LETTER
We have read the original article entitled “The role of rifampicin within the treatment of Mycobacterium avium pulmonary disease” by Schildkraut et al. (1). We would like to contribute to the ongoing discussion on the role of rifampicin in the treatment of non-tuberculous mycobacteria (NTM). The authors showed no advantages in rifampicin use since it lacks bactericidal activity, and no adjunctive role in preventing the emergence of resistance was observed. Additionally, NTM MICs for rifamycins are generally higher than those in Mycobacterium tuberculosis, requiring higher doses (2, 3).
The other relevant point to be considered is rifampicin-mediated reduction in macrolide exposure. It should be mentioned that macrolides are key drugs for the treatment of NTM, and their absence was identified as a significant risk factor for both microbiological and clinical failure (4). Several publications showed that clarithromycin (CRL) exposure is significantly reduced by the concomitant administration of rifampicin, while fewer data are available for azithromycin (AZM) (5–8). Jeong et al. (6) reported that patients treated with regimens containing AZM and rifampicin [for Mycobacterium avium complex (MAC) infections] had lower AZM maximal concentrations (Cmax) than patients with M. abscessus who received rifampicin-free regimens (220 vs 530 ng/mL, P < 0.001); they also identified AZM Cmax above 400 ng/mL as a factor predicting microbiological response at multivariate analysis. Van Ingen et al. reported that 56% and 34% of the patients who received rifampicin failed to reach CLR and AZM plasma target concentrations (200 ng/mL), respectively (7). This observation directly reflects the induction by rifampicin (RIF) of cytochrome 3A4, of which macrolides are the substrate. AZM is metabolized by CYP3A4, albeit to a lesser extent than CLR (9, 10). AZM plasma concentration undergoes a noteworthy reduction although less pronounced than CLR. If the effect of RIF-induced metabolism of CLR has been described in multiple studies (11, 12), likewise van Ingen has shown for the first time how this interaction affects AZM Cmax (7). Another possible mechanism included p-glycoprotein induction, as shown in early works on AZM disposition (13). Therapeutic drug monitoring of anti-NTM drugs is recommended by guidelines in selected patients such as those in which subtherapeutic levels are suspected, in case of delayed sputum conversion or in those reporting tolerability issues (14).
We here report preliminary data on AZM Cmax in serum and in peripheral blood mononuclear cells (PBMC) of patients treated for NTM infections. They were enrolled in an observational study on the effect of anti-NTM pharmacokinetics on treatment outcomes and signed a written informed consent (MOTT Study, Ethics approval number 44132/22). Samples for AZT Cmax were withdrawn 2 hours post dose, and they were analyzed through an LC-MS/MS Kit (from CoQua Lab, Italy; code: TBC100) for quantifying tuberculosis and non-tuberculous mycobacteria plasma drugs, modified for PBMC drug quantifications [process validation described in reference (15)]. Data are reported as median values (interquartile range) and compared through non-parametric tests (Mann-Whitney and Spearman’s tests).
AZM Cmax was assessed in 26 plasma and 13 PBMC samples, withdrawn from 15 participants. A median of 1 (1-2) plasma measurement for each patient was available. The population was mainly composed of female participants (66.7%) with a median age of 64 years (46-75). Median weight and body mass index were 55 kg (48–58) and 17.9 kg/m2 (16.2–18.9). No one had cystic fibrosis, while 66.7% had underlying pulmonary diseases: chronic obstructive pulmonary disease and bronchiectasis were the most commonly reported (33.3% each). M. avium complex represented 60.0% of all the species isolated. Nodular-bronchiectatic NTM-PD was present in 60.0% of the participants. All of the patients enrolled were initially treated with targeted anti-NTM combination therapy according to the ATS/ERS/ESCMID/IDSA Guidelines (14). In 10 patients (66.7%), the regimen was modified or interrupted due to the occurrence of adverse events, drug interactions, or treatment failures. The blood samples for therapeutic drug monitoring (TDM) evaluation were collected after a median time interval of 12.6 weeks (4.1–38.2) after the introduction of the first or new line regimen.
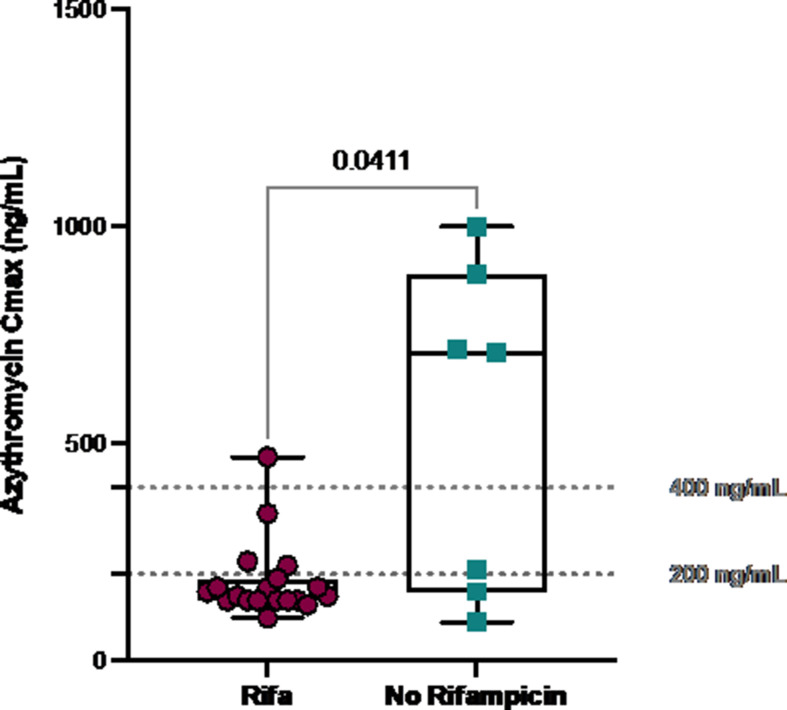
When the plasma AZM Cmax measurements were performed, eight (53.3%) participants received AZM with rifampicin, while seven (46.7%) were receiving rifampicin-free regimens. In the former group, one (12.5%) patient received clofazimine (CLO), while in the latter, CLO was administered in four (57.1%) participants. Ethambutol (ETB) was included in 12 regimens (80%), and in 9 (75%) of them, it was associated to RIF. Moxifloxacin (n = 4, 57.1%), linezolid, and cotrimoxazole (n = 1, 14.3%) were used as an AZM companion in RIF-free regimens. A total of 19 (73.1%) and 7 (26.9%) samples were collected for the two groups, respectively. Median AZM Cmax was 165 ng/mL (140–227) in plasma and 37,466 ng/mL (20,029–59,427) in PBMC, with a median plasma/PBMC ratio of 227 (143–261). Higher dose-per-weight of AZM (mean AZM: 9.0 mg/kg, 8.6–10.2) did not correspond to higher plasma (rho = 0.090, P = 0.690) or intracellular (rho = 0.405, P = 0.319) concentrations, and moreover, higher plasma AZM concentrations were not associated with higher intracellular AZM Cmax (P = 0.553). AZM Cmax was significantly lower in participants with vs without rifampicin coadministration (152 vs 710 ng/mL P = 0.041; Fig. 1). The prevalence of AZM Cmax below 200 ng/mL (78.9% vs 28.6%, P = 0.017) and below 400 ng/mL (94.7% vs 42.9%, P = 0.003) was significantly higher in patients concomitantly receiving rifampicin. Furthermore, a linear reduction of AZM Cmax was observed along with increasing rifampicin dose-per-weight (P = 0.036). Significantly lower intracellular AZM Cmax was observed in rifampicin-receiving participants (27,166 vs 237,778 ng/mL, P = 0.026). Clofazimine-containing regimens (n = 5, 33.3%) were associated with greater AZM plasma Cmax (350 vs 150 ng/mL, P = 0.030) and a higher prevalence of AZM Cmax > 200 ng/mL (75.0% vs 16.7%, P = 0.004). The majority of clofazimine-including regimens did not contain rifampicin and, therefore, were associated with a higher chance of reaching therapeutic AZM exposure, likely due to reduced drug-drug interaction. Recent data support the efficacy of clofazimine-containing regimens (16, 17).
Fig 1.
Azythromycin maximal concentrations in participants receiving and not receiving rifampicin. The statistical analysis was performed using Mann-Whitney’s test. Dashed horizontal lines are proposed thresholds associated with favorable treatment outcomes.
Higher AZM concentration has been described to be predictive for more favorable outcomes in MAC-PD (6, 16), thus emphasizing the need for optimized macrolide doses and the avoidance of interacting drugs. In our population, AZM Cmax above 200 ng/mL was associated with improved radiological outcome (P = 0.045), while a non-significant relation was observed with microbiological response (P = 0.0063).
This study presents major limitations due to its small sample size and limited pharmacokinetic data, restricting the generalizability of the findings. A broad spectrum of confounding factors, unrelated to rifampicin, has not been taken in account. Despite these limitations, our study aims to utilize the available data set to provide preliminary insights and justify the need for a larger, randomized clinical trial with rifampicin-free regimens with bactericidal activity in order to improve the outcomes of patients with NTM-PD (16, 18).
ACKNOWLEDGMENTS
We acknowledge funding from the University of Turin, Local Research Funding (RILO 2021, PI Andrea Calcagno), and from the Ministry of Health—Ricerca corrente to IRCCS Sacro Cuore Don Calabria hospital (Linea 1).
Contributor Information
Alberto Gaviraghi, Email: albertogaviraghi212@gmail.com.
Sean Wasserman, St. George's, University of London, London, United Kingdom.
REFERENCES
- 1. Schildkraut JA, Raaijmakers J, Aarnoutse R, Hoefsloot W, Wertheim HFL, van Ingen J. 2023. The role of rifampicin within the treatment of Mycobacterium avium pulmonary disease. Antimicrob Agents Chemother 67:e0087423. doi: 10.1128/aac.00874-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fröberg G, Maurer FP, Chryssanthou E, Fernström L, Benmansour H, Boarbi S, Mengshoel AT, Keller PM, Viveiros M, Machado D, et al. 2023. Towards clinical breakpoints for non-tuberculous mycobacteria - determination of epidemiological cut offvalues for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect 29:758–764. doi: 10.1016/j.cmi.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 3. Kengo A, Nabisere R, Gausi K, Musaazi J, Buzibye A, Omali D, Aarnoutse R, Lamorde M, Dooley KE, Sloan DJ, Denti P, Sekaggya-Wiltshire C. 2023. Dolutegravir pharmacokinetics in Ugandan patients with TB and HIV receiving standard- versus high-dose rifampicin. Antimicrob Agents Chemother 67:e0043023. doi: 10.1128/aac.00430-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. 2020. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 18:263–273. doi: 10.1080/14787210.2020.1720650 [DOI] [PubMed] [Google Scholar]
- 5. Miwa S, Shirai M, Toyoshima M, Shirai T, Yasuda K, Yokomura K, Yamada T, Masuda M, Inui N, Chida K, Suda T, Hayakawa H. 2014. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. a preliminary study. Ann Am Thorac Soc 11:23–29. doi: 10.1513/AnnalsATS.201308-266OC [DOI] [PubMed] [Google Scholar]
- 6. Jeong B-H, Jeon K, Park HY, Moon SM, Kim S-Y, Lee S-Y, Shin SJ, Daley CL, Koh W-J. 2016. Peak plasma concentration of azithromycin and treatment responses in Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 60:6076–6083. doi: 10.1128/AAC.00770-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, Aarnoutse RE, Heifets LB, Peloquin CA, Daley CL. 2012. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 186:559–565. doi: 10.1164/rccm.201204-0682OC [DOI] [PubMed] [Google Scholar]
- 8. Koh WJ, Jeong BH, Jeon K, Lee SY, Shin SJ. 2012. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 186:797–802. doi: 10.1164/rccm.201206-1088OC [DOI] [PubMed] [Google Scholar]
- 9. Lode H. 1991. The pharmacokinetics of azithromycin and their clinical significance. Eur J Clin Microbiol Infect Dis 10:807–812. doi: 10.1007/BF01975832 [DOI] [PubMed] [Google Scholar]
- 10. Westphal JF. 2000. Macrolide - induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol 50:285–295. doi: 10.1046/j.1365-2125.2000.00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallace RJ, Brown BA, Griffith DE, Girard W, Tanaka K. 1995. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. Intracellulare infection. J Infect Dis 171:747–750. doi: 10.1093/infdis/171.3.747 [DOI] [PubMed] [Google Scholar]
- 12. Peloquin C, Berning SE. 1996. Evaluation of the drug interaction between clarithromycin and rifampin. J Infect Dis Pharmacother 2:19–35. doi: 10.1300/J100v02n02_02 [DOI] [Google Scholar]
- 13. Sugie M, Asakura E, Zhao YL, Torita S, Nadai M, Baba K, Kitaichi K, Takagi K, Takagi K, Hasegawa T. 2004. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob Agents Chemother 48:809–814. doi: 10.1128/AAC.48.3.809-814.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 71:e1–e36. doi: 10.1093/cid/ciaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baietto L, Calcagno A, Motta I, Baruffi K, Poretti V, Di Perri G, Bonora S, D’Avolio A. 2015. A UPLC MS MS method for the simultaneous quantification of first line antituberculars in plasma and in PBMCs. J Antimicrob Chemother 70:2572–2575. doi: 10.1093/jac/dkv148 [DOI] [PubMed] [Google Scholar]
- 16. Zweijpfenning SMH, Aarnoutse R, Boeree MJ, Magis-Escurra C, Stemkens R, Geurts B, van Ingen J, Hoefsloot W. 2023. Clofazimine is a safe and effective alternative for rifampicin in Mycobacterium avium complex pulmonary disease treatment - outcomes of a randomized trial. Chest:S0012-3692(23)05830-0. doi: 10.1016/j.chest.2023.11.038 [DOI] [PubMed] [Google Scholar]
- 17. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. 2016. Long term follow up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 149:1285–1293. doi: 10.1378/chest.15-0543 [DOI] [PubMed] [Google Scholar]
- 18. Nasiri MJ, Calcagno T, Hosseini SS, Hematian A, Nojookambari NY, Karimi-Yazdi M, Mirsaeidi M. 2021. Role of clofazimine in treatment of Mycobacterium avium complex. Front Med (Lausanne) 8:638306. doi: 10.3389/fmed.2021.638306 [DOI] [PMC free article] [PubMed] [Google Scholar]