Abstract
Background
Green foxtail [Setaria viridis (L.)] is one of the most abundant and troublesome annual grass weeds in alfalfa fields in Northeast China. Synthetic auxin herbicide is widely used in agriculture, while how auxin herbicide affects tillering on perennial grass weeds is still unclear. A greenhouse experiment was conducted to examine the effects of auxin herbicide 2,4-D on green foxtail growth, especially on tillers.
Results
In the study, 2,4-D isooctyl ester was used. There was an inhibition of plant height and fresh weight on green foxtail after application. The photosynthetic rate of the leaves was dramatically reduced and there was an accumulation of malondialdehyde (MDA) content. Moreover, applying 2,4-D isooctyl ester significantly reduced the tillering buds at rates between 2100 and 8400 ga. i. /ha. Transcriptome results showed that applying 2,4-D isooctyl ester on leaves affected the phytohormone signal transduction pathways in plant tillers. Among them, there were significant effects on auxin, cytokinin, abscisic acid (ABA), gibberellin (GA), and brassinosteroid signaling. Indeed, external ABA and GA on leaves also limited tillering in green foxtail.
Conclusions
These data will be helpful to further understand the responses of green foxtail to 2, 4-D isooctyl ester, which may provide a unique perspective for the development and identification of new target compounds that are effective against this weed species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10579-6.
Keywords: Setaria viridis; Tillering; Phytohormones; Gene expression; 2,4-D isooctyl ester
Introduction
Green foxtail (Setaria viridis L.) is one of the most abundant and troublesome annual grass weeds in crop and alfalfa fields in China [1, 2]. Green foxtail belongs to the family Gramineae, with the characteristics of drought resistance [3]. Because of its strong adaptability and tillering ability, green foxtail is regarded as a common malignant weed [4]. It has been revealed that when the green foxtail density is above the threshold of 32 plants/m2, it causes loss rate of alfalfa yield in alfalfa fields [5]. Tillering is an important agronomic trait of Poaceae that greatly determines seed output [6]. For crops, such as cucumber and sorghum, strong tillering ability can increase food production [7, 8]. However, it will increase the production of weed seeds, accelerating their reproduction and improving the propagation coefficient [9]. Since weed infestation can cause losses of crop production due to the competition with the water, light, nutrient, or other resources, weed management is important for crop protection [10–12]. Thus, decreasing the number of tillering would be a strategy for controlling green foxtail.
Tillers are generally initiated by the establishment and elongation of tiller buds, which can be manipulated by phytohormones [13]. It is well-known that phytohormones like auxin, cytokinin, gibberellic acid (GA) and strigolactone (SL) play important roles in tillering of monocots and branching of dicots. Auxin is known as the first hormone to regulate shoot branching [14]. Removing the apex abolish the inhibition of axillary bud outgrowth and plants will start branching [15]. External application of auxin inhibit the growth of tiller buds in rice and wheat [16, 17]. Cytokinin plays a positive role in axillary bud outgrowth, and exogenous application of cytokinin promotes axillary bud outgrowth in plants [18]. GA can inhibit tillering, mainly due to its antagonistic interaction with cytokinin [18]. Exogenous SL supply has been reported to inhibit tiller or branch production by reducing the cytokinin content in the tiller or branch buds [19, 20]. Therefore, the interactions of auxin, cytokinin and SL control the regulation of tiller bud development [21, 22]. Thus, synthetic hormones would be a strategy for weeds control by decreasing plant tillers [23].
Synthetic auxins are used as growth regulators for yield improvement in agriculture, and as herbicides for weeds control [23]. The 2,4-dichlorophenoxy acetic acid (2,4-D) is one of the most widely used synthetic auxin herbicides to control weeds in pastures, especially for some Poaceae crops, such as wheat and maize [24, 25]. 2,4-D is developed during World War II and there are over 600 2,4-D products currently on the market including 2,4-D isooctyl ester [26]. It is absorbed through roots, stems, and leaves, and then it is translocated to the meristems of the plant, interfering with plant physiological processes [26, 27]. 2,4-D controls many broadleaf weeds and spare monocots, such as Chenopodium album, Xanthium sibiricum, and Sonchus arvensis [28]. For Euphorbia heterophylla to obtain 90% of control, the required dose was 3710 g a.e./ha [29]. Digitaria insularis and Amaranthus hybridus were more sensitive to the herbicide because 90% control was obtained with doses close to 755, and 269 g a.e./ha, respectively [29]. Monocots, particularly grasses, may perceive or respond differently to exogenous synthetic auxins compared to dicots [26]. It is proposed that there is a difference in vascular tissue structure between dicots and monocots, contributing to the selectivity of auxinic herbicide [26].
The 2,4-D has been reported to be used in controlling green foxtail growth, while many studies have recommended the use of clethodim and glyphosate to control green foxtail rather than 2,4-D [14, 17]. Little is understood regarding the effects of 2,4-D isooctyl ester on plant and tiller bud growth in green foxtail under different application concentrations. Moreover, the mechanism of different tillering responses of 2,4-D isooctyl ester at different concentrations is still unclear.
Therefore, the objectives of this study are to (i) evaluate the effect of 2,4-D isooctyl ester on the outgrowth of tiller buds in S. viridis and (ii) identify metabolic pathways and candidate genes involved in the diverse tillering sensitivities at different concentrations of 2,4-D isooctyl ester by transcriptomic analysis. Undoubtedly, the results will be highly valuable to further understand the regulatory mechanism of auxin on tillering growth and provide new ideas for solving the challenges of plant hormone selectivity for green foxtail and other grass weeds.
Results
Dose-dependent effects of 2,4-D isooctyl ester on plant growth in green foxtail
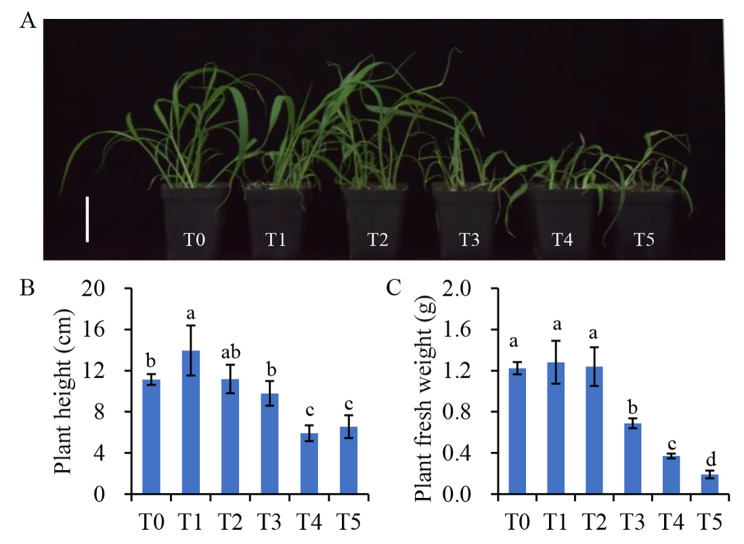
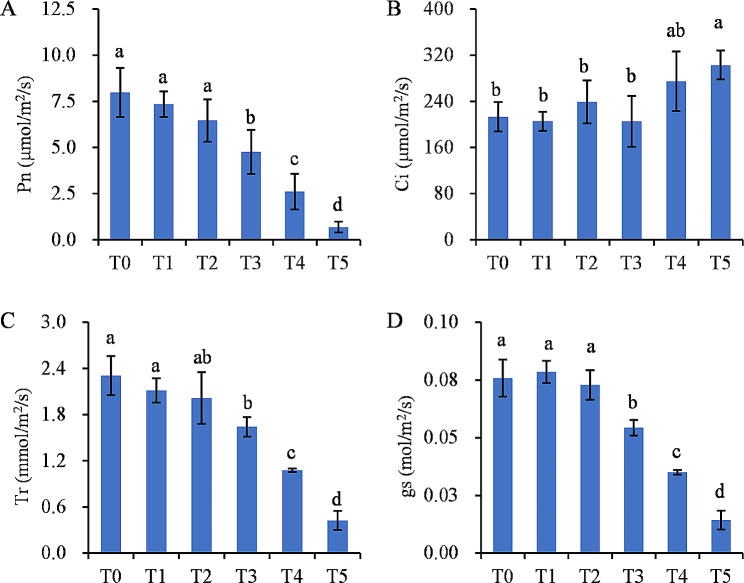
The objective of this study was to evaluate the effects of 2,4-D isooctyl ester on plant growth in green foxtail. In doing so, green foxtail was treated with different dosages of 2,4-D isooctyl ester and plant height, plant fresh weight, and leaf net photosynthetic rate were measured 7 days post-treatment. External 2,4-D isooctyl ester spraying had a significant effect on plant growth (P < 0.05, Fig. 1). The plant height increased and then decreased significantly after high-dose application of 2,4-D isooctyl ester (Fig. 1A and B). There was no significant effect on plant fresh weight at lower concentrations of 525 and 1050 g a.i./ha, but the plant fresh weight dramatically decreased at high concentrations (P < 0.05, Fig. 1B). Since part of the plant leaves were wilted and yellowed after treatment, the photosynthetic parameters were measured. The plant net photosynthetic rate (Pn) displayed a dramatic reduction under 2,4-D isooctyl ester treatment compared with the control, with the stomatal conductance (gs) and the transpiration rate (Tr) also reduced after treatment (P < 0.05, Fig. 2). Indeed, the malondialdehyde (MDA) content accumulated in the leaves of green foxtail after 2,4-D isooctyl ester treatment when the level was above 2100 g a.i. /ha (Additional file 1: Figure S1). The peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) enzyme activities were enhanced in all treatments (P < 0.05, Additional file 1: Figure S1).
Fig. 1.
Effect of 2,4-D isooctyl ester on green foxtail after 7 days treatment. Plant phenotype (A), shoot height (B), and fresh weight (C) were measured after 7 days treatment. T0 to T5 represented concentrations of 0, 525, 1050, 2100, 4200, and 8400 g a.i./ha, respectively. The bar in Fig. 1A indicates 5 cm. Means followed by different lowercase letters are significantly different at P < 0.05
Fig. 2.
Effect of 2,4-D isooctyl ester on leaf net photosynthetic rate (A), intercellular CO2 concentrations (B), transpiration rate (C), and stomatal conductance (D) of green foxtail after 7 days treatment. T0 to T5 represented concentrations of 0, 525, 1050, 2100, 4200, and 8400 g a.i./ha, respectively. Pn, net photosynthetic rate; Ci, intercellular CO2 concentrations; Tr, transpiration rate; gs, stomatal conductance. Means followed by different lowercase letters are significantly different at P < 0.05
Effects of 2,4-D isooctyl ester on tiller production in green foxtail
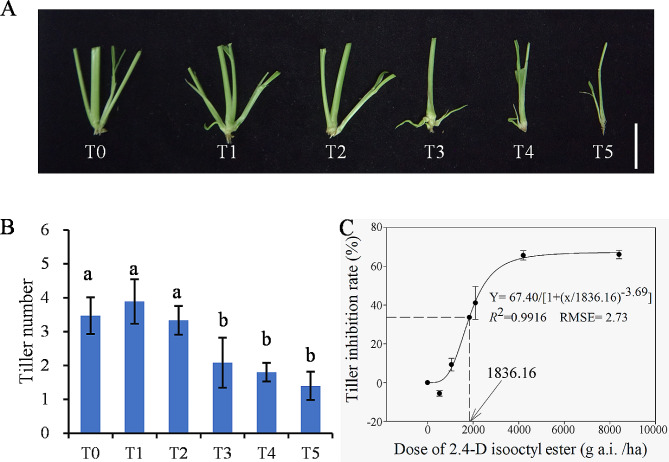
The 2,4-D isooctyl ester treatment affected not only the plant height and fresh weight but also the tiller number (Fig. 3). It had no significant effect on the tiller development at 7 days after treatment (DAT) under 525 and 1050 g a.i./ha compared to the control (Fig. 3A and B). When the dose was increased to 2100 g a.i./ha, it showed a significant inhibition effect on tiller number (Fig. 3A and B). The 2,4-D isooctyl ester dose required for 50% growth inhibition of tiller was estimated to be approximately 1836.16 g a.i./ha in green foxtail (Fig. 3C). Considering the 50% growth inhibition effect and the effects of 2,4-D isooctyl ester on plant growth, T4 was chosen for further analysis of effects of 2,4-D isooctyl ester on green foxtail tillers.
Fig. 3.
Effect of 2,4-D isooctyl ester on tillers in green foxtail after 7 days treatment. Plant tiller phenotype (A), tiller number (B), and dose response of tiller growth (C) were measured after 7 days treatment. T0 to T5 represented concentrations of 0, 525, 1050, 2100, 4200, and 8400 g a.i./ha, respectively. The bar in Fig. 3A indicates 2 cm. Means followed by different lowercase letters are significantly different at P < 0.05. The solid lines in Fig. 3C represent a three-parameter logistic model fitted to the data. Y (%) = 67.40/[1+ (x/1836.16)−3.69], R2 = 0.9916, RMSE = 2.73. Data represent the mean ± standard error of the mean (n = 30)
Differentially expressed genes of 2,4-D isooctyl ester treatment on tiller in green foxtail
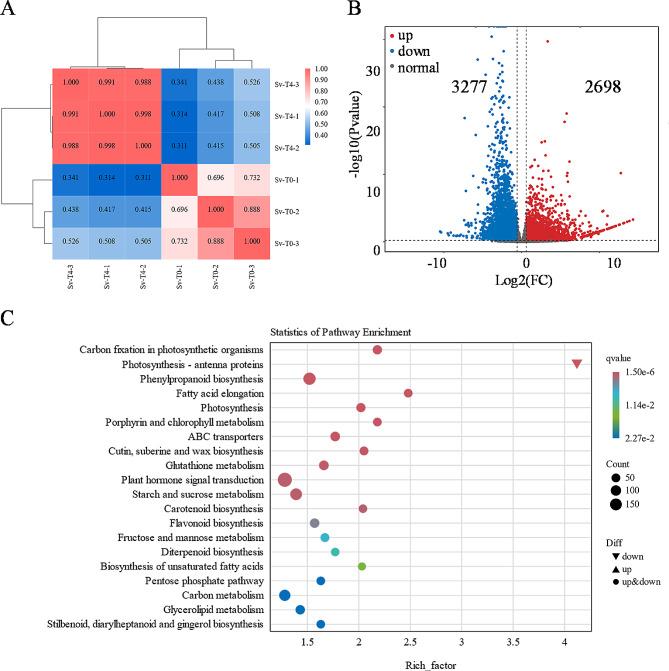
To analyze the 2,4-D isooctyl ester effects on tiller growth, tillers treated with 0 and 4200 g a.i./ha concentrations were used for transcriptome analysis. A total of 40.53 million reads to 56.80 million reads were obtained after RNA-Seq (Additional file 2: Table S1). Among them, 95.09–96.17% of the clean reads were successfully mapped to the green foxtail genome with a unique map ranging from 91.59 to 93.70% (Additional file 2: Table S1). The Pearson’s correlation coefficient heated map showed that samples under treatment clustered together (Fig. 4A). Based on the gene differential expression threshold of |log2fold change| ≥ 1 and false discovery rate (FDR) < 0.01, a total of 5975 differentially expressed genes (DEGs) were detected in tiller buds, of which 2698 DEGs were up-regulated and 3277 were down-regulated under treatment at 7 DAT (Fig. 4B; Additional file 1: Table S2).
Fig. 4.
Pearson’s correlation (A), differentially expressed genes (B), and KEGG enrichment analysis (C) in green foxtail. Fold enrichment in a pathway means the ratio of the number of observed DEGs and expected DEGs. T0 and T4 represented concentrations of 0 and 2100 g a.i./ha, respectively
GO and KEGG Enrichment Analysis
To gain insights into the DEGs functional categories, gene ontology (GO) enrichment analysis was used to evaluate the specific functional role of all DEGs. The “cellular process” and “metabolic process” were the top GO terms in the “biological process” group. The “cellular anatomical entity”, “intracellular” and “protein-containing complex” were enriched in “cellular component” group ranked by gene number. The “binding”, “catalytic activity” and “transporter activity” categories were the most enriched GO terms in the “molecular function” group (Additional file 1: Figure S2).
To understand the functional enrichment classification of DEGs, KEGG analysis was carried out. The top 15 enriched pathways at 7 DAT including Carbon fixation in photosynthetic organisms, Photosynthesis-antenna proteins, Phenylpropanoid biosynthesis, Fatty acid elongation, Photosynthesis, Porphyrin and chlorophyll metabolism, and Plant hormone signal transduction (q < 0.05) (Fig. 4C). The plant net photosynthetic rate was reduced in the T4 (Fig. 2) and the soil and plant analyzer development (SPAD) was also reduced in the leaves (Additional file 1: Figure S3). Although the 2,4-D isooctyl ester was sprayed on the leaves, genes involved in plant hormone signal transduction accumulated after treatment. It was speculated that 2,4-D isooctyl ester might affect the plant hormone signal transduction and disturb the distribution and equilibrium of other plant hormones in the tillers.
Differentially expressed genes analysis
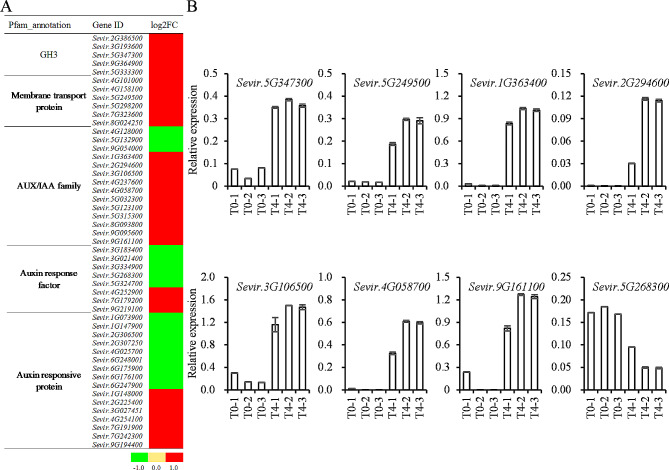
Given the above speculation, plant hormone metabolism and signal transduction pathways were further analyzed. DEGs were highly enriched in the plant hormone signal transduction pathways in tiller buds of green foxtail under treatment according to the KEGG enrichment analysis (Fig. 4C). A total of 49 DEGs related to auxin signal transduction pathway were identified (Fig. 5A). Among them, the expression levels of GH3 genes were highly enhanced after 2,4-D isooctyl ester treatment (Fig. 5A). Clearly, the expression levels of auxin/indoleacetic acid (AUX/IAA), auxin response factor (ARF), and small auxin-up RNA (SAUR) genes were also regulated after 2,4-D isooctyl ester treatment. To verify this result, nine DEGs related to auxin signal transduction were randomly selected and their expression levels were validated by qRT-PCR. Indeed, the RNA-seq DEGs also differentially expressed between treatments via qRT-PCR (Fig. 5B). Genes such as Sevir.5G347300, Sevir.5G249500, Sevir.1G363400, and Sevir.2G294600 were up-regulated after treatment in RNA-seq analysis, and they were also up-regulated in RT-qPCR (Fig. 5). For gene Sevir.5G268300, it was down-regulated after treatment in both RNA-seq and RT-qPCR analysis (Fig. 5).
Fig. 5.
The expression level of DEGs related with auxin signal transduction in green foxtail. (A) DEGs related with auxin signal transduction in green foxtail log2(fold change) are shown. (B) Relative expression of DEGs associated in green foxtail by qRT-PCR. T0-1, T0-2, and T0-3 represented the control with three biological replicates and each was done with three technical replicates. T4-1, T4-2, and T4-3 represented the treatment with three biological replicates and each was done with three technical replicates
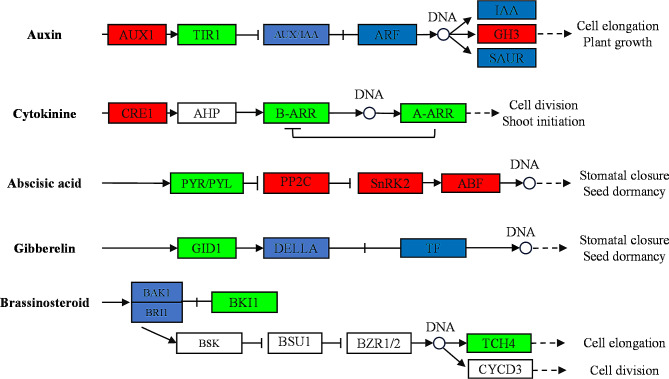
Similar results were also found in the cytokinin (CTK), abscisic acid (ABA), gibberellin (GA), and brassinosteroid (BR) synthesis and signal transduction pathways (Fig. 6; Additional file 1: Table S3). Two DEGs (Sevir.1G319700 and Sevir.4G202400) annotated as cytokinin receptors (histidine kinase 2/3/4, AHK2_3_4) were up-regulated and cytokinin regulators encoding genes of cytokinin signaling response regulators Type-A and type-B were down-regulated. The genes encoding gibberellin receptor GID1 (Sevir.2G045600, Sevir.2G395200, Sevir.2G422700, and Sevir.8G084700) were down-regulated. Genes encoding abscisic acid receptor PYR/PYL family (Sevir.3G213000, Sevir.7G137000, and Sevir.9G438800) were down-regulated and their down-regulated genes encoding ABA responsive element binding factor (ABF; Sevir.2G239700, Sevir.2G452300, and Sevir.4G081100) were up-regulated. In the BR signal transduction pathway, TCH4, encoding a xyloglucan endo-transglycosylase, was down-regulated after 2,4-D isooctyl ester treatment. Therefore, it was speculated that the different impacts of 2,4-D isooctyl ester on tillering growth in green foxtail were related to different plant hormone metabolism and signal transduction responses.
Fig. 6.
Map of the plant hormone signal transduction pathway. The red and green rectangles indicate the DEGs up-regulated and down-regulated in the 2, 4-D isooctyl ester treatment, respectively. The blue rectangle indicates that the DEGs containing both up-regulated and down-regulated
MIR156 regulatory module is well believed to be involved in branching and tillering development in plant species [30]. Besides the above-mentioned genes in the plant hormone signal transduction pathways, transcriptomic analysis showed that six DEG belonging to SPL (SQUAMOSA PROMOTER BINDING PROTEIN-LIKE) family (Sevir.2G276900, Sevir.4G200400, Sevir.2G336300, Sevir.2G336100, Sevir.3G023500, and Sevir.1G065500) was down-regulated.
Effects of phytohormones on tiller in green foxtail
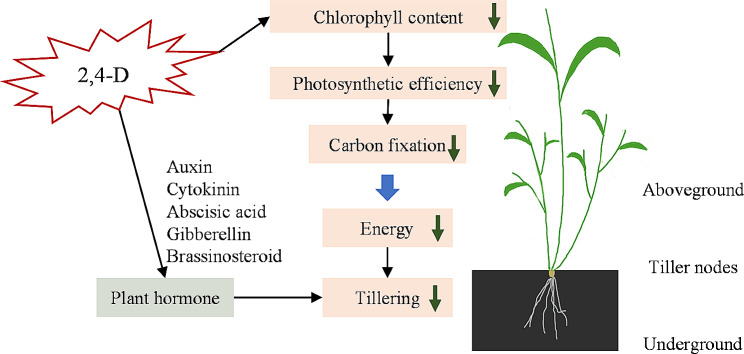
Since genes involved in the phytohormones signal transduction pathways were regulated and the contents of ABA and tZR were changed in the tiller after 2,4-D isooctyl ester treatment, it was speculated that spaying other phytohormones besides auxin might also change tiller growth in green foxtail. To determine effects of different concentrations of other phytohormones (GA3, ABA, and 6-Benzylaminopurine) on tiller development, seedlings were foliar sprayed with 0, 300, 600, and 1200 mg/L. Indeed, spraying GA3 and ABA on leaves inhibited tiller growth (Additional file 1: Figure S4). But there was no significant effect after 6-BA application on tiller number (Additional file 1: Figure S4). Here, spraying 2,4-D isooctyl ester can affect the leaf chlorophyll content and reduce its photosynthetic efficiency, which might reduce plant carbon fixation (Fig. 7). On the other way, the treatments would affect the plant hormone distribution in the tillering buds. The reduced energy and varied plant hormone distribution affected the tiller growth (Fig. 7).
Fig. 7.
The regulatory network of green foxtail tillering response to 2,4-D isooctyl ester
Discussion
Synthetic hormones are used as growth regulators for improving crop yield in agriculture, and as herbicides for controlling weeds [23]. The 2,4-D is one of the most widely used synthetic auxin herbicides for the control of weeds in pastures, especially for some Poaceae crops for the control of broadleaf weeds and spare monocots [24, 25, 28]. But the effects of 2,4-D on green foxtail, especially on tillering, are unclear. Here, the effects on plant growth especially for the tiller bud were analyzed under different dose of 2,4-D isooctyl ester. Our study showed that high dose application of 2,4-D isooctyl ester inhibited plant growth and reduced the number of tiller buds significantly.
Tillering is controlled by genetic factors and environmental factors [31, 32]. Plant carbohydrate status is well known to affect tillering, and therefore reducing carbohydrate supply have been developed to account for how this factor modulates tillering under field conditions [33, 34]. Changes in photosynthetic leaf area affect the propensity of tiller buds for outgrowth in sorghum [35]. In the study, the experiment was conducted in pots with a relatively uniform potting medium, which could supply sufficient nutrient. Also, plants were grown with a same planting density in a controlled environment. Thus, planting density and nutrient scavenging were not the considered factors in the study. Sugars are necessary for the release of axillary buds for their apical dominance [36]. Indeed, in the study the SPAD value and photosynthetic efficiency were dramatically reduced. Application of 2,4-D isooctyl ester reduced the plant photosynthesis, thereby reducing the carbon fixation. The declined carbon fixation and limited energy supply might suppress the growth of tillers. Here, it is speculated that the photosynthesis of green foxtail after 2,4-D isooctyl ester treatment is weakened, unable to provide sufficient energy and produce less sugar for tiller bud development, which would partially explain the decrease in the number of tillers after the application of 2,4-D isooctyl ester.
Phytohormones also play important roles in tiller bud outgrowth. The regulation of plant lateral growth by auxin has been reported in various plant species, but simulative and suppressive effects have also been reported depending on the plant species and auxin concentration [16, 17, 28]. No differences in giant foxtail control were observed among herbicide application methods for glyphosate using either dicamba or 2,4-D isooctyl ester [28]. But the tiller buds are inhibited in crops such as rice and wheat after auxin was applied [16, 17]. Spraying indole-3-acetic acid (IAA) and naphthyl acetic acid (NAA, a synthetic auxin) on rice significantly restricted tiller bud elongation [16]. In wheat, tiller bud development was also completely inhibited by exogenous application of IAA [17]. Here, a relative high concentration of auxin on green foxtail would inhibit the tiller bud growth. Thus, application of synthetic auxin for weeds control depends on the species and the dosage.
Since the auxin pesticides were sprayed on leaves, it was unknown how it affected the tillering of green foxtail. Through the transcriptome data, the auxin transport and response genes were regulated after 2,4-D isooctyl ester application. Clearly, the expression levels of GH3, AUX/IAA, ARF, and SAUR genes were also regulated after 2,4-D isooctyl ester treatment, suggesting that auxin may participate in the regulation of tillering through influencing the balances of endogenous auxin metabolism and signaling. 2,4-D isooctyl ester can be moved throughout the plant through the phloem, and the reduction in tillers might cause by the variation of the phytohormone distribution in seedlings [28].
Besides the auxin response genes, other responses genes of phytohormones like CTK, GA, ABA, and BR were also regulated. Gene TCH4, encoding xyloglucan endotransglucosylase, was down-regulated in tillers after 2,4-D isooctyl ester application. BR is also a typical hormone controlling tiller initiation and there is a cross talk between auxin and BR signaling pathways [37–39]. TCH4 can be regulated by BR because the promoter region of TCH4 contains cis-regulatory element e-boxes binding to the transcription factor BES1 in the BR signaling pathway [40]. Genes involved in auxin were significantly up-regulated in plant roots under BR treatment [37, 39]. CTK plays a positive role in axillary bud outgrowth and the CTK-mediated genes A-ARR have been reported to be required for bud release in Arabidopsis [41, 42]. Exogenous application CTK promotes axillary bud outgrowth, which is accompanied by increases in CK content in buds or adjacent nodes [18]. The CTK signal transduction genes, A-ARR and B-ARR, were found to be down-regulated after 2,4-D isooctyl ester application, which indicated that the application of 2,4-D isooctyl ester could influence genes involved in CTK. Moreover, A-ARR may be the key factor in CTK signal transduction contributing to SLs-induced bud inhibition. SL represses auxin transport from the buds, thus inhibiting bud outgrowth whereas auxin up-regulates SL production to control apical dominance [19, 43]. Moreover, SLs can suppress the A-ARR expression independent of CTK signal transduction. ABA is associated with bud dormancy through an auxin-independent mechanism [44]. Therefore, application 2,4-D isooctyl ester on the leaves affected the expression of several hormone signal transduction genes and might affect distribution of phytohormone levels in tillers. In addition to the hormone signal transduction genes, it is generally believed that the MIR156-SPL module participates in the regulation of plant architecture, including tillering [30]. In treated green foxtail plants, the expression levels of six SPL genes differed from those in untreated plants. It is speculated that variation of distribution of hormone levels would be another main reason affecting the tillering of green foxtail after 2,4-D isooctyl ester treatment.
Conclusion
Tiller production is important for grass weeds control. The 2,4-D isooctyl ester is one of the most widely used synthetic auxin herbicides to control weeds in pastures. However, how 2,4-D isooctyl ester affects tillering in grass weeds is still unclear. This study aimed to elucidate the roles and the underlying mechanisms of auxin in regulating tiller development. Green foxtail seedlings were treated with different concentrations of 2,4 isooctyl ester and dose-dependent inhibitory effects on tiller production were observed. Auxin-inhibition of tillering was mainly due to its effect on inhibition of photosynthesis and the distribution of other plant hormones, as shown by the changed plant hormone signal transduction expression in 2,4 isooctyl ester treated plants. Furthermore, auxin could act through regulating the expression of SPL specifically expressed in axillary buds to induce bud outgrowth. These results provide insights into the regulatory mechanisms of auxin for tiller bud outgrowth through crosstalk with phytohormones.
Materials and methods
Plant Material, growth conditions
Wild-type A10.1 seeds of green foxtail (S. viridis) were germinated in petri dishes for seven days. After emergence, green foxtail seedlings were sown to six uniform seedlings per pot containing a mixture of soil, vermiculite, and organic fertilizer (3:1:1, v/v/v) and grown under greenhouse conditions at a temperature of 25 °C, with a 16 h/8 h photoperiod at a relative humidity of 65%. The plants were watered weekly with Hoagland solution.
2,4-D isooctyl ester treatment
2,4-D isooctyl ester 50% emulsifiable concentrate (EC) was provided by Shandong Zhongshi Pharmaceutical Co., Ltd. (Liaocheng, China). Twenty-five-day-old seedlings, reached the 3- to 4-leaf stage and the length of the first tiller bud was less than 0.5 cm, were treated with 2,4-D isooctyl ester. Based on the recommended concentration approximately 500 to 1000 g a.i./ha (a.i., active ingredient), the treatments consisted of applications of the herbicide 2,4-D isooctyl ester at doses of 0, 525, 1050, 2100, 4200, and 8400 g a.i./ha. Deionized water spray was used as a control. Spraying was performed using a 3WP-2000 Walking Spray Tower (Nanjing Institute of Agricultural Mechanization, Ministry of Agriculture and Rural Affairs, Nanjing, China). The tiller buds were measured in 30 seedlings per treatment at 7 DAT, and the experiment was conducted in three independent replicates.
External application of ABA, GA3, and 6-BA
To ascertain the effects of exogenous ABA, GA and CTK on tiller growth, ABA (catalog number A8060, Solarbio, China), GA3(catalog number G8910, Solarbio, China), and 6-BA (catalog number A8170, Solarbio, China) were applied to green foxtail. Spraying was performed using a 3WP-2000 Walking Spray Tower (Nanjing Institute of Agricultural Mechanization, Ministry of Agriculture and Rural Affairs, Nanjing, China). Uniform 20-day-old seedlings with the length of the first tiller bud less than 0.5 cm were treated with ABA, GA3 or 6-BA at concentrations of 300, 600, and 1200 mg/L. Deionized water was applied as a control. Plants were sprayed twice with a 3WP-2000 Walking Spray Tower. Each treatment included five pots containing six seedlings each, and the experiment was repeated three times.
Measurement of Leaf photosynthetic rate and measurement of enzyme activity assay
The photosynthetic rate of the uppermost expanded leaves was measured with an LI-6800 portable photosynthesis system at 7 DAT. The photosynthetically active radiation was set to 800 µmol/m2/s. The freeze-dried and powered leaf samples were used to analyze the content of MDA and enzyme assay activities of CAT, POD, and SOD [45].
Sample Preparation for RNA sequencing, qPCR validation
Tiller buds of 1–2 cm were collected at 7 DAT and then immediately frozen in liquid nitrogen and stored at − 80 °C and total RNA was extracted with the plant RNA kit (catalog number RC411-01, Vazyme, China) according to the manufacturer’s instruction. RNA concentration and quality were assessed using a NanoDrop2000 and an Agilent 2100. The mRNA was obtained by enriching qualified RNA using magnetic beads equipped with oligo (dT). The library was constructed and the quality of the constructed libraries was strictly monitored before sequencing with an Illumina NovaSeq 6000.
A total of 1 µg RNA was used for the synthesis of first-strand cDNA for qRT-PCR (catalog number R323-01, Vazyme, China). qPCR was carried out according to MIQE guidelines with SYBR Green (Takara, Tokyo, Japan) using the CFX96 (Bio-Rad, USA). The β-actin gene Sevir.7G305900 was used as the reference gene to normalize the target gene expression levels in green foxtail and the 2−ΔΔCT method was used to calculate the gene expression level [46]. All qPCR primers are shown in Table S4.
Differential expression and Gene Ontology enrichment analysis
Quality control of raw reads was performed to remove adapter sequences and clean reads were mapped against the Setaria viridis reference genome (Setaria viridis v4.1, Phytozome) using HISAT2 software (version 2.1.0) [47]. For quality control, raw data were filtered by removing the reads with adapter pollution, unknown nucleotides more than 5%, or Q20 value ≤ 20% (percentage of sequences with sequencing error rates < 1%), and the remaining highly qualitative clean reads were then subjected to further analyses. Gene expression levels were estimated using fragments per kilobase of exon per million fragments mapped (FPKM) values by the Cufflinks software. The comparison of gene expression differences was carried out between treatment (T4) and control (T0) based on the ratio of FPKM values using the DESeq2 software (version 1.22.1) [48]. GO annotation was obtained using Blast2 GO program (version 3.0.8) at a threshold e-value ≤ 1e–5 [49]. All DEGs were subjected to GO enrichment analysis using the GOseq R package based on FDR and fold change (FC) of genes betweenT4 and T0, with a level of | log2FC| ≥ 1 and FDR < 0.05 as the threshold. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation was obtained using KEGG Automatic Annotation Server (KAAS) and KEGG analysis was also performed on the DEGs using cluster Profiler R packages to find KEGG pathway [50, 51].
Statistical analysis
All experiments were performed with three replicates, and the means and standard deviations were calculated. ANOVA was employed to evaluate the effect of external treatment with 2,4-D isooctyl ester using IBM SPSS software. Differences were distinguished by LSD test at the 0.05 probability level.
The tiller bud inhibition rate collected was modelled using logistic models. SigmaPlot (Version 11, Sysat Software, Inc., Point Richmond, CA, USA) was used to perform nonlinear regression analysis to determine how inhibition rate was affected by 2,4-D isooctyl ester dosage. The goodness-of-fit of models was assessed according to their R2 and root mean-square error (RMSE) values. Nonlinear regression analysis was applied to calculate the effect of 2,4-D isooctyl ester concentration on tiller inhibition rate analysis with the following functional three-parameter logistic model:
Y = A/[1+(x/x50)B]
Here, Y is the percentage (%) of inhibition rate under different concentration of 2,4-D isooctyl ester treatments, A is the maximum germination or emergence (%), x50 is the 2,4-D isooctyl ester dosage required for 50% of the highest inhibition rate and B represents the slope [52].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- 2,4-D
2,4-dichlorophenoxy acetic acid
- MDA
Malondialdehyde
- ABA
Abscisic acid
- GA
Gibberellic acid
- SL
Strigolactone
- Pn
net photosynthetic rate
- Gs
stomatal conductance
- Tr
Transpiration rate
- POD
peroxidase
- SOD
superoxide dismutase
- CAT
catalase
- DAT
Days after treatment
- FDR
false discovery rate
- DEG
Differential expression gene
- GO
Gene Ontology
- SPAD
Soil and plant analyzer development
- AUX
Auxin
- IAA
Indole-3-acetic acid
- AFR
Auxin response factor
- SAUR
Small auxin-up RNA
- CTK
Cytokinin
- BR
Brassinosteroid
- ABF
element binding factor
- tZR
trans-Zeatin Riboside
- 6-BA
6-Benzylaminopurine
- NAA
naphthyl acetic acid
- FPKM
Fragments per kilobase of exon model per million mapped reads
- FC
fold change
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KAAS
KEGG Automatic Annotation Server
- RMSE
Root mean-square error
Author contributions
SJ, and XW conducted experimental design and manuscript writing. XW, JX, WQ, ZN, GH conducted the experiments. XW, WQ, and ZL participated in data collection and analysis. All authors approved the submitted version.
Funding
This work was supported by grants from China Agriculture Research System (CARS-34), Natural Science Foundation of Shandong Province (ZR2023MC071), Innovation Training Program for college students in Shandong province (S202310435231), and Qingdao Agricultural University high-level talent research fund (Grant No. 1120007).
Data availability
Sequence data that support the findings of this study have been deposited in NCBI SRA BioProject with accession number of PRJNA1111665.
Declarations
Ethics approval and consent to participate
This study does not address ethical issues.
Wild-type A10.1 seeds of green foxtail (Setaria viridis) were obtained from the GermplasmResources Information Network (GRIN, http://www.ars-grin.gov/), and it was used for research study only. All the study procedures were carried out in accordance with relevant guidelines.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wangdan Xiong and Xinfeng Jia are co-first author.
References
- 1.Zhang Y, Tian J, Wen Y, Zhao J, Sun J. The research on occurrence trends of weeds in alfalfa fields in autumn of Qingdao area. J Hebei Agri Sci. 2013;17(2):47–50. [Google Scholar]
- 2.Yu J, Li Q, Zhang W, Bai P, Huo J, Liu Y, Gao W. First report of causing anthracnose of green foxtail (Setaria viridis) in China. Plant Dis. 2023;107(3):939–939. doi: 10.1094/PDIS-02-22-0352-PDN. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Brutnell TP. Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J Exp Bot 2011; 62(9):3031–3037. [DOI] [PubMed]
- 4.Keshavarzi M, Seifali M. Systematic study of weedy species of Setaria (L.) P. Beauv. (Poaceae) in Iran. Pak J Biol Sci. 2007;10(19):3362–7. doi: 10.3923/pjbs.2007.3362.3367. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wei Y. The study of the pathogen and host of thickspike wildrye stripe mosaic disease. Pratac Sci. 1992;9(6):21–3. [Google Scholar]
- 6.Li C. Toward understanding the stem-cell origin and molecular regulation of rice tillering. J Genet Genomics. 2015;42(2):47–8. doi: 10.1016/j.jgg.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Chen J, Zhang X. Genetic regulation of shoot architecture in cucumber. Hortic Res. 2021;8(1):143. doi: 10.1038/s41438-021-00577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafarge TA, Hammer GL. Tillering in grain sorghum over a wiide range of population densities: modelling dynamics of tiller fertility. Ann Bot. 2002;90(1):99–110. doi: 10.1093/aob/mcf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Cui H, Chen J, Li X. Regulation of Aegilops tauschii coss tiller bud growth by plant density: transcriptomic, physiological and phytohormonal responses. Front Plant Sci. 2020;11:1166. doi: 10.3389/fpls.2020.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scavo A, Mauromicale G. Integrated weed management in herbaceous field crops. Agronomy-Basel 2020; 10(4).
- 11.Singh M, Kukal MS, Irmak S, Jhala AJ. Water Use characteristics of weeds: A Global Review, Best practices, and future directions. Front Plant Sci. 2021;12:794090. doi: 10.3389/fpls.2021.794090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringselle B, Prieto-Ruiz I, Andersson L, Aronsson H, Bergkvist G. Elymus repens biomass allocation and acquisition as affected by light and nutrient supply and companion crop competition. Ann Bot. 2017;119(3):477–85. doi: 10.1093/aob/mcw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Hiroshi F, et al. Control of tillering in rice. Nature. 2003;422(6932):618–21. doi: 10.1038/nature01518. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu-Sato S, Tanaka M, Mori H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol. 2009;69(4):429–35. doi: 10.1007/s11103-008-9416-3. [DOI] [PubMed] [Google Scholar]
- 15.Ongaro V, Leyser O. Hormonal control of shoot branching. J Exp Bot. 2008;59(1):67–74. doi: 10.1093/jxb/erm134. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L) Plant Cell Rep. 2015;34(9):1647–62. doi: 10.1007/s00299-015-1815-8. [DOI] [PubMed] [Google Scholar]
- 17.Cai T, Meng X, Liu X, Liu T, Wang H, Jia Z, Yang D, Ren X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front Plant Sci 2018; 9. [DOI] [PMC free article] [PubMed]
- 18.Zhuang L, Ge Y, Wang J, Yu J, Yang Z, Huang B. Gibberellic acid inhibition of tillering in tall fescue involving crosstalks with cytokinins and transcriptional regulation of genes controlling axillary bud outgrowth. Plant Sci. 2019;287:110168. doi: 10.1016/j.plantsci.2019.110168. [DOI] [PubMed] [Google Scholar]
- 19.Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009;150(1):482–93. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dun EA, Brewer PB, Beveridge CA. Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009;14(7):364–72. doi: 10.1016/j.tplants.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Janssen BJ, Drummond RS, Snowden KC. Regulation of axillary shoot development. Curr Opin Plant Biol. 2014;17:28–35. doi: 10.1016/j.pbi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Xue Z, Liu L, Zhang C. Regulation of shoot apical meristem and axillary meristem development in plants. Int J Mol Sci 2020; 21(8). [DOI] [PMC free article] [PubMed]
- 23.Grossmann K. Auxin herbicides: current status of mechanism and mode of action. Pest Manag Sci. 2010;66(2):113–20. doi: 10.1002/ps.1860. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Li L, Wang S, You X, Jiang S, Liu F. Dissipation and residue of 2,4-D isooctyl ester in wheat and soil. Environ Monit Assess. 2012;184(7):4247–51. doi: 10.1007/s10661-011-2259-4. [DOI] [PubMed] [Google Scholar]
- 25.Anesio AHC, Santos MV, Silveira RR, Ferreira EA, Dos Santos JB, Da Silva LD. Persistence of auxinic herbicides applied on pasture and toxicity for succeeding crops. Acad Bras Cienc. 2018;90(2):1717–32. doi: 10.1590/0001-3765201820170134. [DOI] [PubMed] [Google Scholar]
- 26.Song Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol. 2014;56(2):106–13. doi: 10.1111/jipb.12131. [DOI] [PubMed] [Google Scholar]
- 27.Munro IC, JC GLC, RM OKGS, BS WEK. A comprehensive, integrated review and evaluation of the scientific evidence relating to the safety of the herbicide 2,4-D. J Am Coll Toxicol. 1992;11:560–664. [Google Scholar]
- 28.Merritt LH, Brown-Johnson AE, Meredith AN, Ferguson JC. Comparison of efficacy and detection of clethodim and glyphosate applied with dicamba and 2,4-D through tank mixture and sequential applications. J Agric Food Chem. 2021;69(1):101–11. doi: 10.1021/acs.jafc.0c05541. [DOI] [PubMed] [Google Scholar]
- 29.Gazola T, Costa RN, Carbonari CA, Velini ED. Dynamics of 2,4-D and dicamba applied to corn straw and their residual action in weeds. Plants-Basel 2022; 11(20). [DOI] [PMC free article] [PubMed]
- 30.Wang H, Wang H. The miR156/SPL Module, a Regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol Plant. 2015;8(5):677–88. doi: 10.1016/j.molp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Reviews Mol Cell Biol. 2011;12(4):211–21. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- 32.Kebrom TH, Spielmeyer W, Finnegan EJ. Grasses provide new insights into regulation of shoot branching. Trends Plant Sci. 2013;18(1):41–8. doi: 10.1016/j.tplants.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim HK, van Oosterom E, Dingkuhn M, Luquet D, Hammer G. Regulation of tillering in sorghum: environmental effects. Ann Bot. 2010;106(1):57–67. doi: 10.1093/aob/mcq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam MM, Hammer GL, van Oosterom EJ, Cruickshank AW, Hunt CH, Jordan DR. A physiological framework to explain genetic and environmental regulation of tillering in sorghum. New Phytol. 2014;203(1):155–67. doi: 10.1111/nph.12767. [DOI] [PubMed] [Google Scholar]
- 35.Kebrom TH, Mullet JE. Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ. 2015;38(8):1471–8. doi: 10.1111/pce.12500. [DOI] [PubMed] [Google Scholar]
- 36.Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. Sugar demand, not auxin, is the initial regulator of apical dominance. P Natl Acad Sci USA. 2014;111(16):6092–7. doi: 10.1073/pnas.1322045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Bai Y, Shen C, Wu Y, Zhang S, Jiang D, Guilfoyle TJ, Chen M, Qi Y. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Func Integ Genomic. 2010;10(4):533–46. doi: 10.1007/s10142-010-0174-3. [DOI] [PubMed] [Google Scholar]
- 38.Zou T, Zhang K, Zhang J, Liu S, Liang J, Liu J, Zhu J, Liang Y, Wang S, Deng Q, et al. DWARF AND LOW-TILLERING 2 functions in brassinosteroid signaling and controls plant architecture and grain size in rice. Plant J. 2023;116(6):1766–83. doi: 10.1111/tpj.16464. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Park PJ, Hwang HJ, Lee SY, Oh MH, Kim SG. Brassinosteroid signals control expression of the AXR3/IAA17 gene in the cross-talk point with auxin in root development. Biosci Biotech Bioch. 2006;70(4):768–73. doi: 10.1271/bbb.70.768. [DOI] [PubMed] [Google Scholar]
- 40.Iliev EA, Xu W, Polisensky DH, Oh MH, Torisky RS, Clouse SD, Braam J. Transcriptional and posttranscriptional regulation of Arabidopsis TCH4 expression by diverse stimuli. Roles of cis regions and brassinosteroids. Plant Physiol. 2002;130(2):770–83. doi: 10.1104/pp.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009;149(4):1929–44. doi: 10.1104/pp.109.135475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller D, Waldie T, Miyawaki K, To JP, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O. Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J. 2015;82(5):874–86. doi: 10.1111/tpj.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice. Plant Cell Rep. 2015;34(9):1647–62. doi: 10.1007/s00299-015-1815-8. [DOI] [PubMed] [Google Scholar]
- 44.Gocal GF, Pharis RP, Yeung EC, Pearce D. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. Cv Tender Green. Plant Physiol. 1991;95(2):344–50. doi: 10.1104/pp.95.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Yu L, Song B. Proteomics analysis of Xiangcaoliusuobingmi-treated Capsicum annuum L. infected with Cucumber mosaic virus. Pestic Biochem Phys. 2018;149:113–22. doi: 10.1016/j.pestbp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Martins PK, Mafra V, de Souza WR, Ribeiro AP, Vinecky F, Basso MF, da Cunha BA, Kobayashi AK, Molinari HB. Selection of reliable reference genes for RT-qPCR analysis during developmental stages and abiotic stress in Setaria viridis. Sci Rep. 2016;6:28348. doi: 10.1038/srep28348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love MI, Huber W, Anders S. Moderated estimation of Fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–6. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 50.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2):R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao X, Cai T, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–93. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 52.Seefeldt LC, Rasche ME, Ensign SA. Carbonyl sulfide and carbon dioxide as new substrates, and carbon disulfide as a new inhibitor, of nitrogenase. Biochemistry. 1995;34(16):5382–9. doi: 10.1021/bi00016a009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this study have been deposited in NCBI SRA BioProject with accession number of PRJNA1111665.