Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory arthritis affecting the spine, presenting a considerable morbidity risk. Although evidence consistently indicates an elevated risk of ischemic heart disease among AS patients, debates persist regarding the likelihood of these patients developing left ventricular dysfunction (LVD). Our investigation aimed to determine whether individuals with AS face a greater risk of LVD compared to the general population. To accomplish this, we identified studies exploring LVD in AS patients across five major databases and Google Scholar. Initially, 431 studies were identified, of which 30 met the inclusion criteria, collectively involving 2933 participants. Results show that AS patients had: (1) poorer Ejection Fraction (EF) [mean difference (MD): −0.92% (95% CI: −1.25 to −0.59)], (2) impaired Early (E) and Late (atrial—A) ventricular filling velocity (E/A) ratio [MD: −0.10 m/s (95% CI: −0.13 to −0.08)], (3) prolonged deceleration time (DT) [MD: 12.30 ms (95% CI: 9.23–15.36)] and, (4) a longer mean isovolumetric relaxation time (IVRT) [MD: 8.14 ms (95% CI: 6.58–9.70)] compared to controls. Though AS patients show increased risks of both systolic and diastolic LVD, we found no significant differences were observed in systolic blood pressure [MD: 0.32 mmHg (95% Confidence Interval (CI): −2.09 to 2.73)] or diastolic blood pressure [MD: 0.30 mmHg (95% CI: −0.40 to 1.01)] compared to the general population. This study reinforces AS patients' susceptibility to LVD without a notable difference in HTN risk.
Keywords: ankylosing spondylitis, cardiovascular disease, hypertension (HTN), inflammatory arthritis, left ventricular dysfunction
1. INTRODUCTION
Ankylosing spondylitis (AS) represents a chronic inflammatory disorder, primarily affecting the axial skeleton, with pronounced involvement of the spinal facet and sacroiliac joints. 1 AS predominantly affects young males, and its ramifications extend beyond the musculoskeletal system. 2 Notably, while cardiac involvement in AS patients remains relatively rare, manifestations such as diastolic dysfunction—often concomitant with heart failure maintaining preserved ejection fraction (EF)—have been documented. 3 , 4 Dilated and restrictive cardiomyopathy patterns, conduction anomalies, and aortic insufficiency are other cardiac sequelae associated with AS. 4 , 5
The overarching cardiovascular mortality in AS patients is approximately double that of the general populace, with heart failure emerging as a pivotal contributor. 5 , 6 Intriguingly, chronic inflammatory pathways, previously linked with cardiac disturbances such as coronary artery disease in rheumatoid arthritis (RA), are believed to also underlie the cardiac dysfunction in AS. 5 , 6 , 7 , 8 A multitude of research underscores the shared pathophysiological underpinnings of cardiac dysfunction in RA and AS. 5 , 6 , 7 , 8
Considering these insights, our endeavor is a comprehensive meta‐analysis, targeting studies that underscore a heightened incidence and prevalence of left ventricular dysfunction (LVD) amidst AS patients.
2. METHODS
2.1. Objective
The study aims to assess the risk of LVD in patients with AS compared to the general population. This updated meta‐analysis synthesizes data on LVD parameters such as EF, Early (E) and Late (atrial—A) ventricular filling velocity (E/A) ratio, Deceleration Time (DT), and Isovolumetric Relaxation Time (IVRT).
2.2. Search strategy
A comprehensive literature search was conducted in PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science, along with Google Scholar. The search was restricted to articles published between January 1, 2000, and December 31, 2022. Search terms included combinations and variants of “Ankylosing Spondylitis,” “Left Ventricular Dysfunction,” and related keywords. Comprehensive search strategies with detailed inclusion and exclusion criteria can be found in the supplemental material.
2.3. Study selection and data extraction
Inclusion criteria encompassed studies that examined LVD parameters in AS patients using either cohort, case‐control, or cross‐sectional designs. Exclusion criteria included case reports, review articles, and studies not explicitly focusing on LVD in AS patients. Two independent reviewers screened the titles and abstracts, proceeding to a full‐text review for eligible studies using the Covidence online platform. A standardized data extraction form was utilized to capture study details, patient demographics, and LVD metrics. Discrepancies were resolved through consensus or by involving a third reviewer.
2.4. Statistical analysis
A meta‐analysis was performed using a random‐effects model to accommodate potential heterogeneity across studies. Heterogeneity was assessed using the I 2 statistic. Mean differences (MD) were calculated for systolic and diastolic blood pressure, EF, E/A ratio, DT, and IVRT. All analyses were conducted using the RevMan and R software package, version 4.0.
3. RESULTS
Database search yielded 431 records. After removing duplicates, 104 full‐text articles were reviewed from 350 screened titles and abstracts (See Figure 1).
FIGURE 1.
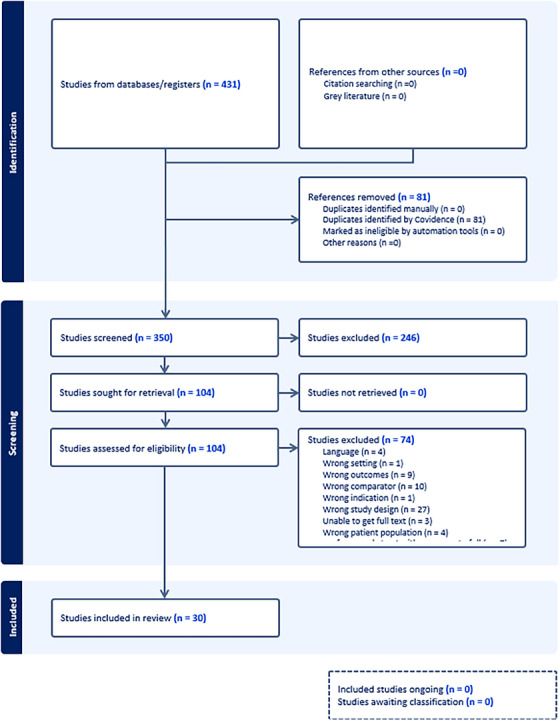
Prisma chart summarizing the method's process.
Our comprehensive analysis involved 2,933 participants assimilated from 30 individual studies from the full‐text screening. The characteristics of these selected studies are systematically detailed in Table 1.
TABLE 1.
Features of included studies.
| Authors | AS (n) | Control (n) | Mean age of AS (years) | Mean age of control (years) | Quality | Findings | Conclusion | Sex ratio |
|---|---|---|---|---|---|---|---|---|
| Midtbø H et al. 13 | 139 | 126 | 49.1 ± 11.7 | 52.1 ± 11.4 | 4/2/2 | Hypertension and diabetes were similar among patients and controls, but the prevalence of LVH was higher in patients with AS compared to controls | AS is linked to an increased prevalence of (LVH), independent of cardiovascular disease (CVD) risk factors. | 60% AS males vs. 58% male controls |
| Gould BA et al. 25 | 21 | 24 | 33 ± 6 | 35.7 ± 6 | 3/1/2 | Individuals with AS exhibited lower peak filling rates and higher time‐to‐peak filling rates during exercise, alongside significantly elevated angiographic scores compared to normal subjects. | Improvement with anti‐inflammatory treatment may indicate the inflammatory nature of this myocardial tissue. | 81% AS males, and 83% male controls |
| Sun JP et al. 26 | 20 | 25 | 45 ± 11 | 44 ± 11 | 3/2/2 | Atrial, ventricular, and aortic dimensions were comparable between AS patients and controls, with normal ejection indexes and systolic wall motion observed in both groups; however, AS patients exhibited diastolic dysfunction characterized by a shortened diastolic filling period, decreased velocity of early mitral inflow, and lower ratios of early/late inflow velocities. | AS can result in changes to ventricular diastolic function. | 85% AS males vs. 80% male controls |
| Francisco Javier Jiménez‐Balderas et al. 27 | 31 | 20 | 21 ± 1.2 | NA | 3/0/2 | Cardiomyopathy was more prevalent in individuals with adult‐onset ankylosing spondylitis (AOAS) compared to juvenile‐onset ankylosing spondylitis (JOAS) and controls. Patients with JOAS exhibited a higher mitral valve gradient than AOAS patients and controls. Abnormal aortic ring reflectance was observed in 19% of AOAS, with no abnormalities in JOAS and controls. Aortic root diameter was significantly increased in 58% of AOAS, 30% of JOAS, and 0% of controls. | Cardiopulmonary asymptomatic spondylitis patients exhibited an increased frequency of 2DECHO abnormalities. JOAS had a lower occurrence of aortic abnormalities compared to AOAS. Both JOAS and AOAS showed mitral valve gradients. | 100% AS males vs. 100% male controls |
| Aylin Yildirir et al. 10 | 88 | 31 | 33 ± 9 | 33 ± 9 | 4/2/2 | AS patients had a decreased peak of E‐wave velocity (E) and E/A ratio, an increased peak of A‐wave velocity (A) and acceleration rate of the A‐wave, along with an extended deceleration time of E‐wave velocity and isovolumic relaxation time. However, the mean filtered P‐wave duration (PWD) was similar in both groups. | Cardiac involvement in AS may occur without apparent clinical manifestations. Early detection enables aggressive anti‐inflammatory treatment, potentially slowing disease progression. | 50% AS males vs. 52% male controls |
| Peregud‐Pogorzelska et al. 23 | 38 | 25 | 51 ± 11 | 47 ± 12 | 4/2/2 | Echocardiographic abnormalities were found in 67% of the study group and 32% of controls, with aortic incompetence in 21% of patients and 4% of controls. No statistical differences were noted in ejection fraction, fractional shortening, or chamber diameters, except for the left atrium. Mitral valve prolapse occurred in 8% of study group patients, and interatrial septum aneurysm in 13%. | Patients with AS often experience cardiovascular abnormalities that progress with the disease duration. | 92% AS males vs. 88% male controls |
| Okan T 14 | 49 | 33 | 38 ± 11 | 36 ± 9 | 4/2/2 | No right ventricular function involvement was observed. However, AS patients had a 3.7 times higher risk of developing left ventricular diastolic dysfunction. | Diastolic dysfunction is common in AS without prior cardiovascular disease, possibly impacting cardiovascular mortality. | 51% AS males vs. 52% male controls |
| Caliskan M et al. 8 | 40 | 35 | 38.9 ± 10.2 | 37.5 ± 6.4 | 3/2/2 | The coronary flow reserve (CFR) was reduced in AS group. Left ventricular diastolic function, including the mitral A‐wave and E/A ratio, showed borderline significance, while mitral E‐wave deceleration time and isovolumic relaxation time were significantly different between the two groups. | Reduced CFR could indicate early sign of cardiac involvement in AS. | 65% AS males vs. 66% male controls |
| Acar G et al. 29 | 40 | 42 | 37.82 ± 10.22 | 35.74 ± 9.98 | 2/1/2 | In AS patients, mitral A‐wave, E/A ratio, mitral E‐wave deceleration time (DT), Am, and Em/Am, demonstrated significant differences when compared to control. | AS patients exhibit impaired left ventricular diastolic function. | 55% AS males vs. 52% male controls |
| Ioannis Moyssakis et al. 3 | 57 | 78 | 41.78 ± 10.02 | 39.92 ± 9.11 | 3/0/2 | Aortic distensibility (AoD) in AS was reduced compared to controls, indicating increased aortic stiffness. AS patients also showed higher left ventricular Tei index, while the EF remained comparable. AoD showed significant associations with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and LV isovolumic relaxation time (IVRT), while the LV Tei index was associated with BASDAI and the LV mass index. | AS without clinical cardiac disease shows increased aortic stiffness and reduced myocardial performance, correlating with disease activity. The abnormal Tei index may indicate early cardiac dysfunction. | 95% AS males vs. 94% male controls |
| Aksoy et al. 30 | 28 | 30 | 28.7 ± 5.7 | 29.3 ± 5.8 | 3/2/2 | The AS and control groups showed similarity in terms LVEF. However, patients with AS exhibited significantly higher P‐wave duration (PWD) compared to controls. Additionally, interatrial electromechanical delay (Inter‐AEMD) and intra‐left atrial electromechanical delay (Intra‐left AEMD) were significantly higher in AS patients. There was no statistically significant difference in Intra‐right AEMD between the two groups. | In patients with AS, prolonged PWD, Inter‐AEMD, and intra‐left AEMD suggest an association with atrial electromechanical abnormalities. | 86% AS males vs. 87% male controls |
| Kaya EB et al. 31 | 28 | 30 | 28.7 ± 5.7 | 29.3 ± 5.8 | 3/2/2 | LVEF was similar in both AS and control groups. However, AS showed increased Low frequency and Low frequency / High frequency compared to control. | Cardiac autonomic functions may be affected in AS, even without cardiac symptoms. | 86% AS males vs. 87% male controls |
| Park SH et al. 32 | 70 | 25 | 30.3 ± 5.3 | 27.0 ± 2.5 | 4/2/2 | In AS, aortic and mitral valve thickness is significantly increased, particularly in the aortic valve (AV), where thickness is >1.3 mm can predict AS. AV thickening is higher in AS, and is linked to longer disease duration, high blood pressure, disease activity, and inflammatory markers. | In early AS, aortic and mitral valve thickening without regurgitation is seen, despite negative cardiac symptoms. | 100% AS males vs. 100% male controls |
| Kırış A et al. 33 | 77 | 40 | 36.4 ± 10 | 39.1 ± 8.2 | 4/0/2 | AS echocardiographic characteristics at baseline were similar to controls. In AS patients, all time‐to‐peak tissue synchrony imaging (TSI) parameters indicating LV asynchrony were extended compared to controls. Notably, these asynchrony parameters displayed significant correlations with the index of myocardial performance (Tei index) and peak systolic mitral annular velocity. | TSI revealed the existence of LV systolic asynchrony in AS, potentially contributing to their cardiovascular complications. | 79% AS males vs. 86% male controls |
| Kuloglu O et al. 34 | 30 | 30 | 37.2 ± 10.23 | 33.2 ± 8.12 | 3/0/2 | Echocardiography indicated higher end‐diastolic interventricular septum and posterior wall diameters in AS. AS exhibited a significantly lower E/A ratio, prolonged deceleration time, lower Em, Em/Am ratio, and CTm, longer IVRTm, and higher MPI. | AS patients exhibit impaired diastolic but preserved systolic function. | 60% AS males vs. 53% male controls |
| Ercan S et al. 35 | 66 | 21 | 30.9 ± 6 | 27.1 ± 4 | 4/2/2 | Mitral early diastolic flow speed (mE) and late diastolic flow speed (mA) scores were reduced in AS. The Em ratio in AS was notably lower than in control subjects. Differences in mA and mE/mA ratios were significant between patients with a BASDAI score of > 4 and those with a BASDAI score of <4. However, aortic elasticity did not show significant differences between the groups. | AS patients exhibited significantly impaired myocardial diastolic functions, which were associated with disease activity. | 76% AS males vs. 76% male controls |
| Nilgün Üstün et al. 36 | 26 | 26 | 43.7 ± 11.8 | 42.5 ± 9.5 | 3/1/2 | In AS, the mitral early/late diastolic inflow velocity ratio and the mitral E‐wave velocity were lower than in controls. However, EF was similar between the two groups. Additionally, AS patients exhibited a significant reduction in LV diastolic and systolic strain values across all segments compared to healthy controls. | AS patients may have impaired LV systolic function despite no clinical evidence of cardiovascular disease. | 81% AS males vs. 85% male controls |
| Sabri Onur Çağlar et al. 37 | 42 | 40 | 39.4 ± 8.5 | 37.3 ± 8.7 | 4/2/2 | In AS, both interatrial left and right intraatrial electromechanical delay (EMD) intervals were prolonged. Also, AS patients exhibited higher values of left and right carotid intima‐media thickness (CIMT) and epicardial fat thickness (EFT). | Atrial EMD, CIMT, and EFT, indicative of cardiovascular involvement, were higher in AS patients. | 67% AS males vs. 60% male controls |
| Ümit İnci et al. 38 | 60 | 40 | 32 ± 7.8 | 30.0 ± 7.3 | 3/1/2 | No significant difference in left ventricular diastolic dysfunction was observed between AS and control groups, as assessed by both conventional and tissue Doppler echocardiography. | Increased ADMA levels suggest impaired nitric oxide metabolism in young AS patients without classical cardiovascular risk factors. | % AS males vs. % male controls |
| Gür et al. 39 | 75 | 30 | 41 ± 9.18 | 37.53 ± 10.69 | 3/1/2 | In AS patients compared to controls, systolic aortic diameter, diameter at the level of sinus valsalva, and central pulse wave velocity (cPWV) increased, while transverse strain of anterior and posterior aortic walls decreased. Disease activity (BASDAI) correlated with aortic strain, compliance, distensibility, and elastic modulus, and cPWV correlated with various aortic parameters. Logistic regression identified cPWV, transverse strain of the posterior wall (TS of PW), and transverse strain of the anterior wall (TS of AW) as independent predictors of AS. | AS patients showed elevated aortic diameters, cPWV, and impaired TS of AW and PW, indicating aortic vasculopathy. | 63% AS males vs. 60% male controls |
| Murathan Kucuk et al. 40 | 30 | 30 | 41.0 ± 9.2 | 37.5 ± 10.7 | 4/2/2 | The mean deceleration time (DT) was extended in ankylosing spondylitis (AS) patients compared to the control group. Additionally, in AS patients, left atrial (LA) S‐S, LA S‐E, and LA S‐A values were statistically lower than those in the control group. Furthermore, a negative correlation was noted between the Bath Ankylosing Spondylitis Metrology Index (BASMI) and LA S‐S. | 2D‐STE is valuable for detecting left atrial involvement in AS patients without clinical cardiovascular disease. | 73% AS males vs. 63% male controls |
| Law et al. 41 | 92 | 38 | 53 ± 12 | 57 ± 10 | 3/1/2 | In patients, LVEF, posterior wall (PW) thickness, and transmitral E/A ratio were significantly reduced, while left atrial (LA) and left ventricular (LV) volumes were significantly increased compared to controls. Despite the majority of individual patient results falling within standard reference ranges, measured global longitudinal strain (GLS) values showed that 53% of individuals had a mean GLS below the lower limit of the normal range, in contrast to controls. | GLS analysis demonstrated reduced LV long‐axis function in AS patients, highlighting its utility as a measure for cardiac involvement in this population. | 67% AS males vs. 42% male controls |
| Mustafa Zungur et al. 42 | 64 | 70 | 55.7 ± 9.2 | 54.9 ± 8.5 | 4/2/2 | While standard TTE showed no significant differences in right ventricular (RV) function parameters, speckle tracking echocardiography (STE), revealed impaired RV function in AS patients. Lower values were observed in RV‐free wall longitudinal strain, RV‐free wall longitudinal systolic strain rate, RV‐free wall longitudinal early diastolic strain rate, and RV‐free wall longitudinal late diastolic strain rate, with a higher RV‐early diastolic strain rate/RV‐late diastolic strain rate ratio for AS patients. | AS is linked to impaired RV function, as demonstrated by STE, even in the absence of clinical or laboratory signs of cardiac abnormalities. | 83% AS males vs. 79% male controls |
| Emren SV et al. 43 | 72 | 56 | 39.2 ± 9.9 | 40.9 ± 7.2 | 4/2/2 | Although the AS, non‐radiographic axial spondyloarthritis (nr‐axSpA), and control groups exhibited similar EF values, there was a difference in global longitudinal peak systolic strain (GLS) between the groups. In a post‐hoc analysis, GLS was not different between the nr‐axSpA and control groups, but it was significantly lower in patients with AS. | Subclinical myocardial dysfunction, as evaluated by GLS, was observed in AS patients but not in nr‐axSpA. | 72% AS males vs. 54% male controls |
| Ozen et al. 44 | 55 | 20 | 43.33 ± 7.87 | 44.60 ± 8.06 | 3/1/2 | AS patients exhibited elevated aortic stiffness compared to controls. LV global longitudinal strain (LVGLS) values were worse in AS patients. | Subclinical cardiac dysfunction, manifested through increased aortic stiffness and deteriorated LVGLS, is evident in AS patients even when their musculoskeletal disease is well‐controlled. | % AS males vs. % male controls |
| Demet Özkaramanlı Gür et al. 45 | 75 | 30 | 41.7 ± 10.1 | 38.5 ± 9.9 | 4/2/2 | In AS, reductions were observed in carotid‐to‐femoral pulse wave velocity (cPWV), E and e' velocities, longitudinal strain, strain rates at all myocardial layers, and transverse strains of both anterior and posterior aortic walls. Gal‐3 levels, along with strain and strain rates at circumferential and radial axes, were similar between the groups. Independently, AS was associated with LV dysfunction, expressed by longitudinal strain, and aortic impairment, expressed by transverse strain of the anterior wall. | Functional impairment in AS manifests early in the disease course, and strain imaging proves to be an effective tool for discriminating involvement. | 63% AS males vs. 57% male controls |
| Almasi S et al. 46 | 67 | 40 | 35 (26‐59) | 33.5 (20.5‐59) | 4/2/2 | LV systolic dysfunction was significantly higher in AS patients compared to controls, as was LV diastolic dysfunction. AS patients also showed a significantly higher occurrence of left‐axis deviation and left anterior fascicular block compared to the control group. However, the number of patients with aortic valve involvement was comparable between the two groups. | Common cardiac involvement in AS includes LV dysfunction, rhythm disturbances, and aortic valve insufficiency, independent of age, AS severity, and disease duration. | 75% AS males vs. 84% male controls |
| Turkmen S et al. 47 | 40 | 40 | 32.1 ± 5.5 | 31.2 ± 3.9 | 4/2/2 | AS patients, in comparison to controls, showed lower early (Em)/late (Am) diastolic myocardial velocities, mitral annular plane systolic excursion, and end‐diastolic distance from the mitral annulus to the LV apex. Conversely, AS patients exhibited higher systolic myocardial velocity (Sm), isovolumetric relaxation time, and displacement index. Elevated hs‐cTnT levels in AS patients independently predicted LV diastolic dysfunction. | AS patients demonstrated impaired LV functions and elevated hs‐cTnT levels. Tissue Doppler imaging proves to be a useful tool for detecting early functional LV abnormalities, and hs‐cTnT emerges as a valuable biomarker for diastolic LV dysfunction in AS patients. | 58% AS males vs. 50% male controls |
| Baniaamam M et al. 12 | 193 | 70 | 60 ± 7 | 63 ± 7 | 3/1/2 | The prevalence of diastolic dysfunction and Conduction disturbances in AS patients and controls were trivial and comparable, with limited clinical relevance. However, AS patients exhibited a significantly higher prevalence of aortic valve regurgitation (AVR) compared to controls while the prevalence of mitral valve regurgitation (MVR) was similar. | AS patients have up to a fivefold increased odds of developing aortic valve regurgitation (AVR) compared to controls. | 72% AS males vs. 58% male controls |
| Kenan Demır et al. 48 | 60 | 60 | 46.68 ± 8.72 | 44.90 ± 8.93 | 4/2/2 | There were no differences in basal characteristics and echocardiographic parameters between patients with AS and the control group. However, epicardial adipose tissue thickness (EATT) and pulse wave velocity (PWV) were higher in AS patients. Additionally, PWV showed significant correlations with EATT, age, and central blood pressure in AS patients. | In patients with AS, markers of atherosclerosis and cardiovascular disease, including EATT and PWV, were significantly higher than in the control group. Furthermore, the study revealed a significant relationship between PWV and EATT in patients with AS. | 60% AS males vs. 60% male controls |
Note: AS: Ankylosing spondylitis, Interquartile range, Quality Assessment by the Newcastle‐Ottawa scale.
Upon meticulous examination of the data:
3.1. Blood pressure metrics
The analysis showed no statistically significant difference in systolic and diastolic blood pressures between the groups under comparison. Specifically, the MD for systolic blood pressure was 0.32 mmHg with a 95% Confidence Interval (CI) ranging from −2.09 to 2.73. The diastolic blood pressure showed an MD of 0.30 mmHg, with a 95% CI of −0.40 to 1.01.
3.2. Ejection fraction
A pivotal finding was that the AS patients exhibited a markedly reduced EF as compared to the control group (Figure 2). The MD is −0.92%, confirmed by a 95% CI ranging between −1.25% and −0.59%.
FIGURE 2.
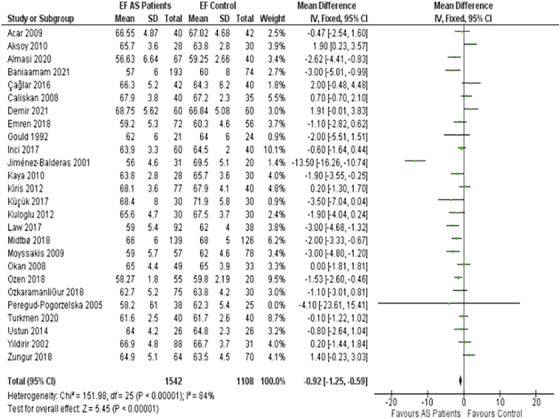
Forest plot depicting the distribution of Ejection Fractions (EF) among the included studies.
3.3. Ventricular filling velocities (E/A ratio)
Our findings illustrated that the Early (E) to Late (atrial—A) ventricular filling velocity ratio, commonly referred to as the E/A ratio, was considerably compromised in AS patients (Figure 3). The ratio exhibited an MD of −0.10 m/s, with a 95% CI of −0.13 to −0.08.
FIGURE 3.
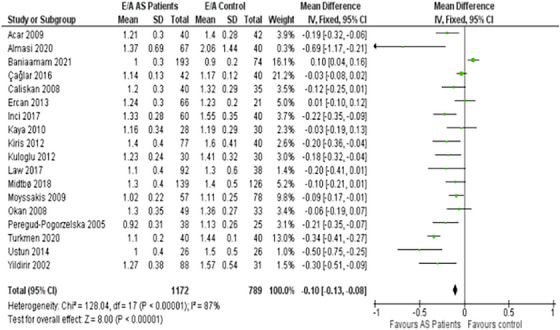
Forest plot depicting the distribution of Early (E) and Late (atrial—A) ventricular filling velocity (E/A) ratio among the included studies.
3.4. DT & IVRT
Another significant observation was the AS patients' cardiac timings. The DT in this group was prolonged by a mean of 12.30 ms (95% CI: 9.23–15.36) compared to controls (Figure 4). Additionally, the mean IVRT was extended, with an MD of 8.14 ms, statistically supported by a 95% CI ranging from 6.58 to 9.70 (Figure 5).
FIGURE 4.
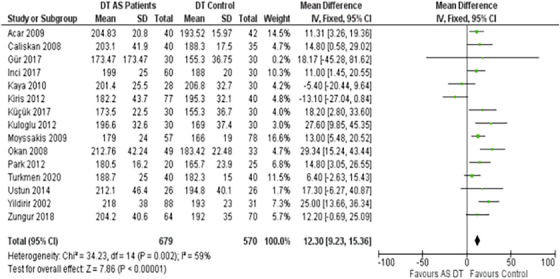
Forest plot depicting the distribution of Deceleration Time (DT) among the included studies.
FIGURE 5.
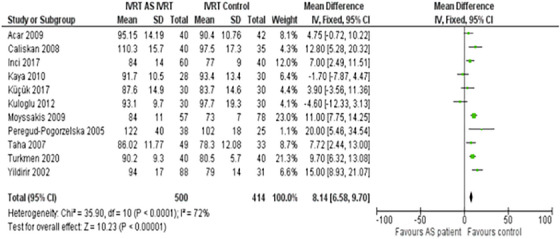
Forest plot depicting the distribution of Isovolumetric Relaxation Time (IVRT) among the included studies.
3.5. Assessment of publication bias
The integrity of our meta‐analytic conclusions was evaluated through a funnel plot analysis, complemented by Egger's test for publication bias. Visually, the funnel plot (Figure 6) displayed a symmetrical distribution of studies around the pooled effect size estimate, which is indicative of minimal publication bias. The precision of included studies, reflected by the inverse of standard errors, did not show the expected dispersion, with many studies clustering at the top of the plot. This unusual pattern prompted further investigation through Egger's regression test.
FIGURE 6.
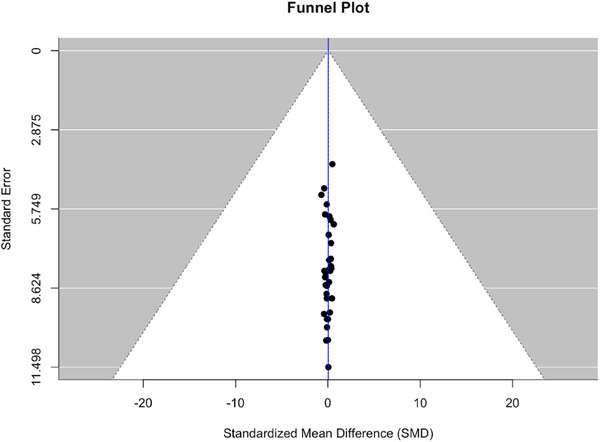
Funnel plot for assessment of bias.
Egger's test statistically examines the funnel plot asymmetry by regressing the standard normal deviation against precision. The results indicated no significant asymmetry (t = −0.5852, and p = .5623), with the limit estimate for the intercept as the precision approaches zero being b = 0.1880 (95% CI: −0.3146, 0.6906). The non‐significant p‐value (p > .05) suggests that the effect sizes of the included studies are not influenced by publication bias. This reinforces the reliability of our meta‐analytic findings and supports the notion that the literature analyzed provides a comprehensive view of the research landscape without overrepresentation of studies with more favorable outcomes.
The funnel plot, in conjunction with Egger's test, provides a methodological safeguard against the dissemination of biased conclusions. The absence of publication bias as indicated by the statistical tests ensures that our meta‐analysis reflects an unbiased estimate of the effect size, which is crucial for evidence‐based practice.
4. DISCUSSION
While the link between AS and cardiovascular diseases has been established, 9 our understanding of the specific impact of AS on left ventricular function remains limited. This meta‐analysis, encompassing 30 studies and a significant sample size of 2933 patients, aimed to shed light on this relationship by examining cardiovascular parameters like blood pressure, EF, and echocardiographic measures among AS patients.
4.1. Left ventricular function and echocardiographic parameters
Our study highlights a significant finding of compromised EF in AS patients compared to controls, displaying an MD of −0.92% [95% CI: −1.25 to −0.59]. Historically, diastolic LV dysfunction has been more associated with AS than systolic LV dysfunction. 10 The progression to LV dysfunction is believed to involve a sequence of events: systemic inflammation triggers coronary endothelium activation, resulting in alterations in cardiomyocytes, infiltration of monocytes, collagen production, microvascular rarefaction, interstitial fibrosis, and increased stiffness of cardiomyocytes, collectively contributing to the impairment of ventricular relaxation. 11 , 12
However, our analysis revealed inconsistencies among studies in their echocardiographic findings. While Baniaamam and colleagues (2021) detected minimal variances between AS patients and controls regarding both systolic and diastolic left ventricular (LV) dysfunction, 12 Midtbø and colleagues (2018) indicated a reduced left ventricular EF in AS patients. 13 These inconsistencies could be attributed to the evolving guidelines set forth by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI), as well as the inherent variability in measuring diastolic dysfunction. 14 , 15 According to one study, the prevalence of diastolic LV dysfunction in AS patients was 53% using the 2009 ASE/EACVI algorithm, but decreased notably to 3.8% with the application of the 2016 ASE/EACVI criteria. 12
Further reinforcing the notion of LV dysfunction in AS, Caliskan and colleagues (2008) identified differences in parameters reflecting LV diastolic function between AS patients and controls. 8 Our findings were consistent with this, revealing impaired diastolic function in AS patients. Indicators like the decreased E/A ratio, prolonged DT, and extended IVRT in AS patients collectively underscore the challenges these patients face during the cardiac cycle's diastolic phase.
While our study primarily focuses on the relationship between AS and LVD, it is important to acknowledge that various other factors can contribute to the development of LVD. Valvular heart disease, particularly aortic stenosis and mitral regurgitation, has been identified as a significant cause of LVD. 16 In a recent study by Généreux and colleagues (2017), severe aortic stenosis was found to be associated with a higher prevalence of LVD, with a reduced EF observed in 24% of patients. 17 Similarly, mitral regurgitation has been linked to LVD, as chronic volume overload leads to progressive left ventricular remodeling and dysfunction. 18
Moreover, hypertension (HTN) and diabetes mellitus are well‐established risk factors for LVD. 19 A meta‐analysis by Huang and colleagues (2020) demonstrated that HTN was associated with a 2.1‐fold increased risk of LVD, while diabetes mellitus conferred a 1.7‐fold increased risk. 20 These comorbidities can contribute to the development of LVD through various mechanisms, such as increased afterload, myocardial fibrosis, and microvascular ischemia. 21
The strength of this study includes its comprehensive approach in encompassing a diverse array of studies including a large patient population and thus leading to a robust statistical power to our meta‐analysis. The meticulous scrutiny of baseline characteristics across individual studies, coupled with the utilization of major databases and stringent inclusion criteria, ensures the reliability and validity of our findings. Moreover, the unprecedented scale of this meta‐analysis, representing the largest exploration of the relationship between AS and LVD to date, offers unparalleled insights. These findings could potentially reshape the management strategies for AS patients, potentially ameliorating cardiovascular outcomes owing to the depth and breadth of the data examined and analyzed.
Limitations of this study include heterogeneity among individual studies, such as measurement of EF, as some studies reported measuring EF through both biplane and visual methods, while others did not specify the exact technique used in their EF measurement. Other potential limitations include some studies having incomplete data on confounders, the potential biases of individual study quality, and the absence of conclusive causative evidence between AS and LVD.
As a meta‐analysis, due to the variability in parameters across individual studies, we were unable to evaluate other common associated diseases of AS, such as its association with aortic valve disease, stroke, and diabetes. Also, most of the articles that we analyzed in our studies did not contain the newly proposed indices such as E/e' ratio. We acknowledge that at the time of publication of these articles, these novel indices were not included in most guidelines. This limitation was also taken into consideration during our research.
Although our study primarily focused on the association between AS and LVD, we acknowledge the multifactorial nature of LVD and the potential contributions of other cardiovascular and systemic diseases. Future studies should consider these factors and their interactions with AS to provide a more comprehensive understanding of LVD in this patient population.
4.2. Blood pressure
Historically, a heightened risk of HTN in AS patients was suggested. 22 , 23 However, our findings, corroborated by studies like Midtbø and colleagues (2018), indicated comparable HTN prevalence between AS patients and controls. 13 Despite this similarity in blood pressure readings, the increase in left ventricular (LV) hypertrophy prevalence in AS patients hints at profound implications on the LV's structure and function. 13 , 24 This emphasizes that the effects of AS on cardiac health might be more intricate and extend beyond just blood pressure metrics.
5. CONCLUSIONS
Our updated meta‐analysis underscores the pronounced cardiovascular alterations in AS patients, with marked deviations in EF, ventricular filling velocities, and specific cardiac timings compared to controls. These revelations have pivotal clinical ramifications, emphasizing the necessity of comprehensive cardiovascular assessment in AS patients. However, considering the limitations, the findings should be interpreted judiciously, and further rigorous studies are warranted to corroborate our results.
AUTHOR CONTRIBUTIONS
The following statements specify the individual contributions of each author to the systematic review and meta‐analysis, in accordance with the CRediT (Contributor Roles Taxonomy) guidelines: Olayiwola Bolaji: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. Osejie Oriaifo: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. Olanrewaju Adabale: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Review & Editing, Visualization. Arthur Dilibe: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing—Review & Editing, Visualization. Faizal Ouedraogo: Software, Validation, Formal analysis, Data Curation, Writing—Original Draft, Visualization. Ebubechukwu Ezeh: Software, Validation, Writing—Original Draft, Visualization. Titilope Olanipekun: Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing. Ambica Nair: Investigation, Resources, Data Curation. Sula Mazimba: Conceptualization, Writing—Review & Editing, Project administration. Krishna Kuruvada: Conceptualization, Writing—Review & Editing, Supervision. Chadi Alraies: Methodology, Software, Validation, Writing—Original Draft, Project administration. All authors have read and agreed to the published version of the manuscript. Each author certifies that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, all authors have reviewed the final checklist and have ensured that the specific details pertaining to their contributions are accurately recorded. Each author contributes to the study's intellectual content and approves the final manuscript as submitted.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
In accordance with ethical publication standards, this manuscript reports on a systematic review and meta‐analysis that synthesizes findings from pre‐existing, publicly accessible data sources. The nature of this research is such that it involves secondary data analysis of de‐identified information where individual patient consent for inclusion in the original studies was obtained by the primary investigators at the time of the initial research. As this study did not involve the collection, use, or transmittal of individual patient data by the authors, no additional patient consent was required for the purposes of our analysis. This research complies with all relevant national regulations and institutional policies, and is in accordance with the tenets of the Declaration of Helsinki. The authors affirm that this article does not contain any personal information that could lead to the identification of any individuals and maintains the confidentiality of all data in line with the original sources' terms of use. We have conducted a thorough review of the studies included in our analysis to ensure that each complied with ethical standards concerning patient consent. Our research design and reporting strategies have been carefully developed to respect the privacy of participants and uphold the integrity of the data presented.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors wish to confirm that there were no funding sources supporting this work. Each author acknowledges that the systematic review and meta‐analysis was conducted without any external financial support. The contributions were made through the authors' personal and institutional resources. All authors concur with this statement and have collectively decided to disclose the absence of funding to maintain transparency and adhere to ethical reporting standards.
Bolaji O, Oriaifo O, Adabale O, et al. A meta‐analysis of left ventricular dysfunction in ankylosing spondylitis. J Clin Hypertens. 2024;26:772–788. 10.1111/jch.14827
DATA AVAILABILITY STATEMENT
For the purpose of this systematic review and meta‐analysis, all underlying evidence is sourced from publicly accessible datasets. These datasets were utilized under the applicable open data licenses without any restrictions on their use. The complete data extracted for the meta‐analysis, including study characteristics and outcomes, are provided within this manuscript and its supplementary materials. No proprietary or confidential data were used in this study. As this research is based entirely on previously published data, no new datasets were generated. The synthesized data used for the meta‐analysis are available within the article's supplementary information files.
REFERENCES
- 1. Rudwaleit M, Landewé R, Sieper J. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;375(13):1302‐1303. [DOI] [PubMed] [Google Scholar]
- 2. Luckie M, Irion L, Khattar RS. Severe mitral and aortic regurgitation in association with ankylosing spondylitis. Echocardiography. 2009;26(6):705‐710. [DOI] [PubMed] [Google Scholar]
- 3. Moyssakis I, Gialafos E, Vassiliou VA, et al. Myocardial performance and aortic elasticity are impaired in patients with ankylosing spondylitis. Scand J Rheumatol. 2009;38(3):216‐221. [DOI] [PubMed] [Google Scholar]
- 4. Ozkan Y. Cardiac involvement in ankylosing spondylitis. J Clin Med Res. 2016;8(6):427‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koivuniemi R, Paimela L, Suomalainen R, Leirisalo‐Repo M. Cardiovascular diseases in patients with rheumatoid arthritis. Scand J Rheumatol. 2013;42(2):131‐135. [DOI] [PubMed] [Google Scholar]
- 6. Mirjafari H, Al‐Husain A, Bruce IN. Cardiovascular risk factors in inflammatory arthritis. Curr Opin Lipidol. 2011;22(4):296‐301. [DOI] [PubMed] [Google Scholar]
- 7. Kerola AM, Kerola T, Kauppi MJ, et al. Cardiovascular comorbidities antedating the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 2013;72(11):1826‐1829. [DOI] [PubMed] [Google Scholar]
- 8. Caliskan M, Erdogan D, Gullu H, et al. Impaired coronary microvascular and left ventricular diastolic functions in patients with ankylosing spondylitis. Atherosclerosis. 2008;196(1):306‐312. [DOI] [PubMed] [Google Scholar]
- 9. Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population‐based study. Ann Intern Med. 2015. [DOI] [PubMed] [Google Scholar]
- 10. Yildirir A, Aksoyek S, Calguneri M, Oto A, Kes S. Echocardiographic evidence of cardiac involvement in ankylosing spondylitis. Clin Rheumatol. 2002;21(2):129‐134. [DOI] [PubMed] [Google Scholar]
- 11. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263‐271. [DOI] [PubMed] [Google Scholar]
- 12. Baniaamam M, Heslinga SC, Boekel L, et al. The prevalence of cardiac diseases in a contemporary large cohort of Dutch elderly ankylosing spondylitis patients‐The CARDAS study. J Clin Med. 2021;10(21):5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Midtbø H, Gerdts E, Berg IJ, Rollefstad S, Jonsson R, Semb AG. Ankylosing spondylitis is associated with increased prevalence of left ventricular hypertrophy. J Rheumatol. 2018;45(9):1249‐1255. [DOI] [PubMed] [Google Scholar]
- 14. Okan T, Sari I, Akar S, et al. Ventricular diastolic function of ankylosing spondylitis patients by using conventional pulsed wave Doppler, myocardial performance index and tissue Doppler imaging. Echocardiography. 2008;25(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 15. Prasad SB, Holland DJ, Atherton JJ, Whalley G. New diastology guidelines: evolution, validation and impact on clinical practice. Heart Lung Circ. 2019;28(9):1411‐1420. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739‐2791. [DOI] [PubMed] [Google Scholar]
- 17. Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38(45):3351‐3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mentias A, Naji P, Gillinov AM, Rodriguez LL, Reed G, Mihaljevic T, et al. Strain echocardiography and functional capacity in asymptomatic primary mitral regurgitation with preserved ejection fraction. J Am Coll Cardiol. 2016;68(18):1974‐1986. [DOI] [PubMed] [Google Scholar]
- 19. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 20. Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta‐analysis. BMJ. 2020;370:m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Treibel TA, López B, González A, Menacho K, Schofield RS, Ravassa S, et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non‐invasive study in 133 patients. Eur Heart J. 2018;39(8):699‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han C, Robinson DW Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167‐2172. [PubMed] [Google Scholar]
- 23. Heslinga SC, Van den Oever IA, Van Sijl AM, et al. Cardiovascular risk management in patients with active ankylosing spondylitis: a detailed evaluation. BMC Musculoskelet Disord. 2015;16:80. Published 2015 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CH, Chen HA, Liao HT, Chou CT, Chen CH. Association of blood pressure and hypertension with radiographic damage among the patients with ankylosing spondylitis. Medicine (Baltimore). 2022;101(38):e30811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gould BA, Turner J, Keeling DH, Hickling P, Marshall AJ. Myocardial dysfunction in ankylosing spondylitis. Ann Rheum Dis. 1992;51(2):227‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun JP, Khan MA, Farhat AZ, Bahler RC. Alterations in cardiac diastolic function in patients with ankylosing spondylitis. Int J Cardiol. 1992;37(1):65‐72. [DOI] [PubMed] [Google Scholar]
- 27. Jiménez‐Balderas FJ, García‐Rubi D, Pérez‐Hinojosa S, et al. Two‐dimensional echo doppler findings in juvenile and adult onset ankylosing spondylitis with long‐term disease. Angiology. 2001;52(8):543‐548. [DOI] [PubMed] [Google Scholar]
- 28. Przepiera‐Bedzak H, Peregud‐Pogorzelska M, Brzosko M, et al. Activity of the disease and selected echocardiographic abnormalities in ankylosing spondylitis. Pol Merkur Lekarski. 2006;20(117):296‐298. [PubMed] [Google Scholar]
- 29. Acar G, Sayarlioglu M, Akcay A, et al. Assessment of atrial electromechanical coupling characteristics in patients with ankylosing spondylitis. Echocardiography. 2009;26(5):549‐557. [DOI] [PubMed] [Google Scholar]
- 30. Aksoy H, Okutucu S, Kaya E, et al. Assessment of atrial conduction in patients with ankylosing spondylitis via P wave dispersion and atrial electromechanical delay. Int J Cardiol. 2010;29:0167‐5273. [Google Scholar]
- 31. Kaya EB, Okutucu S, Aksoy H, et al. Evaluation of cardiac autonomic functions in patients with ankylosing spondylitis via heart rate recovery and heart rate variability. Clin Res Cardiol. 2010;99(12):803‐808. [DOI] [PubMed] [Google Scholar]
- 32. Park SH, Sohn IS, Joe BH, et al. Early cardiac valvular changes in ankylosing spondylitis: a transesophageal echocardiography study. J Cardiovasc Ultrasound. 2012;20(1):30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kırış A, Karkucak M, Karaman K, et al. Patients with ankylosing spondylitis have evidence of left ventricular asynchrony. Echocardiography. 2012;29(6):661‐667. [DOI] [PubMed] [Google Scholar]
- 34. Kuloglu O, Bayram NA, Erten Ş, et al. Assessment of left ventricular function by tissue Doppler imaging in patients with ankylosing spondylitis. Rheumatol Rep. 2012;4(1):e6‐e6. [Google Scholar]
- 35. Ercan S, Goktepe F, Kisacik B, et al. Subclinical cardiovascular target organ damage manifestations of ankylosing spondylitis in young adult patients. Mod Rheumatol. 2013;23(6):1063‐1068. [DOI] [PubMed] [Google Scholar]
- 36. Ustun N, Kurt M, Nacar AB, Karateke HP, Guler H, Turhanoglu AD. Left ventricular systolic dysfunction in patients with ankylosing spondylitis without clinically overt cardiovascular disease by speckle tracking echocardiography. Rheumatol Int. 2015;35(4):607‐611. [DOI] [PubMed] [Google Scholar]
- 37. Çağlar SO, Boyraz İ, Erdem F, et al. Evaluation of atrial conduction times, epicardial fat thickness and carotid intima‐media thickness in patients with ankylosing spondylitis. Arch Rheumatol. 2016;31(4):353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inci U, Yildiz A, Batmaz I, Tekbas E. Assessment of serum asymmetric dimethylarginine levels and left ventricular diastolic function in patients with ankylosing spondylitis. Int J Rheum Dis. 2017;20(2):238‐244. [DOI] [PubMed] [Google Scholar]
- 39. Gür D, Özaltun D, Akyüz A, et al. Impaired aortic biomechanics in early diagnosis of cardiovascular involvement in Ankylosing Spondylitis. Anatol J Cardiol. 2017;18(1):1‐109. [Google Scholar]
- 40. Kucuk M, Korucuk N, Tosun V, Cavusoglu M, Basarici İ. Assessment of left atrial function using speckle tracking echocardiography in ankylosing spondylitis: a case‐control study. Int J Cardiovasc Imaging. 2018;34(12):1863‐1868. [DOI] [PubMed] [Google Scholar]
- 41. Law L, Henein M, Smeds J, et al. Left ventricular function using 2D strain echocardiography in patients with ankylosing spondylitis. esc365.escardio.org. Published December 9, 2017. Accessed January 28, 2024.
- 42. Zungur M, Gul I, Kobak S. Evaluation of right ventricular function by speckle‐tracking echocardiography in patients with ankylosing spondylitis: a case‐control study. Acta Cardiol Sin. 2018;34(2):159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emren SV, Gerçik O, Özdemir E, et al. Evaluation of subclinical myocardial dysfunction using speckle tracking echocardiography in patients with radiographic and non‐radiographic axial spondyloarthritis. Eur J Rheumatol. 2019;7(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ozen S, Ozen A, Unal EU, Tufekcioglu O, Ataman S, Yalcin AP. Subclinical cardiac disease in ankylosing spondylitis. Echocardiography. 2018;35(10):1579‐1586. [DOI] [PubMed] [Google Scholar]
- 45. Ozkaramanli Gur D, Ozaltun DN, Guzel S, et al. Novel imaging modalities in detection of cardiovascular involvement in ankylosing spondylitis. Scand Cardiovasc J. 2018;52(6):320‐327. [DOI] [PubMed] [Google Scholar]
- 46. Almasi S, Farahani B, Samiei N, Rezaei Y, Mahmoodi H, Qorbani M. Echocardiographic and electrocardiographic findings in patients with ankylosing spondylitis without cardiovascular risk factors. J Tehran Heart Cent. 2020;15(2):43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turkmen S, Askin L, Uzel KE, et al. Association of high‐sensitivity troponin t with left ventricular dysfunction in ankylosing spondylitis. J Clin Rheumatol. 2020;26(3):87‐93. [DOI] [PubMed] [Google Scholar]
- 48. Demir K, Avcı A, Esmen S, et al. Assessment of arterial stiffness and epicardial adipose tissue thickness in predicting the subclinical atherosclerosis in patients with ankylosing spondylitis. Clin Exp Hypertens. 2021;43(2):169‐174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
For the purpose of this systematic review and meta‐analysis, all underlying evidence is sourced from publicly accessible datasets. These datasets were utilized under the applicable open data licenses without any restrictions on their use. The complete data extracted for the meta‐analysis, including study characteristics and outcomes, are provided within this manuscript and its supplementary materials. No proprietary or confidential data were used in this study. As this research is based entirely on previously published data, no new datasets were generated. The synthesized data used for the meta‐analysis are available within the article's supplementary information files.


