ii. Summary/abstract:
The Drosophila egg chamber is a powerful system to study epithelial cell collective migration and polarized basement membrane secretion. A strength of this system is the ability to capture these dynamic processes in ex vivo organ culture using high resolution live imaging. Ex vivo culture also allows acute pharmacological or labeling treatments, extending the versatility of the system. However, many current ex vivo egg chamber culture set-ups do not permit easy medium exchange, preventing researchers from following individual egg chambers through multiple treatments. Here we present a method to immobilize egg chambers in an easy-to-construct flow chamber that permits imaging of the same egg chamber through repeated solution exchanges. This will allow researchers to take greater advantage of the wide variety of available pharmacological perturbations and other treatments like dyes to study dynamic processes in the egg chamber.
Keywords: Drosophila, egg chamber, follicle, collective cell migration, secretion, morphogenesis, live imaging, flow chamber, drug treatment
1. Introduction
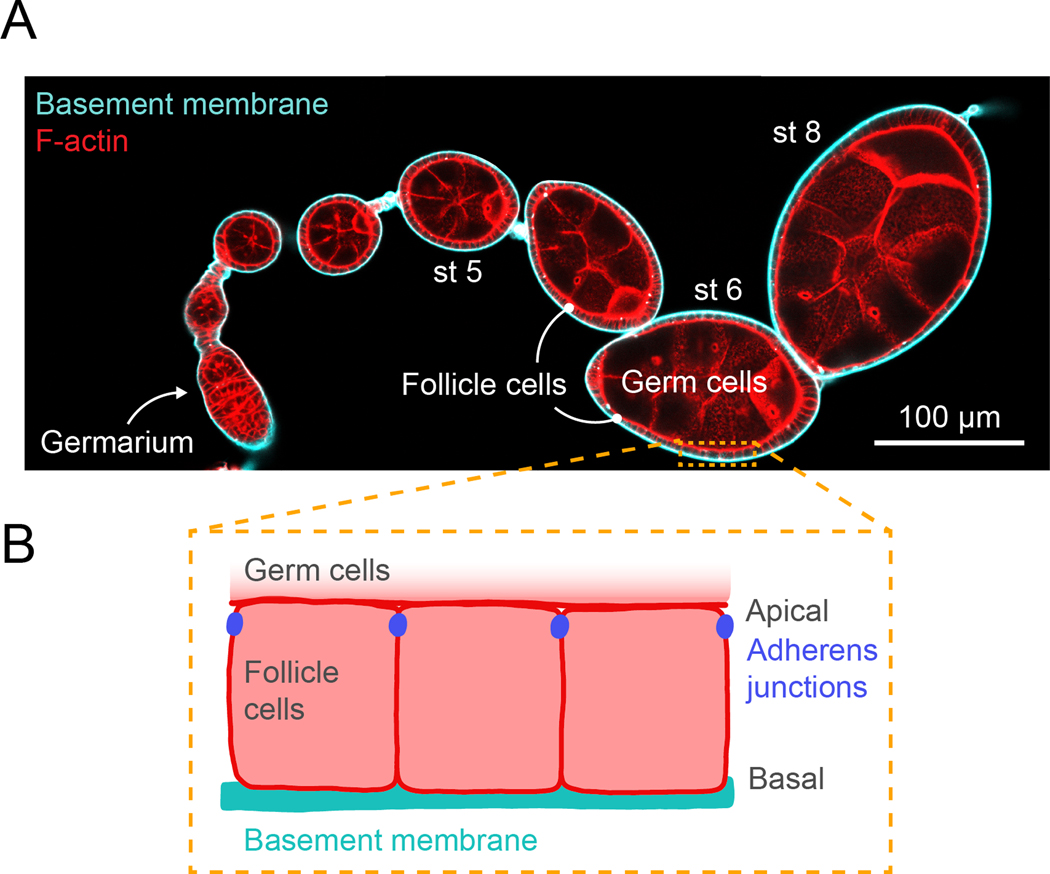
The follicular epithelium of the Drosophila egg chamber has emerged as a powerful system to investigate the dynamics of collective cell migration and extracellular matrix (ECM) secretion and assembly. The egg chamber consists of a central cluster of germ cells surrounded by a single layer of somatic follicular epithelia cells (follicle cells, Fig. 1A) [1]. The follicle cells are polarized such that their apical surfaces contact the germ cells and their basal surfaces contact a sheet of basement membrane (BM) ECM that wraps the tissue (Fig. 1B). During the early stages of development (up to stage 8), the follicle cells undergo a collective migration along the BM [2, 3]. During this time, they are also actively secreting new BM proteins and depositing new layers of BM as they migrate [2, 4]. The geometry of the egg chamber places much of the machinery involved in collective cell migration and BM secretion near the exterior of the tissue. This useful property has allowed cutting-edge imaging techniques to be applied to study these dynamic processes in the intact, developing tissue [2–17].
Fig. 1. Drosophila egg chamber organization.
A. Confocal cross-section image through an ovariole stained for F-actin (phalloidin) and expressing GFP-Collagen IV to label the basement membrane. Note that different sizes of egg chambers require different levels of compression to hold them in place in the flow chamber. B. Illustration of the organization of the follicular epithelial cells that surround the egg chamber. The apical surfaces of follicle cells contact the germline cells and the basal surfaces contact the basement membrane on the exterior of the tissue.
Genetic screens have been useful for identifying the machinery involved in collective cell migration and BM secretion. However, genetic perturbations often result in the cells completely failing to migrate or secrete BM proteins and leave open many questions about the normal dynamics of these processes. In dynamic systems, being able to make acute perturbations using pharmacological inhibitors, and to watch recovery from these perturbations after washing out the treatment, can provide important mechanistic information. Since a variety of pharmacological inhibitors are available that target machinery involved in cell migration and secretion, this approach is likely to provide new insights into the dynamics of these processes.
Although conditions for ex vivo culture are established [2, 18–20], it has been technically challenging to mount egg chambers in a way that allows imaging of a single egg chamber through rounds of solution exchange. Attachment of egg chambers to glass-bottomed dishes would allow easy exchange. However, past studies have found that, although using an adhesive coating like Concavalin A can immobilize egg chambers, this coating also stops the migration of cells [9]. An additional imaging challenge is the egg chamber’s curved surface. To view a larger surface area of an egg chamber, we gently compress it between two pieces of glass, which makes solution exchange challenging. In past work, our lab used small fragments of glass to compress egg chambers in a dish, which does allow new solutions to be added to the bulk medium [3, 20]. However, this approach still has the drawbacks of not allowing rapid mixing of the treatment into the bulk medium, and not allowing for easy wash-out of the drug-treated medium.
Here we report a simple modification to our current mounting protocol that creates a flow chamber and allows multiple rounds of solution exchange while imaging the same egg chamber. Many single molecule in vitro studies use hand-made flow chambers built from a slide and a coverslip that have a channel with walls created by tape or vacuum grease [21]. We have combined this approach with our standard mounting of egg chambers between a slide and a coverslip that is supported by spacer beads [13, 20]. This is an easy-to-make flow chamber that uses widely available materials and is compatible with both upright and inverted microscopes. This flow chamber allows multiple rounds of solution exchange for drug treatment or pulse-labeling of endocytic tracers or other labels.
Finally, we want to bring the reader’s attention to two alternative approaches to immobilize egg chambers in either gels [22] or fibrin clots [23, 24]. These methods also permit solution exchanges and may be preferable depending on the experimental goals.
In this chapter, we describe how to dissect and prepare ovariole strands containing stage 1–8 egg chambers and immobilize them in a flow chamber suitable for repeated media exchanges. We have previously published a chapter in Methods in Molecular Biology on live imaging of follicle cell migration [20]. The protocol for dissection is shared between these chapters, and the chapter by Anderson et al., on F-Actin staining in the current edition, and sections are duplicated here for convenience. We refer the reader to the live migration imaging chapter [20] for detailed images and movies of the egg chamber dissection process, which are not included here.
2. Materials
2.1. Preparing Female Flies for Dissection
Vial with fly food.
Yeast powder: dry active yeast ground to a fine powder in coffee grinder.
2.2. Flow Chamber Preparation
Glass microscope slide, 3” x 1” x 1 mm.
10 ml syringe.
High-vacuum silicone grease.
100 or 200 μl pipette tip.
2.3. Egg Chamber Dissection
Acidified water: 1 μl concentrated (12.1 N) HCl in 1 ml deionized water.
Insulin: 1 mg dissolved in 100 μl acidified water (see Note 1).
Live imaging medium: Schneider’s S2 medium, 0.6X Pen/Strep (Pen/Strep: penicillin G- sodium 10,000 U/ml, streptomycin sulfate 10,000 μg/ml in 0.85% saline), 15% vol/vol fetal bovine serum, 0.1 mg/ml insulin (see Note 2).
Pyrex 9-Cavity Spot Plate.
Dumont forceps: #5, 0.1 × 0.06 mm tip, and #55, 0.05 × 0.02 mm tip (see Note 3).
Eyelash tool: insert an eyelash into a slightly melted p1000 pipettor tip.
Disposable needle, 27G x ½ in.
Glass pasteur pipets, 5 ¾ in.
5 ml pipet pump.
Stereomicroscope with magnification of at least 10X.
Dye to visualize plasma membranes, for example CellMask (see Note 4).
10 μl pipette and tips.
Glass microscope slide, 3” x 1” x 1 mm.
CO2 pad to anesthetize flies.
2.4. Egg Chamber Mounting in Flow Chamber
2.5. Exchanging Media and Imaging
Live imaging medium.
Desired treatments: pharmacological inhibitors, dyes, etc.
100 or 200 μl pipette and tips.
Filter paper or delicate task wiper.
Vaseline.
Heat block set to 65°C.
Paintbrush.
3. Methods
3.1. Prepare Female Flies for Dissection
Sprinkle yeast powder on fly food in a vial, covering about one half of the surface. Add up to six 1–2 day old females and at least 1–2 males to the vial. Yeast is essential for proper egg chamber production. Incomplete nutrition will slow egg chamber production by inducing cell death in the germarium and in stage 8 egg chambers [25–27].
Age females for 1–3 days. Move flies to a new vial with fresh yeast every two days before dissection (see Note 8).
3.2. Prepare Flow Chamber
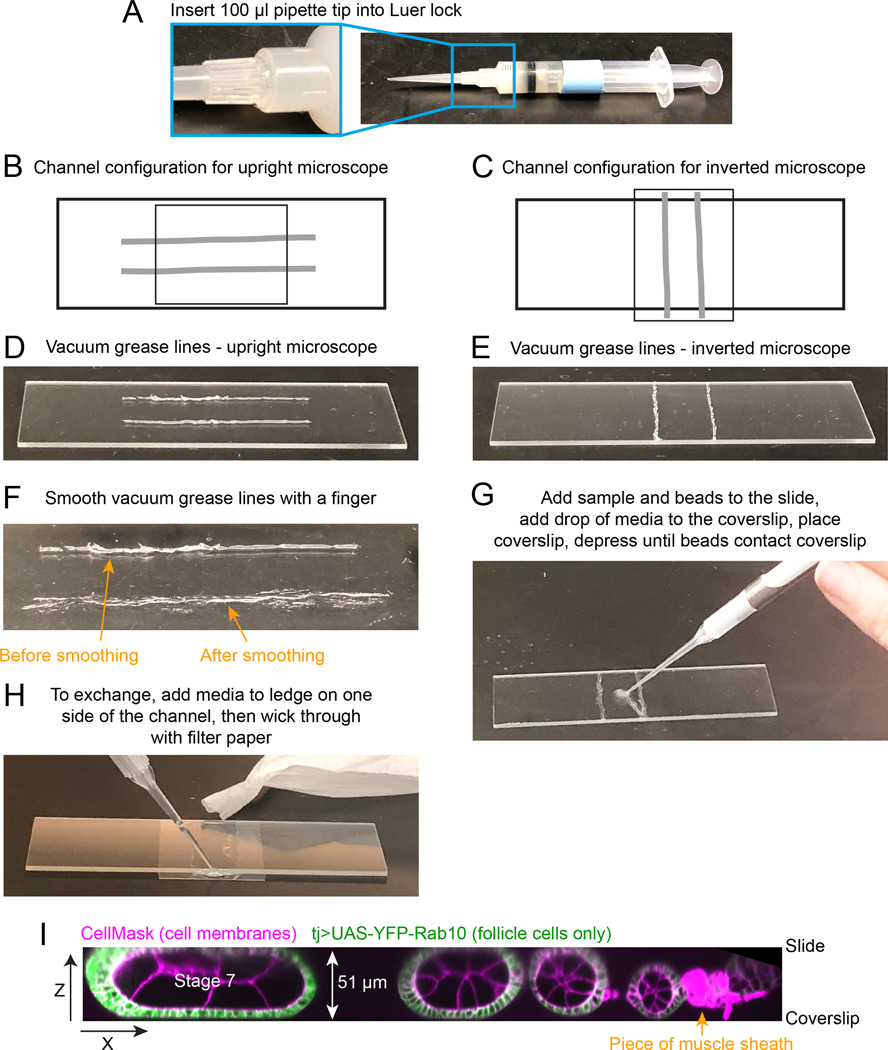
Make a vacuum grease-filled 10 ml syringe (save for repeated use indefinitely) by removing the plunger and squeezing vacuum grease tube into the barrel. Use the plunger to push the vacuum grease into the syringe (with no attached needle). Attach a 100 or 200 μl pipette tip to the Luer lock of the syringe in place of a needle (Fig. 2A).
- Make a vacuum grease channel on the slide. The pipette tip can detach from the Luer lock of the syringe while you are depressing the plunger, so hold it in place while drawing vacuum grease lines. The channel opening needs to be accessible to exchange media with the slide mounted on the coverslip. Therefore, the geometry of the flow chamber will be different depending on whether you are using an inverted or an upright microscope (Fig. 2B–E).
Smooth the vacuum grease lines by running a finger over each line to partially flatten it and make sure there are no gaps in the line, as gaps will cause leaks (Fig. 2F; see Note 9). Set aside for use after the dissection.
Fig. 2. How to construct flow chamber.
A. Vacuum grease-filled 10 ml syringe with a 100 μl pipette tip attached to the Luer lock in place of a needle. B and C. Diagram of flow chamber construction for an inverted or upright microscope. Light grey thick lines indicate where vacuum grease lines should be drawn on slide. Smaller rectangle indicates position of the 20 × 30 coverslip. D and E. Image of the desired spacing and thickness of vacuum grease lines. Note that these should be thin and you will likely have some gaps in the original lines. F. Image of the same vacuum grease line after smoothing it with finger (bottom). Now it is flatter and will require less manual compression when you add the coverslip. You should make sure there are no gaps that will allow leaks in the final line. G. Image showing where to initially add egg chambers and beads to slide. H. Image of the assembled and compressed flow chamber, showing where to add media to the coverslip ledge that extends past one edge of the channel, and image of torn filter paper being used to wick media through flow chamber. I. Zeiss Lattice Light Sheet volume of an ovariole compressed between slide and coverslip, supported by 51 μm diameter beads. A x-z projection is shown to highlight the compression of the stage 7 egg chamber that will hold it in place during medium exchanges.
3.3. Egg Chamber Dissection
In this section we describe how to isolate egg chambers from the ovary, which requires a basic understanding of the ovary’s structure. Each ovary is composed of 15–18 developmental arrays of egg chambers called ovarioles. At the anterior of each ovariole is the germarium, the site of egg chamber production. Moving posteriorly are successively older egg chambers, connected to one another like beads on a string by stalk cells. At the posterior of each ovariole are the oldest egg chambers or mature eggs waiting to be laid. Each ovariole, as well as the entire ovary, is surrounded by a muscle sheath that needs to be removed during dissection. Egg chambers can be staged by eye based on both their overall size and shape, as well as the relative size and shape of the oocyte. Older egg chambers are also distinguished by their partly opaque appearance, caused by the onset of vitellogenesis at stage 8.
Based on your experimental goals, you may choose egg chambers of various stages to dissect. Dissection techniques vary based on which stage egg chambers you wish to collect, and dissection will damage egg chambers of other stages, so it is important to tailor your dissection approach to your stage of interest. Finally, we caution that the entire dissection process described below not last for longer than ~10 min to ensure that you can stain, mount, and image your egg chambers with minimal time in ex vivo culture. We usually limit ex vivo culture of egg chambers to < 1 hr and recommend dissecting only 1–2 females at a time. In genotypes with healthy ovaries, this will yield plenty of ovarioles for an hour of imaging. In addition to the methods below, we refer the reader to a previously published chapter in Methods in Molecular Biology on live imaging of follicle cell migration [20].
If staining cell membranes for live imaging is desired, dilute CellMask 1:2000 or alternative dyes in live imaging medium. This staining step is also helpful to allow you to assess damage to egg chambers, as damaged regions will accumulate dye.
Add live imaging medium to the Pyrex 9-Cavity Spot Plate and allow medium to come to room temperature prior to dissection. If you are not staining cell membranes, you will need 1 well with 500 μl and 1 well with 250 μl of live imaging medium per dissection. If you are staining with CellMask, you will need 1 well with 500 μl live imaging medium for dissection, 1 well of 250 μl live imaging medium with CellMask, and 2 wells of 250 μl live imaging medium for washes.
Position a Spot Plate well containing live imaging medium underneath a dissecting microscope. Adjust magnification to ~16X if microscope allows, and focus to the bottom of the well.
Dissect ovaries. First, anesthetize the flies. We normally use CO2. Using the #5 forceps in your non-dominant hand, pinch a female fly at the anterior-most part of the abdomen (see Note 10). While still pinching, transfer the fly to the live imaging media and completely submerge. Using the #55 forceps in your dominant hand, pinch the abdomen between the two posterior-most segments and pull posteriorly. The ovaries should release from the abdomen (if ovaries do not release, see Note 11). Remove all non-ovary tissue from the well. To obtain stage 1–5 egg chambers, see step 5. To obtain stage 5–8 egg chambers, see step 6.
To obtain stage 1–5 egg chambers, pinch the posterior of an ovary with the #5 forceps and pin it to the bottom of the well. Use the #55 forceps to pinch a single ovariole just anterior to where the ovary is pinned. Pull the ovariole orthogonally and then anteriorly to remove it from the muscle sheath and isolate it from the ovary. Ovarioles that have been removed from their muscle sheaths will look and move like beads on a string, rather than like a single packaged unit. Repeat this process until ovarioles do not easily separate from the ovary, as repeated pulling risks damaging egg chambers. Repeat for the second ovary.
To obtain stage 5–8 egg chambers, pinch the posterior of an ovary with the #5 forceps and pin it to the bottom of the well. Use the #55 forceps to pinch single ovarioles at the anterior tip of the ovary and pull anteriorly to remove ovarioles from their muscle sheaths and isolate them from the ovary. Ovarioles that have been removed from their muscle sheaths will look and move like beads on a string, rather than like a single packaged unit. Repeat this process until ovarioles do not easily separate from the ovary, as repeated pulling risks damaging egg chambers. Repeat for the second ovary.
Separate healthy ovarioles from any other unwanted material using an eyelash tool or 27- gauge needle. Do not take ovarioles that retained their muscle sheaths, as this sheath will contract in culture and move the egg chambers.
Trim ovarioles containing egg chambers older than the stage you plan to image by placing the needle on the stalk between two egg chambers and pressing down using a slicing motion. Older egg chambers will prop up the coverslip and prevent the needed compression of younger egg chambers under the coverslip. This will both reduce the area of the basal surface available for single-plane imaging and cause egg chambers to move when fluids are exchanged. This step takes practice (see Note 12).
Gather trimmed ovarioles in a cluster using the eyelash tool. Use a 10–20 μl pipette to transfer ovarioles to a well of fresh media (either with CellMask for staining or fresh medium if not staining), and cover with a slide to slow evaporation. It is important to transfer the desired, healthy-looking ovarioles to new medium as quickly as you can. We notice even undamaged egg chambers can have failed cell migration if they spend extended times in medium containing debris from damaged egg chambers. If staining with CellMask, incubate for ~10 min in medium with stain.
To wash egg chambers from CellMask, collect egg chambers in the center of the Spot Plate by swirling. Use a 10–20 μl pipette to transfer ovarioles to the first well with 250 μl of live imaging medium. Minimize the amount of stain-containing medium transferred with the egg chambers. Swirl the dish to facilitate rinsing. Use a fresh 10–20 μl tip to transfer ovarioles to the second 250 μl well of live imaging media. Cover with a slide to slow evaporation until ready to mount (see Note 13).
3.4. Flow Chamber Construction and Egg Chamber Mounting
Transfer ovarioles to the flow chamber slide. Use a 10 μl pipette to transfer 1–5 ovarioles in 10 μl of medium, creating a drop in the center of the 2 lines of vacuum grease. Add a small drop of spacer beads from your bead-media stock (see Note 6)–the goal is ~20–100 beads per slide. Choose bead size based on the desired compression and stage/size of egg chambers you are imaging (see Note 5). Use the eyelash tool to arrange ovarioles in center of slide surrounded by beads that will support the weight of the coverslip (Fig. 2G).
Add a coverslip to complete the flow chamber. Add a 10 μl drop of live imaging medium to the center of a 22×30 mm coverslip. This will reduce bubbles when you lower it onto the sample. For an inverted microscope, the coverslip needs to extend past the slide to create a platform to allow you to exchange the fluid in the vacuum grease channel; see Fig. 2C for desired geometry. For an upright microscope, the coverslip will lie parallel to the long axis of the slide, but it is important that the vacuum grease lines extend past the coverslip on both sides to create a full channel (Fig. 2B).
Once you have lowered the coverslip into place, cover it with a delicate task wiper to prevent fingerprints, and press gently to compress the vacuum grease to the same height as the spacer beads. Wick away excess medium with filter paper or a delicate task wiper. Check under a dissecting scope that your egg chambers are sufficiently compressed to stop egg chamber movement due to fluid flow (Fig. 2H). This step requires practice. A sufficiently compressed Stage 7 egg chamber is shown in Fig. 2I.
3.5. Exchange Fluids in Flow Chamber
To dislodge egg chambers that are not compressed well enough, you can exchange media in the chamber for fresh, untreated media before beginning your imaging (optional). Have torn pieces of filter paper or delicate task wipers ready to use. Add a 20 μl drop of fresh live imaging medium to one side of the flow chamber (Fig. 2H). The drop must contact the liquid in the channel. Put the piece of filter paper/delicate task wiper on the opposite side and wick the medium through (Fig. 2H). Make sure the channel remains filled with media so the sample does not dry out during pre-treatment imaging.
Put the slide onto the microscope. Be sure to anchor the slide against 1 corner of the slide holder so that if it is bumped during liquid exchange, or if you choose to exchange media off the microscope, you can quickly move the slide back to its initial position and find the same egg chamber. Perform “pre-treated” imaging of egg chambers compressed in the flow chamber.
Exchange medium to add the experimental treatment (drug, etc). To make it easier to access the slide, you may want to pull back the microscope condenser head. Add a 20 μl drop of experimental medium and wick to other side (Fig. 2H). Repeat ~2x. There will be a short (1–2 min) time gap where the slide is out of focus while you exchange the medium. Restart imaging. Repeat exchanges with media containing different treatments as desired. If you are imaging for more than a few minutes after the last medium exchange, seal the two ends of the flow chamber by using a paintbrush to place a drop of Vaseline (melted in a 65°C heat block) over the openings on both sides of the channel. Be careful not to damage the microscope with the medium or Vaseline. If you cannot do this easily with the slide on the microscope, remove the slide, exchange the medium on the bench and seal with Vaseline, and replace the slide on the microscope.
Acknowledgments
American Heart Association 16POST2726018, American Cancer Society 132123-PF-18-025-01-CSM, and Chicago Biomedical Consortium FP064171-01-PR postdoctoral fellowships to A.L.Z., NIH T32 HD055164 to A.M.W., and work in the Horne-Badovinac lab is supported by NIH R01s GM126047 and GM136961 to S.H-B.
Footnotes
Notes
Insulin solution can be stored at 4 °C for up to one week. Insulin solution should be added to the live imaging medium immediately before use.
Schneider’s S2 medium and Pen/Strep can be combined and stored at 4 °C. Fetal bovine serum can also be combined with the Schneider’s S2 medium and Pen/Strep mixture and stored at 4 °C for up to one week. We make 10 mL of the S2, Pen/Strep, fetal bovine serum mixture at a time.
To minimize tissue damage, maintain forceps with care and sharpen as needed.
We regularly use CellMask dyes to label the plasma membrane. An alternative plasma membrane label is FM 4–64 [20].
Both glass and polystyrene beads work well. Experiment with different sizes depending on the amount of compression you need.
We resuspend beads in live imaging medium (without insulin) to make it easy to add them to the imaging chamber. Add dry beads to an Eppendorf tube. Wash beads with live imaging medium before use: add 500 μl of live imaging media, spin briefly to sediment, exchange to new media, repeat 2x. You can store beads in medium at 4 °C until you notice contamination.
Our microscope slide holder can only accommodate a 30 mm-long coverslip. If you have more space, and are using an inverted microscope, using a longer coverslip will be easier because it will create a longer platform for medium exchanges.
Depending on the genotype, age, and temperature, the rate of oogenesis may vary, and flies may need to be aged for different numbers of days to achieve an optimal number of egg chambers of your desired stage for dissection. Younger females produce more eggs than older females, colder temperatures slow oogenesis, and warmer temperatures speed oogenesis. Flies must be moved to fresh yeast regularly or females will cease laying eggs and retain them in their ovaries, resulting in unhealthy egg chambers and fewer younger egg chambers. Certain genetic conditions can result in round eggs that will block the oviduct, which results in unhealthy egg chambers; dissecting younger flies prior to oviduct blockage is often useful in these backgrounds.
You will have to experiment with making these vacuum grease lines. The goal is to have a thin, unbroken line that can be compressed to the height of the beads you are using. The most common mistakes made are using lines of vacuum grease that are too thick or having gaps in the lines.
Proper pinching of the fly will ensure the rest of your dissection proceeds smoothly. Pinching the thorax increases the risk that attempting to open the abdomen to remove the ovaries will instead remove the entire abdomen without opening it. Pinching a more posterior part of the abdomen increases the risk that you will damage young egg chambers.
If the ovaries do not initially release from the abdomen, there are several methods to remove them. If the oviduct is visible, first attempt to tug posteriorly on the oviduct with your #55 forceps to remove the ovaries, as this is the least likely method to damage egg chambers. If the oviduct is not visible or tugging did not work, there are two other options. If late-stage egg chambers are desired, the abdomen can be gently squeezed like a tube of toothpaste in an anterior-to-posterior direction to extrude the ovaries. If younger egg chambers are desired, the posterior of the ovaries can be pulled on directly.
Cutting the stalks between egg chambers takes practice and can result in damage to egg chambers even with experience. If you are using a plasma membrane dye, you will see dye uptake in damaged regions of egg chambers. If you are not using a dye, check egg chambers for other signs of tissue damage or abnormal cell migration, and, if present, exclude them from analysis. An alternative to using a needle is a thinned tungsten wire tool [20].
It is important to wash out excess dye to prevent it from sticking to the coverslip and creating high background fluorescence. Fingerprints or dust on the coverslip will pick up excess dye so be careful to not leave coverslips uncovered or touch them.
References
- 1.Horne-Badovinac S, Bilder D (2005) Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn 232:559–74. 10.1002/dvdy.20286 [DOI] [PubMed] [Google Scholar]
- 2.Haigo SL, Bilder D (2011) Global Tissue Revolutions in a Morphogenetic Movement Controlling Elongation. Science 331:1071–1074. 10.1126/science.1199424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cetera M, Juan GRR-S, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S (2014) Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat Commun 5:5511. 10.1038/ncomms6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isabella AJ, Horne-Badovinac S (2016) Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Dev Cell 38:47–60. 10.1016/j.devcel.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA, Horne- Badovinac S (2013) A Rab10-Dependent Mechanism for Polarized Basement Membrane Secretion during Organ Morphogenesis. Dev Cell 24:159–168. 10.1016/j.devcel.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewellyn L, Cetera M, Horne-Badovinac S (2013) Misshapen decreases integrin levels to promote epithelial motility and planar polarity in Drosophila. J Cell Biol 200:721–729. 10.1083/jcb.201209129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viktorinová I, Dahmann C (2013) Microtubule Polarity Predicts Direction of Egg Chamber Rotation in Drosophila. Curr Biol 23:1472–1477. 10.1016/j.cub.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 8.Chen D-Y, Lipari KR, Dehghan Y, Streichan SJ, Bilder D (2016) Symmetry Breaking in an Edgeless Epithelium by Fat2-Regulated Microtubule Polarity. Cell Reports 15:1125–1133. 10.1016/j.celrep.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squarr AJ, Brinkmann K, Chen B, Steinbacher T, Ebnet K, Rosen MK, Bogdan S (2016) Fat2 acts through the WAVE regulatory complex to drive collective cell migration during tissue rotation. J Cell Biol 212:591–603. 10.1083/jcb.201508081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viktorinová I, Henry I, Tomancak P (2017) Epithelial rotation is preceded by planar symmetry breaking of actomyosin and protects epithelial tissue from cell deformations. Plos Genet 13:e1007107. 10.1371/journal.pgen.1007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alégot H, Pouchin P, Bardot O, Mirouse V (2018) Jak-Stat pathway induces Drosophila follicle elongation by a gradient of apical contractility. Elife 7:e32943. 10.7554/elife.32943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent LG, Manning SA, Kroeger B, Williams AM, Hilmi AJS, Crea L, Kondo S, Horne- Badovinac S, Harvey KF (2019) The dPix-Git complex is essential to coordinate epithelial morphogenesis and regulate myosin during Drosophila egg chamber development. Plos Genet 15:e1008083. 10.1371/journal.pgen.1008083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stedden CG, Menegas W, Zajac AL, Williams AM, Cheng S, Özkan E, Horne-Badovinac S (2019) Planar-Polarized Semaphorin-5c and Plexin A Promote the Collective Migration of Epithelial Cells in Drosophila. Curr Biology 29:908–920.e6. 10.1016/j.cub.2019.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos FC, Dennis C, Alégot H, Fritsch C, Isabella A, Pouchin P, Bardot O, Horne- Badovinac S, Mirouse V (2020) Oriented basement membrane fibrils provide a memory for F- actin planar polarization via the Dystrophin-Dystroglycan complex during tissue elongation. Dev 147:dev186957. 10.1242/dev.186957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherrard KM, Cetera M, Horne-Badovinac S (2021) DAAM mediates the assembly of long- lived, treadmilling stress fibers in collectively migrating epithelial cells in Drosophila. Elife 10:e72881. 10.7554/elife.72881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams AM, Donoughe S, Munro E, Horne-Badovinac S (2022) Fat2 polarizes the WAVE complex in trans to align cell protrusions for collective migration. Biorxiv 2022.01.17.476701. 10.1101/2022.01.17.476701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zajac AL, Horne-Badovinac S (2022) Kinesin-directed secretion of basement membrane proteins to a subdomain of the basolateral surface in Drosophila epithelial cells. Curr Biol 32:735–748.e10. 10.1016/j.cub.2021.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad M, Jang AC-C, Montell DJ (2007) A protocol for culturing Drosophila melanogaster egg chambers for live imaging. Protoc Exch. 10.1038/nprot.2007.233 [DOI] [PubMed] [Google Scholar]
- 19.Prasad M, Montell DJ (2007) Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell 12:997–1005. 10.1016/j.devcel.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 20.Cetera M, Lewellyn L, Horne-Badovinac S (2016) Drosophila, Methods and Protocols. Methods Mol Biology Clifton N J 1478:215–226. 10.1007/978-1-4939-6371-3_12 [DOI] [PubMed] [Google Scholar]
- 21.Dixit R, Ross JL (2010) Chapter 27 Studying Plus-End Tracking at Single Molecule Resolution Using TIRF Microscopy. Methods Cell Biol 95:543–554. 10.1016/s0091-679x(10)95027-9 [DOI] [PubMed] [Google Scholar]
- 22.Chanet S, Huynh J-R (2020) Collective Cell Sorting Requires Contractile Cortical Waves in Germline Cells. Curr Biol. 10.1016/j.cub.2020.08.045 [DOI] [PubMed] [Google Scholar]
- 23.Wilcockson SG, Ashe HL (2019) Drosophila Ovarian Germline Stem Cell Cytocensor Projections Dynamically Receive and Attenuate BMP Signaling. Dev Cell 50:296–312.e5. 10.1016/j.devcel.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcockson SG, Ashe HL (2021) Live imaging of the Drosophila ovarian germline stem cell niche. Star Protoc 2:100371. 10.1016/j.xpro.2021.100371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond-Barbosa D, Spradling AC (2001) Stem Cells and Their Progeny Respond to Nutritional Changes during Drosophila Oogenesis. Dev Biol 231:265–278. 10.1006/dbio.2000.0135 [DOI] [PubMed] [Google Scholar]
- 26.Mazzalupo S, Cooley L (2006) Illuminating the role of caspases during Drosophila oogenesis. Cell Death Differ 13:1950–1959. 10.1038/sj.cdd.4401892 [DOI] [PubMed] [Google Scholar]
- 27.Pritchett TL, Tanner EA, McCall K (2009) Cracking open cell death in the Drosophila ovary. Apoptosis 14:969. 10.1007/s10495-009-0369-z [DOI] [PMC free article] [PubMed] [Google Scholar]