Abstract
Background
Cardiomyopathy is a leading cause of pregnancy-related mortality and the number one cause of death in the late postpartum period. Delay in diagnosis is associated with severe adverse outcomes.
Objective
To evaluate the performance of an artificial intelligence–enhanced electrocardiogram (AI-ECG) and AI-enabled digital stethoscope to detect left ventricular systolic dysfunction in an obstetric population.
Methods
We conducted a single-arm prospective study of pregnant and postpartum women enrolled at 3 sites between October 28, 2021, and October 27, 2022. Study participants completed a standard 12-lead ECG, digital stethoscope ECG and phonocardiogram recordings, and a transthoracic echocardiogram within 24 hours. Diagnostic performance was evaluated using the area under the curve (AUC).
Results
One hundred women were included in the final analysis. The median age was 31 years (Q1: 27, Q3: 34). Thirty-eight percent identified as non-Hispanic White, 32% as non-Hispanic Black, and 21% as Hispanic. Five percent and 6% had left ventricular ejection fraction (LVEF) <45% and <50%, respectively. The AI-ECG model had near-perfect classification performance (AUC: 1.0, 100% sensitivity; 99%–100% specificity) for detection of cardiomyopathy at both LVEF categories. The AI-enabled digital stethoscope had an AUC of 0.98 (95% CI: 0.95, 1.00) and 0.97 (95% CI: 0.93, 1.00), for detection of LVEF <45% and <50%, respectively, with 100% sensitivity and 90% specificity.
Conclusion
We demonstrate an AI-ECG and AI-enabled digital stethoscope were effective for detecting cardiac dysfunction in an obstetric population. Larger studies, including an evaluation of the impact of screening on clinical outcomes, are essential next steps.
Keywords: Cardiomyopathies, ECG, Heart failure, Obstetrics, Pregnancy, Postpartum
Graphical abstract
Introduction
Cardiomyopathy is a leading cause of pregnancy-related mortality and the number one cause of death in the late postpartum period.1,2 In addition, any form of pregnancy-associated cardiomyopathy is known to be associated with a high risk of severe complications.3 Antepartum diagnosis of cardiomyopathy is challenging owing to the overlap between pregnancy physiology–related symptoms and those suggestive of cardiomyopathy or heart failure,4,5 and a delay in diagnosis is believed to be an important contributor to mortality.6 As such, the need to develop scalable and effective tools for routine cardiomyopathy screening in the obstetric population is imperative.
Prior studies have demonstrated the effectiveness of an artificial intelligence (AI)–enabled electrocardiogram (AI-ECG) for detection of left ventricular dysfunction in unselected patient populations.7, 8, 9, 10, 11 This AI-ECG model, based on a standard 12-lead ECG recording, has been validated in an emergency room setting,12 racial and ethnic subgroups,13 a retrospective community-based cohort,14 a retrospective sample of pregnant and postpartum women,15 and in a clinical trial among patients seen for routine primary care.16
In a bid to evaluate potentially scalable and portable cardiomyopathy screening options, an AI-enabled single-lead ECG recording from a digital stethoscope has also been shown to be effective for detection of left ventricular dysfunction,17,18 and more recently, the digital stethoscope single-lead AI-ECG model has been revised to incorporate data from recorded phonocardiograms as well. In addition, a smartwatch-enabled ECG demonstrated similar performance19 in a prospective study.
However, these AI models and digital tools have yet to be prospectively evaluated in the obstetric population. Pregnant and postpartum women are an ideal group for targeted screening owing to reported delays in cardiomyopathy diagnosis and a high risk of cardiomyopathy-associated mortality with the current standard of care. In addition, failure to identify symptoms of heart disease during pregnancy, which can be difficult to distinguish from normal physiological changes of pregnancy, is an important driver of health inequities in the obstetric population.5 The goal of this study was to prospectively evaluate the effectiveness of an AI-ECG and AI-enabled digital stethoscope to screen for left ventricular systolic dysfunction (LVSD) in a pilot study of pregnant and postpartum women.
Methods
Study design
We conducted a single-arm, prospective study among pregnant and postpartum women (up to 12 months following delivery) between October 28, 2021, and October 27, 2022, with follow-up for outcomes through November 21, 2022. The study was approved by the Mayo Clinic Institutional Review Board (IRB). A written or electronic consent was obtained from each study participant in accordance with IRB guidance.
Study population
Consecutive consenting study participants were enrolled at 3 sites: Mayo Clinic, Jacksonville, Florida; Agape Family Health Clinic, Jacksonville, Florida; and Mayo Clinic, Rochester, Minnesota, to ensure that a racially and ethnically diverse group of women were included. To meet this goal, we aimed to have at least 30% of the study sample be Black/African American. As such, enrollment of White participants was halted after 38 were enrolled in the study. Study participants included patients receiving care, hospital/clinic staff, and visitors. Inclusion criteria were female sex, aged 18–49 years, and pregnant or within 12 months postpartum. We excluded participants with complex congenital heart disease (status post complex cardiac surgery, single-ventricle physiology, or significant shunts with cardiac structural changes) or significant conduction abnormalities on a resting ECG (paced ECGs or presence of a left ventricular assist device). Study participants completed a standard 12-lead ECG recording, a digital stethoscope ECG recording, a questionnaire (including demographic variables and clinical symptoms), and a comprehensive transthoracic echocardiogram on the same day or within 24 hours. Over a 12-month enrollment period, 126 women agreed to participate and 101 were able to make it to a scheduled research appointment. We excluded 1 participant who did not complete all required baseline tests or the questionnaire (Figure 1).
Figure 1.
Flow diagram for study participants.
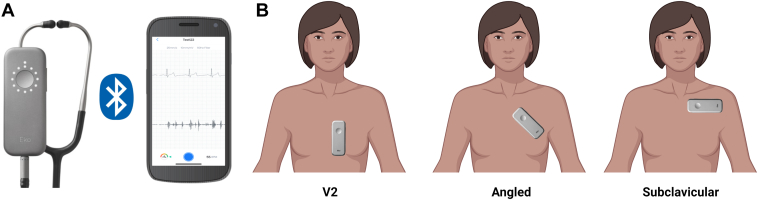
Standard 12-lead ECGs were acquired by trained personnel at a sampling rate of 500 Hz using GE ECG machines (MAC 5500, MAC 7, and MAC VU360), were stored and clinically interpreted using the MUSE ECG data management system (GE Healthcare, Chicago, IL), and digital XML files were exported for AI analysis. Digital stethoscope ECG and phonocardiogram recordings were performed by study staff; 15-second recordings at 3 locations across the chest were obtained, as follows: (1) placed vertically at the left sternal border (V2), (2) angled across the left upper chest (angled), and (3) placed horizontally over the left subclavicular area (subclavicular) (Figure 2).
Figure 2.
Digital stethoscope positions. A: Digital stethoscope connects to mobile app via Bluetooth. B: Fifteen-second single-lead electrocardiogram recordings were obtained at 3 locations across the chest: placed vertically at the left sternal border (V2), angled across the left upper chest (angled), and placed horizontally over the left subclavicular area (subclavicular).
All echocardiograms were performed by trained sonographers at a Mayo Clinic site using standard image acquisition protocols and clinically interpreted by a board-certified cardiologist according to the American Society of Echocardiography guidelines.20 All echocardiogram images and measurements were also reviewed by the study lead (DA). Quantitative measures including left ventricular ejection fraction (LVEF) were extracted from the echocardiogram reports.
ECG preprocessing
Standard 12-lead ECGs
Standard 12-lead ECGs were uploaded from GE ECG machines to the MUSE ECG database management system (GE Healthcare). Following clinical interpretation, digital XML files were exported to a secure research server, after which they were processed and decoded from GE’s proprietary format to a NumPy object using an open source R package21 and Mayo Clinic’s AI-ECG algorithm for detection of left ventricular dysfunction7 was used to generate predictions.
Digital stethoscope ECG and phonocardiogram recordings
Single-lead digital ECGs and phonocardiograms were extracted from the cloud server by Eko Health (Emeryville, CA). The phonocardiogram signal is run through a Butterworth low-pass filter with a cutoff frequency of 800 Hz and then through a Butterworth high-pass filter with a cutoff frequency of 30 Hz. The single-lead ECG signal is first run through a notch filter to remove interference from the main power supply (60 Hz in the United States or 50 Hz in the UK/other countries), then it is run through a bandpass filter with a lower cutoff of 0.5 Hz and an upper cutoff of 40 Hz. AI predictions based on the ECG combined with phonocardiogram recordings were provided by Eko Health to the Mayo Clinic study team for each study participant (up to 3 for each participant based on recording location on the chest wall, Figure 2). Digital stethoscope data linkage to echocardiogram results and area under the receiver operating characteristic curve analyses were performed at Mayo Clinic.
Measures
Our primary study endpoint was detection of LVSD, defined as LVEF <50% using the AI-ECG (based on a standard 12-lead ECG recording) and an AI-enabled digital stethoscope. Our secondary study endpoint was detection of LVEF <45% using the AI-ECG (based on a standard 12-lead ECG recording) and an AI-enabled digital stethoscope. LVEF <45% was selected as a secondary outcome, being a clinically relevant threshold for identification of peripartum cardiomyopathy in the obstetric population. LVEF measurements were assessed using multiple methods in this order: 2D-biplane > 2D-linear > visual assessment. For echocardiographic images with more than 1 segment not clearly seen, an ultrasound-enhancing agent was used.
Statistical analysis
This study was designed as a single-arm study to provide an understanding of subject accrual rate, feasibility of obtaining standard 12-lead and portable ECGs using US Food and Drug Administration–approved devices in pregnant and postpartum women, and the performance of the AI-enabled ECG algorithm in this patient population. For sample size justification, assuming a ratio of positive to negative of 1:19 (based on a retrospective analysis of obstetric patients who had cardiovascular testing at Mayo Clinic15, a sample size of 100 cases would be expected to have >90% power to reject the null hypothesis that the area under the curve (AUC) = 0.5 at the alpha = 0.05 level of significance provided the AUC is at least 0.90. From prior AI algorithms for left ventricular dysfunction, this level of discrimination was expected.
Diagnostic performance for detection of LVSD was evaluated for the AI-ECG and digital stethoscope using the AUC and other measures of diagnostic accuracy, including sensitivity, specificity, positive and negative predictive values, with 95% confidence intervals calculated. We used a previously validated AI-ECG deep learning model originally developed for identification of LVEF ≤35%7,16,22 without any modifications to analyze the 12-lead ECGs obtained in this study. The digital stethoscope had up to 3 separate AI model predictions based on the device position on the chest wall at the time of data acquisition (Figure 2). Model performance was summarized for each lead configuration along with 2 global measures: the mean of the model outputs and the maximum model output. The maximum value was taken as the primary summary for the digital stethoscope, as it would represent the most sensitive summary using the device. The research reported in this paper adhered to the Standards for Reporting of Diagnostic Accuracy Studies guidelines. P values <.05 were considered statistically significant. All analysis were performed using R version 4.0.3 (Vienna, Austria).23
Results
One hundred pregnant and postpartum women were included in the final analysis. At the time of enrollment, 78% were pregnant and 22% were within 12 months postpartum. Summary sample characteristics are provided in Table 1. The median age of participants was 31 years (Q1: 27, Q3: 34). Thirty-eight percent identified as non-Hispanic White, 32% as non-Hispanic Black, 21% as Hispanic, and 9% as other (comprising 6% Asian, 2% multiracial, and 1% Native Hawaiian/Pacific Islander). Five percent and 6% had LVEF <45% and <50%, respectively.
Table 1.
Demographics and clinical characteristics of the study sample
| Normal LV systolic function (n=94) | LV systolic dysfunction† (n=6) | Overall | P values | |
|---|---|---|---|---|
| Age | 31.6 (27.5, 34.8) | 30.9 (25.6, 35.2) | 31.6 (27.5, 34.9) | .828 |
| Body mass index | 29.0 (24.1, 32.8) | 26.4 (24.2, 28.4) | 28.5 (24.1, 32.7) | .411 |
| Race/ethnicity | ||||
| Non-Hispanic White | 34 (36.2%) | 4 (66.7%) | 38 (38.0%) | .204 |
| Non-Hispanic Black | 30 (31.9%) | 2 (33.3%) | 32 (32.0%) | |
| Hispanic or Latino | 21 (22.3%) | 0 (0%) | 21 (21.0%) | |
| Asian | 6 (6.4%) | 0 (0%) | 6 (6.0%) | |
| Native Hawaiian/Pacific Islander | 1 (1.1) | 0 (0%) | 1 (1.0%) | |
| Multiracial | 2 (2.1%) | 0 (0%) | 2 (2.0%) | |
| Recruitment site | ||||
| Mayo Clinic Florida | 46 (48.9%) | 5 (83.3%) | 51 (51.0%) | .067 |
| AGAPE Clinic | 41 (43.6%) | 0 (0%) | 41 (41.0%) | |
| Mayo Clinic Rochester | 7 (7.5%) | 1 (16.7%) | 8 (8.0%) | |
| Pregnancy status | ||||
| Pregnant | 76 (80.9%) | 2 (33.3%) | 78 (78.0%) | .02‡ |
| - First trimester | 9 (9.6%) | 1 (16.7%) | 10 (10.0%) | |
| - Second trimester | 37 (39.4%) | 0 (0.0%) | 37 (37.0%) | |
| - Third trimester | 30 (31.9%) | 1 (16.7%) | 31 (31.0%) | |
| Postpartum | 18 (19.2%) | 4 (66.7%) | 22 (22.0%) | |
| Vital signs | ||||
| Systolic blood pressure | 113.0 (105.3, 120.0) | 106.0 (93.3, 118.0) | 113.0 (104.8, 120.0) | .186 |
| Diastolic blood pressure | 70.0 (64.0, 75.0) | 68.5 (62.3, 76.3) | 70.0 (63.0, 75.3) | .788 |
| Heart rate | 76.0 (68.3, 85.0) | 98.5 (84.3, 109.0) | 76.5 (69.0, 86.3) | .01 |
| Echocardiographic parameters | ||||
| LV end-diastolic diameter (mm) | 46.0 (43.0, 49.0) | 60.0 (57.3, 64.3) | 46.0 (43.0, 50.0) | <.001 |
| LV end-systolic diameter (mm) | 30.0 (28.0, 33.0) | 54.0 (49.3, 59.5) | 30.0 (28.0, 33.0) | <.001 |
| LV septal wall thickness (mm) | 8.0 (8.0, 9.0) | 7.5 (7.0, 8.8) | 8.0 (8.0, 9.0) | .283 |
| LV relative wall thickness (%) | 38.0 (33.3, 42.0) | 27.0 (25.3, 28.0) | 37.0 (33.0, 42.0) | <.001 |
| LV mass index (g/m2) | 73.0 (61.0, 80.0) | 98.5 (86.3, 119.0) | 73.5 (61.8, 82.3) | .008 |
| LV posterior wall thickness (mm) | 9.0 (8.0, 10.0) | 8.0 (8.0, 8.0) | 9.0 (8.0, 10.0) | .244 |
| Mitral valve early diastolic filling velocity – E (m/s) | 0.8 (0.7, 0.9) | 1.1 (0.9, 1.3) | 0.8 (0.7, 1.0) | .033 |
| Medial mitral annulus velocity by tissue Doppler – eʹ (m/s) | 0.11 (0.09, 0.12) | 0.07 (0.07, 0.08) | 0.10 (0.08, 0.12) | .001 |
| Mitral valve E/eʹ ratio | 7.8 (6.4, 9.0) | 16.3 (11.3, 18.6) | 7.8 (6.4, 9.2) | .001 |
| Cardiac output – Doppler method (L/min) | 5.6 (4.1, 6.2) | 5.3 (4.9, 5.5) | 5.6 (4.9, 6.1) | .437 |
| Cardiac index – Doppler method (L/min/m2) | 3.0 (2.7, 3.4) | 2.9 (2.7, 3.0) | 3.0 (2.7, 3.4) | .379 |
| Peak tricuspid valve regurgitation velocity (m/s) | 2.0 (1.8, 2.3) | 2.9 (2.7, 3.1) | 2.1 (1.8, 2.3) | .007 |
| Left atrial volume index (mL/m2) | 25.0 (21.0, 28.3) | 28.0 (26.0, 31.0) | 25.0 (21.0, 29.0) | .266 |
| Right atrial volume index (mL/m2) | 19.0 (15.0, 23.1) | 16.5 (14.0, 29.0) | 18.9 (15.0, 23.2) | .943 |
| LV global longitudinal systolic strain | -19.0 (-21.0, -18.0) | -9.5 (-10.8, -8.0) | -19.0 (-21.0, -18.0) | .001 |
| LV geometry | ||||
| Normal | 61 (64.9%) | 2 (33.3%) | 63 (63.0%) | .006 |
| Concentric remodeling | 23 (24.5%) | 0 (0%) | 23 (23.0%) | |
| Eccentric hypertrophy | 9 (9.6%) | 4 (66.7%) | 13 (13.0%) | |
| Concentric hypertrophy | 1 (1.1%) | 0 (0%) | 1 (1.0%) | |
| Diastolic function | ||||
| Normal | 87 (92.6%) | 2 (33.3%) | 89 (89.0%) | .001 |
| Abnormal | 4 (4.3%) | 2 (33.3%) | 6 (6.0%) | |
| Indeterminate | 3 (3.2%) | 2 (33.3%) | 5 (5.0%) | |
| Medical history | ||||
| Chronic hypertension | 3 (3.2%) | 0 (0%) | 3 (3.0%) | 1 |
| Gestational hypertension | 1 (1.1%) | 2 (33.3%) | 3 (3.0%) | .009 |
| Preeclampsia | 2 (2.1%) | 2 (33.3%) | 4 (4.0%) | .017 |
| Gestational diabetes | 5 (5.3%) | 2 (33.3%) | 7 (7.0%) | .055 |
| Small for gestational age | 3 (3.2%) | 0 (0%) | 3 (3.0%) | 1 |
| Preterm birth | 2 (2.1%) | 1 (16.7%) | 3 (3.0%) | .171 |
| Other | 22 (23.4%) | 2 (33.3%) | 24 (24.0%) | .628 |
| Pregnancy outcome | ||||
| Live birth (single) | 77 (81.9%) | 5 (83.3%) | 82 (82.0%) | .139 |
| Live birth (multiple) | 1 (1.1%) | 1 (16.7%) | 2 (2.0%) |
Median, 25th percentile (Q1), and 75th percentile (Q3) reported for all numeric variables.
Frequency counts and percentages reported for categorical variables.
LV = left ventricular.
LV systolic dysfunction is defined as left ventricular ejection fraction <50%.
Fisher's exact P value computed for the comparison of pregnant vs postpartum. The enumeration of the stage of pregnancy was not used in the analysis.
Twelve-lead ECG
The AI-ECG model had excellent classification performance for detection of cardiomyopathy at both LVEF categories (<45% and <50%), correctly identifying all cases of LVSD in our study sample and achieving near-perfect discrimination (Figure 3A–3D, Supplemental Table 1). At the LVEF <50% threshold, all 100 participants were correctly classified (AUC 1.0, sensitivity and specificity were 100%). At LVEF <45%, AUC was 1.0, sensitivity was 100%, and specificity was 98.9%. Overall accuracy was 99% (99/100; 95% CI: 94.6%–100.0%), with the 1 misclassified individual having an LVEF of 49% and classified as a false positive. Standard 12-lead ECG tracings are shown in Figure 4 for a patient with LVEF of 35% and another with LVEF 66%. These are shown side by side to demonstrate the similarity between both ECGs on visual appreciation. Although minor differences are appreciated on the ECGs, a formal cardiologist’s clinical interpretation of the ECGs would be insufficient for definite determination of low LVEF status.
Figure 3.
Twelve-lead electrocardiogram (ECG) receiver operating characteristic (ROC) curves and confusion matrix. A, B: ROC curve (A) and confusion matrix (B) for detection of left ventricular ejection fraction (LVEF) <50% using a 12-lead artificial intelligence–enabled ECG (AI-ECG) algorithm among pregnant and postpartum women. C, D: ROC curve (C) and confusion matrix (D) for detection of LVEF <45% using 12-lead AI-ECG algorithm among pregnant and postpartum women. The 1 false-positive case had an ejection fraction of 49%.
Figure 4.
Standard 12-lead electrocardiogram (ECG) examples. ECG tracings for 2 patients in our study sample, with clinical interpretations: 1 with left ventricular ejection fraction (LVEF) of 35% (AI-predicted probability 0.796) and the other with LVEF of 66% (AI-predicted probability 0.180). The previously determined AI-predicted probability threshold for a positive flag is 0.256.
Digital stethoscope with AI-enabled single-lead ECG and phonocardiogram
Across all 3 stethoscope positions, diagnostic performance was robust. The number of evaluable ECGs by position, however, varied, with 99, 98, and 96 participants providing ECG signals for the V2, angled, and subclavicular positions, respectively. Of the 3 positions, the angled position for the digital stethoscope provided the numerically highest discrimination, with AUC of 0.987 (95% CI: 0.967, 1.00) for both the detection of LVEF <50% (Supplemental Table 2) and LVEF <45% (Supplemental Table 3). To provide predictions for all participants, the diagnostic performance for the mean and maximum model outputs over the 3 positions was also considered. For the maximum model output, which would have the highest sensitivity of any of the combinations of positions, the AUC, sensitivity, and specificity were 0.968 (95% CI: 0.934, 1.000), 100.0% (95% CI: 54.1%, 100.0%), and 90.4% (85/94; 95% CI: 82.6%, 95.5%) for LVEF <50% (Supplemental Figure 1A and 1B). Model performance metrics for all other stethoscope positions and LVEF thresholds are provided in Supplemental Tables 2–4. A subgroup analysis was performed and showed stable performance of the AI model by age and race/ethnicity categories (Supplemental Figure 2) at both LVEF thresholds (<45% and <50%). The model outputs from both the 12-lead ECG and digital stethoscope recordings appear to be appropriately correlated with LVEF and other lead/device configurations (Supplemental Figure 3).
Discussion
This pilot prospective study of an AI-powered screening tool for LVSD among pregnant and postpartum women had 3 main findings. First in this US-based population, pregnancy-related cardiomyopathy was relatively common, impacting 6% of consecutive women enrolled in the study (4 diagnosed with peripartum cardiomyopathy and 2 with dilated cardiomyopathy). Second, a standard 12-lead ECG, a ubiquitous, inexpensive test that is integrated in clinical workflows, was a powerful screening test for LVSD among pregnant and postpartum women with the addition of AI analysis, demonstrating near-perfect discrimination in this cohort (AUC = 1.0). Third, a digital stethoscope with single-lead ECG recording capability combined with a phonocardiogram recording had a similarly strong performance (AUC = 0.987). These findings, if confirmed in larger studies, would support widespread screening for this important condition that impacts young women and children.24
The frequency and clinical impact of LVSD occurring during pregnancy or postpartum is incompletely understood. Studies have suggested that peripartum cardiomyopathy is likely under-recognized and underdiagnosed.25 Its incidence varies in different patient populations, with the highest estimates reported in predominantly Black populations (1 in 100 deliveries in Nigeria and 1 in 300 deliveries in Haiti).25,26 The incidence of peripartum cardiomyopathy in the United States was estimated at approximately 1 in 850 live births based on the National Inpatient Sample (NIS) data from 2011, which is up from approximately 1 in 1200 in 2004.26 Among women aged 40 and older, the incidence is reported to be as high as 1 in 270 live births.27 It is important to detect and initiate appropriate therapy for cardiomyopathy early owing to the high risk (up to 13%) for severe maternal morbidity (left ventricular assist device placement or heart transplantation) and mortality,28 with important implications for the infant as well. Novel clinical approaches and tools, such as the AI-powered ECG, are needed to help identify disease sooner in an effort to help decrease maternal and fetal morbidity and mortality related to this condition. The presented screening approach, which leverages the ECG enhanced with AI and confirmatory evaluation by echocardiogram if an AI-positive flag is obtained, has been demonstrated to be cost effective.29
Current guidelines from the American College of Cardiology and the American College of Obstetrics and Gynecology (ACOG) do not recommend routine screening for any cardiomyopathy in the obstetric population. This may in part reflect the current need for expensive imaging tests (echocardiogram, computed tomography, or magnetic resonance imaging) that can include radiation or high-magnetic-force exposure to mother and fetus, making such screening impractical. The availability of an ECG-based AI screen may change the equation. This study provides important preliminary data supporting the use of AI-powered tools for cardiovascular screening during pregnancy.
The performance of both the 12-lead ECG and digital stethoscope significantly exceeded the strong performance previously reported in retrospective7 and prospective16 studies of the same algorithm that included older, general patient populations. In contrast to previous studies, in which individuals with normal ventricular function (control) had many comorbidities such as hypertension, diabetes, hyperlipidemia, and renal dysfunction, the control subjects in the present study were younger and healthier. This likely facilitated the ability of the AI-ECG to powerfully classify normal from abnormal ventricular function. We have previously demonstrated the ability of AI analysis of a single-lead ECG from a watch to detect ventricular dysfunction,19 with only minimal loss in test performance compared to the 12-lead model. Thus, it was not surprising to find a similar very modest degradation of test performance using the digital single-lead ECG stethoscope recording. Prior to the implementation of AI models in clinical practice, it is essential that they be thoroughly validated in populations in which they are intended for use,30 with prospective validation studies being an important step, as well as ensuring dataset diversity for model training and validation.31,32
While routine screening is not recommended, the ACOG guidelines do recommend a cardiovascular toolkit be used to risk-stratify patients with suspected cardiovascular disease.5 This toolkit uses a combination of symptoms, vital signs, risk factors from medical history, and physical examination findings to determine risk, and, based on a combination of these, an ECG and natriuretic peptide measurement are the recommended first-line screening tests.33,34 Clinical interpretation of the ECG alone is insufficient for identification of LVSD, and natriuretic peptide levels are known to vary by pregnancy trimester35 and can be altered by obesity36,37 or preeclampsia.38
Our study leverages the ECG, a simple, effective, scalable, and cost-effective screening tool to screen for LVSD in the obstetric population. Our team has previously evaluated the effectiveness of the AI-ECG for detection of LVSD in a retrospective sample of pregnant and postpartum women seen at Mayo Clinic, demonstrating its effectiveness (AUC = 0.92) and that it outperformed natriuretic peptides.15 In addition, the current study evaluated a portable device, the digital stethoscope, as an alternative to the standard 12-lead ECG and showed it remained effective, thus providing an alternate option for obstetric cardiovascular screening in clinical settings, nonclinical settings, and low-resource settings. While 12-lead ECGs are readily available in standard cardiovascular outpatient and in-patient practices, this may not necessarily be the case in obstetric care settings, particularly in the outpatient clinics. As such, the ability to use a portable device in various health care settings as well as for remote/virtual care or in the community can have a profound impact as a public health intervention. The diagnostic performance of the AI-ECG for detection of left ventricular dysfunction is notably impressive when compared to screening tests used in routine clinical care, such as mammography,39 pap smear,40,41 and natriuretic peptide screening for heart failure,19 with AUC values ranging from 0.71 to 0.85. The AI-ECG also demonstrates superior diagnostic performance when compared to commonly used diagnostic tests in cardiovascular medicine, such as SPECT myocardial perfusion imaging for detection of obstructive coronary disease (AUC = 0.87 based on a meta-analysis)42 and stress echocardiography (AUC = 0.73–0.84).43
A key limitation of this study is the relatively small sample size with a low number of participants having LVSD, as such model performance needs to be interpreted cautiously in this context. In addition, the low number of cases limits subgroup analysis (including age and race categories), with less precise confidence intervals around calculated estimates of diagnostic performance. The sample for this study was intentionally small to allow for the initial examination of the AI-ECG algorithm in pregnant and postpartum women. The sample size justification used a lower threshold of AUC = 0.5 to allow for any discrimination above chance to be considered evidence that the algorithm shows some diagnostic performance in this population. As such, a positive signal in this study would warrant a larger, more comprehensive study.
Strengths include the use of standard and portable ECG recordings for AI-based cardiomyopathy prediction, targeted enrollment of a racially and ethnically diverse group of women, and performance of the ECGs on the same day as or (in 1 patient) within 24 hours of the echocardiogram, which allows for an almost simultaneous assessment of the AI-based predictions with echo-based left ventricular function assessment.
Conclusion
In this pilot prospective study, we found that a 12-lead AI-ECG and an AI-enabled digital stethoscope (single-lead ECG + phonocardiogram) are powerful screening tools for LVSD in obstetric patients, a treatable, under-recognized condition with significant morbidity and mortality. This suggests AI-ECG tools may provide powerful point-of-care screening for pregnant and postpartum women.
Acknowledgments
We would like to extend our sincere appreciation to all study participants, without whom this study would not have been possible. We also want to thank all Agape Family Health Clinic staff in Jacksonville, FL, and all staff including nursing, administrative, sonographers (many of whom volunteered to stay after work hours to ensure research echocardiograms were performed), physicians, and other allied health staff at Mayo Clinic Florida and Mayo Clinic Rochester; the department of cardiovascular medicine and digital innovation laboratory at Mayo Clinic Florida; the Eko Health team; and Miami Heart Research Institute, who all contributed to the success of this study. Some figure illustrations were created with biorender.com.
Funding Sources
This study was funded by the Miami Heart Research Institute, Florida Heart Research Foundation. Dr Adedinsewo is also supported by the Mayo Clinic Women’s Health Research Center and the Mayo Clinic Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program funded by the National Institutes of Health (grant number K12AR084222). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Digital stethoscope data extraction and AI analysis of stethoscope ECG and phonocardiogram recordings were performed by Eko Health. The funder and Eko Health had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures
Drs Attia and Friedman are co-inventors of several AI algorithms (including screening for low LVEF, QT tool, aortic stenosis, atrial fibrillation detection during normal sinus rhythm). These have been licensed to Anumana, AliveCor, and Eko. Mayo Clinic, Dr Attia, and Dr Friedman may benefit from their commercialization. Dr Noseworthy, Dr Lopez-Jimenez, and Mayo Clinic have filed patents related to the application of AI to the ECG for diagnosis and risk stratification and have licensed several AI-ECG algorithms to Anumana. Dr Noseworthy and Mayo Clinic are involved in potential equity/royalty relationship with AliveCor. Dr Noseworthy is a study investigator in an ablation trial sponsored by Medtronic and has served on an expert advisory panel for OptumLabs. All other authors have no conflicts to disclose.
Role of the Funding Source
Miami Heart Research Institute and Eko Health had no role in the study design and conduct; data collection, management, analysis, and interpretation; manuscript preparation, review, or approval; and decision to submit the manuscript for publication.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author. All requests for raw and analyzed data and related materials, excluding programming code, will be reviewed by the Mayo Clinic legal department and Mayo Clinic Ventures to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for patient-related data not included in the paper will not be considered. Any data and materials that can be shared will be released via a Material Transfer Agreement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.cvdhj.2024.03.005.
Appendix. Supplementary data
References
- 1.Petersen E.E., Davis N.L., Goodman D., et al. Vital signs: pregnancy-related deaths, United States, 2011-2015, and strategies for prevention, 13 states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–429. doi: 10.15585/mmwr.mm6818e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDorman M.F., Thoma M., Declcerq E., Howell E.A. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016‒2017. Am J Public Health. 2021;111:1673–1681. doi: 10.2105/AJPH.2021.306375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: the Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 4.Germain S., Nelson-Piercy C. Common symptoms during pregnancy. Obstetrics, Gynaecology & Reproductive Medicine. 2011;21:323–326. [Google Scholar]
- 5.ACOG ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–e356. doi: 10.1097/AOG.0000000000003243. [DOI] [PubMed] [Google Scholar]
- 6.Hameed A.B., Lawton E.S., McCain C.L., et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. 2015;213:379.e1–379.e10. doi: 10.1016/j.ajog.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Attia Z.I., Kapa S., Lopez-Jimenez F., et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 8.Kwon J.M., Kim K.H., Jeon K.H., et al. Development and validation of deep-learning algorithm for electrocardiography-based heart failure identification. Korean Circ J. 2019;49:629–639. doi: 10.4070/kcj.2018.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J.Y., Qiu Y., Guo H.C., et al. A method to screen left ventricular dysfunction through ECG based on convolutional neural network. J Cardiovasc Electrophysiol. 2021;32:1095–1102. doi: 10.1111/jce.14936. [DOI] [PubMed] [Google Scholar]
- 10.Katsushika S., Kodera S., Nakamoto M., et al. The effectiveness of a deep learning model to detect left ventricular systolic dysfunction from electrocardiograms. Int Heart J. 2021;62:1332–1341. doi: 10.1536/ihj.21-407. [DOI] [PubMed] [Google Scholar]
- 11.Bjerkén L.V., Rønborg S.N., Jensen M.T., Ørting S.N., Nielsen O.W. Artificial intelligence enabled ECG screening for left ventricular systolic dysfunction: a systematic review. Heart Fail Rev. 2023;28:419–430. doi: 10.1007/s10741-022-10283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adedinsewo D., Carter R.E., Attia Z., et al. Artificial intelligence-enabled ECG algorithm to identify patients with left ventricular systolic dysfunction presenting to the emergency department with dyspnea. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008437. [DOI] [PubMed] [Google Scholar]
- 13.Noseworthy P.A., Attia Z.I., Brewer L.C., et al. Assessing and mitigating bias in medical artificial intelligence: the effects of race and ethnicity on a deep learning model for ECG analysis. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attia I.Z., Tseng A.S., Benavente E.D., et al. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. Int J Cardiol. 2021;329:130–135. doi: 10.1016/j.ijcard.2020.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adedinsewo D.A., Johnson P.W., Douglass E.J., et al. Detecting cardiomyopathies in pregnancy and the postpartum period with an electrocardiogram-based deep learning model. Eur Heart J Digit Health. 2021;2:586–596. doi: 10.1093/ehjdh/ztab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao X., Rushlow D.R., Inselman J.W., et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27:815–819. doi: 10.1038/s41591-021-01335-4. [DOI] [PubMed] [Google Scholar]
- 17.Bachtiger P., Petri C.F., Scott F.E., et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health. 2022;4:e117–e125. doi: 10.1016/S2589-7500(21)00256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attia Z.I., Dugan J., Rideout A., et al. Automated detection of low ejection fraction from a one-lead electrocardiogram: application of an AI algorithm to an electrocardiogram-enabled Digital Stethoscope. Eur Heart J Digit Health. 2022;3:373–379. doi: 10.1093/ehjdh/ztac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attia Z.I., Harmon D.M., Dugan J., et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat Med. 2022;28:2497–2503. doi: 10.1038/s41591-022-02053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Popa T., Mocanu A. Medical data storage, visualization and interpretation: a case study using a proprietary ECG XML format. Annals of the University of Craiova Series: Automation, Computers, Electronics and Mechatronics. 2011;8:44–49. [Google Scholar]
- 22.Harmon D.M., Carter R.E., Cohen-Shelly M., et al. Real-world performance, long-term efficacy, and absence of bias in the artificial intelligence enhanced electrocardiogram to detect left ventricular systolic dysfunction. Eur Heart J Digit Health. 2022;3:238–244. doi: 10.1093/ehjdh/ztac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing. [Google Scholar]
- 24.Gunderson E.P., Croen L.A., Chiang V., Yoshida C.K., Walton D., Go A.S. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118:583–591. doi: 10.1097/AOG.0b013e318229e6de. [DOI] [PubMed] [Google Scholar]
- 25.Davis M.B., Arany Z., McNamara D.M., Goland S., Elkayam U. Peripartum cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:207–221. doi: 10.1016/j.jacc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 27.Honigberg M.C., Givertz M.M. Peripartum cardiomyopathy. BMJ. 2019;364 doi: 10.1136/bmj.k5287. [DOI] [PubMed] [Google Scholar]
- 28.DeFilippis E.M., Haythe J.H., Walsh M.N., Kittleson M.M. Intersection of heart failure and pregnancy: beyond peripartum cardiomyopathy. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.008223. [DOI] [PubMed] [Google Scholar]
- 29.Tseng A.S., Thao V., Borah B.J., et al. Cost effectiveness of an electrocardiographic deep learning algorithm to detect asymptomatic left ventricular dysfunction. Mayo Clin Proc. 2021;96:1835–1844. doi: 10.1016/j.mayocp.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Siontis K.C., Noseworthy P.A., Arghami A., et al. Vol. 14. Circ Cardiovasc Qual Outcomes; 2021. Use of artificial intelligence tools across different clinical settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adedinsewo D.A., Pollak A.W., Phillips S.D., et al. Cardiovascular disease screening in women: leveraging artificial intelligence and digital tools. Circ Res. 2022;130:673–690. doi: 10.1161/CIRCRESAHA.121.319876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matheny M.E., Whicher D., Thadaney Israni S. Artificial intelligence in health care: a report from the National Academy of Medicine. JAMA. 2020;323:509–510. doi: 10.1001/jama.2019.21579. [DOI] [PubMed] [Google Scholar]
- 33.Hameed A., Morton C., Moore A. 2017. Improving health care response to cardiovascular disease in pregnancy and postpartum. The Cardiovascular Disease in Pregnancy and Postpartum Task Force California Maternal Quality Care Collaborative, Stanford University Maternal, Child and Adolescent Health Division, Center For Family Health California Department of Public Health. [Google Scholar]
- 34.Hameed A.B., Tarsa M., Graves C.R., et al. Cardiovascular risk assessment as a quality measure in the pregnancy and postpartum period. JACC Adv. 2023;2 doi: 10.1016/j.jacadv.2022.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minhas A.S., Rooney M.R., Fang M., et al. Prevalence and correlates of elevated NT-proBNP in pregnant women in the general U.S. population. JACC Adv. 2023;2 doi: 10.1016/j.jacadv.2023.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madamanchi C., Alhosaini H., Sumida A., Runge M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–617. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Afshani N., Moustaqim-Barrette A., Biccard B.M., Rodseth R.N., Dyer R.A. Utility of B-type natriuretic peptides in preeclampsia: a systematic review. Int J Obstet Anesth. 2013;22:96–103. doi: 10.1016/j.ijoa.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Pisano E.D., Gatsonis C., Hendrick E., et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 40.Katki H.A., Schiffman M. A novel metric that quantifies risk stratification for evaluating diagnostic tests: the example of evaluating cervical-cancer screening tests across populations. Prev Med. 2018;110:100–105. doi: 10.1016/j.ypmed.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung M.H., McKenzie K.P., De Vuyst H., et al. Comparing Papanicolau smear, visual inspection with acetic acid and human papillomavirus cervical cancer screening methods among HIV-positive women by immune status and antiretroviral therapy. AIDS. 2013;27:2909–2919. doi: 10.1097/01.aids.0000432472.92120.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaarsma C., Leiner T., Bekkers S.C., et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease. J Am Coll Cardiol. 2012;59:1719–1728. doi: 10.1016/j.jacc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 43.Peteiro J., Piñon P., Perez R., Monserrat L., Perez D., Castro-Beiras A. Comparison of 2- and 3-dimensional exercise echocardiography for the detection of coronary artery disease. J Am Soc Echocardiogr. 2007;20:959–967. doi: 10.1016/j.echo.2007.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. All requests for raw and analyzed data and related materials, excluding programming code, will be reviewed by the Mayo Clinic legal department and Mayo Clinic Ventures to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for patient-related data not included in the paper will not be considered. Any data and materials that can be shared will be released via a Material Transfer Agreement.