Abstract
The prophylactic application of inactivated parapox ovis viruses (Baypamun; Bayer AG, Leverkusen, Germany) has been shown to reduce efficiently the outbreak of stress-mediated diseases in different species. However, little is known about the basic mechanism behind this observed stimulatory property. We therefore tested eight inactivated poxvirus strains belonging to three different genera (Orthopoxvirus, Avipoxvirus, and Parapoxvirus) for their capacity to activate cells of the porcine innate and specific immune systems in vitro. The results indicated that poxviruses failed to induce increased phagocytosis, oxidative burst, or natural killer cell activity in swine. In contrast, enhanced release of interleukin-2, alpha interferon, and gamma interferon, as well as strong proliferation, could be measured. Flow cytometric analyses and cell sorting experiments identified T-helper cells as the main target responding to inactivated poxviruses: the activated cells had a CD4high CD25+ major histocompatibility complex type II-positive phenotype and were the major source of secreted cytokines. Together, the results demonstrated that all tested poxviruses possessed immunostimulating capacity. These in vitro poxvirus-induced effects may be responsible at least in part for the in vivo immunostimulating capacity of inactivated poxviruses.
The Poxviridae are the largest of the known human and animal viruses (23). Due to their complex genetic structure (2) and thereby their strong immunogenic properties, poxviruses developed strategies of immune evasion that are distinct from those of smaller viruses. Instead of latency, slow replication, or high mutation rates (38), poxviruses utilize a variety of genes encoding proteins that counteract host immune responses. These viral proteins include soluble host growth factor homologues (35) as well as soluble receptors for tumor necrosis factor alpha (TNF-α) (36), interleukin-1β (IL-1β) (1), gamma interferon (IFN-γ) (37), alpha/beta interferon (IFN-α/β) (34), and various chemokines (11). If the viruses fail to secrete these proteins, as when the respective genes are deleted or the viruses are inactivated, the strong immunogenicity of the viruses may induce host immune reactions which are no longer inhibited (15, 25). There is a growing evidence that such immune reactions may result in more than elimination of the viruses. A more general stimulating effect on the immune system may ensue.
In 1978, it was first noted that inactivated poxviruses may possess immunostimulating capacity. Prophylactic application of parapox ovis viruses (PPOV) or fowl poxviruses (FPV) that had been inactivated (iPPOV and iFPV, respectively) clearly diminished the rate of mortality of Pseudomonas aeruginosa-infected mice (21). Subsequently, various in vitro experiments, mainly focusing on early effects (before day 2), indicated that iFPV, iPPOV, and modified vaccinia virus Ancara (MVA) that had been inactivated (iMVA) could induce enhanced phagocytosis, natural killer (NK) cell activity, and release of IFN-α (5, 10, 18). Moreover, the secretion of TNF-α, IL-2, and granulocyte-macrophage colony-stimulating factor could also be enhanced by iFPV and iPPOV (17, 19, 32). In addition, several in vivo studies demonstrated that a prophylactic or metaphylactic application of iPPOV (strain D1701; Baypamun; Bayer AG, Leverkusen, Germany) efficiently reduced susceptibility to infectious diseases in different species (6, 16, 33, 39).
The three viruses—MVA, PPOV, and FPV—that have been predominantly analyzed for their immunostimulating capacity so far are (in part) highly attenuated compared to the respective wild-type viruses (20, 22). For example, MVA has lost about 15% of its original genetic information, including genes that encode host growth factor homologues and soluble receptors for chemokines, TNF-α, IFN-γ, and IFN-α/β (2). Although iMVA, iPPOV, and iFPV belong to different genera (Orthopoxvirus, Avipoxvirus, and Parapoxvirus), being characterized by different morphological, chemical, and genetical properties (23), immunostimulating capacity has been found for all three. This finding suggests that the capacity to stimulate the immune system is a general feature of poxviruses.
The aim of the present study was to characterize further the poxvirus-induced immunostimulating effects. As a suitable model, pigs offer several advantages in comparison to other species. Different in vivo studies have indicated that iPPOV can stimulate the porcine immune system. This virus may also be helpful in the prevention of economically important stress-mediated diseases, such as mastitis metritis agalactia syndrome (13), postweaning diarrhoea syndrome (16), or wasting pig syndrome (16). In addition, in vitro studies have revealed that porcine peripheral blood mononuclear cells (PBMC) respond to iFPV, iMVA, and iPPOV stimulation with increased IFN-α and IL-2 release (5, 32). Our intention was to examine further inducible effects within the innate and specific immune systems. As markers for early immunological reactions, phagocytosis, oxidative burst, and NK cell activity were chosen. Markers for late observable reactions were the quantification of proliferation and of IL-2, IFN-α, and IFN-γ. The investigations began with characterization of the immunostimulating property of iPPOV, because this virus is the best characterized in terms of in vivo and in vitro immunostimulating capacities (13, 16, 32, 33). These studies were then extended to other poxviruses to determine whether the stimulating capacity is a common feature of poxviruses, even those belonging to different genera. Together, these experiments should help to elucidate the basic mechanisms responsible for the immunostimulating capacity of inactivated poxviruses.
MATERIALS AND METHODS
Animals.
A total of 50 conventionally reared, healthy, 3- to 6-month-old German Landrace swine of both sexes, housed at the Federal Research Centre for Virus Diseases of Animals, were used for the experiments. With the exception of a prophylactic deworming (Citarin; Bayer AG), animals were not medically treated. They had no contact with any poxviruses and were thus regarded as non-poxvirus-primed animals. PBMC were isolated from heparinized blood (0.1 mg/ml) taken by anterior vena cava puncture. PBMC from all 50 animals were tested for reactivity to iPPOV in proliferation assays; PBMC from 10 animals were used for other experiments.
Cells.
PBMC were isolated by Ficoll-Hypaque density gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum, 2 mM l-glutamine, 100 IU of penicillin per ml, 0.1 mg of streptomycin sulfate per ml, and 0.05 mM mercaptoethanol. The medium for the murine IL-2-dependent cell line HT-2 (American Type Culture Collection [ATCC], Manassas, Va.) was additionally supplemented with 10 IU of recombinant human IL-2 (rhIL-2; Roche, Basel, Switzerland) per ml. The bovine kidney cell lines MDBK (ATCC) and BKK (Bayer AG), as well as the monkey kidney cell line Vero (ATCC), were cultured in Dulbecco's modified Eagle's medium supplemented with 5% (vol/vol) fetal calf serum, 100 IU of penicillin per ml, and 0.1 mg of streptomycin sulfate per ml.
Viruses.
The immunomodulator Baypamun served as the source of iPPOV. Baypamun was produced by growing PPOV strain D1701 in bovine kidney cells and titrating the virus on the same cell line. After the removal of cell debris, the virus harvest was inactivated with binary ethyleneimine and concentrated by ultrafiltration. The concentrated bulk material was reconstituted in medium and adjusted to the nominal product titer of 106.75 tissue culture infective doses (TCID50)/ml. Thus, approximately 99% of the cell culture supernatant could be substituted. For stabilization of the virus preparation, 25 mg of Polygeline per ml was added before lyophilization. Cell culture supernatant from mock-infected bovine kidney cells, treated in the same manner as the virus preparation, served as negative control material. Both the Baypamun material and the control material were kindly provided by Bayer AG.
FPV strain HP-1 (Pind Avi) and MVA preparations were generous gifts from A. Mayr (Veterinary Faculty, University of Munich, Munich, Germany). Both virus preparations were produced and inactivated as previously described (19). Briefly, FPV-infected chicken embryo fibroblasts and MVA-infected Vero cells were harvested when the cytopathic effect was 100% (3 to 5 days postinfection). The viruses were liberated from the cells by freezing-thawing and sonication, followed by low-speed centrifugation to remove cellular debris. After determination of the virus titers (FPV, 108.5 TCID50/ml; MVA, 107.5 TCID50/ml), the preparations were inactivated with β-propriolactone (Boehringer Ingelheim, Ingelheim, Germany), stabilized with gelatin (2.5% [wt/vol]), and lyophilized in 1-ml aliquots. Mock-infected chicken embryo fibroblasts and Vero cells treated in the same manner as the virus preparations served as negative controls. Before use, virus stocks and controls were dialyzed for 48 h to eliminate any possible toxic effects due to residual β-propriolactone.
Vaccinia virus Lister, originally obtained from the ATCC, was grown and titrated on BKK cells as described above. Vaccinia viruses Kopenhagen and Tian Tan, parapox bovis virus I, and wild-type PPOV were provided by M. Büttner (Federal Research Centre for Virus Diseases of Animals) and propagated and titrated on Vero cells as described above. Before heat inactivation (56°C, 30 min), the titers of these virus stocks ranged between 107 and 108 TCID50/ml. Preparations of mock-infected cells treated in a manner similar to that used for the respective poxviruses served as controls.
Inactivation of all virus preparations was verified by assessment of the TCID50 after 7 days of culturing. Whereas binary ethyleneimine- and β-propriolactone-inactivated viruses showed no residual infectivity (TCID50, <101/ml), heat-inactivated virus preparations retained residual infectivity ranging between 101 and 103 TCID50/ml. Heat-inactivated classical swine fever virus (CSFV) and β-propriolactone-inactivated pseudorabies virus (PRV) and foot-and-mouth-disease virus (FMDV) served as controls; these were produced in our own laboratory.
MAbs and antisera.
Murine monoclonal antibodies (MAbs) against porcine CD4 (MAb 74-12-4; immunoglobulin G2b [IgG2b] [26]) and major histocompatibility complex (MHC) class II (MHCII) (SLA-DR; MAb MSA3; IgG2b [12]) were received from J. K. Lunney (Agricultural Research Service, U.S. Department of Agriculture, Beltsville, Md.). A murine MAb against CD25 (MAb K231-3B2; IgG1 [4]), recognizing an epitope of the IL-2 receptor-α-chain expressed only on activated lymphocytes, was provided by C. R. Stokes (Department of Veterinary Medicine, University of Bristol, Bristol, United Kingdom). Murine MAb G47 and rabbit antiserum no. 652, specific for porcine IFN-γ, and a murine MAb against porcine IFN-α were generous gifts from B. Charley and C. LaBonnadiere (Institut National de la Recherche Agronomique, Jouy-en-Josas, France).
Lymphocyte proliferation assays.
Poxvirus-induced stimulation of PBMC was achieved by incubation of 2 × 105 PBMC/well with inactivated poxviruses in a final volume of 200 μl. The number of added inactivated virus particles depended on the optimal stimulating capacity of each poxvirus lot and ranged between 2 × 105 TCID50 (measured before inactivation) for iFPV and 5 × 104 TCID50 for iPPOV. After 7 days of culturing, cells were pulsed with 1 μCi of 3H-thymidine (ICN Biomedicals GmbH, Eschwege, Germany) per microculture for 18 h; cells were then harvested onto glass fiber filters, which were analyzed in a scintillation counter as described previously (30). Results were expressed as mean counts per minute of triplicate cultures or as a stimulation index (SI), calculated as follows: SI = mean of inactivated-poxvirus-specific proliferation/mean of spontaneous proliferation. An SI of >2 was considered positive.
Cytolytic assays.
For the quantification of spontaneous cytolytic activity, PBMC (105/well) were treated with inactivated poxviruses, mock-infected cell culture supernatant, or 25 IU of rhIL-2 and then cultured with 51Cr-labeled K562 cells (103/well) for 18 h. The percent specific cytolytic activity was calculated as described previously (28).
Measurement of phagocytosis and the oxidative burst.
For the investigation of poxvirus-induced phagocytosis or oxidative burst, 100 μl of heparinized blood was incubated with 100 μl of poxvirus suspension (TCID50, 0.5 × 105 to 2 × 105, depending on the virus strain) for 30 min at 37°C. Phagocytosis and the oxidative burst were measured with commercial available test kits (Phagoburst and Phagotest; Orpegen Pharma, Heidelberg, Germany) according to the manufacturer's instructions.
Measurement of cytokine secretion.
The content of IL-2, IFN-α, and IFN-γ in either supernatants of poxvirus-stimulated PBMC (2 × 105/well) or CD4-defined, separated cell fractions collected at various times was measured.
IL-2 production was assayed by the capacity of supernatants to support the proliferation of the IL-2-dependent cell line HT-2.
IFN-α secretion as determined by an indirect plaque reduction bioassay as described previously (27). Briefly, the antiviral activity of IFN-α was quantified by the reduction of vesicular stomatitis virus-induced cytopathic effect on MDBK cells after preincubation of the cell line with a serial dilution of supernatants from poxvirus-stimulated PBMC. The assay was calibrated with an internal laboratory standard of recombinant porcine IFN-α (generous gift of C. LaBonnadiere, Institut National de la Recherche Agronomique) that was equivalent to 50 IU of recombinant human IFN-α. Specificity was conferred by blocking with an MAb against porcine IFN-α.
IFN-γ production was quantified by an enzyme-linked immunosorbent assay (ELISA) using an MAb and an antiserum specific for porcine IFN-γ. Cytokine concentrations were calculated using the linear portion of the curve obtained with recombinant standards run on each ELISA plate and were expressed in nanograms per milliliter.
Multicolor flow cytometric analysis.
For determination of the poxvirus-stimulated cell fraction, PBMC (4 × 106) were cultured with inactivated poxviruses (106 TCID50) for 9 days in six-well plates. After quantification of the total cell number by trypan blue exclusion, cells were stained with an MAb against CD4 (IgG2b) in combination with an anti-CD25 (IgG1) or an anti-MHCII (IgG2a) antibody, followed by labeling with isotype-specific antisera (fluorescein isothiocyanate [FITC]-conjugated anti-IgG2b, phycoerythrin [PE]-conjugated anti-IgG1, or PE-conjugated anti-IgG2a; Southern Biotechnology Associates, Birmingham, Ala.). All measurements were obtained with a dual-LASER FACStar plus (Becton Dickinson, Mountain View, Calif.) as described previously (29). Relative cell numbers of the single-cell fractions were converted into absolute cell numbers by use of the total cell number.
The list mode data were processed using PC-lysis and Corel draw software.
Cell sorting.
For determination of the major cytokine-secreting cell fraction in response to iPPOV, PBMC were separated into CD4-defined lymphocyte fractions as described previously (30). In brief, PBMC were stained in four 20-min incubation steps with an MAb against CD4, biotinylated goat anti-mouse immunoglobulin (Jackson Laboratories), FITC-conjugated streptavidin (Jackson Laboratories), and biotinylated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) on ice. They were then passed over magnetic cell separation columns to retain the bead-coated CD4+ cells. The bound cells were eluted by removing the magnet. The purity of the CD4+ and CD4− cell fractions was verified by fluorescence-activated cell sorting (FACS) analysis (CD4+, 90%; CD4−, 99.8%).
RESULTS
Influence of iPPOV on cells assigned to the innate immune system.
Previous studies suggested that poxvirus-mediated activation of innate immune reactions is responsible for the observed immunostimulating capacity (18, 19). Consequently, our investigations of poxvirus-induced immunostimulating effects began by analyzing the influence of iPPOV on the cytolytic activity of NK cells and phagocytosis by monocytes and granulocytes.
The cytolytic activity of NK cells was determined by an 18-h 51Cr release assay using 51Cr-labeled K562 cells as target cells. Table 1 shows the reactivity of 1 out of 10 tested animals and the influence of iPPOV on NK cell cytolytic activity at an effector-to-target ratio of 100:1. Compared to spontaneous lysis, no increased lysis of K562 cells could be seen in any of the cultures that were supplemented with different numbers of iPPOV particles for the duration of the assay. Similarly, no response could be found for any animal when cells were cultured with an inactivated cell culture supernatant derived from mock-infected BKK cells. In contrast, the addition of rhIL-2 enhanced spontaneous cytolytic activity to 72% (Table 1, positive control). From nine more animals, the spontaneous lysis of iPPOV-stimulated PBMC was comparable to control spontaneous lysis, ranging from 25 to 62%. Together, these results provide evidence that iPPOV has no capacity to enhance porcine NK cell cytolytic activity.
TABLE 1.
Influence of iPPOV on reactions of the innate immune system
| Stimulus | Influence of iPPOV on:
|
||
|---|---|---|---|
| NK cell activity (% specific lysis) | Phagocytosis (% phagocytes) | E. coli-induced oxidative burst (% oxidized cells) | |
| Spontaneous | 39 | 21.8 | 35.7 |
| Mock-infected control | 39 | 22.1 | 36.4 |
| Positive controla | 72 | 35.4 | 44.6 |
| iPPOV particles | |||
| 5 × 104 | 40 | 18.9 | 31.1 |
| 1 × 104 | 38 | 21.3 | 33.9 |
| 5 × 103 | 41 | 20.2 | 35.8 |
| 1 × 103 | 40 | 19.9 | 34.3 |
| 2 × 102 | 39 | 21.6 | 34.6 |
Positive controls were rhIL-2 for NK cell activity, pooled swine serum for phagocytosis, and PMA for the oxidative burst.
In order to study the influence of iPPOV on the phagocytosis of neutrophils and monocytes, heparinized porcine blood was incubated for 30 min with iPPOV, mock-infected cell culture supernatant, phosphate-buffered saline, or pooled swine serum. Thereafter, the uptake of FITC-labeled Escherichia coli was quantified by flow cytometry. A typical example of the results obtained is presented in Table 1 for one animal. From these data it can be seen that independent of the number of added particles, iPPOV failed to enhance the rate of phagocytosis over that obtained with phosphate-buffered saline or mock-infected cell culture supernatant. Only in the presence of pooled swine serum, serving as a positive control, was the uptake of bacteria increased by 14%. The data presented could be reproduced with nine more animals (spontaneous phagocytosis, 8 to 24%). This result indicated that iPPOV possessed no capacity to modulate the phagocytic uptake of E. coli by monocytes or granulocytes.
This finding was further confirmed by analysis of the oxidative burst, which follows the phagocytic uptake of antigen (24). The studies were performed in a manner similar to that used for the phagocytosis assays, with the exceptions that the oxidation of the fluorogenic substrate dihydrorhodamine by reactive O2 metabolites was analyzed and phorbol myristate acetate (PMA) served as a positive control. For the experiments, heparinized blood was supplemented with iPPOV or the respective controls for 30 min before opsonized E. coli bacteria were added for another 10 min to induce the oxidative burst. The results obtained with blood cells from 1 out of 10 tested animals are presented in Table 1 as a typical example. These data show that opsonized E. coli bacteria can trigger an oxidative burst in a certain percentage of the cells, increasing from less than 10% to approximately 40%. The addition of different numbers of iPPOV particle led to a slight decrease, whereas preincubation of the samples with PMA increased the induction of reactive O2 metabolites by an additional 8%. The fact that iPPOV did not show any costimulatory influence on an E. coli-induced oxidative burst supports the finding that iPPOV has no apparent stimulating influence on porcine leukocyte phagocytosis.
Taken together, these functional analyses indicate that iPPOV possesses the capacity neither to modify porcine NK cell cytolytic activity nor to increase the phagocytic uptake or release of reactive O2 metabolites by porcine neutrophils and monocytes. An iPPOV-induced modulation of innate immune reactions being responsible for the observed immunostimulating property therefore seems to be unlikely.
iPPOV-induced proliferation of porcine PBMC.
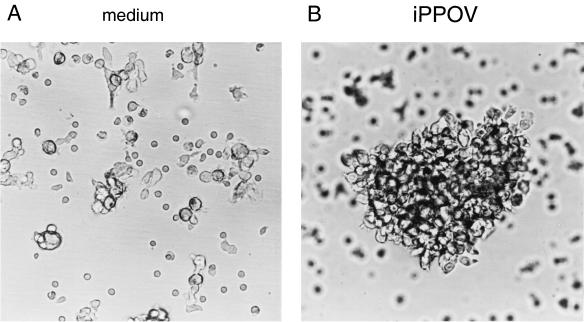
With regard to the fact that iPPOV apparently had no immunostimulating influence on innate immune reactions in swine, it was surprising to find morphological changes in resting non-poxvirus-primed PBMC upon cultivation with iPPOV. As early as 1 day after the beginning of stimulation, the formation of small clusters involving approximately 25% of the PBMC was observed. This characteristic property of activated cells could not be detected in cultures incubated without iPPOV. By day 9, the proportion of clustering cells had increased, as had both the number of cells in the clusters and the number of clusters (Fig. 1B). In contrast, only minimal aggregation could be detected for the adherent cells of the control cultures (Fig. 1A).
FIG. 1.
iPPOV-induced cell aggregation by porcine PBMC. PBMC (4 × 106) were cultured for 9 days with iPPOV (106 TCID50) (B) or inactivated cell culture supernatant of mock-infected BKK cells (diluted 1:2) (A). Cells were cultured in a final volume of 3 ml of cell culture medium in six-well plates. Magnification, ∼×46.
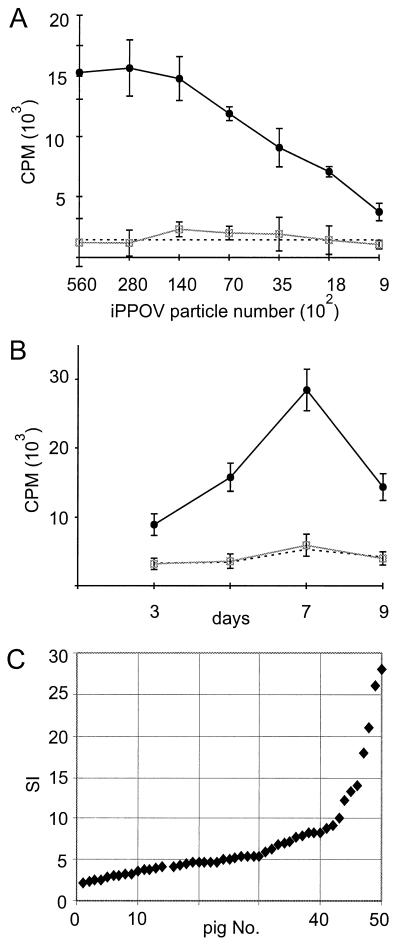
This microscopic finding was evidence for an activation of PBMC. Consequently, the proliferation of PBMC was analyzed. With resting porcine PBMC, an increase in proliferation of up to 10-fold was detected in the presence of iPPOV, compared to spontaneous proliferation or proliferation after the addition of a cell culture supernatant from mock-infected BKK cells. As illustrated in Fig. 2A, this response was dependent on the number of added virus particles, the highest 3H-thymidine incorporation being obtained at a ratio of 0.1 iPPOV particle per lymphocyte. In further assays, the time course of proliferation was analyzed, as was the heterogeneity of the response within a larger animal population. The proliferative response of PBMC usually started 2 to 3 days after iPPOV stimulation, reaching its maximum as late as 7 to 8 days (Fig. 2B). PBMC from 50 tested animals all showed increased reactivity in response to cultivation with iPPOV. However, by analysis of SIs, which ranged between 2 and 28, with an average of approximately 5 (Fig. 2C), a rather heterogeneous response to iPPOV became apparent. This result indicated that not all animals responded equally to iPPOV stimulation.
FIG. 2.
iPPOV-dependent proliferation of porcine PBMC. PBMC (2 × 105 cells/well) were cultured with iPPOV (closed circles), inactivated cell culture supernatant from mock-infected BKK cells (open squares), or medium (broken line). The proliferative response of the microcultures was quantified by 3H-thymidine incorporation. The standard deviation of triplicate cultures in single experiments is indicated by error bars. (A) Dose-response curve after 7 days of cultivation. (B) Time course of iPPOV stimulation (5 × 104 TCID50). (C) SI of iPPOV-stimulated PBMC (5 × 104 TCID50) derived from 50 animals.
Identification of iPPOV-responding porcine PBMC.
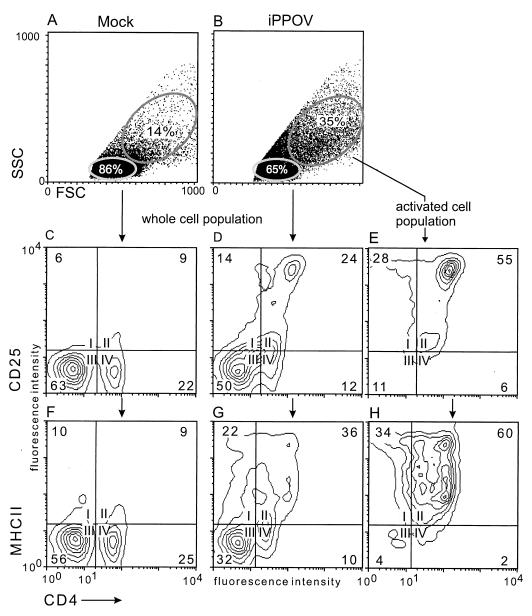
The phenotype of the activated lymphoblasts was identified by flow cytometric analyses. For this purpose, PBMC were cultured for 9 days in the presence of iPPOV or inactivated cell culture supernatant from mock-infected cells. After determination of the absolute cell numbers in the respective cultures, cells were stained with an MAb against CD4 in combination with an MAb against CD25 or MHCII (MAb directed against SLA-DR). In agreement with the microscopic findings, the dot plots in Fig. 3A and B) illustrated that iPPOV-stimulated PBMC contained a significantly higher proportion of activated cells. These were characterized by increased size (high forward scatter) and granularity (high side scatter) (Fig. 3B) (35%) compared with the results for the control (Fig. 3A) (14%). The majority of these iPPOV-activated cells (Fig. 3B) (35%) were T-helper cells with a high CD4 surface antigen density, expressing the activation marker CD25 and surface MHCII molecules (Fig. 3E and H). Resting PBMC cultured in the presence of iPPOV (Fig. 3B) (65%) contained only a minor proportion of CD4low T-helper cells and were characterized by almost no expression of CD25 and MHCII molecules (data not shown). In view of this antigen expression pattern, resting iPPOV-cultured PBMC strongly resembled cells from the control (Fig. 3C and F).
FIG. 3.
Phenotypic identification of the iPPOV-responding cell fraction. PBMC were cultured in the presence of iPPOV or cell culture supernatant from mock-infected BKK cells for 9 days as described in the legend to Fig. 2. (A and B) In the dot plots of forward scatter (FSC) versus side scatter (SSC) (A and B), the size and granularity of both cell cultures are shown. The selective analysis of nonactivated PBMC and PBMC activated with iPPOV (B) was achieved by setting electronic windows on cells with low FSC and SSC (65%) or high FSC and SSC (35%). (C to H) Contour plots of expression of CD4 versus CD25 (C to E) and CD4 versus MHCII (F to H) for all mock-cultured cells (C and F), all iPPOV-cultured cells (D and G), and the iPPOV-activated cell population (E and H). Numbers in the corners of the contour plots indicate percentages of cells.
A comparison was made of the relative cell numbers in all iPPOV-stimulated PBMC and all mock-cultured PBMC. This result showed that iPPOV-stimulated cells had an approximately threefold increase in CD25+ T-helper cells (24 versus 9%) and a fourfold increase in MHCII-positive T-helper cells (36 versus 9%) compared with the T-helper cells from the control. The increased proportion of activated T-helper cells was due both to the death of other cells in the culture over time and to a threefold absolute increase in the CD4+ cell numbers compared to those in the control (control, 8.89 × 104/ml of T-helper cells; iPPOV, 21.26 × 104/ml of T-helper cells). These experiments were repeated with PBMC from nine more animals, all showing a two- to fourfold absolute increase in T-helper-cell numbers upon iPPOV stimulation compared to the results obtained with cultivation with mock-infected cell culture supernatant (data not shown). Together, these experiments demonstrate that mainly T-helper cells responded to stimulation with iPPOV.
Functional characterization of the iPPOV-responding cell fraction.
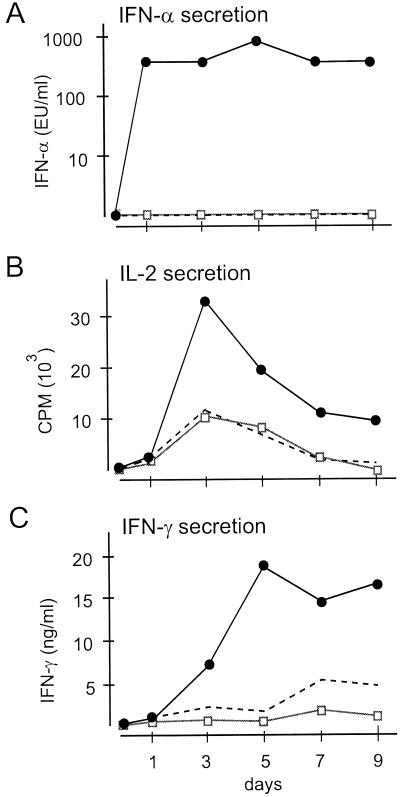
Functional analysis of the effector cells generated upon activation of PBMC with iPPOV should contribute to the elucidation of the basic mechanism responsible for the stimulation of the immune system. In this context, analyses focused particularly on the secretion of the cytokines IL-2, IFN-γ, and IFN-α, because they play an important role in the response against infectious agents. As shown in Fig. 4, it was possible to detect increased concentrations of all tested cytokines in cell culture supernatants from iPPOV-stimulated PBMC. Inactivated control supernatants from mock-infected cells had no effect. When the time course of the induced reactions was monitored, increased secretion of IFN-α measured after the first day of stimulation was the earliest detectable event (Fig. 4A). Thereafter, maximal IL-2 release at day 3 and maximal IFN-α and IFN-γ release at day 5 were detected. In combination with the observed maximal proliferation at day 7 (Fig. 2B), the results were indicative of the classical pathway of T-helper-cell activation and differentiation. The analyses of cytokine production could be reproduced with PBMC from nine more animals, with similar heterogeneous reactivities, as already described for proliferation. Compared to the data for the control supernatants, a 2- to 10-fold increase in the levels of all cytokines was detected (data not shown).
FIG. 4.
Kinetics of iPPOV-induced cytokine release. Cytokine release time courses for PBMC cultured in the presence of iPPOV (5 × 104 TCID50) (closed circles), inactivated cell culture supernatant from mock-infected BKK cells (diluted 1:2) (open squares), or medium (broken line). Results are shown for one representative animal. The standard deviation of triplicate cultures in single experiments was less than 10%. At different times, supernatants were collected. The IFN-α antiviral activity in the supernatants was calculated by determination of dilutions causing a 50% reduction of the vesicular stomatitis virus-induced cytopathic effect in MDBK cells. The IL-2 concentration was measured by a bioassay using the IL-2-dependent cell line HT-2. The IFN-γ concentration was quantified by an ELISA.
By iPPOV stimulation of immunomagnetically sorted CD4+ and CD4− cells, it was possible to show that CD4+ cells, as the main iPPOV-responding cell fraction, were also the major source for the secreted cytokines. In these experiments, the amount of IL-2 secreted by the iPPOV-stimulated CD4+ cell fraction was twice that of the CD4− cell fraction (58 × 103 cpm versus 28 × 103 cpm). The amount of IFN-γ secreted by the CD4+ T cells was increased nearly threefold (196 versus 75 ng/ml), and that of IFN-α was increased up to fourfold (320 versus 80 experimental units [EU] of IFN-α/ml). In summary, these data indicated that the stimulating capacity of iPPOV is effected primarily through the activation of T-helper cells, which in turn secrete important immunomodulatory cytokines.
Investigation of the stimulating capacities of inactivated poxviruses other than iPPOV.
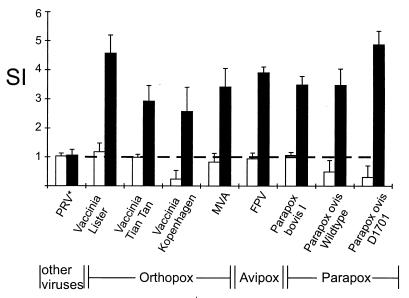
The experiments described so far were performed with iPPOV. However, the finding that iFPV and iMVA may also have immunostimulating effects (5, 10) suggested that the capacity to stimulate the porcine immune system represents a property common to all poxviruses. We therefore analyzed the reactivity of porcine PBMC to seven more poxvirus strains belonging to the genera Orthopoxvirus, Avipoxvirus, and Parapoxvirus. In order to exclude inhibitory effects caused by the secretion of viral immunomodulating proteins, such as soluble receptors for IFN-α/β, IFN-γ, and TNF-α, the experiments were performed only with heat- or β-propriolactone-inactivated viruses. As shown in Fig. 5, all tested poxviruses were capable of enhancing the proliferative response of PBMC compared to spontaneous proliferation, whereas inactivated cell culture supernatants from mock-infected cells or other tested viruses (PRV, FMDV, CSFV) had no effect. The optimal SI of the poxviruses, as determined with PBMC from the same animal, ranged between 2.5 and 5. This result indicated that only minor differences existed in the stimulating capacities of the different poxviruses.
FIG. 5.
Proliferative response of porcine PBMC upon stimulation with different inactivated poxvirus strains. PBMC (2 × 105 cells/well) were cultured with different poxvirus strains (orthopoxviruses and parapoxviruses, 0.5 × 105 to 1 × 105 TCID50; avipoxvirus, 2 × 105 TCID50), other viruses (PRV, 105 TCID50; similar results were obtained with FMDV and CSFV), the respective controls, or medium alone. After 7 days, proliferation was determined by measurement of 3H-thymidine incorporation. The data presented show the SI of poxvirus-stimulated (black bars) or mock-cultured (white bars) PBMC in comparison with spontaneous proliferation (broken line). The standard deviation of triplicate cultures in single experiments is indicated by error bars.
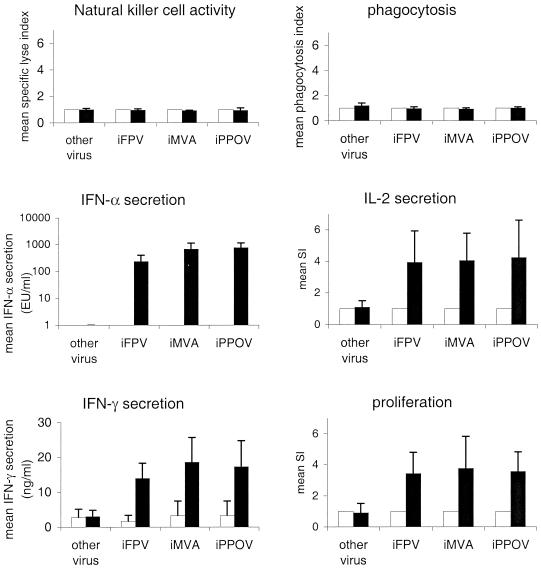
Clearly, the proliferative response of PBMC upon iPPOV stimulation was caused mainly by the activation of T-helper cells. It appeared that the proliferative response of PBMC against other poxviruses might also be based primarily on the stimulation of T-helper cells. In order to directly prove this hypothesis, we repeated the iPPOV experiments with one member of each tested poxvirus genus. Like the data obtained with iPPOV, the experiments shown in Fig. 6 indicate that iFPV and iMVA failed to influence NK cell activity and phagocytosis, whereas both viruses were capable of increasing IFN-α, IL-2, and IFN-γ secretion and cell proliferation. All experiments were performed with PBMC from 10 animals, and the mean SI (or the mean concentration, if no effect was detectable within the medium controls) was calculated for each poxvirus. The approximately fourfold-increased reaction in response to each poxvirus (Fig. 6) supports the finding that different virus genera showed no significant differences in their capacities to induce immunostimulatory effects. Further flow cytometric analyses identified T-helper cells as the main reacting cell fraction in response to iMVA and iFPV (data not shown). It may thus be concluded that the capacity to stimulate the immune system is not specific for the Parapoxvirus genus but may also be observed in virus strains of other poxvirus genera. The basic mechanism responsible for the immunostimulatory effects seems to be the same for all poxviruses.
FIG. 6.
Comparison of the immunostimulating capacities of different inactivated poxviruses. The influence of iFPV (2 × 105 TCID50), iMVA (1 × 105 TCID50) iPPOV (5 × 104 TCID50), and FMDV (other virus; 1 × 106 TCID50) on NK cell activity and phagocytosis was determined as described in the text. The concentrations of IFN-α, IL-2, and IFN-γ in cell culture supernatants and the proliferation of PBMC (2 × 105/well) were quantified as described in the legend to Fig. 4. The data presented show the mean SI relative to the control (or the mean concentrations if no effect was detectable within the medium control) and the means ± standard deviations for PBMC from 10 tested animals. Different inactivated poxviruses are indicated as black bars; controls are indicated as white bars.
DISCUSSION
As shown in several in vivo studies, the prophylactic application of iPPOV can strengthen host immune defense, resulting in reduced susceptibility to invading pathogens (6, 16, 33, 39). In agreement with earlier in vitro studies (5, 18), we showed that this immunostimulating capacity is not iPPOV specific but is common to poxviruses of different genera. Considering that poxviruses are widely used as vectors in vaccine development, it seems surprising that only a few studies exist on this immunostimulating property. One reason might be the synthesis of viral immunomodulating proteins, such as soluble receptors for IFN-α/β, IFN-γ, and TNF-α, at early times during poxvirus (vector) infections (25). These secreted proteins may in turn impair the immunological response against the poxvirus vectors. This hypothesis is supported by the reported finding that inactivated but not infectious vaccinia viruses may induce IFN-α production in bovine or porcine PBMC (6). In contrast, the addition of MVA, which lacks the respective immunomodulatory genes and is not permissive for porcine and bovine PBMC, induces the secretion of high levels of IFN-α (6). Thus, the detection of immunostimulating properties might be expected only if poxviruses are inactivated or their genome is deficient in the respective immunomodulating genes.
On the basis of investigations of the basic mechanism for the immunostimulating capacity of poxviruses, it was suggested that poxvirus-mediated activation of innate immune reactions was responsible for the observed immunostimulating capacity (18, 19). This assumption was supported by earlier studies revealing increased phagocytosis and oxidative burst in rats and humans and increased NK cell activity in mice under the influence of iFPV, iMVA, and iPPOV (10, 19). When we analyzed the reaction of porcine PBMC to inactivated poxviruses from different genera, no such early detectable reactions could be found. We believe that the different results seen within the innate immune system may be due to the different species involved or to different experimental approaches. Unlike investigators in former studies (10), we did not isolate phagocytes for the phagocytosis assays, so that any preactivation of these cells during the course of the isolation process (as described in the literature) could be ruled out (8, 9). The poxvirus-induced secretion of inflammatory cytokines, such as TNF-α and granulocyte-macrophage colony-stimulating factor, which can function as costimulatory signals for preactivated (isolated) phagocytes, was minimized by reduction of the experimental incubation time from up to 4 h (10) to 30 min. Similarly, incubation times in the NK cell assays were kept as short as possible to avoid indirect stimulation of NK cells, e.g., by cytokine secretion. Taken together, our experiments gave no evidence for direct poxvirus-induced stimulation of innate immune reactions in swine.
While former studies have focused only on early detectable poxvirus-induced effects (until day 2) (5, 10, 18), the experiments presented here included investigations of late observable effects (until day 9). This difference might explain the finding that mainly T-helper cells responded to stimulation by inactivated poxviruses. The question arises as to how specific T cells might be stimulated by poxviruses in an in vitro culture system. A secondary (memory) response seems unlikely, because our studies were performed exclusively with PBMC from non-poxvirus-treated (unprimed) animals. However, it may be considered that memory T-helper cells previously primed to unrelated but poxvirus-cross-reactive antigens responded to activation with the poxviruses. A primary poxvirus-specific immune response also appears to be unusual. Nevertheless, it might be possible that the extensive immunogenic properties of poxviruses could have increased the frequency of reacting T cells, resulting in a measurable primary response, even in an in vitro system. In any case, further experiments are needed to answer this question.
In this study, T-helper cells were identified not only as the main activated and proliferating cell fraction but also as the major source of the increased levels of IL-2, IFN-α, and IFN-γ All three secreted cytokines have important antiviral and immunomodulatory functions (3, 31). In consequence, their usage as therapeutic agents in cancer research and in the treatment of viral infectious diseases or autoimmune diseases is becoming more attractive (7, 14, 31). It is thus understandable that increased poxvirus-induced secretion of these cytokines also might prove therapeutically beneficial. It might even be considered a basic mechanism for the finding that animals pretreated with poxviruses had reduced susceptibility or milder symptoms and more rapid recovery from infectious diseases than the respective, untreated control animals (33, 39). Poxvirus-preactivated T-helper cells might promote the enhanced secretion of cytokines during the development of a specific host defense against an unrelated antigen. Additionally, the possibility cannot be ruled out that other important immunomodulatory cytokines might be secreted by lymphocytes during stimulation with inactivated poxviruses. Genes encoding soluble, viral receptors for TNF-α, IL-1β, or different chemokines (25) indicate the importance of these cytokines, at least within poxvirus infections.
The late onset of poxvirus-induced cytokine release follows the pattern of typical T-helper-cell activation and differentiation. With regard to IFN-γ secretion, it provides further evidence for a cell-mediated immune reaction, at least in part, in response to stimulation with inactivated poxviruses. At the same time, it explains why only a prophylactic or metaphylactic application of inactivated poxviruses provides efficiently reduced susceptibility to infectious diseases, whereas a therapeutic application has almost no effect (16). It is likely that a minimum of 5 days is required to create an effective barrier against invading pathogens by poxvirus-mediated T-helper-cell activation and cytokine secretion. Consequently, it is understandable that a therapeutic application of inactivated poxviruses would take place later than the development of a specific immune response against an unrelated antigen.
In summary, the results presented here indicate that iPPOV primarily stimulates cells of the specific immune system. T-helper cells were identified as the main activated and cytokine-secreting cell fraction. Moreover, other poxviruses seem capable of stimulating T-helper cells by the same mechanism, providing evidence that all tested poxviruses possess similar stimulating capacities. Further studies are required to determine whether this stimulating property seen in vitro relates to the increased protection against infectious diseases observed in vivo.
ACKNOWLEDGMENTS
We thank A. Mayr and M. Büttner for generously supplying inactivated poxviruses and K. McCullough and B. Ober for critical review of the manuscript.
This work was supported by R&D/BIO Research, Bayer AG Animal Health, Leverkusen, Germany.
REFERENCES
- 1.Alcami A, Smith G L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 2.Antoine G, Scheiflinger F, Dorner F, Falkner F G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 3.Bach E A, Aguet M, Schreiber R D. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 4.Bailey M, Stevens K, Bland P W, Stokes C R. A monoclonal antibody recognising an epitope associated with pig interleukin-2 receptors. J Immunol Methods. 1992;153:85–91. doi: 10.1016/0022-1759(92)90309-h. [DOI] [PubMed] [Google Scholar]
- 5.Büttner M, Czerny C P, Lehner K H, Wertz K. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet Immunol Immunopathol. 1995;46:237–250. doi: 10.1016/0165-2427(94)05357-x. [DOI] [PubMed] [Google Scholar]
- 6.Castrucci G, Frigeri F, Osburn B I, Ferrari M, Barreca F, Salvatori D. Further investigations on the efficacy of a non-specific defence inducer evaluated in calves exposed to infectious bovine rhinotracheitis virus. Comp Immunol Microbiol Infect Dis. 1998;21:155–163. doi: 10.1016/s0147-9571(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 7.Clark J I, Gaynor E R, Martone B, Budds S C, Manjunath R, Flanigan R C, Waters W B, Sosman J A. Daily subcutaneous ultra-low-dose interleukin 2 with daily low-dose interferon-alpha in patients with advanced renal cell carcinoma. Clin Cancer Res. 1999;5:2374–2380. [PubMed] [Google Scholar]
- 8.Elbim C, Bailly S, Chollet-Martin S, Hakim J, Gougerot-Pocidalo M A. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect Immun. 1994;62:2195–2201. doi: 10.1128/iai.62.6.2195-2201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbim C, Chollet-Martin S, Bailly S, Hakim J, Gougerot-Pocidalo M A. Priming of polymorphonuclear neutrophils by tumor necrosis factor alpha in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993;82:633–640. [PubMed] [Google Scholar]
- 10.Förster R, Wolf G, Mayr A. Highly attenuated poxviruses induce functional priming of neutrophils in vitro. Arch Virol. 1994;136:219–226. doi: 10.1007/BF01538831. [DOI] [PubMed] [Google Scholar]
- 11.Graham K A, Lalani A S, Macen J L, Ness T L, Barry M, Liu L Y, Lucas A, Clark-Lewis I, Moyer R W, McFadden G. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229:12–24. doi: 10.1006/viro.1996.8423. [DOI] [PubMed] [Google Scholar]
- 12.Hammerberg C, Schurig G G. Characterization of monoclonal antibodies directed against swine leukocytes. Vet Immunol Immunopathol. 1986;11:107–121. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- 13.Hammerl J, Wolf G, Berner H. Klinische Untersuchungen zur Wirkung des Paramunitätsinducers Baypamun als Prophylaxe beim MMA-Komplex der Sau. Tieraerztl Umsch. 1995;50:383–386. [Google Scholar]
- 14.Harris D T, Matyas G R, Gomella L G, Talor E, Winship M D, Spitler L E, Mastrangelo M J. Immunologic approaches to the treatment of prostate cancer. Semin Oncol. 1999;26:439–447. [PubMed] [Google Scholar]
- 15.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 16.Kyriakis S C, Tzika E D, Lyras D N, Tsinas A C, Saoulidis K, Sarris K. Effect of an inactivated Parapoxvirus based immunomodulator (Baypamun) on post weaning diarrhoea syndrome and wasting pig syndrome of piglets. Res Vet Sci. 1998;64:187–190. doi: 10.1016/s0034-5288(98)90122-9. [DOI] [PubMed] [Google Scholar]
- 17.Marsig E, Stickl H. The effectiveness of immunomodulators from microorganisms and of animal pox preparations against tumor cell lines in vitro. Zentbl Veterinaermed. 1988;35:601–609. [PubMed] [Google Scholar]
- 18.Mayr A, Büttner M, Pawlas S, Erfle V, Mayr B, Brunner R, Osterkorn K. Comparative studies of the immunostimulating (paramunizing) effectiveness of BCG, levamisole, Corynebacterium parvum and preparations of pox viruses in various in vivo and in vitro tests. Zentbl Veterinaermed. 1986;33:321–339. [PubMed] [Google Scholar]
- 19.Mayr A, Büttner M, Wolf G, Meyer H, Czerny C. Experimental detection of the paraspecific effects of purified and inactivated poxviruses. Zentbl Veterinaermed. 1989;36:81–99. [PubMed] [Google Scholar]
- 20.Mayr A, Herlyn M, Mahnel H, Danco A, Zach A, Bostedt H. Bekämpfung des Ecthyma contagiosum (Pustulardermatitis) der Schafe mit einem neuen Parenteral-Zellkultur-Lebendimpfstoff. Zentbl Veterinaermed. 1981;28:535–552. [PubMed] [Google Scholar]
- 21.Mayr A, Himmer B, Baljer G, Sailer J. Erregerunspezifische Prophylaxe und Therapie von Pseudomonas-aeruginosa-Wundinfektion mittels Paramunisierung im Mäusemodell. Zentbl Bakteriol Hyg Parasitenkd Infektionskr Abt 1 Orig Reihe A. 1978;176:506–514. [PubMed] [Google Scholar]
- 22.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 23.Moss B. Poxviridae and their replication. In: Fields B N, Knipe D M, Howley P, editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2079–2111. [Google Scholar]
- 24.Murray H W, Cohn Z A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980;152:1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nash P, Barrett J, Cao J X, Hota-Mitchell S, Lalani A S, Everett H, Xu X M, Robichaud J, Hnatiuk S, Ainslie C, Seet B T, McFadden G. Immunomodulation by viruses: the myxoma virus story. Immunol Rev. 1999;168:103–120. doi: 10.1111/j.1600-065x.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 26.Pescovitz M D, Lunney J K, Sachs D H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985;134:37–44. [PubMed] [Google Scholar]
- 27.Rubinstein S, Familletti P C, Pestka S. Convenient assay for interferons. J Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saalmüller A, Hirt W, Maurer S, Weiland E. Discrimination between two subsets of porcine CD8+ cytolytic T lymphocytes by the expression of CD5 antigen. Immunology. 1994;81:578–583. [PMC free article] [PubMed] [Google Scholar]
- 29.Saalmüller A, Hirt W, Reddehase M J. Phenotypic discrimination between thymic and extrathymic CD4−CD8− and CD4+CD8+ porcine T lymphocytes. Eur J Immunol. 1989;19:2011–2016. doi: 10.1002/eji.1830191107. [DOI] [PubMed] [Google Scholar]
- 30.Saalmüller A, Maurer S. Major histocompatibility antigen class II expressing resting porcine T lymphocytes are potent antigen-presenting cells in mixed leukocyte culture. Immunobiology. 1994;190:23–34. doi: 10.1016/S0171-2985(11)80281-0. [DOI] [PubMed] [Google Scholar]
- 31.Sen G, Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992;267:5017–5020. [PubMed] [Google Scholar]
- 32.Steinmassl M, Wolf G. Formation of interleukin-2 and interferon alpha by mononuclear leukocytes of swine after in vitro stimulation with different virus preparations. Zentbl Veterinaermed. 1990;37:321–331. [PubMed] [Google Scholar]
- 33.Strube W, Kretzdorn D, Grunmach J, Bergle R D, Thein P. The effectiveness of the paramunity inducer Baypamun (PIND-ORF) for the prevention and metaphylaxis of an experimental infection with the infectious bovine rhinotracheitis virus in cattle. Tieraerztl Prax. 1989;17:267–272. [PubMed] [Google Scholar]
- 34.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 35.Tzahar E, Moyer J D, Waterman H, Barbacci E G, Bao J, Levkowitz G, Shelly M, Strano S, Pinkas-Kramarski R, Pierce J H, Andrews G C, Yarden Y. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upton C, Macen J L, Schreiber M, McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- 37.Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 38.Whitton J L, Oldstone M B. Immune response to viruses. In: Fields B N, Knipe D, Howley P, editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 345–374. [Google Scholar]
- 39.Ziebell K L, Steinmann H, Kretzdorn D, Schlapp T, Failing K, Schmeer N. The use of Baypamun N in crowding associated infectious respiratory disease: efficacy of Baypamun N (freeze dried product) in 4–10 month old horses. Zentbl Veterinaermed. 1997;44:529–536. doi: 10.1111/j.1439-0450.1997.tb01004.x. [DOI] [PubMed] [Google Scholar]