorize sites based on the observations of sites across data sets at different thresholds; Gold, Silver, and Bronze. Gold represents sites seen in n data set with <1% FLR, Silver represents sites seen in m data sets with <1% FLR, and Bronze represents any other sites passing <5% FLR. The values for n and m can be set empirically in a PTM “build” based on the number of data sets and the counts of decoys following the aggregation of multiple data sets, and application of possible values of n and m. As our rice build contains eight data sets, we categorized Gold sites as seen in more than one data set with <1% FLR and Silver as only one data set with <1% FLR. We then calculated the counts of pAla sites within these sets, allowing us to estimate the resulting FLR following data set merging in the different categories.
Data Set Deposition and Visualization
The reprocessed data has been deposited in PRIDE in mzIdentML format,47 as well as SDRF-Proteomics files, tab-separated text formatted files (one per data set) containing sites detected per PSM, and sites detected for each peptidoform, following the collapse processed described above (PRIDE ID: PXD046188). To load all phosphosites into UniProtKB, the identified peptides were mapped to the canonical protein sequences within the proteome (UP000059680) using an exact peptide sequence match, following theoretical tryptic digest. The phophosites can be viewed in the Protein APIs, in the Feature Viewer under Proteomics track and in the entry page. Decoy sites were not loaded to avoid misinterpretation.
All results have also been loaded as a PeptideAtlas build, available at https://peptideatlas.org/builds/rice/phospho/. The PeptideAtlas interface allows browsing of all modified peptides from these data sets, including those passing and not passing the above thresholds. All localization probabilities for all PSMs are displayed, along with links to the original spectra that may be visualized in the PeptideAtlas interface. The corresponding mass spectra in PeptideAtlas and PRIDE (https://www.ebi.ac.uk/pride/archive/usi) can be referenced and accessed via their Universal Spectrum Identifiers.48
Downstream Analysis
Motif and Pathway Enrichment Analysis
All eight data sets were further investigated using motif and pathway enrichment analysis. Once the confident phosphosites (5% pAla FLR) from each of the data sets had been combined and given an FLR ranking (Gold, Silver, Bronze), the enriched motifs surrounding phosphosites were identified using the R package rmotifx.49 15mer peptides were generated surrounding each of the identified phosphosites. These phosphopeptides were compared against a background of 15mer peptides with STY at the central position of the 15mer, and matched to the central residue of the phosphosite motif, to identify the enriched motifs seen around the confident phosphosites.
The proteins containing these enriched motifs were then carried forward for pathway enrichment analysis using ClusterProfiler.50 The proteins containing each enriched motif was compared against all phosphoproteins in the search database. Similarly, a comparison was made between all phosphoproteins containing any enriched motif, for each of the FLR ranking categories, against the background of all phosphorylated proteins in the search database.
SAAV Analysis
We also explored the phosphosites across all data sets we have reanalyzed and how SAAVs may potentially affect these sites. We compiled a list of unique phosphosites (by sites on unique peptides) from the confident phosphosites (5% pAla FLR) across all eight searches and created a matrix showing which sites were seen in each data set. These were then also mapped to the relevant protein sites in the three search databases: MSU, RAP-DB, and UniProtKB. We mapped this list of unique phosphosites to known SAAV positions for the 3,000 rice varieties using the Rice SNP-Seek Database API (Application Programming Interface), for those sites that mapped to the MSU database. We categorized the phosphosites with relation to the SAAV sites; where “Category 1” = SAAV at the same position as a phosphosite, “Category 2” = SAAV at the +1 position to a phosphosite, “Category 3” = SAAV at the −1 position to a phosphosite and “Category 4” = SAAV at ±5 amino acids from a phosphosite (and not in Category 1, 2, or 3). All other sites were assigned “Category 0”. For each phosphosite in the unique list across all data sets, the nearest SAAV to each phosphosite was identified and categorized. For those protein phosphosites with SAAV data available, we then investigated which alleles carried the SAAV and the minor allele frequencies for each site. We also added in annotation to show the genes involved, obtained using the Oryzabase database.51 From this analysis, we could identify candidate sites of potential biological importance, which may be disrupted due to SAAVs.
A protein multiple sequence alignment was created using Clustalx 2.1, for the example protein Os09g0135400, versus the same locus in 15 other Oryza sativa genomes, which have been annotated and deposited in Ensembl Plants and Gramene.52,53 The association of gene models from different cultivars to be the same locus (a “pan gene cluster”) was created using the GET_PANGENES pipeline.54
Results
Phosphosite Identification
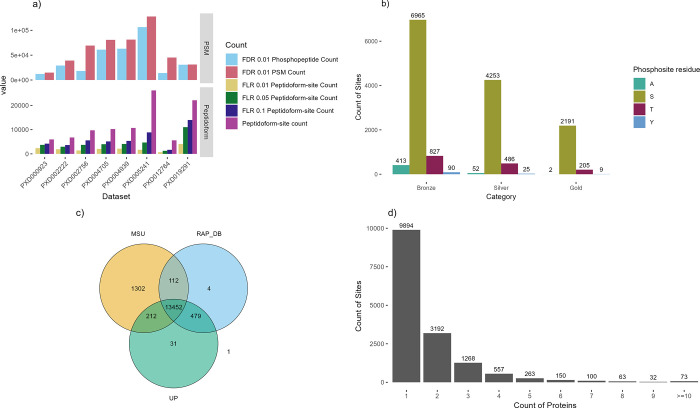
First, we reanalyzed each of the rice data sets identified as suitable for phosphosite identification using TPP as explained in Methods (Figure 1). Our scripts were used to calculate the FLR across confident PSMs identified (filtered for 1% FDR). We next collapsed the sites by protein position and remove duplicated hits. The FLR estimation was then recalculated on these collapsed sites, ordering by the calculated probability that a site had been observed, considering evidence across multiple PSMs (see Methods). The counts of phosphosites passing at different FLR thresholds are shown in Table 2 and Figure 1a (counts are derived from the unique combinations of a peptidoform and phosphosites within those peptidoforms, i.e. not accounting for some peptidoforms mapping to more than one genomic locus (gene)).
Figure 1.
Overview of phosphosite counts. a) Counts of phosphosites before and after (peptidoform) collapse for removing redundancy at three FLR levels per data set. b) The count of phosphosites in each of the three categories (Gold–Silver–Bronze) per residue where A is the decoy Alanine. c) The overlap of phosphosites reported per protein database: MSU, RAP-DB, or UniProtKB (UP). d) Counts of phosphosites observed across different protein counts.
Table 2. PSM Counts for Each Data Set at 1% FDR, Phosphopeptide PSM Counts at 1% FDR, Site Counts (Excluding pA Decoy Sites) for all PSMs Collapsed by Peptidoform Position and at Each of the FLR Thresholds; 1%, 5%, and 10%.
| Data set | 1% FDR PSM count | 1% FDR Phospho-peptide count | Peptidoform-site count | 1% FLR Peptidoform site count | 5% FLR Peptidoform site count | 10% FLR Peptidoform site count |
|---|---|---|---|---|---|---|
| PXD002222 | 39092 | 29086 | 6762 | 1935 | 2951 | 3649 |
| PXD004939 | 81254 | 62935 | 10701 | 2156 | 4094 | 5348 |
| PXD005241 | 127696 | 106459 | 26190 | 1701 | 4687 | 8872 |
| PXD004705 | 80741 | 61029 | 10258 | 2050 | 3989 | 5133 |
| PXD002756 | 69232 | 18203 | 9734 | 1421 | 3708 | 5581 |
| PXD012764 | 45184 | 14020 | 5600 | 695 | 1269 | 1677 |
| PXD000923 | 14852 | 12167 | 5965 | 2366 | 3708 | 4221 |
| PXD019291 | 31094 | 30764 | 22128 | 4072 | 11034 | 13984 |
Different data sets contributed between ∼700 and ∼4,000 sites at the strictest FLR 1% threshold, and between 1,700 and ∼14,000 sites at 10% FLR. When performing PTM site localization, there is a steep drop off in sensitivity when applying strict FLR thresholding (at say 1%), compared to weak thresholding (10% FLR) or say performing no explicit FLR thresholding–indicative of the challenge of confident site localization. Table 1 displays the counts of sites observed in the original studies and the statistical controls performed. Site counts ranged from ∼2,000 up to ∼9,000. In no original analysis (as published originally) was global FLR estimated, although this is not surprising since methods for accurate FLR estimation have not been well described until recently. While some ad hoc score thresholds were applied for local (i.e., per PSM) site scoring, e.g., PhosphoRS > 0.9, this does not easily translate to a global (i.e., across the entire data set) FLR, and thus it is reasonable to assume that there were variable (sometimes high) rates of FLR in different studies.
The tissues used for each data set are shown in Table 1. These include flower, leaf, anther, shoot, panicles (young and mature), root and pollen samples. Although we can make no quantitative claims about site occupancy in a given tissue, by showing the tissues present in each data set, a reader can infer if a given site has been seen in specific tissues.
We next performed a simple meta-analysis by combining all data sets, and assigned sites labels based on their scores and occurrences in data sets: Gold–Silver–Bronze (Table 3, Figure 1b). Decoy identifications of pAla were carried forward, enabling validation of the false reporting within these subsets. There are only two pAla hits within the Gold set, indicating that the overall FLR is very low in this subset. The meta-analysis also demonstrates that within each set, the reported counts for pTyr are relatively similar to pAla (taking into account that Ala is more abundant than Tyr in the proteome), indicating that pTyr hits reported for these data sets are likely to be mostly/entirely false positives, and should be treated with caution when interpreting any reported observations of pTyr from these data sets in rice. We recorded five unique Gold category pTyr sites. When looking at the scores of the spectra supporting these sites (Supplementary Figure 1), it was seen that most of these had only weak evidence supporting them and may be false positives.
Table 3. Count of Sites (Uniquely Mapped to Genomic Loci), Per Category for Gold, Silver, and Bronzea.
| Gold | Silver | Bronze | Total | |
|---|---|---|---|---|
| A | 2 | 53 | 420 | 475 |
| S | 2248 | 4397 | 7233 | 13878 |
| T | 212 | 499 | 850 | 1561 |
| Y | 9 | 25 | 92 | 126 |
| Total target sites | 2469 | 4921 | 8175 | 15565 |
Totals target sites for categories are excluding alanine sites.
In Figure 1c, we display the counts of sites depending in the source database—13,452 sites were identified on peptides within proteins from all three databases (MSU, RAP-DB, and UniProtKB). The original source of UniProtKB proteins is RAP-DB (with some later manual curation)—and we can observe 479 sites observed in RAP-DB and UniProtKB, but not in MSU, giving indications of peptides where the source RAP-DB gene model is likely superior to the MSU alternative. For the 212 sites that are common to MSU and UniProtKB, but not present in RAP-DB, would indicate that UniProtKB curators have altered gene models, such that they contain peptides identical to MSU. For 112 sites identified in MSU and RAP-DB, but not in UniProtKB, it is possible that UniProtKB curation has removed correct sections of gene models, or these sequences are entirely absent from UniProtKB. There are few phosphosites unique to RAP-DB or to UniProtKB, but 1,302 unique to MSU-derived protein sequences. The MSU annotation contains a larger count of protein sequences (48,237) than RAP-DB (46,665), and many gene models different to the RAP-DB annotation. The identification of many phosphosites unique to MSU sequences gives evidence for gene models that should be added or updated in the RAP-DB source.
In our mapping process from peptidoforms to proteins, we take the approach that if a peptide can be matched to proteins from multiple different locus, then all mappings should be accepted (unlike traditional proteomics approaches where parsimony in reporting protein identifications is preferred). The rationale is that the evidence presented is that a given peptidoform has been observed with a phosphosite, although due to the nature of tandem MS/MS, it is not possible to say definitely which protein was actually observed (when the peptidoforms matches multiple). If two proteins with highly similar sequences overall (and in this case an identical peptide sequence that has been identified), it seems probable that both can be phosphorylated on the identified position. The counts of phosphosites mapped onto one or more proteins is displayed in Figure 1d—the vast majority of sites are mapped to one or two proteins only, with a small count of sites mapped to multiple proteins, including 73 sites mapped to ≥10 proteins. This happens in cases of very expanded gene families in rice, with paralogues of near identical sequence—it is not possible to determine which protein was actually observed in the experiment.
Data Visualization
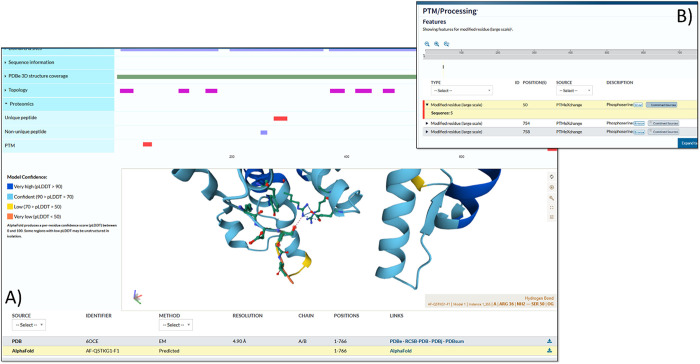
The Gold–Silver–Bronze classified data has been loaded into UniProtKB for visualization alongside other data sets and information available for rice. As one example, the phosphopeptides can be viewed in the context of AlphaFold2 (AF2)55 predicted protein structures (Figure 2). The protein visualized in this case is OSCA1.2 (hyperosmolality-gated calcium-permeable channel 1.2, UniProtKB: Q5TKG1, MSU: LOC_Os05g51630, RAP-DB: Os05t0594700) and has a pSer at position 50. The AlphaFold prediction suggests that the serine forms a hydrogen bond with Arg36. It has been shown that phosphorylation can strengthen hydrogen bonds with Arg residues,56 and thus the pSer may have a structural role. With the widespread availability (now) of both phosphosite and structural data from AF2 models in UniProtKB, this provides a significant resource for rice cell signaling research.
Figure 2.
UniProtKB visualization. A display in UniProtKB of protein Q5TKG1 (RAP-DB: Os05t0594700-01; LOC_Os05g51630.1) showing A) three identified phosphosites in the tabular view and the B) structural context of the site on an AlphaFold2 prediction.
The build has also been loaded into PeptideAtlas, enabling browsing or searching the evidence for individual sites, peptides and proteins. An example of evidence supporting a phosphosite identified on a peptide is shown in Supplementary Figure 2. We also demonstrate how Universal Spectrum Identifiers (USIs) can be used to visualize spectra supporting modification positions and can be a valuable tool to investigate the evidence supporting identified modifications (Supplementary Figure 3). The USI with the highest site probability for identified sites can be located in Supp Data File 3. Loading one of these USIs via https://proteomecentral.proteomexchange.org/usi/ imports the spectra and the claimed identification. By altering the position of the modification, it is possible to test which ions support an alternative hypothesis (site position in the peptide).
Motif and Pathway Enrichment Analysis
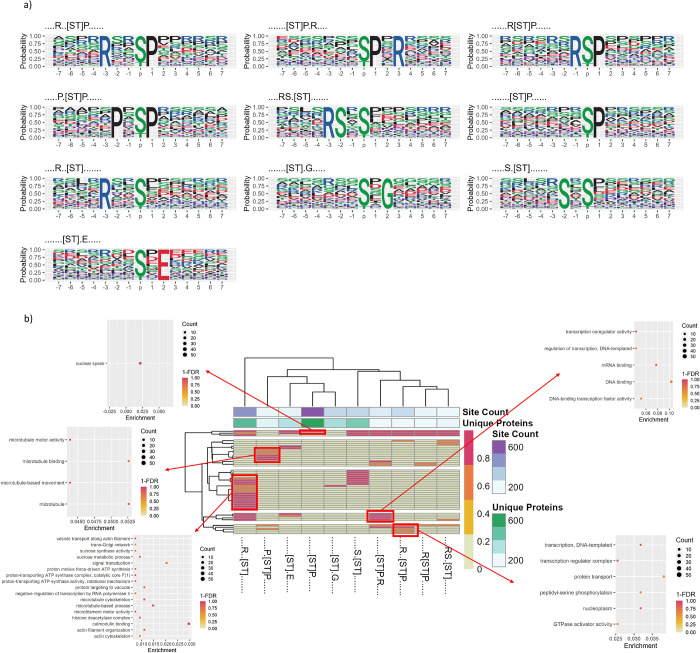
We ran motif analysis on the full set of identified pSer or pThr phosphosites (Gold+Silver+Bronze) using rmotifx (Figure 3), to identify enrichment of amino acids proximal to phosphosites potentially indicative of kinase families responsible for those sites (Supplementary Data File 1). Supplementary Figure 4 displays plots of the most enriched amino acids at each position, relative to the phosphosite for significant motifs. Numerous motifs are identified, with commonly enriched amino acids being P at +1 (relative to the target site), D/E at −1, +1, +2, and several others.
Figure 3.
Motif analysis. a) Motif logos showing the probability of particular amino acids to be present, surrounding the S/T phosphosites within Gold, Silver, and Bronze data sets (motifs were filtered to be seen in at least 100 unique proteins). b) Heat map displaying significant motifs versus GO term clusters from Cluster Profiler (y-axis), displaying 1-FDR color scale for pathway enrichment from proteins containing that phosphorylation, annotated with the count of unique proteins containing each motif and the count of phosphorylation sites supporting each motif.
There is a trade-off where having a higher overall count of true sites is likely to improve discovery of significant motifs, but too many false positive sites will weaken statistical power. As such, we also ran rmotifx analysis on “Gold+Silver” and “Gold only” sets (Supplementary Figures 4 and 5). Supplementary Table 2 displays a comparison of motifs discovered on different subsets of data (“Gold+Silver+Bronze” Gold+Silver” vs “Gold only”). The largest number of significant phosphorylation motifs was found for “Gold+Silver+Bronze”, which were thus used for the main analysis. We filtered those motifs to those found in at least 100 proteins, as shown in Figure 3a—indicating five motifs with proline (P) at the +1 position, four motifs with arginine (R) in a minus position (−1 or −3), and several others.
We next wished to explore whether such signatures related to differences in the pathways in which phosphorylated proteins act. We performed enrichment analysis to identify the pathways in which proteins containing significant motifs were acting (against a background of all rice phosphoproteins), using clusterProfiler (summarized in Figure 3b for motifs found in at least 100 proteins, results for all motifs shown in Supplementary Figure 6 and Supplementary Data File 2). Distinct enrichment of significant terms was obtained for different motifs. As examples, [ST]P.R motif-containing proteins were enriched for GO terms related to microtubules (“microtubule motor activity” (GO:0003777) and “microtubule-based movement” (GO:0007018)), compared to similar motif R..[ST]P was enriched for GO terms related to regulation of transcription (“transcription coregulator activity” (GO:0003712)), DNA and mRNA binding (“DNA polymerase III complex” (GO:0009360) and “mRNA splicing, via spliceosome” (GO:0000398)). P.[ST]P motif-containing proteins were enriched for “transcription regulator complex” (GO:0005667). R..[ST] motif-containing proteins were enriched in many GO terms, including “calmodulin binding” (GO:0005516), microtubule related terms, including “microtubule binding” (GO:0008017), “microtubule motor activity” (GO:0003777) and “microtubule cytoskeleton” (GO:0015630), among others), proton transport (“proton-transporting ATP synthase complex” (GO:0045261)), and “histone deacetylase complex” (GO:0000118). The rice kinome contains ∼1,500 kinases57 much larger than the kinome in mammalian systems (humans have around 600 kinases for example). Even in humans, accurate assignment of kinase-substrate relationships is especially challenging, and for rice, given the sparsity of experimental data on kinase-substrate relationships, it is not possible to make accurate predictions about the kinases responsible for individual sites. However, the motif groups and downstream pathways identified here provided a starting point for interpreting the high-level different signaling pathways, which presumably correspond to different families of kinases.
Single Amino Acid Variation Analysis
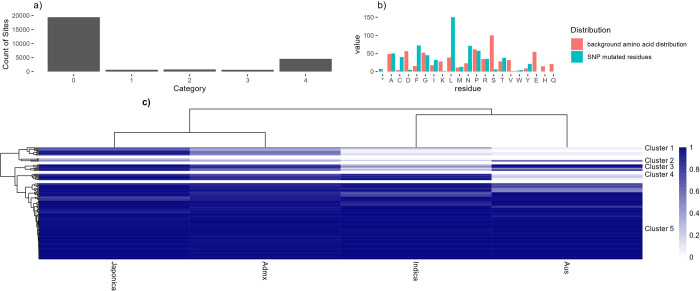
We next assigned all phosphosites into five categories, determined in relationship to known nonsynonymous SNPs (i.e., single amino acid variants—SAAVs) from the rice 3,000 genome set,58 whereby a category 1 site has an amino acid polymorphism in the reference genome (Nipponbare), causing a loss of this phosphosite in some other varieties (Figure 4). In the whole data set, excluding pA decoy sites, there are 388 category 1 sites (Figure 4a), which are further explored in Figure 4b showing the most commonly substituted amino acid. Over-represented substitutions included S → L. Under-represented substitutions were D/E/H/K/Q/V. S/T → D mutations are potentially of great interest as Asp can mimic pSer/pThr as a constitutively active phosphorylation site, which could be a dominant allele for breeding. However, in our data, we saw only a single phosphosite (Bronze FLR category) with a S → D mutation (in LOC_Os10g32980.1/“Cellulose synthase A7”). The implied amino acid substitution only observed at very low minor allele frequency (0.00033, i.e., one single cultivar in the 3,000 set), which could also be a sequencing error. We thus conclude that pSer → Asp phospho-mimetic substitutions are exceedingly rare in the rice proteome. Within the data, there are 25 cases of S → T and 4 T → S phosphosite SAAVs (which would likely not disrupt phosphorylation).
Figure 4.
SAAV analysis. A) Bar chart showing counts of phosphosite by SAAV category. B) Counts of substituted amino acid in category 1 phosphosites, including the normalized background distribution of that amino acid (* = stop codon). C) A heat map to show the assumed major allele frequency (i.e., frequency of Ser/Thr within the 3K “pseudo-proteins” from the rice 3K SNP set) of the phosphosite in four rice family groups: Japonica, Admixed, Indica, and Aus. Allele frequencies are filtered for total difference between families >0.05 to remove genes showing the same frequency across all families.
We also note five observations where the pSer has apparently been substituted with a stop codon (*) in proteins: LOC_Os03g17084.1 (Gold site on position 21, RAP-DB ID = Os03t0279000, annotated as “Similar to Histone H2B.1”; LOC_Os02g52780.1 (Gold site on position 98, RAP-DB ID = Os02t0766700-01, annotated as “BZIP transcription factor”), Bronze sites are seen on proteins LOC_Os02g07420.1, LOC_Os05g39730.1 and LOC_Os01g31010.1. However, in all cases, SNPs were at very low minor allele frequencies (1–3 cultivars only out of the entire 3,000 set), indicating that phosphosite mutation to a stop codon is an exceptionally rare event in the rice pan genome. Supplementary Data File 3 shows the genomic location of all identified phosphorylation sites along with the SAAV positions and gene annotations.
We converted category 1 SAAV data into a heat map (Figure 4C), with clustering of sites by major allele frequency in four rice families: Japonica, Indica, Admixed and Aus, with a tree cut method to split the dendrogram into subgroups (Supplementary Data File 4). Five distinct clusters can be observed (as labeled): 1) high in Japonica and Admixed, low in Indica and Aus families; 2) variable pattern cluster, mostly medium to low conservation in all families; 3) high in three families, lower in Indica ; 4) high in three families, low in Aus; and 5) high in all families. Supplementary Data File 4 contains the source data, enabling the data to be filtered to find alleles of interest, where there are likely significant differences between major varietal groups. Cluster 4 (Table 4) contains phosphosites that are mostly conserved in Japonica, Indica, and Admixed varieties, but lowly conserved in Aus. Aus variety rice cultivars are generally considered to be resistant to biotic stress, like drought. Source genes mostly have limited annotation, although genes with annotations include part of the Tho complex (involved with mRNA transcription, processing, and nuclear export) and a Zinc finger protein (members within this large family of proteins have been implicated in transcriptional regulation and responses to stress). Phosphosites in proteins lacking annotation may be good candidates for further study, for potential roles in Aus-specific phenotypic responses.
Table 4. Phosphosites Identified in Cluster 4 on Figure 4, Defined by the Pattern of Major Allele Frequencies (AFs) across Four Varietal Groups: Japonica (jap), Indica (ind), Admixed (admx), and Ausa.
| Protein accession | FLR cat | PTM pos | PTM res | SAAV | jap major AF | ind major AF | admx major AF | aus major AF | Annotation(s) |
|---|---|---|---|---|---|---|---|---|---|
| LOC_Os01g42010.1 | Bronze | 7 | S | S→N | 0.732 | 0.925 | 0.728 | 0.184 | Similar to cDNA clone:001–043-A08, full insert sequence.:PF03661.6;UPF0121;Family_7 |
| LOC_Os06g36940.1 | Gold | 238 | S | S→Y | 0.835 | 0.820 | 0.689 | 0.035 | Conserved hypothetical protein.:_238 |
| LOC_Os05g22920.1 | Gold | 53 | S | S→A | 0.993 | 0.829 | 0.854 | 0.234 | Hypothetical conserved gene.:PF06862.5;DUF1253;Family_53 |
| LOC_Os01g12530.1 | Silver | 581 | S | S→G | 0.857 | 0.925 | 0.806 | 0.055 | PF08590.3;DUF1771;Domain_581 |
| LOC_Os03g15940.1 | Bronze | 196 | S | S→F | 0.961 | 0.978 | 0.854 | 0.224 | Zinc finger, LIM-type domain containing protein.:PF00412.15;LIM;Domain_196 |
| LOC_Os04g51120.1 | Silver | 220 | S | S→G | 0.975 | 0.736 | 0.738 | 0.179 | Similar to ENTH1 protein (Fragment).:PF01417.13;ENTH;Domain_220 |
| LOC_Os08g06360.1 | Bronze | 69 | S | S→G | 0.905 | 0.941 | 0.816 | 0.055 | Tho complex subunit 7 domain containing protein.:PF05615.6;THOC7;Family_69 |
| LOC_Os05g43670.1 | Bronze | 365 | S | S→G | 0.997 | 0.959 | 0.874 | 0.060 | IQ motif, EF-hand binding site domain containing protein.:PF02179.9;BAG;Family_365 |
Annotations are sourced by merging any data held in MSU or RAP-DB databases for the corresponding gene. Cluster 4 is mostly characterized by high AF in Japonica, Indica, Admixed, but low in Aus.
Cluster 1 phosphosites are those present at high allele frequencies in Japonica and Admixed but lower in Indica and Aus type rices—indicating potential cell signaling differences across the two major branches of Oryza sativa (summarized in Table 5). Proteins of potential interest for further study include LGD1 (“Lagging Growth and Development”), “Cullin 1” (LOC_Os03g44900.1), and HSP40. LGD1 has been implicated in regulation of plant growth and yield.59
Table 5. Phosphosites Identified in Cluster 1 in Figure 4, Defined by the Pattern of Major Allele Frequencies (AFs) across Four Varietal Groups: Japonica (jap), Indica (ind), Admixed (admx), and Ausa.
| Protein accession | FLR cat | PTM pos | PTM res | SAAV | jap major AF | ind major AF | admx major AF | aus major AF | Annotation(s) |
|---|---|---|---|---|---|---|---|---|---|
| LOC_Os09g04990.1 | Gold | 427 | S | S→P | 0.977 | 0.040 | 0.612 | 0.015 | Similar to octicosapeptide/Phox/Bem1p (PB1) domain-containing protein/tetratricopeptide repeat (TPR)-containing protein.:PF00564.17; PB1; Domain_427 |
| LOC_Os09g32540.1 | Gold | 86 | S | S→C | 0.916 | 0.071 | 0.573 | 0.095 | LGD1; LAGGING GROWTH AND DEVELOPMENT 1; Von Willebrand factor type A (VWA) domain containing protein, RNA binding protein, Regulation of vegetative growth and development:_86 |
| LOC_Os02g12850.1 | Bronze | 53 | S | S→G | 0.859 | 0.090 | 0.476 | 0.060 | Nucleotide-binding, alpha-beta plait domain containing protein.:PF00076.15; RRM_1; Domain_53 |
| LOC_Os03g44900.1 | Silver | 387 | S | S→P | 0.652 | 0.497 | 0.612 | 0.055 | CUL1; CULLIN 1Not CCR4-Not complex component, N-terminal domain containing protein.PF04153.11; NOT2_3_5; Family_387 |
| LOC_Os03g15580.1 | Silver | 71 | T | T→A | 0.732 | 0.051 | 0.359 | 0.025 | Hypothetical conserved gene.:PF03215.8; Rad17; Family_71 |
| LOC_Os10g37340.1 | Bronze | 86 | S | S→A | 0.710 | 0.236 | 0.417 | 0.104 | RRJ1; Cystathionine γ -lyase: PF01053.13; Cys_Met_Meta_PP; Domain_86 |
| LOC_Os01g11952.1 | Bronze | 339 | T | T→P | 0.608 | 0.108 | 0.340 | 0.005 | SET1; SET PROTEIN 1:SDG721; SET-domain group protein 721; TRITHORAX-like protein, Regulation of H3K4 methylation, Regulation of plant height and pollen grain development:PF00856.21; SET; Family_339 |
| LOC_Os04g51080.1 | Bronze | 84 | S | S→G | 0.980 | 0.078 | 0.524 | 0.060 | Scramblase family protein.:PF03803.8; Scramblase; Family_84 |
| LOC_Os11g11490.1 | Bronze | 81 | S | S→P | 0.815 | 0.201 | 0.544 | 0.085 | PF00069.18; Pkinase; Domain_81 |
| LOC_Os01g70250.1 | Bronze | 252 | S | S→L | 0.784 | 0.067 | 0.437 | 0.000 | Molecular chaperone, heat shock protein, Hsp40, DnaJ domain containing protein.:PF00226.24; DnaJ; Domain_252 |
Annotations are sourced by merging any data held in MSU or RAP-DB databases for the corresponding gene. Cluster 1 is mostly characterized by high AF in Japonica, and Admixed, but low in Aus and Indica.
The gene annotated in OryzaBase as OsCullin1 (LOC_Os03g44900.1) appears to have been misnamed in this publication,60 based on apparent shared homology to Arabidopsis thaliana Cullin 1 (annotated in TAIR61 to act as a component of ubiquitin ligase, with roles in response to auxin and jasmonic acid). However, LOC_Os03g44900.1 has high homology to NOT family transcriptional regulators, and has characteristic domains of this family, and should be renamed in OryzaBase.
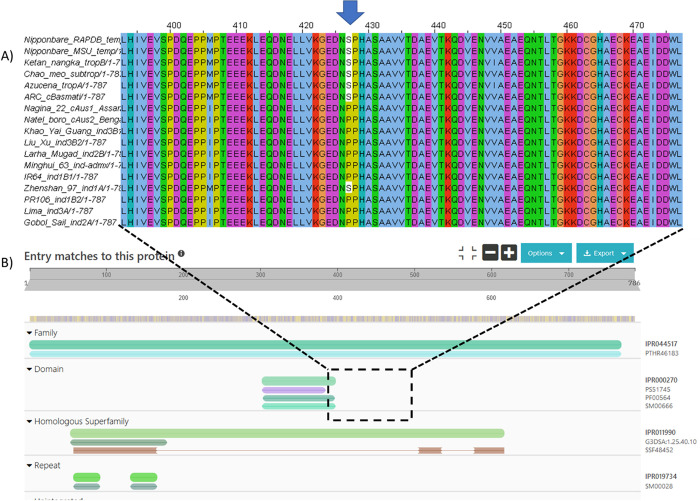
The SAAV analysis presented above generated “pseudo-protein” sequences by substituting amino acids, based on short DNA read data from the 3K rice genome set, which have been mapped against the Nipponbare reference genome. There is thus potential for assumed SAAVs to be incorrect, due to sequencing errors (as the 3K set does not always have high depth of coverage), if gene structure genuinely differs across different varietal groups, or if the RAP-DB or MSU gene model for Nipponbare is not correct. To validate the SAAV data, we also mapped the phosphosites to recently released gene models for 16 new rice varieties, called the “MAGIC-16”.62 In Figure 5, we display the protein sequence alignment across orthologs for cluster 1 protein LOC_Os09g04990.1 (Os09g0135400) position 427, with the position of an identified phosphosite marked. It can be observed that the pSer is present in tropical, subtropical, temperate, and aromatic varieties, but absent in all indica varieties, except Zhenshan 97. The equivalent allele frequencies for this pSer site are trop_ref_freq = 0.99; temp_ref_freq = 0.99; admix_ref_freq = 0.61; japx_ref_freq = 0.99; subtrop_ref_freq = 0.97; aus_ref_freq = 0.01; aro_ref_freq = 0.93; ind2_ref_freq = 0.02; indx_ref_freq = 0.05; ind1B_ref_freq = 0.08; ind3_ref_freq = 0.01; ind1A_ref_freq = 0.03, which appears to be in-line with the genuine protein sequences from the MAGIC-16 set. Protein sequences for all the MAGIC-16 set are available from Ensembl Plants and Gramene, enabling any phosphosites identified in this resource, to be cross-referenced to protein sequences annotated from high-quality whole genome assemblies, prior to any experimental work being conducted to validate PTM site differences.
Figure 5.
Sequence alignment of rice varieties. a) Protein sequence alignment of Os09g0135400 (RAP-DB), LOC_Os09g04990 (MSU) alongside protein sequences from other recently Oryza sativa varieties. b) The results of searching the protein sequence in InterProScan. The pSer site is nearby to PB1 protein binding domain (IPR000270, InterPro), and the protein is part of the Tetratricopeptide-like helical domain superfamily (IPR011990).
Discussion
In this work, we have performed a meta-analysis of phosphoproteomics data sets for rice, mapped onto the reference Nipponbare proteome. The analysis pipeline comprised the use of an open source search engine (Comet), with modules embedded in TPP for calculation of statistics at the level of peptides and site localization. We next calculated the global FLR, using an established approach with a decoy phosphorylated amino acid per data set to avoid reporting excessive false positives. To combine results across data sets, we applied a simple Gold–Silver–Bronze metric based on the counts of high-quality site observations across data sets at different FLR thresholds. We acknowledge that it would theoretically be possible to achieve higher sensitivity (more sites detected), for example by using multiple search engines or multiple packages for site localization, but with a downside of higher computational cost, and making results potentially more difficult for downstream users to interpret. As such, we applied a pipeline that is robust, delivering simple metrics and a full evidence trail back to source evidence (using Universal Spectrum Identifiers), to support various user groups.
The data set has been deposited into UniProtKB enabling sites to be analyzed alongside any other data held there about protein structure/function, including AlphaFold2 predictions for all rice proteins. The data is also available in PeptideAtlas and PRIDE, enabling detailed exploration of scores and visualization of source mass spectra, as a full evidence trail.
We have also mapped the data to variation coming from the 3,000 genome set, creating a resource for allele mining, where phosphosites are likely to have lost function due to amino acid substitutions in some rice varieties, with alterations to downstream cell signaling pathways. Phosphosites have been implicated in multiple phenotypic responses in rice. For example, it has recently been demonstrated that phosphorylation at a key position of a WRKY transcription factor is implicated in immune response to fungal infection in rice.63 Phosphorylation has also been implicated in abiotic stress response, e.g., to cold.64 As such, we expect that our data sets will make it easier for rice researchers to identify variation in and around phosphosites, assisting in hypothesis formation, and potentially identifying alleles within particular rice varieties conveying desirable phenotypic traits. All data sets are fully open and available for reanalysis, through multiple user-focused databases.
Acknowledgments
We are grateful to all the groups that generated MS data and deposited into PRIDE/ProteomeXchange to allow for reanalyses, and those who generated the 3K and MAGIC-16 rice resources.
Data Availability Statement
Reprocessed data files and search database were deposited into PRIDE database: https://www.ebi.ac.uk/pride/archive/projects/PXD046188. The code used for the FLR analysis can be found in GitHub: https://github.com/PGB-LIV/mzidFLR. The code for the downstream analysis and figure generation can be found in GitHub: https://github.com/PGB-LIV/Rice_Phospho_Manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.4c00187.
Supplementary Figure 1: Histograms showing scores of potential phosphosites on five peptidoforms identified with pTyr Gold scored sites. Supplementary Figure 2: Score histograms of peptide modification positions and spectra for a given peptide “IAVQNAETLSLASPR.” Supplementary Figure 3: Spectrum visualization comparing the potential phosphorylation positions in the peptide IAVQNAETLSLASPR from the “Rice_phos_Nip_0h_20per_F1_R3” experiment file within the data set PXD004939. Supplementary Figure 4: Significant motifs surrounding the S/T phosphosites for the gold and silver category sites. Supplementary Figure 5: Significant motifs surrounding the S/T phosphosites for the gold category sites. Supplementary Table 2: Table showing the presence/absence of significant motifs in each of the category groupings; Gold, Gold and Silver, and Gold, Silver and Bronze. Supplementary Figure 6: Enriched terms for proteins containing each enriched motif across all Gold–Silver–Bronze data sets against all phosphoproteins. (PDF)
Supplementary Data File 1—All_motifs: motifs found in each of the three categories (“Gold, Silver and Bronze”, “Gold and Silver”, and “Gold only”) with enrichment scores and protein counts. Supplementary Data File 2—Genomic_site_data_w_SNP_annotation: All phosphosites identified in the study, along with their mapped genomic position, data on SNPs, single amino acid variants and functional annotations. Supplementary Data File 3—ClusterProfiler GO enrichment GSB motifs: Enriched GO terms for each motif identified around phosphosites scored in the “Gold, Silver and Bronze” category. Supplementary Data File 4—Figure_4b_heatmap_clusters: Clusters of genes seen in the heatmap shown in Figure 4b. Supplementary Tables and Figures—Supplementary Table 1: Summary of all rice phosphoproteomics data sets identified via the PRIDE repository. (ZIP)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. K.A.R. performed MS data curation, searching, analysis, and manuscript writing. A.P. supported MS data curation. Y.P.R. supported PRIDE data upload and curation. O.M.C. facilitated statistical data analysis. D.J.K. assisted with metadata data set curation. E.B.-B., M.M., and J.F. assisted with data loading into UniProtKB. D.C. and K.L.M. assisted with rice 3K data analysis. E.W.D. assisted with MS data searching and project supervision. Z.S. assisted with MS data search and the loading into PeptideAtlas. J.A.V. assisted with PRIDE data loading and project supervision. A.R.J. coordinated the research, assisted with data analysis and writing the manuscript. We acknowledge funding from BBSRC [BB/T015691/1, BB/S017054/1, BB/S01781X/1, BB/T019670/1]. J.A.V., D.J.K., and Y.P.R. acknowledge funding from Wellcome [223745/Z/21/Z] and EMBL core funding. E.W.D. and Z.S. acknowledge funding from National Institutes of Health grants R01 GM087221 and R24 GM148372, and by the National Science Foundation grant DBI-193331.1
The authors declare no competing financial interest.
Supplementary Material
References
- Fornasiero A.; Wing R. A.; Ronald P. Rice domestication. Curr. Biol. 2022, 32 (1), R20–R24. 10.1016/j.cub.2021.11.025. [DOI] [PubMed] [Google Scholar]
- Sasaki T. The map-based sequence of the rice genome. Nature 2005, 436 (7052), 793–800. 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- Yu J.; Hu S.; Wang J.; Wong G. K.; Li S.; Liu B.; Deng Y.; Dai L.; Zhou Y.; Zhang X.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 2002, 296 (5565), 79–92. 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
- Koizumi T.; Gay S. H.; Furuhashi G.. Reviewing Indica and Japonica rice market developments; OECD, 2021. 10.1787/0c500e05-en. [DOI]
- Ouyang S.; Zhu W.; Hamilton J.; Lin H.; Campbell M.; Childs K.; Thibaud-Nissen F.; Malek R. L.; Lee Y.; Zheng L.; et al. The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res. 2007, 35 (Database issue), D883–D887. 10.1093/nar/gkl976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H.; Lee S. S.; Tanaka T.; Numa H.; Kim J.; Kawahara Y.; Wakimoto H.; Yang C. C.; Iwamoto M.; Abe T.; et al. Rice Annotation Project Database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol 2013, 54 (2), e6 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A. D.; Allen J.; Amode R. M.; Azov A. G.; Barba M.; Becerra A.; Bhai J.; Campbell L. I.; Carbajo Martinez M.; Chakiachvili M.; et al. Ensembl Genomes 2022: an expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50 (D1), D996–D1003. 10.1093/nar/gkab1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello-Ruiz M. K.; Stein J.; Wei S.; Youens-Clark K.; Jaiswal P.; Ware D. Gramene: A Resource for Comparative Analysis of Plants Genomes and Pathways. Methods Mol. Biol. 2016, 1374, 141–163. 10.1007/978-1-4939-3167-5_7. [DOI] [PubMed] [Google Scholar]
- UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51 (D1), D523–D531. 10.1093/nar/gkac1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Mauleon R.; Hu Z.; Chebotarov D.; Tai S.; Wu Z.; Li M.; Zheng T.; Fuentes R. R.; Zhang F.; et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 2018, 557 (7703), 43–49. 10.1038/s41586-018-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Chebotarov D.; Kudrna D.; Llaca V.; Lee S.; Rajasekar S.; Mohammed N.; Al-Bader N.; Sobel-Sorenson C.; Parakkal P.; et al. A platinum standard pan-genome resource that represents the population structure of Asian rice. Sci. Data 2020, 7 (1), 113. 10.1038/s41597-020-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. M.; Xie W. Z.; Wang S.; Guo Y. X.; Koo D. H.; Kudrna D.; Gong C.; Huang Y.; Feng J. W.; Zhang W.; et al. Two gap-free reference genomes and a global view of the centromere architecture in rice. Mol. Plant 2021, 14 (10), 1757–1767. 10.1016/j.molp.2021.06.018. [DOI] [PubMed] [Google Scholar]
- Blanco-Tourinan N.; Serrano-Mislata A.; Alabadi D. Regulation of DELLA Proteins by Post-translational Modifications. Plant Cell Physiol 2020, 61 (11), 1891–1901. 10.1093/pcp/pcaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosa-Puente B.; Leftley N.; von Wangenheim D.; Banda J.; Srivastava A. K.; Hill K.; Truskina J.; Bhosale R.; Morris E.; Srivastava M.; et al. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 2018, 362 (6421), 1407–1410. 10.1126/science.aau3956. [DOI] [PubMed] [Google Scholar]
- Rigbolt K. T.; Blagoev B. Quantitative phosphoproteomics to characterize signaling networks. Semin Cell Dev Biol. 2012, 23 (8), 863–871. 10.1016/j.semcdb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Ramsbottom K. A.; Prakash A.; Riverol Y. P.; Camacho O. M.; Martin M. J.; Vizcaino J. A.; Deutsch E. W.; Jones A. R. Method for Independent Estimation of the False Localization Rate for Phosphoproteomics. J. Proteome Res. 2022, 21 (7), 1603–1615. 10.1021/acs.jproteome.1c00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho O. M.; Ramsbottom K. A.; Collins A.; Jones A. R. Assessing Multiple Evidence Streams to Decide on Confidence for Identification of Post-Translational Modifications, within and Across Data Sets. J. Proteome Res. 2023, 22 (6), 1828–1842. 10.1021/acs.jproteome.2c00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnyy A.; Eyers P. A.; Eyers C. E.; Bowler-Barnett E.; Martin M. J.; Sun Z.; Deutsch E. W.; Jones A. R. Profiling the Human Phosphoproteome to Estimate the True Extent of Protein Phosphorylation. J. Proteome Res. 2022, 21 (6), 1510–1524. 10.1021/acs.jproteome.2c00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P.; Horne A.; Van Parys T.; Goormachtig S.; De Smet I.; Botzki A.; Van Breusegem F.; Gevaert K. The Plant PTM Viewer, a central resource for exploring plant protein modifications. Plant J. 2019, 99 (4), 752–762. 10.1111/tpj.14345. [DOI] [PubMed] [Google Scholar]
- Heazlewood J. L.; Durek P.; Hummel J.; Selbig J.; Weckwerth W.; Walther D.; Schulze W. X. PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res. 2007, 36 (Database issue), D1015–D1021. 10.1093/nar/gkm812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y.; Bai J.; Bandla C.; Garcia-Seisdedos D.; Hewapathirana S.; Kamatchinathan S.; Kundu D. J.; Prakash A.; Frericks-Zipper A.; Eisenacher M.; et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50 (D1), D543–D552. 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch E. W.; Bandeira N.; Sharma V.; Perez-Riverol Y.; Carver J. J.; Kundu D. J.; Garcia-Seisdedos D.; Jarnuczak A. F.; Hewapathirana S.; Pullman B. S.; et al. The ProteomeXchange consortium in 2020: enabling ’big data’ approaches in proteomics. Nucleic Acids Res. 2019, 48 (D1), D1145–D1152. 10.1093/nar/gkz984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiere F.; Deutsch E. W.; King N. L.; Nesvizhskii A. I.; Mallick P.; Eng J.; Chen S.; Eddes J.; Loevenich S. N.; Aebersold R. The PeptideAtlas project. Nucleic Acids Res. 2006, 34 (Database issue), D655–D658. 10.1093/nar/gkj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk K. J.; Leppert T.; Sun Q.; Boguraev S. S.; Sun Z.; Mendoza L.; Deutsch E. W. The Arabidopsis PeptideAtlas: Harnessing worldwide proteomics data to create a comprehensive community proteomics resource. Plant Cell 2021, 33 (11), 3421–3453. 10.1093/plcell/koab211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino J. A.; Deutsch E. W.; Wang R.; Csordas A.; Reisinger F.; Rios D.; Dianes J. A.; Sun Z.; Farrah T.; Bandeira N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32 (3), 223–226. 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens L.; Hermjakob H.; Jones P.; Adamski M.; Taylor C.; States D.; Gevaert K.; Vandekerckhove J.; Apweiler R. PRIDE: the proteomics identifications database. Proteomics 2005, 5 (13), 3537–3545. 10.1002/pmic.200401303. [DOI] [PubMed] [Google Scholar]
- Wang K.; Zhao Y.; Li M.; Gao F.; Yang M. K.; Wang X.; Li S.; Yang P. Analysis of phosphoproteome in rice pistil. Proteomics 2014, 14 (20), 2319–2334. 10.1002/pmic.201400004. [DOI] [PubMed] [Google Scholar]
- Lu Q.; Ding S.; Reiland S.; Rodiger A.; Roschitzki B.; Xue P.; Gruissem W.; Lu C.; Baginsky S. Identification and characterization of chloroplast casein kinase II from Oryza sativa (rice). J. Exp Bot 2015, 66 (1), 175–187. 10.1093/jxb/eru405. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Qiu J.; Tong X.; Wei X.; Nallamilli B. R.; Wu W.; Huang S.; Zhang J. A comprehensive quantitative phosphoproteome analysis of rice in response to bacterial blight. BMC Plant Biol. 2015, 15, 163. 10.1186/s12870-015-0541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Yin X.; Sakata K.; Yang P.; Komatsu S. Proteomic Analysis of Phosphoproteins in the Rice Nucleus During the Early Stage of Seed Germination. J. Proteome Res. 2015, 14 (7), 2884–2896. 10.1021/acs.jproteome.5b00215. [DOI] [PubMed] [Google Scholar]
- Qiu J.; Hou Y.; Wang Y.; Li Z.; Zhao J.; Tong X.; Lin H.; Wei X.; Ao H.; Zhang J. A Comprehensive Proteomic Survey of ABA-Induced Protein Phosphorylation in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2017, 18 (1), 60. 10.3390/ijms18010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Tong X.; Qiu J.; Li Z.; Zhao J.; Hou Y.; Tang L.; Zhang J. A phosphoproteomic landscape of rice (Oryza sativa) tissues. Physiol Plant 2017, 160 (4), 458–475. 10.1111/ppl.12574. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Qiu J.; Wang Y.; Li Z.; Zhao J.; Tong X.; Lin H.; Zhang J. A Quantitative Proteomic Analysis of Brassinosteroid-induced Protein Phosphorylation in Rice (Oryza sativa L.). Front Plant Sci. 2017, 8, 514. 10.3389/fpls.2017.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.; Zhang Z.; Long H.; Zhang Z.; Hong Y.; Zhang X.; You C.; Liang W.; Ma H.; Lu P. Proteomic and phosphoproteomic analyses reveal extensive phosphorylation of regulatory proteins in developing rice anthers. Plant J. 2015, 84 (3), 527–544. 10.1111/tpj.13019. [DOI] [PubMed] [Google Scholar]
- Yang J.; Xie M. Y.; Yang X. L.; Liu B. H.; Lin H. H. Phosphoproteomic Profiling Reveals the Importance of CK2, MAPKs and CDPKs in Response to Phosphate Starvation in Rice. Plant Cell Physiol 2019, 60 (12), 2785–2796. 10.1093/pcp/pcz167. [DOI] [PubMed] [Google Scholar]
- Al-Momani S.; Qi D.; Ren Z.; Jones A. R. Comparative qualitative phosphoproteomics analysis identifies shared phosphorylation motifs and associated biological processes in evolutionary divergent plants. J. Proteomics 2018, 181, 152–159. 10.1016/j.jprot.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata T.; Baslam M.; Masui T.; Koshu T.; Takamatsu T.; Kaneko K.; Pozueta-Romero J.; Mitsui T. Proteomics Analysis Reveals Non-Controlled Activation of Photosynthesis and Protein Synthesis in a Rice npp1Mutant under High Temperature and Elevated CO(2) Conditions. Int. J. Mol. Sci. 2018, 19 (9), 2655. 10.3390/ijms19092655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.; Zou T.; Xiao Q.; Yuan G.; Liu M.; Tao Y.; Zhou D.; Zhang X.; Deng Q.; Wang S. An L-type lectin receptor-like kinase promotes starch accumulation during rice pollen maturation. Development 2021, 148 (6), dev196378. 10.1242/dev.196378. [DOI] [PubMed] [Google Scholar]
- Dai C.; Fullgrabe A.; Pfeuffer J.; Solovyeva E. M.; Deng J.; Moreno P.; Kamatchinathan S.; Kundu D. J.; George N.; Fexova S.; et al. A proteomics sample metadata representation for multiomics integration and big data analysis. Nat. Commun. 2021, 12 (1), 5854. 10.1038/s41467-021-26111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa J. M.; Guan S.; Moran M. F.; Ma B. Repeat-Preserving Decoy Database for False Discovery Rate Estimation in Peptide Identification. J. Proteome Res. 2020, 19 (3), 1029–1036. 10.1021/acs.jproteome.9b00555. [DOI] [PubMed] [Google Scholar]
- Deutsch E. W.; Mendoza L.; Shteynberg D.; Slagel J.; Sun Z.; Moritz R. L. Trans-Proteomic Pipeline, a standardized data processing pipeline for large-scale reproducible proteomics informatics. Proteomics Clin Appl. 2015, 9 (7–8), 745–754. 10.1002/prca.201400164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch E. W.; Mendoza L.; Shteynberg D. D.; Hoopmann M. R.; Sun Z.; Eng J. K.; Moritz R. L. Trans-Proteomic Pipeline: Robust Mass Spectrometry-Based Proteomics Data Analysis Suite. J. Proteome Res. 2023, 22 (2), 615–624. 10.1021/acs.jproteome.2c00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J. K.; Jahan T. A.; Hoopmann M. R. Comet: an open-source MS/MS sequence database search tool. Proteomics 2013, 13 (1), 22–24. 10.1002/pmic.201200439. [DOI] [PubMed] [Google Scholar]
- Keller A.; Nesvizhskii A. I.; Kolker E.; Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74 (20), 5383–5392. 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Shteynberg D.; Deutsch E. W.; Lam H.; Eng J. K.; Sun Z.; Tasman N.; Mendoza L.; Moritz R. L.; Aebersold R.; Nesvizhskii A. I. iProphet: multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol. Cell Proteomics 2011, 10 (12), M111.007690 10.1074/mcp.M111.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteynberg D. D.; Deutsch E. W.; Campbell D. S.; Hoopmann M. R.; Kusebauch U.; Lee D.; Mendoza L.; Midha M. K.; Sun Z.; Whetton A. D.; et al. PTMProphet: Fast and Accurate Mass Modification Localization for the Trans-Proteomic Pipeline. J. Proteome Res. 2019, 18 (12), 4262–4272. 10.1021/acs.jproteome.9b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. R.; Eisenacher M.; Mayer G.; Kohlbacher O.; Siepen J.; Hubbard S. J.; Selley J. N.; Searle B. C.; Shofstahl J.; Seymour S. L.; et al. The mzIdentML data standard for mass spectrometry-based proteomics results. Mol. Cell Proteomics 2012, 11 (7), M111.014381-1 10.1074/mcp.M111.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch E. W.; Perez-Riverol Y.; Carver J.; Kawano S.; Mendoza L.; Van Den Bossche T.; Gabriels R.; Binz P. A.; Pullman B.; Sun Z.; et al. Universal Spectrum Identifier for mass spectra. Nat. Methods 2021, 18 (7), 768–770. 10.1038/s41592-021-01184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagih O.; Sugiyama N.; Ishihama Y.; Beltrao P. Uncovering Phosphorylation-Based Specificities through Functional Interaction Networks. Mol. Cell Proteomics 2016, 15 (1), 236–245. 10.1074/mcp.M115.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.; Hu E.; Xu S.; Chen M.; Guo P.; Dai Z.; Feng T.; Zhou L.; Tang W.; Zhan L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021, 2 (3), 100141 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata N.; Yamazaki Y. Oryzabase. An integrated biological and genome information database for rice. Plant Physiol 2006, 140 (1), 12–17. 10.1104/pp.105.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Chen L. L.; Xing F.; Kudrna D. A.; Yao W.; Copetti D.; Mu T.; Li W.; Song J. M.; Xie W.; et al. Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (35), E5163–5171. 10.1073/pnas.1611012113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. C.; Yu Y.; Copetti D.; Zwickl D. J.; Zhang L.; Zhang C.; Chougule K.; Gao D.; Iwata A.; Goicoechea J. L.; et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza. Nat. Genet. 2018, 50 (2), 285–296. 10.1038/s41588-018-0040-0. [DOI] [PubMed] [Google Scholar]
- Contreras-Moreira B.; Saraf S.; Naamati G.; Casas A. M.; Amberkar S. S.; Flicek P.; Jones A. R.; Dyer S. GET_PANGENES: calling pangenes from plant genome alignments confirms presence-absence variation. Genome Biol. 2023, 24 (1), 223. 10.1186/s13059-023-03071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Zidek A.; Potapenko A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596 (7873), 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell D. J.; Chorny I.; Groban E. S.; Wong S. E.; Levine E.; Rapp C. S.; Jacobson M. P. Strengths of hydrogen bonds involving phosphorylated amino acid side chains. J. Am. Chem. Soc. 2007, 129 (4), 820–827. 10.1021/ja063019w. [DOI] [PubMed] [Google Scholar]
- Dardick C.; Chen J.; Richter T.; Ouyang S.; Ronald P. The rice kinase database. A phylogenomic database for the rice kinome. Plant Physiol 2007, 143 (2), 579–586. 10.1104/pp.106.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov N.; Tai S.; Wang W.; Mansueto L.; Palis K.; Fuentes R. R.; Ulat V. J.; Chebotarov D.; Zhang G.; Li Z.; et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res. 2015, 43 (Database issue), D1023–1027. 10.1093/nar/gku1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Tang T.; Tang H.; Huang J.; Shi S.; Wu C. I. The accumulation of deleterious mutations in rice genomes: a hypothesis on the cost of domestication. Trends Genet 2006, 22 (3), 126–131. 10.1016/j.tig.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Vicente N.; Cardoso L.; Barros L.; Carrilho F. Antithyroid Drug-Induced Agranulocytosis: State of the Art on Diagnosis and Management. Drugs R D 2017, 17 (1), 91–96. 10.1007/s40268-017-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardini T. Z.; Reiser L.; Li D.; Mezheritsky Y.; Muller R.; Strait E.; Huala E. The Arabidopsis information resource: Making and mining the ″gold standard″ annotated reference plant genome. Genesis 2015, 53 (8), 474–485. 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Yu Z.; Chebotarov D.; Chougule K.; Lu Z.; Rivera L. F.; Kathiresan N.; Al-Bader N.; Mohammed N.; Alsantely A.; et al. Pan-genome inversion index reveals evolutionary insights into the subpopulation structure of Asian rice. Nat. Commun. 2023, 14 (1), 1567. 10.1038/s41467-023-37004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Han S.; Zhou X.; Zhao C.; Guo L.; Zhang J.; Liu F.; Huo Q.; Zhao W.; Guo Z.; et al. Phosphorylation and ubiquitination of OsWRKY31 are integral to OsMKK10–2-mediated defense responses in rice. Plant Cell 2023, 35 (6), 2391–2412. 10.1093/plcell/koad064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M.; Meng X.; Song X.; Zhang D.; Kou L.; Zhang J.; Jing Y.; Liu G.; Liu H.; Huang X.; et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov 2022, 8 (1), 71. 10.1038/s41421-022-00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reprocessed data files and search database were deposited into PRIDE database: https://www.ebi.ac.uk/pride/archive/projects/PXD046188. The code used for the FLR analysis can be found in GitHub: https://github.com/PGB-LIV/mzidFLR. The code for the downstream analysis and figure generation can be found in GitHub: https://github.com/PGB-LIV/Rice_Phospho_Manuscript.