Abstract
Background
Fatal coronary heart disease (FCHD) is often described as sudden cardiac death (affects >4 million people/year), where coronary artery disease is the only identified condition. Electrocardiographic artificial intelligence (ECG-AI) models for FCHD risk prediction using ECG data from wearable devices could enable wider screening/monitoring efforts.
Objectives
To develop a single-lead ECG–based deep learning model for FCHD risk prediction and assess concordance between clinical and Apple Watch ECGs.
Methods
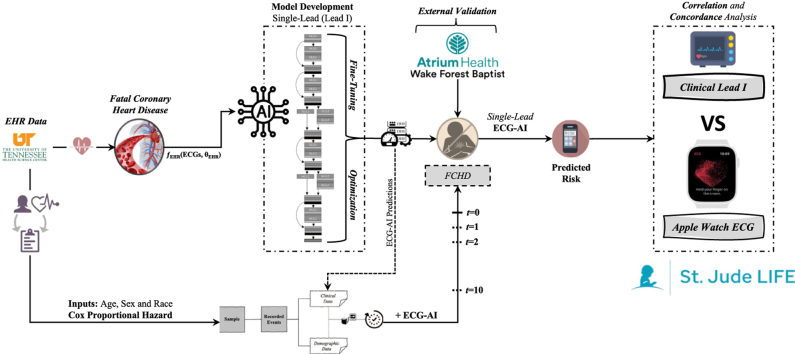
An FCHD single-lead (“lead I” from 12-lead ECGs) ECG-AI model was developed using 167,662 ECGs (50,132 patients) from the University of Tennessee Health Sciences Center. Eighty percent of the data (5-fold cross-validation) was used for training and 20% as a holdout. Cox proportional hazards (CPH) models incorporating ECG-AI predictions with age, sex, and race were also developed. The models were tested on paired clinical single-lead and Apple Watch ECGs from 243 St. Jude Lifetime Cohort Study participants. The correlation and concordance of the predictions were assessed using Pearson correlation (R), Spearman correlation (ρ), and Cohen’s kappa.
Results
The ECG-AI and CPH models resulted in AUC = 0.76 and 0.79, respectively, on the 20% holdout and AUC = 0.85 and 0.87 on the Atrium Health Wake Forest Baptist external validation data. There was moderate-strong positive correlation between predictions (R = 0.74, ρ = 0.67, and κ = 0.58) when tested on the 243 paired ECGs. The clinical (lead I) and Apple Watch predictions led to the same low/high-risk FCHD classification for 99% of the participants. CPH prediction correlation resulted in an R = 0.81, ρ = 0.76, and κ = 0.78.
Conclusion
Risk of FCHD can be predicted from single-lead ECGs obtained from wearable devices and are statistically concordant with lead I of a 12-lead ECG.
Keywords: Fatal coronary heart disease, Artificial intelligence, ECG-AI, Apple Watch, Risk prediction, Concordance
Graphical abstract
Introduction
Fatal coronary heart disease (FCHD) leads to sudden cardiac failure and is attributed to having a history of cardiovascular disease, history of nonfatal myocardial infarction, or underlying coronary disease risk factors.1, 2, 3 Owing to similarities in its outcomes, researchers have suggested that FCHD is highly correlated with sudden cardiac death (SCD).4,5 While the proximate cause of FCHD is difficult to ascertain owing to its typically unwitnessed nature, the underlying cause is often coronary artery disease (CAD),5,6 ventricular arrhythmias,7 heart failure (HF),8 congenital and acquired cardiomyopathies,9,10 or other complications of atherosclerotic disease.11 In addition, FCHD risk increases by age, but is now also more observed in 30- to 40-year-olds,12,13 males,14,15 African Americans,16,17 and athletes,18, 19, 20 highlighting the need for routine risk assessment. However, assessment tools remain suboptimal.1,21, 22, 23
Primary prevention for FCHD without evidence of clinical symptoms or conditions is difficult, with interventions provided only to those with underlying risk factors such as HF, CAD, hypertrophic cardiomyopathy, or certain ventricular arrhythmias.24, 25, 26, 27 Interventions in these populations include implantable cardioverter-defibrillators or treatment of other pharmacological therapies,28 evaluation for anomalous coronary arteries,29,30 or, for less severe cases, recommendations for lifestyle modifications. Noninvasive screening for potential FCHD has been elusive. Artificial intelligence (AI) methods are now available to detect and predict risk for a variety of cardiovascular outcomes, given advances in computational capacity and in machine learning.31,32 Nevertheless, AI methods require data, heretofore only available in patients presenting to a clinical setting to receive screening. However, the increasing number of individuals wearing smart devices, including wearables with health monitoring applications, has potential for implementation of AI predictive tools that can leverage a single-lead electrocardiogram (ECG) for screening and risk monitoring, providing an easy and cost-effective pathway to facilitate preventive and remedial interventions.
Electrocardiographic AI (ECG-AI) models assessing cardiovascular disease risk have been developed, yet virtually none exist for FCHD. Accurate ECG-AI models based on single-lead ECG signal data from wearable devices would enable scalable screening and/or monitoring of larger populations. Raw single-lead ECG data may, therefore, be useful to predict risk of FCHD, but there is a need for assessment of correlation and concordance between AI models developed on single-lead (typically “lead I” of a 12-lead clinical ECG) ECGs with those obtained from smartwatches (since smartwatch ECGs typically mimic lead I).33
The main objective of this study was to assess the feasibility of using single-lead ECG–based deep learning models for FCHD risk prediction by assessing the correlation and concordance between predictions obtained from paired clinical single-lead and Apple Watch ECGs.
Materials and methods
FCHD ECG-AI model development
Data sources and ECG signal data
We obtained demographic and clinical data (eg, comorbidities) from the electronic health records (EHR) and standard raw 12-lead 10-second time-voltage supine ECG data from patients at the University of Tennessee Health Science Center/Medical Center in Memphis, TN (UTHSC). The study was approved by the institutional review board of both UTHSC and Atrium Health Wake Forest Baptist, Winston-Salem, NC.
The 12-lead ECG data were in either 500 Hz or 250 Hz voltage. All ECGs were downsampled to 250 Hz by removing amplitudes at every other time point. In addition, the first second of the 10-second ECG was removed to reduce noise associated with ECG initiation, resulting in a 9-second ECG with 225 Hz sampling rate. A single-lead version using lead I of the 12-lead ECG was developed, since this lead is often mimicked in wearable devices with ECG functionality, such as smartwatches. Lead I was extracted from the 12-lead ECG and replicated 12 times, to retain consistency between the model architectures.
Inclusion/exclusion criteria
Patients aged 18 or older with at least 1 ECG recording were included in the initial dataset. For initial analysis, there were no time restrictions between ECGs and FCHD event (cases). Controls were matched by age, sex, and race and had no sudden fatal event. In the case of controls, the date last seen in the EHR system was used as the anchor point, and any previous ECGs were extracted.
FCHD outcome definition
FCHD refers to sudden cardiovascular failure with the only identifiable cause being CAD.1,2 FCHD events were derived from a combination of ICD-9 codes 410, 427.5, and 799 and ICD-10 codes I46.2, I46.9, I21, and I25.1,34
Study design and model development
The single-lead ECG dataset was split (and stratified) into 80% for derivation and 20% as a holdout dataset. The 20% holdout dataset was only used for final testing and performance assessment. Five-fold cross-validation was employed on the 80% derivation data. The ECG-AI model was developed based on an adjusted ResNet convolutional neural network deep learning architecture.35 The Adam optimizer was used with default parameters. The batch size was 1024 and ran over 100 epochs. The single-lead ECG-AI models were assessed using the area under the receiver operating characteristic curve (ROC AUC) on the 20% holdout data.
In addition to the ECG-AI framework, we also developed a Cox proportional hazards (CPH) model that incorporated the predictions from the single-lead ECG-AI model for FCHD with simple demographic variables: age at time of ECG, sex, and race. The CPH model was assessed on the same 20% holdout data using the concordance index (C-index) and AUC.
The St. Jude Lifetime Cohort Study
The St. Jude Lifetime Cohort Study (SJLIFE) is an institutional review board–approved (NCI U01CA195547, MPI Hudson & Ness) pediatric cancer cohort established to longitudinally evaluate health outcomes among survivors previously treated for childhood cancer at St. Jude Children’s Research Hospital who had survived ≥5 years following cancer diagnosis.36 The SJLIFE study involves an ongoing prospective cohort study for patients diagnosed and treated over 5 decades from 1962 until June 30, 2012.36 Participants return to St. Jude for comprehensive clinical assessments approximately every 5 years. By 2020, 6560 participants had agreed to participate in the study, of whom 5223 had completed a baseline clinical assessment.
As a part of an existing ancillary SJLIFE study (NCI 1R01CA261834, MPI Akbilgic & Hudson), and following informed consent, paired clinical 12-lead and Apple Watch single-lead ECGs were recorded from participants during their SJLIFE evaluation since December 2022. The availability of the 2 ECG modalities from the same patient, taken at the same time, provided a unique resource to test for correlation of FCHD predictions using both ECG modalities: the lead I of 12-lead clinical ECGs and single-lead Apple Watch ECGs.
Following model development, we aimed to prospectively test the ECG-AI and CPH models on ECG and demographic data obtained from SJLIFE. At each visit, a 12-lead clinical ECG, an Apple Watch ECG, and demographic information are recorded. The 12-lead clinical ECGs were obtained as XML files, from which the patient ID and demographic data are parsed out and de-identified. The Apple Watch ECGs are transferred to a secure cloud-based storage system and linked to the paired clinical ECG via the participant’s study ID code.
Paired electrocardiogram data (clinical and Apple Watch ECGs)
For the single-lead clinical ECG model, the first lead (lead I) of the 12-lead ECG was extracted and replicated 12 times to maintain the same input structure into the previously developed model. All clinical ECGs were downsampled to 250 Hz and the first second was removed, resulting in ECGs at 225 Hz of 9-second length.
ECGs obtained from the Apple Watch had a sampling rate of 512 Hz. The middle 9 seconds (downsampled to 250 Hz) of the 30-second Apple Watch ECGs were used in the analysis.
Prediction of FCHD using ECG-AI and CPH
This research employed an adjusted ResNet convolutional neural network deep learning architecture from Akbilgic and colleagues35 for ECGs to predict risk of FCHD. The input into this deep learning architecture is a 1-dimensional ECG signal and the output was the predicted risk of FCHD. Similarly, the developed CPH models, with inputs of age at time of ECG, sex, and race, as well as the ECG-AI were employed on data from single-lead and Apple Watch ECGs.
ECG-AI and CPH correlation of predictions
Correlation and concordance between the predictions from the ECG-AI model single-lead clinical and Apple Watch was assessed using coefficient of determination, Pearson correlation coefficient, Spearman correlation coefficient, and Cohen’s kappa. In addition, we assessed concordance of the ECG-AI model predictions using the paired single-lead ECGs and the Apple Watch ECGs. For the CPH models, we followed the same protocol for assessment of correlation and concordance using predicted risk scores from the CPH models between single-lead and Apple Watch ECGs.
Model development and all additional analyses were performed using the Python programming language.
Results
Clinical characteristics
The analytical UTHSC cohort data, on which model development and internal testing was performed, included a total of 167,662 ECGs collected from a total of 50,132 patients after applying inclusion and exclusion criteria. From these, 29,093 were controls with 78,472 ECGs and 21,039 were cases for FCHD with 89,190 ECGs. The average age at time of ECG was 62.58 ± 14.0. A total of 51.69% were African American and 45.72% were white, and 53.09% were male. The median time between ECG and FCHD diagnosis in the derivation cohort was 1.38 years. A patient cohort from Atrium Health Wake Forest Baptist (AHWFB) was selected for external validation. A total of 2305 patients were included for validation, with 461 patients as cases for FCHD. The mean age of the validation cohort was 63.04 ± 16.90, with 18.79% being of African American race, and76.31% of white race; 51.02% were male.
The SJLIFE cohort, from which the paired clinical and Apple Watch ECGs were prospectively obtained, included a total of 243 SJLIFE participants. The average age of this cohort was 35.4 ± 10.0. A total of 13.7% were of African American race and 83.1% were white, and 49.6% were male. Table 1 summarizes the clinical characteristics for both cohorts.
Table 1.
Demographics and clinical characteristics of the patient cohort from UTHSC EHR
| Demographics | UTHSC cohort | AHWFB external cohort | St. Jude LIFE cohort |
|---|---|---|---|
| Npatients = 50,132 | Npatients = 2,305 | Npatients = 243 | |
| NECGs = 167,662 | NECGs = 2,305 | NECGs = 243 | |
| Age at ECG taken (y) ± SD | 62.58±13.98 | 63.04±16.89 | 35.4±10.0 |
| Sex | |||
| Male (0), n (%) | 26,616 (53.1) | 1176 (51.02) | 123 (49.6) |
| Female (1), n (%) | 23,513 (46.9) | 1129 (48.98) | 125 (50.4) |
| Race/ethnicity | |||
| African American, n (%) | 25,911 (51.7) | 433 (18.79) | 34 (13.7) |
| White, n (%) | 22,918 (45.7) | 1746 (75.75) | 206 (83.1) |
| Other, n (%) | 1303 (2.6) | 126 (5.5) | 8 (3.2) |
AHWFB = Atrium Health Wake Forest Baptist; ECG = electrocardiogram; LIFE = [St. Jude] Lifetime Cohort Study; UTHSC = University of Tennessee Health Science Center/Medical Center in Memphis, TN.
FCHD risk prediction model on 20% UTHSC holdout data for ECG-AI
The single-lead ECG-AI model, when tested on the UTHSC holdout data, resulted in an AUC of 0.76 (0.76–0.77).37 The accuracy of the lead I–based ECG-AI was 70% with a sensitivity of 67% and specificity of 71%. The positive predicted (PPV) and negative predicted (NPV) values were 55% and 81% respectively. The CPH model, when tested on the same holdout data set, resulted in a C-Index of 0.70 (0.69-0.71) and an AUC of 0.79 (0.78-0.80).
3.1 FCHD model external validation on the AHWFB cohort
The single-lead ECG-AI model, when tested on the AHWFB external cohort, resulted in an AUC of 0.85 (0.83-0.87) while the CPH model resulted in an AUC of 0.87 (0.84-0.89). The accuracy of the Lead I-based ECG-AI was 82% with a sensitivity of 71% and specificity of 84%. The positive predicted and negative predicted values were 53% and 92%, respectively. The CPH model, when tested on the same holdout dataset, resulted in a C-index of 0.83 (0.80–0.85) and an AUC of 0.87 (0.85–0.89).
Concordance between the single-lead clinical and Apple Watch FCHD predictions
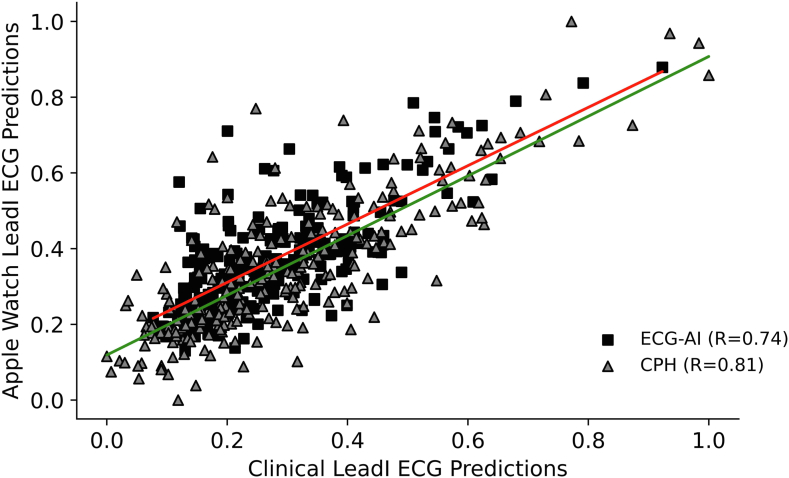
Correlation and concordance between predictions from the single-lead clinical ECG with those using the Apple Watch ECGs were performed. The correlation (Figure 1) and concordance results were favorable, with a Pearson correlation (R) of 0.74, Spearman correlation (ρ) of 0.67, and Cohen’s kappa (κ) of 0.58. The correlation between predictions of the CPH model tested on single-lead and Apple Watch ECGs resulted in R = 0.81 and ρ = 0.76, and concordance resulted in κ = 0.78.
Figure 1.
Linear correlation of fatal coronary heart disease predictions from clinical single-lead electrocardiograms (ECGs) vs Apple Watch ECGs using electrocardiographic artificial intelligence (ECG-AI; boxes) and Cox proportional hazards (CPH; triangles). Red trendline is for single-lead ECG-AI and green trendline is for CPH.
To further compare predictions from the single-lead and Apple Watch ECGs, the predictions were stratified into low and high risk for FCHD (Table 2). Overall, using just the ECG-AI predictions, 99% of the study participants were predicted to be in the same risk group when using both ECG modalities. Two study participants out of 4 (50%) were predicted as high risk for FCHD by both the single-lead and Apple Watch ECGs. Two participants were predicted to be at high risk by ECG-AI using the Apple Watch ECGs but at low risk when using the corresponding single-lead clinical ECG. In addition, no participants were predicted to be at high risk for FCHD from their single-lead clinical ECG and at low risk from the Apple Watch ECG. Assessment of agreement between these patient classifications resulted in a Cohen’s kappa of 0.66.
Table 2.
Risk classification of predictions from paired single-lead ECG predictions and Apple Watch predictions
| Apple Watch single-lead ECG-based predictions |
Total | ||
|---|---|---|---|
| Low risk | High risk | ||
| Clinical lead I ECG-based predictions | |||
| Low risk | 239 | 2 | 241 |
| High risk | 0 | 2 | 2 |
| Total | 239 | 4 | 243 |
ECG = electrocardiogram.
Discussion
FCHD is becoming a major health concern owing to its association with multiple risk factors and can lead to sudden cardiac arrest. While there are efforts to reduce its prevalence, often relying on lifestyle changes or pharmacological therapeutics to manage such associated risks (eg, hypertension, diabetes, left ventricular hypertrophy, HF, etc), the suddenness of the fatal event requires timely warning. Therefore, there is a need for prediction models that can be easily accessible within routine clinical care as well as for remote monitoring. In this research, externally validated ECG-based models were used to assess the power of ECGs to predict risk of FCHD and assess correlation and concordance with data obtained from a single ECG lead and Apple smartwatches. Results show moderate-to-good correlation and concordance between single-lead and Apple Watch ECGs, with R = 0.74, ρ = 0.67, and κ= 0.58. In addition, when assessing ECG-AI classification of high- and low-risk groups using paired ECGs, there was moderate concordance between the risk groups with a kappa of 0.66. When including age, race, and sex with ECG-AI predictions within CPH models, correlation and concordance resulted in R = 0.81, ρ = 0.76, and κ= 0.78. This serves as proof of concept that ECG-based AI models developed on clinical ECGs can indeed be used with ECG signals obtained from smartwatch devices. This increases the potential utility for remote monitoring and clinical consultations as well as facilitates opportunities for early intervention.
The most common smartwatches, for example the Apple Watch (from Apple Inc, Cupertino, CA), Samsung watch, Fitbit (Fitbit LLC, San Francisco, CA), and the Google Pixel Watch, enable the wearer to perform a single-lead electrocardiogram. Supporting our assertion that single-lead ECG analysis can have significant clinical applications, certain smartwatches have recently garnered FDA clearance for disease detection (eg, atrial fibrillation within Apple Watches), which leverages ECG sensors.38,39 Most of the measures and calculations are performed within the watch itself, but a health application on the linked iPhone provides associated detailed information. This increases the usability of such wearable devices in remote monitoring and also paves the way for additional research and prediction of multiple cardiovascular diseases when single-lead ECG is used, with possible incorporation of demographic and health data/information, such as height, weight, level of physical activity, and heart rate, which can be extracted from the phone application (for example the Apple HealthKit).40,41
There is an increasing need for ECG-AI methods that use raw ECGs to predict severe and fatal cardiovascular outcomes. Even more so, the rise in use of wearable devices with ECG monitoring capabilities can be an asset to both clinicians and the general population.38,39,41, 42, 43 AI models may be incorporated into future clinical care in aid to risk stratification of different cardiovascular diseases as well as outcomes, such as fatal coronary heart disease and SCD. The current status of ECG functionality in multiple smartwatch brands supports their use for remote monitoring.44 Such models have already been incorporated into smartphones (as an application) to obtain ECGs and predict risk for HF on the device.45 Incorporating use of such AI-based models has the potential to help with clinical assessment and develop patient-centered risk management schemes.
As part of the SJLIFE prospective study, both clinical and Apple Watch ECGs are being collected for comprehensive testing on risk prediction for different cardiovascular diseases. Owing to the efforts performed at SJLIFE as well as the important resource of collecting paired ECGs, this research made use of such a resource to test the ECG-AI model’s capabilities using real smartwatch ECGs to showcase their clinical application and utility.
Results from this research show that an AI deep learning model trained on clinical single-lead ECGs is correlated with predictions from the single-lead ECG obtained from Apple watches. Importantly, when the single-lead ECG-AI was tested on the clinical ECGs and ECGs obtained from the Apple Watch, followed by participant stratification into low or high risk groups, 94% of the predictions from the two ECG modalities matched the same risk group. Most of the study participants were, as expected, placed in the low-risk group when ECG-AI was tested on the clinical single-lead ECG and the paired Apple Watch ECG. Two participants were placed in the high-risk group by both ECG modalities, representing a group that may benefit from further clinical ECG assessment and evaluation of comorbidities linked with high risk of FCHD, such as CAD, heart failure, diabetes, and hypertension.1,34,46,47 Furthermore, while the single-lead models did not overestimate predicted risk from the 12-lead models (ie, all participants classified at low FCHD risk by the 12-lead model were also classified at low risk by the single-lead model), half of the participants predicated to be at high FCHD risk by the 12-lead model were classified as low FCHD risk by the single-lead model. In future works it will, therefore, be important to assess whether this represents improved discrimination (since the positive predicted value was 55% in the UTHSC sample and 53% on the AHWFB data) or underestimated risk.
Monitoring patients with coronary heart disease for risks for FCHD via consumer-level wearables with ECG functionality may offer improved outcomes as well as new challenges. People identified at medium or high risk for FCHD, depending on the false-positive rates, may be referred for clinical visits to confirm results and additional testing. If the risk is confirmed, appropriate course of action can be taken to reduce the identified FCHD risk. These preventive actions can range from simple lifestyle changes to consideration for implantable cardioverter-defibrillator, depending on the patient profile and clinical testing/imaging results. However, development of such systems also brings some challenges inherent to full EHR integration of wearable data generated outside hospital settings, including concerns of data quality, information security, and alarm fatigue. Additional clinical studies are needed to assess the clinical utility of such tools before bringing them into clinical workflow.
This research can extend to other remote monitoring options, especially other smart watches with ECG functionality. In addition, results from this research and planned future work pave the way for the integration of such models within mobile AI platforms such as ECG-Air,45 which can collect ECG from wearable devices and offer an easy-to-use, simple, and cost-effective framework for prescreening at the population level. The availability of tools for prescreening is important, since FCHD are also highly correlated with other cardiac diseases, such as hypertrophic cardiomyopathy, heart failure, and left ventricular hypertrophy, as well as additional underlying risk factors (eg, diabetes or hypertension).20,48,49 Having a simple and usable AI model that can be integrated within a smart device, especially a smartwatch with single-lead ECG capabilities, can be an asset to the clinical workflow and help decision-making for triaging, testing, and risk assessment.
Limitations, potential challenges, and future studies
This study has some limitations. Predominantly, while FCHD has been reported to be correlated to SCD, true SCD data can substantially increase the utility of such AI models. In addition, our study showed a strong correlation between clinical lead I and Apple Watch single-lead ECGs. Yet, the cohort (SJLIFE) used to assess correlation and concordance of lead I of clinical ECG with single-lead Apple Watch ECG was a significantly younger cohort compared to the UTHSC derivation and AHWFB external validation cohorts. Therefore, there were not many SJLIFE participants predicted to be at high risk for FCHD. The correlation/concordance analysis in this study would require validation on a cohort more representative of UTHSC and AHWFB cohorts. Finally, the resolution (signal depth) of Apple Watch ECGs might need further adjustments to increase their concordance with clinical ECGs (via analog-digital conversion), yet such documentation is presently lacking. We also plan to work with other institutes and clinical settings to obtain ECGs from different smart wearable technologies to increase and ensure generalizability in model performance.
Despite promising results from this research, there are also challenges in implementation of ECG-AI models into clinical workflow, both for standard clinical ECG and for wearable ECG. Embedding ECG-AI models into the EHR for automated execution is not a straightforward task. The raw waveform ECG data in clinical settings almost never make it to EHR and are typically collected and stored in cardiology information systems in an encrypted fashion.50,51 Accessing the waveform ECG data requires specific technical expertise and paying service fees to vendors to decrypt the data, and in some cases these data are never made available for research. Owing to such challenges, such data are only available for research in only a very small number of institutes.50 However, with appropriate investments, it is possible to automatically transfer waveform ECGs from the cardiology information systems to a computing environment that hosts ECG-AI models. The models are then executed, and the results are returned to the EHR in a matter of seconds. Furthermore, we also acknowledge that there could be challenges in execution of ECG-AI models on smartphones using data generated from smartwatches or fitness trackers. These challenges include data quality, information security, and accessibility. Yet, these challenges are no more than the challenges one might experience when using atrial fibrillation detection on smartwatches that have been FDA cleared owing to their ECG functionality.
Conclusion
The results from this research show that FCHD events can be predicted, with high accuracy, from both single-lead clinical and Apple Watch ECGs using state-of-the-art ECG-AI methods. In addition, we show that FCHD predictions from single-lead clinical ECGs and ECGs from Apple Watches are concordant. Such models can be implemented on Apple Watch ECGs and can help screen large populations for FCHD with ease and at low cost.
Acknowledgments
Funding Sources
This work was partially supported by the National Institute of Health [grant number: 1R01CA261834-01 (Akbilgic & Hudson) and grant number: U01CA195547 (MPI Hudson & Ness)], the American Lebanese Syrian Associate Charities (ALSAC), Memphis, TN and a Wake Forest Center for Artificial Intelligence Research.
Disclosures
The authors have no relevant conflict of interest or relationship with industry to declare.
Ethics Statement
The research reported in this paper adhered to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guidelines.
Disclaimer
Given his role as Editor-in-Chief, David McManus had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to David Duncker.
References
- 1.Perez-Alday E.A., Bender A., German D., et al. Dynamic predictive accuracy of electrocardiographic biomarkers of sudden cardiac death within a survival framework: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2019;19:1–19. doi: 10.1186/s12872-019-1234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogle B.M., Sotoodehnia N., Kucharska-Newton A.M., Rosamond W.D. Vital exhaustion and sudden cardiac death in the Atherosclerosis Risk in Communities Study. Heart. 2018;104:423–429. doi: 10.1136/heartjnl-2017-311825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenland P., Knoll M.D., Stamler J., et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 4.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg R.J., Junttila M.J. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 7.John R.M., Tedrow U.B., Koplan B.A., et al. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012;380:1520–1529. doi: 10.1016/S0140-6736(12)61413-5. [DOI] [PubMed] [Google Scholar]
- 8.Masarone D., Limongelli G., Ammendola E., Verrengia M., Gravino R., Pacileo G. Risk stratification of sudden cardiac death in patients with heart failure: an update. J Clin Med. 2018;7:436. doi: 10.3390/jcm7110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H., Dong S.-Y., Ren M.-S., Wang R. Ventricular arrhythmia and sudden cardiac death in hypertrophic cardiomyopathy: from bench to bedside. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.949294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo S., Carturan E., De Gaspari M., Pilichou K., Thiene G., Basso C. Update on cardiomyopathies and sudden cardiac death. Forensic Sci Res. 2019;4:202–210. doi: 10.1080/20961790.2019.1631957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micić J., Nikolić S., Savić S. Sudden cardiac death caused by complicated atherosclerosis of the anterior intraventricular branch of the left coronary artery with a myocardial muscle bridge. Srp Arh Celok Lek. 2003;131:173–175. doi: 10.2298/sarh0304173m. [DOI] [PubMed] [Google Scholar]
- 12.Wisten A., Messner T. Symptoms preceding sudden cardiac death in the young are common but often misinterpreted. Scand Cardiovasc J. 2005;39:143–149. doi: 10.1080/14017430510009168. [DOI] [PubMed] [Google Scholar]
- 13.Vaartjes I., Hendrix A., Hertogh E.M., et al. Sudden death in persons younger than 40 years of age: incidence and causes. Eur J Cardiovasc Prev Rehabil. 2009;16:592–596. doi: 10.1097/HJR.0b013e32832d555b. [DOI] [PubMed] [Google Scholar]
- 14.Winkel B.G., Risgaard B., Bjune T., et al. Gender differences in sudden cardiac death in the young-a nationwide study. BMC Cardiovasc Disord. 2017;17:1–8. doi: 10.1186/s12872-016-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skjelbred T., Rajan D., Svane J., Lynge T.H., Tfelt-Hansen J. Sex differences in sudden cardiac death in a nationwide study of 54 028 deaths. Heart. 2022;108:1012–1018. doi: 10.1136/heartjnl-2021-320300. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D., Post W.S., Blasco-Colmenares E., et al. Racial differences in sudden cardiac death: atherosclerosis risk in communities study (ARIC) Circulation. 2019;139:1688–1697. doi: 10.1161/CIRCULATIONAHA.118.036553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fender E.A., Henrikson C.A., Tereshchenko L. Racial differences in sudden cardiac death. J Electrocardiol. 2014;47:815–818. doi: 10.1016/j.jelectrocard.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan N.T., Schilling R.J. Sudden cardiac death and arrhythmias. Arrhythm Electrophysiol Rev. 2018;7:111. doi: 10.15420/aer.2018:15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chugh S.S., Reinier K., Teodorescu C., et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynge T.H., Svane J., Pedersen-Bjergaard U., et al. Sudden cardiac death among persons with diabetes aged 1–49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur Heart J. 2020;41:2699–2706. doi: 10.1093/eurheartj/ehz891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraishi Y., Goto S., Niimi N., et al. Improved prediction of sudden cardiac death in patients with heart failure through digital processing of electrocardiography. Europace. 2023;25:922–930. doi: 10.1093/europace/euac261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Mahony C., Jichi F., Pavlou M., et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 23.Wilson P.W., D’Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 24.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 25.De Lio F., Andreis A., De Lio G., et al. Cardiac imaging for the prediction of sudden cardiac arrest in patients with heart failure. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razavi A.C., Uddin S.I., Dardari Z.A., et al. Coronary artery calcium for risk stratification of sudden cardiac death: the coronary artery calcium consortium. Cardiovasc Imaging. 2022;15:1259–1270. doi: 10.1016/j.jcmg.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vähätalo J., Holmström L., Pakanen L., et al. Coronary artery disease as the cause of sudden cardiac death among victims< 50 years of age. Am J Cardiol. 2021;147:33–38. doi: 10.1016/j.amjcard.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Wallace E., Howard L., Liu M., et al. Long QT syndrome: genetics and future perspective. Pediatr Cardiol. 2019;40:1419–1430. doi: 10.1007/s00246-019-02151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296–1305. doi: 10.1161/CIRCULATIONAHA.106.618082. [DOI] [PubMed] [Google Scholar]
- 30.Gentile F., Castiglione V., De Caterina R. Coronary artery anomalies. Circulation. 2021;144:983–996. doi: 10.1161/CIRCULATIONAHA.121.055347. [DOI] [PubMed] [Google Scholar]
- 31.Shameer K., Johnson K.W., Glicksberg B.S., Dudley J.T., Sengupta P.P. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156–1164. doi: 10.1136/heartjnl-2017-311198. [DOI] [PubMed] [Google Scholar]
- 32.Soudan B., Dandachi F.F., Nassif A.B. Attempting cardiac arrest prediction using artificial intelligence on vital signs from Electronic Health Records. Smart Health. 2022 [Google Scholar]
- 33.Butler L., Celik T., Karabayir I., et al. Feasibility of remote monitoring for fatal coronary heart disease from single lead ECG. Cardiovasc Digit Health J. 2023;4:S1. [Google Scholar]
- 34.White A.D., Folsom A.R., Chambless L.E., et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 35.Akbilgic O., Butler L., Karabayir I., et al. ECG-AI: electrocardiographic artificial intelligence model for prediction of heart failure. Eur Heart J Digit Health. 2021;2:626–634. doi: 10.1093/ehjdh/ztab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell C.R., Bjornard K.L., Ness K.K., et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50:39–49. doi: 10.1093/ije/dyaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler L., Ivanov A., Celik T., et al. Time-dependent ECG-AI prediction of fatal coronary heart disease. medRxiv. 2023;2023 10.11.23296910. [Google Scholar]
- 38.Isakadze N., Martin S.S. How useful is the smartwatch ECG? Trends Cardiovasc Med. 2020;30:442–448. doi: 10.1016/j.tcm.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Pepplinkhuizen S., Hoeksema W.F., van der Stuijt W., et al. Accuracy and clinical relevance of the single-lead Apple Watch electrocardiogram to identify atrial fibrillation. Cardiovasc Digit Health J. 2022;3(6):S17–S22. doi: 10.1016/j.cvdhj.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.North F., Chaudhry R. Apple healthkit and health app: patient uptake and barriers in primary care. Telemed J E Health. 2016;22:608–613. doi: 10.1089/tmj.2015.0106. [DOI] [PubMed] [Google Scholar]
- 41.Taylor A. Apress; 2015. Get Fit with Apple Watch: Using the Apple Watch for Health and Fitness. [Google Scholar]
- 42.Abu-Alrub S., Strik M., Ramirez F.D., et al. Smartwatch electrocardiograms for automated and manual diagnosis of atrial fibrillation: a comparative analysis of three models. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.836375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raja J.M., Elsakr C., Roman S., et al. Apple watch, wearables, and heart rhythm: where do we stand? Ann Transl Med. 2019;7:417. doi: 10.21037/atm.2019.06.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farshchian B.A., Vilarinho T., editors. Ambient Intelligence: 13th European Conference, AmI 2017, Malaga, Spain, April 26–28, 2017, Proceedings 13. Springer; 2017. Which mobile health toolkit should a service provider choose? A comparative evaluation of Apple HealthKit, Google Fit, and Samsung Digital Health Platform. [Google Scholar]
- 45.McCraw C.A., Karabayir I., Akbilgic O. ECG-AIR: an AI platform for remote smartwatch ECG-based cardiovascular disease detection and prediction. Cardiovasc Digit Health J. 2022;3:S7. [Google Scholar]
- 46.Waks J.W., Sitlani C.M., Soliman E.Z., et al. Global electric heterogeneity risk score for prediction of sudden cardiac death in the general population: the atherosclerosis risk in communities (ARIC) and cardiovascular health (CHS) studies. Circulation. 2016;133:2222–2234. doi: 10.1161/CIRCULATIONAHA.116.021306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosamond W.D., Chang P.P., Baggett C., et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schirmer H., Lunde P., Rasmussen K. Prevalence of left ventricular hypertrophy in a general population; The Tromsø Study. Eur Heart J. 1999;20:429–438. doi: 10.1053/euhj.1998.1314. [DOI] [PubMed] [Google Scholar]
- 49.Norrish G., Ding T., Field E., et al. Relationship between maximal left ventricular wall thickness and sudden cardiac death in childhood onset hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemingway H., Asselbergs F.W., Danesh J., et al. Big data from electronic health records for early and late translational cardiovascular research: challenges and potential. Eur Heart J. 2018;39:1481–1495. doi: 10.1093/eurheartj/ehx487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathai N., Shiratudin M., Sohel F. Electronic health record management: expectations, issues, and challenges. Journal of Health & Medical Informatics. 2017;8:1–5. [Google Scholar]