Abstract
The prevalence of isolated systolic hypertension (ISH) has doubled between 2002−2005 and 2014 among the oldest‐old population in China. However, the prevalence and characteristics of ISH among the oldest‐old population in southwestern China remain less known. This study aimed to investigate the prevalence of ISH among the oldest‐old population in Chengdu and identify associated factors to provide valuable information for disease etiology and prevention. We recruited 1,312 participants aged over 80 years by using a stratified cluster sampling method between September 2015 and June 2016, from three districts (Jinjiang, Qingyang, and Longquanyi) of Chengdu, the largest city of southwest China. A structured questionnaire, anthropometric data, and blood pressure were collected according to the standard method. Blood pressure was measured three times by using a standardized mercury sphygmomanometer after a 10‐minute seated rest. Of 1312 participants, 53.0% (n = 695) had ISH. The prevalence of ISH in men and women was 54.7% and 51.3%, respectively, with no significant sex difference (P = .222). The prevalence of ISH increased with advanced age in men (P for trend = 0.029), 52.5% for the 80−84 years group, 55.2% for the 85−89 years group, and 70.4% for the 90–98 years group, respectively. Multivariable logistic regression analyses found that drinking (OR = 1.85, 95%CI = 1.26−2.71), being overweight (OR = 1.88, 95%CI = 1.19−2.96), and having a higher heart rate (OR = 0.66, 95%CI = 0.51−0.86) were associated with ISH. Stratified by sex, these three factors remained significant in men. Our work highlights that the burden of ISH is substantial among the oldest‐old population in southwestern China.
Keywords: cross‐sectional study, isolated systolic hypertension, prevalence, the oldest‐old
1. INTRODUCTION
Elevated systolic blood pressure (SBP), the predominant risk factor for cardiovascular disease, has contributed to 29.3% and 21.6% of the risk of cardiovascular disease for women and men, respectively. 1 The prevalence of isolated systolic hypertension (ISH) has increased from 3.04% in 1991 to 3.30% in 2011 among Chinese adults obtained from the China Health and Nutrition Survey, 2 while it has doubled from 14.3% in 2002 to 2005 to 30.7% in 2014 among the oldest‐old population (aged over 80 years) in China obtained from the Chinese Longitudinal Healthy Longevity Survey. 3 As the rapid trend of industrialization and population aging occurring almost all countries across Asia, the burden of ISH will rise substantially. 4 Findings from the China Kadoorie Biobank study involving 0.5 million adults with a median 10‐year follow‐up have demonstrated a 104% (HR = 2.04, 95%CI = 1.91−2.19) increasing risk of cardiovascular diseases in individuals with ISH compared with those with normal BP. 5 The Systolic Hypertension in the Elderly Program (SHEP) trial has demonstrated a lower risk of cardiovascular death (HR = 0.89, 95%CI = 0.80−0.99) and 0.56 years longer life expectancy in individuals with ISH who received active antihypertensive therapy compared with placebo. 6 Alarmingly, approximately 86.7% of individuals with ISH younger than 50 years had not received treatment in China, 7 yet ISH control rates remain largely inadequate in elderly patients regardless of antihypertensive treatment. 8 Understanding the epidemiological characteristics of ISH helps guide effective prevention and control strategies.
However, the prevalence of ISH among the oldest‐old population in southwestern China and how individual characteristics may vary across diverse subgroups of the population remained less understood. 3 Therefore, we conducted a community‐based cross‐sectional study to investigate the prevalence of ISH among the oldest‐old population in Chengdu and identify associated factors to provide valuable information for disease etiology and prevention.
2. METHODS
2.1. Study design and participants
As this study design has been reported previously, 9 participants were recruited using a community‐based cross‐sectional survey conducted between September 2015 and June 2016, from three districts (Jinjiang, Qingyang, and Longquanyi) of Chengdu, the largest city of southwest China. Inclusion criteria were: (1) more than three years of residence in the community; (2) aged 80 years or above. Excluded criteria were: those who were diagnosed as having neurologic or psychologic diseases, renal failure, or end‐stage cancer or refused to provide written informed consent or answer the questionnaire or measure their blood pressure. A total of 1391 oldest‐old participants were enrolled. After excluding 65 individuals without blood sample collection and 14 individuals without BP measures, 1,312 participants were included in the final analysis. This study was approved by the Second People's Hospital of Chengdu Ethics Committee (No. 2015030), and all participants provided written informed consent.
2.2. Data collection
Data collection was completed by 20 well‐trained research staff. 9 General demographic characteristics, medical conditions history, family history of hypertension, and lifestyle risk factors were collected using a structured questionnaire. Anthropometric data and blood pressure were collected according to the standard examination. Height and weight were measured when participants took off their coats and shoes. After a 10‐min seated rest, blood pressure was measured three times using standardized mercury sphygmomanometers to record the SBP and diastolic blood pressure (DBP). The mean of three readings was calculated for the final analysis. A 5 mL fasting venous blood was collected from each participant. All blood samples were sent to the Clinical Laboratory Center of Second People's Hospital for biochemistry analysis. Total cholesterol (TC), triglycerides (TG), and blood glucose were measured by the oxidase method. Uric acid (UA) was measured by the phosphotungstic acid method.
2.3. Variable definitions
Smoking and drinking status were clarified as “never” or “former and current.” Given the fact that body composition changes with aging, overweight was defined as BMI > 27 kg/m2, acknowledged for the elderly (> = 60 years). 10 Heart rate was dichotomized as heart rate > = 75 beats/min and heart rate <75 beats/min. 9 ISH was defined as SBP > = 140 mmHg and DBP < 90 mmHg, regardless of the participant's hypertension diagnosis history. 3 , 7 Isolated diastolic hypertension (IDH) was defined as SBP < 140 mmHg and DBP > = 90 mmHg, and systolic‐diastolic hypertension (SDH) was defined as SBP > = 140 mmHg and DBP > = 90 mmHg, regardless of the participant's hypertension diagnosis history. 7 Antihypertensive medication was identified by participant's self‐reporting medication history. According to the Seventh Report of the Joint National Committee, patients with SBP > = 140 mmHg and/or DBP > = 90 mmHg, and/or a history of hypertension and currently receiving antihypertensive drug treatment were diagnosed as hypertension. 11 Diabetes mellitus was defined as fasting plasma glucose (FPG) > = 7.0 mmol/L or 2‐h postload glucose (2hPG) > = 11.1 mmol/L or a self‐reported history or medication of diabetes. Hyperlipidemia was defined as TG > = 2.3 mmol/L, or TC > = 6.2 mmol/L, or low‐density lipoprotein cholesterol (LDL‐C) > = 4.1 mmol/L, or high‐density lipoprotein cholesterol (HDL‐C) < 1.0 mmol/L, or a self‐reported history or medication of hyperlipidemia. 12 Hyperuricemia was defined as serum uric acid (SUA) > 420 µmol/L in men and SUA > 360 µmol/L in women, based on the dietary guide for hyperuricemia and gout patients (Chinese standard, WS/T 560−2017). 13
2.4. Statistical analysis
Categorical variables were presented as frequency (percentage), and the Chi‐square test or Fisher exact test was used for intergroup comparisons. A Cochran–Armitage test was used to test the trend of ISH prevalence. For continuous variables, mean ± standard deviation (SD) was used to present data with the normal distribution, and the student's t‐test or analysis of variance (ANOVA) was used for intergroup comparisons. A univariable logistic regression model and a multivariable logistic regression model were used to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of the risk factors associated with ISH compared with normal BP. A P value of <.05 was considered statistically significant. All analyses were conducted using Statistical Product and Service Solutions (SPSS, version 28.0.0.0) software.
3. RESULTS
3.1. Characteristics of study participants
As shown in Table 1, the mean age was 83.9 ± 3.5 years in all participants. Of 1,312 participants, 48.6% (n = 638) were men and 51.4% (n = 674) were women. Men had a higher prevalence of smoking (44.0% vs. 12.9%, P < .001), drinking (29.3% vs. 5.0%, P < .001), and diabetes mellitus (30.4% vs. 23.6%, P = .021) than women, while women had a higher prevalence of overweight (16.6% vs. 10.2%, P = .002) and hyperlipidemia (31.0% vs. 23.7%, P = .012) than men. Moreover, men had a higher rate of antihypertensive medication use than women (31.3% vs. 25.5%, P = .019). As for continuous measures, men had higher BMI (P = .018), FBG (P = .017), and SUA (P < .001) than women. However, women had higher SBP, TG, TC, LDL‐C, and HDL‐C than men (P < .001). DBP, hypertension, heart rate, 2hPG, and hyperuricemia had no significant sex difference (P > .05). Sex‐specific distributions of SBP and DBP levels are present in Figure 1.
TABLE 1.
General and clinical characteristics of the study participants.
| Characteristics | Overall (n = 1,312) | Men (n = 638) | Women (n = 674) | P value |
|---|---|---|---|---|
| Age (years) | 83.9 ± 3.5 | 84.0 ± 3.4 | 83.8 ± 3.5 | .331 |
| Former or current smoking | 368 (28.0%) | 281 (44.0%) | 87 (12.9%) | <.001 |
| Former or current drinking | 221 (16.8%) | 187 (29.3%) | 34 (5.0%) | <.001 |
| BMI (kg/m2) * | 23.0 ± 3.9 | 23.3 ± 3.6 | 22.8 ± 4.1 | .018 |
| Overweight * | 177 (13.5%) | 65 (10.2%) | 112 (16.6%) | .002 |
| Heart rate (beats/min) * | 76.1 ± 12.4 | 76.3 ± 13.7 | 75.9 ± 11.0 | .490 |
| Heart rate ≥ 75 beats/min * | 657 (50.1%) | 312 (48.9%) | 345 (51.2%) | .506 |
| SBP (mmHg) | 149.3 ± 21.7 | 147.2 ± 20.1 | 151.2 ± 23.0 | <.001 |
| DBP (mmHg) | 74.0 ± 12.1 | 74.5 ± 11.5 | 73.6 ± 12.7 | .221 |
| Hypertension | 957 (72.9%) | 466 (73.0%) | 491 (72.8%) | .938 |
| Antihypertensive medication | 372 (28.4%) | 200 (31.3%) | 172 (25.5%) | .019 |
| FPG (mmol/L) * | 5.6 ± 2.0 | 5.8 ± 2.3 | 5.5 ± 1.8 | .017 |
| 2hPG (mmol/L) * | 8.6 ± 3.7 | 8.7 ± 2.7 | 8.4 ± 4.4 | .233 |
| Diabetes mellitus * | 353 (26.9%) | 194 (30.4%) | 159 (23.6%) | .021 |
| TG (mmol/L) * | 1.4 ± 0.7 | 1.3 ± 0.6 | 1.4 ± 0.7 | <.001 |
| TC (mmol/L) * | 4.8 ± 0.9 | 4.6 ± 0.9 | 5.0 ± 0.9 | <.001 |
| LDL‐C (mmol/L) * | 2.6 ± 0.7 | 2.5 ± 0.7 | 2.7 ± 0.8 | <.001 |
| HDL‐C (mmol/L) * | 1.6 ± 0.4 | 1.5 ± 0.4 | 1.7 ± 0.4 | <.001 |
| Hyperlipidemia * | 360 (27.4%) | 151 (23.7%) | 209 (31.0%) | .012 |
| SUA (µmol/L) * | 334.1 ± 117.9 | 359.1 ± 118.4 | 310.4 ± 112.4 | <.001 |
| Hyperuricemia * | 375 (28.6%) | 177 (27.7%) | 198 (29.4%) | .748 |
Note: Data are presented as mean ± standard deviation (SD) or frequency (percent).
There were missing values.
FIGURE 1.
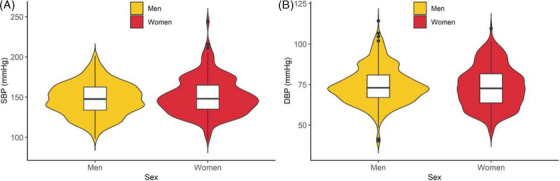
Sex‐specific distributions of systolic blood pressure and diastolic blood pressure levels among the oldest‐old population in southwestern China.
3.2. Age‐specific BP levels
The SBP levels in all participants were 148.8 ± 20.1 mmHg for the 80−84 years group, 149.3 ± 23.6 mmHg for the 85−89 years group, and 152.9 ± 27.1 mmHg for the 90−98 years group, respectively. The SBP levels in men were 147.1 ± 19.6 mmHg for the 80−84 years group, 146.4 ± 20.2 mmHg for the 85−89 years group, and 151.0 ± 23.5 mmHg for the 90−98 years group, respectively. As for women, the SBP levels for the 80−84 year group, 85−89 year group, and 90‐98 year group were 150.3 ± 20.4 mmHg, 152.3 ± 26.6 mmHg, and 154.3 ± 29.7 mmHg, respectively. No significant association of SBP with aging was observed in all participants (P = .147), men (P = .325), or women (P = .327) (Figure 2A). As for DBP levels (Figure 2B), No significant association of DBP with aging was observed in either men (P = .057) or women (P = .319).
FIGURE 2.

Age‐specific systolic blood pressure and diastolic blood pressure levels among the oldest‐old population in southwestern China. The box denotes the mean blood pressure level, and the error bar denotes the standard deviation.
3.3. Prevalence of ISH
Of 1,312 participants, 53.0% (n = 695) had ISH, 0.2% (n = 3) had IDH, 11.4% (n = 149) had SDH, and 35.4% (n = 465) had normotension. According to the Seventh Chengdu Population Census in 2020, there were 341,809 people aged over 80 years who lived in urban areas, which means 181,065 (95%CI = 171,817−190,253) have ISH. The prevalence of ISH in men and women was 54.7% and 51.3%, respectively, with no significant sex difference (P = .222). The prevalence of ISH in the 80−84 years group, 85−89 years group, and 90−98 years group was 52.1%, 52.5%, and 60.2%, respectively, with no significantly increasing trend for the prevalence with aging (P for trend = 0.172) (Table 2). Notably, the prevalence of ISH increased with advanced age in men (P for trend = 0.029), 52.5% for the 80−84 years group, 55.2% for the 85−89 years group, and 70.4% for the 90−98 years group, respectively. Moreover, men who had drinks (P = .041) or were overweight (P = .011) had a higher prevalence of ISH. However, men with a higher heart rate (≥75 beats/min) had a lower prevalence of ISH than those with a lower heart rate (<75 beats/min) (P < .001).
TABLE 2.
Prevalence of isolated systolic hypertension among the oldest‐old population in southwestern China.
| Characteristics | Overall | Men | Women |
|---|---|---|---|
| Age group (years) | |||
| 80−84 | 454 (52.1%) | 220 (52.5%) | 234 (51.8%) |
| 85−89 | 167 (52.5%) | 91 (55.2%) | 76 (49.7%) |
| 90‐98 | 74 (60.2%) | 38 (70.4%) | 36 (52.2%) |
| P value for trend | .172 | .029 | .874 |
| Former or current smoking | |||
| No | 488 (51.7%) | 187 (52.4%) | 301 (51.3%) |
| Yes | 207 (56.3%) | 162 (57.7%) | 45 (51.7%) |
| P value | .138 | .184 | .938 |
| Former or current drinking | |||
| No | 564 (51.7%) | 235 (52.1%) | 329 (51.4%) |
| Yes | 131 (59.3%) | 114 (61.0%) | 17 (50.0%) |
| P value | .040 | .041 | .873 |
| Overweight b | |||
| No | 591 (53.4%) | 300 (54.0%) | 291 (52.8%) |
| Yes | 95 (53.7%) | 44 (67.7%) | 51 (45.5%) |
| P value | .083 | .011 | .226 |
| Heart rate b | |||
| <75 beats/min | 389 (60.6%) | 212 (66.0%) | 177 (55.1%) |
| ≥75 beats/min | 299 (45.5%) | 132 (42.3%) | 167 (48.4%) |
| P value | <.001 | <.001 a | <.069 a |
| Diabetes mellitus b | |||
| No | 504 (53.2%) | 239 (54.6%) | 265 (52.0%) |
| Yes | 188 (53.3%) | 108 (55.7%) | 80 (50.3%) |
| P value | 0.230 | 0.578 a | 0.371 a |
| Hyperlipidemia b | |||
| No | 507 (53.9%) | 262 (54.5%) | 245 (53.3%) |
| Yes | 185 (51.4%) | 85 (56.3%) | 100 (47.8%) |
| P value | .166 | .552 a | .160 a |
| Hyperuricemia b | |||
| No | 479 (53.3%) | 242 (54.6%) | 237 (52.1%) |
| Yes | 202 (53.9%) | 99 (55.9%) | 103 (52.0%) |
| P value | .094 | .646 | .106 |
Fisher's exact test.
There were missing values.
3.4. Associated factors of ISH
The univariable logistic regression analysis (model 1) shows drinking (OR = 1.83, 95%CI = 1.25−2.67), being overweight (OR = 1.92, 95%CI = 1.23−3.01), diabetes mellitus (OR = 1.40, 95%CI = 1.03−1.90) was positively associated with ISH, but having a higher heart rate (OR = 0.65, 95%CI = 0.50−0.84) was negatively associated with ISH (Table 3). The association of drinking (OR = 1.85, 95%CI = 1.26−2.71), being overweight (OR = 1.88, 95%CI = 1.19−2.96), and having a higher heart rate (OR = 0.66, 95%CI = 0.51−0.86) with ISH remained significant in the multivariable logistic regression model. Stratified by sex, the association of drinking (OR = 2.01, 95%CI = 1.28−3.17), being overweight (OR = 6.54, 95%CI = 2.26−18.91), and having a higher heart rate (OR = 0.43, 95%CI = 0.29−0.64) with ISH remained significant in men. Compared with men aged 80−84 years, men aged 90−98 years had a higher prevalence of ISH (OR = 2.19, 95%CI = 1.03−4.65). As for women, diabetes mellitus was positively associated with ISH (OR = 1.63, 95%CI = 1.01−2.62). When restricting to participants without antihypertensive medication use, drinking (OR = 2.20, 95%CI = 1.39−3.48) was positively associated with ISH while smoking (OR = 0.61, 95%CI = 0.42−0.88) and having a higher heart rate (OR = 0.58, 95%CI = 0.44−0.78) were negatively associated with ISH (Table 4).
TABLE 3.
Odds ratios for prevalence of isolated systolic hypertension among the study participants.
| Variable | Overall | Men | Women | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Sex | ||||||
| Women | 1.00 (reference) | – | – | – | – | – |
| Men | 1.07 (0.83‐1.39) | – | – | – | – | – |
| Age groups | ||||||
| 80‐84 years | 1.00 (reference) | – | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | – |
| 85‐89 years | 0.88 (0.65‐1.18) | – | 0.93 (0.61‐1.40) | 0.83 (0.54‐1.29) | 0.82 (0.53‐1.27) | – |
| 90‐98 years | 1.08 (0.70‐1.68) | – | 1.93 (0.93‐3.03) | 2.19 (1.03‐4.65) | 0.73 (0.42‐1.28) | – |
| Smoking | 1.14 (0.86‐1.52) | – | 1.29 (0.89‐1.87) | – | 0.86 (0.52‐1.45) | – |
| Drinking | 1.83 (1.25‐2.67) | 1.85 (1.26‐2.71) | 1.90 (1.23‐2.93) | 2.01 (1.28‐3.17) | 1.84 (0.67‐5.07) | – |
| Overweight * | 1.92 (1.23‐3.01) | 1.88 (1.19‐2.96) | 6.01 (2.12‐17.03) | 6.54 (2.26‐18.91) | 1.21 (0.71‐2.06) | – |
| Heart rate ≥ 75 beats/min * | 0.65 (0.50‐0.84) | 0.66 (0.51‐0.86) | 0.43 (0.29‐0.62) | 0.43 (0.29‐0.64) | 0.97 (0.68‐1.40) | – |
| Diabetes mellitus * | 1.40 (1.03‐1.90) | – | 1.24 (0.82‐1.86) | – | 1.63 (1.01‐2.62) | – |
| Hyperlipidemia * | 1.22 (0.90‐1.65) | – | 1.43 (0.90‐2.27) | – | 1.08 (0.72‐1.62) | – |
| Hyperuricemia * | 1.30 (0.97‐1.75) | – | 1.42 (0.92‐2.18) | – | 1.21 (0.80‐1.82) | – |
Notes: Data are presented as odds ratios (95%CI).
Model 1 was an univariable logistic regression model.
Model 2 was a multivariable logistic regression model using a backward‐stepwise selection method (Likelihood Ratio) to specify how independent variables are entered into the model.
For missing values, a dummy variable was created.
TABLE 4.
Odds ratios for prevalence of isolated systolic hypertension among the study participants without antihypertensive medication use.
| Variable | Model 1 | Model 2 |
|---|---|---|
| Sex | ||
| Women | 1.00 (reference) | – |
| Men | 0.96 (0.73‐1.27) | – |
| Age groups | ||
| 80‐84 years | 1.00 (reference) | – |
| 85‐89 years | 0.93 (0.67‐1.29) | – |
| 90‐98 years | 1.27 (0.80‐2.01) | – |
| Smoking | 0.85 (0.61‐1.16) | 0.61 (0.42‐0.88) |
| Drinking | 1.70 (1.13‐2.55) | 2.20 (1.39‐3.48) |
| Overweight * | 1.57 (0.96‐2.56) | – |
| Heart rate ≥ 75 beats/min * | 0.59 (0.45‐0.79) | 0.58 (0.44‐0.78) |
| Diabetes mellitus * | 1.27 (0.91‐1.77) | – |
| Hyperlipidemia * | 0.92 (0.66‐1.28) | – |
| Hyperuricemia * | 1.14 (0.83‐1.58) | – |
Notes: Data are presented as odds ratios (95%CI).
Model 1 was an univariable logistic regression model.
Model 2 was a multivariable logistic regression model using a backward‐stepwise selection method (Likelihood Ratio) to specify how independent variables are entered into the model.
For missing values, a dummy variable was created.
4. DISCUSSION
In this study, we conducted a community‐based cross‐sectional study to investigate the prevalence of ISH among 1,312 participants aged 80 years or above in the largest city of southwestern China, Chengdu. We observed a substantial burden of ISH in the oldest‐old population, with more than half suffering from ISH. Multivariable analyses found that drinking, being overweight, and having a higher heart rate were associated with ISH, which remained significant in men rather than in women. Our findings provide valuable information on disease burden and disease prevention of ISH among the oldest‐old population.
The prevalence of ISH (53.0%) among the oldest‐old population in Chengdu we observed was higher than the national prevalence (30.7%) in 2014 from the Chinese Longitudinal Healthy Longevity Survey. 3 Regional differences in the prevalence of ISH among the oldest‐old population may be attributed to socioeconomic status. Chengdu is the center of commerce, finance, science, and technology, as well as the hub of transport and communication in southwestern China. 13 Furthermore, our results of multivariable analysis appear in agreement with those of previous studies and extended their findings. 7 , 14 First, being overweight was positively associated with the prevalence of ISH. A cross‐sectional study that compared 1,747 ISH with 8,917 healthy controls aged 35 years and older found a positive association between being overweight and ISH (OR = 3.48, 95%CI = 2.97−4.07). 14 Another cross‐sectional study from the China Patient‐Centered Evaluative Assessment of Cardiac Events Million Persons Project that compared 62,819 ISH with 663,791 healthy controls aged 35–49 years found a positive association between being obesity (BMI ≥ 28 kg/m2) and ISH (OR = 2.10, 95%CI = 2.06−2.14). 7 Furthermore, a causal effect of BMI on SBP was confirmed by Mendelian randomization analysis. 15 Different from and extending those findings, we further found this positive association only remained significant with more magnitude in men (OR = 6.54, 95%CI = 2.26−18.91) among the oldest‐old population. Second, drinking was positively associated with the prevalence of ISH. Findings from the China Patient‐Centered Evaluative Assessment of Cardiac Events Million Persons Project also found a positive association between current alcohol use and ISH (OR = 1.21, 95%CI = 1.18−1.25). 7 A Mendelian randomization analysis that used aldehyde dehydrogenase 2 (ALDH2) as instrumental variables found a causal effect on SBP (increasing 7.44 mmHg, P = 1.1×10−12). 16 Stratified by sex, we further found this positive association remained significant in men rather than women, substantiated by our sensitivity analysis conducted in participants without antihypertensive medication use and previous Mendelian randomization analyses. 17 A Mendelian randomization analysis involving 2349 participants in southeastern China that used rs671 on the gene ALDH2 as instrumental variables found a causal effect on SBP (increasing 8.28 mmHg, P = 1.37×10−3) in men, but not in women (increasing 13.44 mmHg, P = .135). 17 Third, having a higher heart rate was negatively associated with the prevalence of ISH. In contrast, a prospective cohort study totaling 2,530 participants in an Inner Mongolian population with a 10‐year follow‐up found an increased risk of hypertension (OR = 1.51, 95%CI = 1.06−2.15) for the highest levels of heart rate (≥ 84 beats/min) compared with the lowest levels of heart rate (<69 beats/min). 18 Another prospective cohort study totaling 31,507 participants conducted in the Kailuan community of Tangshan China with a mean follow‐up period of 3.5 years confirmed the increased risk of hypertension (HR = 1.26, 95%CI = 1.17−1.36) for the highest levels of heart rate (≥ 90 beats/min) compared with the lowest levels of heart rate (< 66 beats/min). 19 This contradictory finding may be attributed to survival bias that the oldest‐old population with the highest levels of heart rate (≥ 84 beats/min) had an increased risk of death (HR = 1.51, 95%CI = 1.29−1.77) compared with the lowest levels of heart rate (≤ 66 beats/min). 20 Further high‐quality studies (e.g., Mendelian randomization) are needed to reveal the intrinsic relationship between heart rate and ISH.
Our findings suggest that keeping a healthy lifestyle including a healthy BMI and quitting drinking may help reduce the burden of ISH among the oldest‐old population. Our study has several limitations. First, all participants were recruited from the urban areas of Chengdu, limiting the generalizability of our findings to the rural oldest‐old population. Second, the dichotomized classification for smoking and drinking may distort the potential relationships between smoking, drinking, and ISH, although this method referred to the widely accepted definition of smoking initiation (current or former vs. never) in genome‐wide association studies. Future research with quantitative measures on smoking and drinking is needed. 21 , 22 Third, the significant associations observed in this study resulted from a cross‐sectional study, which is unlikely to make causal inferences due to its inherent limitations including confounding biases and reverse causality. However, previous Mendelian randomization studies have confirmed the causality underlying the associations we observed. Of note, this is the first comprehensive study to evaluate the prevalence of ISH and identify factors associated with ISH among the oldest‐old population in southwestern China.
5. CONCLUSIONS
In conclusion, this study demonstrated that the burden of ISH is substantial among the oldest‐old population in southwestern China. Drinking and being overweight are associated with the increasing prevalence of ISH, while having a higher heart rate is associated with the decreasing prevalence of ISH. Keeping a healthy lifestyle may help reduce the burden of ISH among the oldest‐old population.
AUTHOR CONTRIBUTIONS
X.H., Q.Z., X.Z., and J.W. contributed to study concept and design, acquisition of data, analysis and interpretation of data, and preparation of the manuscript. X.H. and L.Q. contributed to statistical analysis and preparation of the manuscript. T.W., Q.Y., J.L., and R.X. contributed to interpretation of data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
All participants provided written informed consent.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not available.
CLINICAL TRIAL REGISTRATION
Not available.
ACKNOWLEDGMENTS
We sincerely thank all the staff and participants for their contributions. This study was supported by the project of Chengdu Municipal Science and Technology Bureau (10YTYB272SF).
Huang X, Qiu L, Wang T‐D, et al. Prevalence and risk factors for isolated systolic hypertension among the oldest‐old population in southwestern China: A community‐based cross‐sectional study. J Clin Hypertens. 2024;26:757–764. 10.1111/jch.14826
Contributor Information
Qingkun Zheng, Email: zqk_971607@163.com.
Xingping Zhang, Email: 194955495@qq.com.
Jinhui Wu, Email: Wujinhui@scu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Xiaobo Huang (drhuangxiaobo@126.com), upon reasonable request.
REFERENCES
- 1. Global Cardiovascular Risk Consortium , Magnussen C, Ojeda FM, et al, Global Cardiovascular Risk Consortium . Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. 2023;389(14):1273‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi SF, Zhang B, Wang HJ, et al. Prevalence of hypertension subtypes in 2011 and the trends from 1991 to 2011 among Chinese adults. J Epidemiol Community Health. 2016;70(5):444‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du J, Zhu G, Yue Y, et al. Blood pressure and hypertension prevalence among oldest‐old in China for 16 year: based on CLHLS. BMC Geriatr. 2019;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsai TY, Cheng HM, Chuang SY, et al. Isolated systolic hypertension in Asia. J Clin Hypertens (Greenwich). 2021;23(3):467‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo J, Lv J, Guo Y, et al. Association between blood pressure categories and cardiovascular disease mortality in China. PLoS One. 2021;16(7):e0255373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kostis JB, Cabrera J, Cheng JQ, et al. Association between chlorthalidone treatment of systolic hypertension and long‐term survival. JAMA. 2011;306(23):2588‐2593. [DOI] [PubMed] [Google Scholar]
- 7. Mahajan S, Feng F, Hu S, et al. Assessment of prevalence, awareness, and characteristics of isolated systolic hypertension among younger and middle‐aged adults in China. JAMA Netw Open. 2020;3(12):e209743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, Giannattasio C. Diagnostic and therapeutic problems of isolated systolic hypertension. J Hypertens. 2015;33(1):33‐43. [DOI] [PubMed] [Google Scholar]
- 9. Liang B, Tang WW, Zhang WQ, et al. Prevalence and associated factors of diabetes mellitus in a very elderly Chinese population: a cross‐sectional study. Biomed Environ Sci. 2020;33(5):315‐322. [DOI] [PubMed] [Google Scholar]
- 10. Lipschitz DA. Screening for nutritional status in the elderly. Prim Care. 1994;21(1):55‐67. [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 12. Joint committee for guideline r . 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X‐J, Zhang W, Yuan R‐L, et al. Hyperuricaemia and associated factors among the oldest‐old population in the urban areas of Chengdu, China: a community‐based cross‐sectional study. BMJ Open. 2021;11(12):e055881. [Google Scholar]
- 14. Liu F, Ma YT, Yang YN, et al. The prevalence of isolated systolic hypertension in adult populations from the Han, Uygur and Kazakh ethnic groups in Xinjiang, China. Blood Press. 2014;23(3):154‐159. [DOI] [PubMed] [Google Scholar]
- 15. Fall T, Hagg S, Magi R, et al. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10(6):e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Smith GD, Harbord RM, et al. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 2008;5(3):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao PP, Xu LW, Sun T, et al. Relationship between alcohol use, blood pressure and hypertension: an association study and a Mendelian randomisation study. J Epidemiol Community Health. 2019;73(9):796‐801. [DOI] [PubMed] [Google Scholar]
- 18. Wang T, Zhang W, Zhang M, et al. Higher heart rates increase risk of diabetes and cardiovascular events: a prospective cohort study among Inner Mongolians. Diabetes Metab. 2020;46(1):20‐26. [DOI] [PubMed] [Google Scholar]
- 19. Wang A, Liu X, Guo X, et al. Resting heart rate and risk of hypertension: results of the Kailuan cohort study. J Hypertens. 2014;32(8):1600‐1605. discussion 1605. [DOI] [PubMed] [Google Scholar]
- 20. Wang A, Chen S, Wang C, et al. Resting heart rate and risk of cardiovascular diseases and all‐cause death: the Kailuan study. PLoS One. 2014;9(10):e110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saunders GRB, Wang X, Chen F, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612(7941):720‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Xiaobo Huang (drhuangxiaobo@126.com), upon reasonable request.


