Abstract
Background and Aims:
The multisociety consensus nomenclature has renamed NAFLD to steatotic liver disease (SLD) with various subclassifications. There is a paucity of data regarding how the new nomenclature modifies our understanding of disease prevalence and patient phenotypes.
Approach and Results:
Using the National Health and Nutrition Examination Survey from January 2017 to March 2020, we included all participants aged 18 years or above with complete vibration-controlled transient elastography measures. SLD and its subclassifications [metabolic dysfunction-associated SLD (MASLD), MASLD + increased alcohol intake (MetALD), alcohol-associated liver disease (ALD), etiology-specific/cryptogenic] were defined according to consensus nomenclature. National SLD prevalence and subclassifications were estimated, and among key subgroups [age, sex, race/ethnicity, advanced liver fibrosis (liver stiffness measurement [LSM] ≥ 11.7 kPa)]. Among 7367 participants, 2549 had SLD (mean age 51 y, 57.7% male, 63.2% non-Hispanic White). The estimated prevalence of SLD was 34.2% (95% CI 31.9%–36.5%): MASLD 31.3% (29.2%–33.4%), MetALD 2% (1.6%–2.9%), ALD 0.7% (0.5–0.9%), etiology-specific/cryptogenic 0.03% (0.01%–0.08%). In exploratory analyses, participants classified as non-SLD with (vs. without) advanced fibrosis had a higher mean number of metabolic risk factors [2.7 (2.3–3.1) vs. 2.0 (1.9–2.0)] and a higher proportion with average alcohol use ≥ 20 g/d (women)/ ≥ 30 g/d (men) [20.9% (6.2%–51.3%) vs. 7.2% (6.1%–8.4%)]. In another exploratory analysis, increasing quantities of alcohol use remaining below the threshold for MASLD + increased alcohol intake were associated with advanced liver fibrosis in men, but not women. There was 99% overlap in cases of NAFLD and MASLD.
Conclusions:
Our findings highlight the utility of the new consensus nomenclature to address deficiencies present with the old nomenclature, and identify areas that require research to further refine classifications of SLD.
INTRODUCTION
Steatotic liver disease (SLD) reflects a new nomenclature to replace “NAFLD”, intended to represent less stigmatizing language and classification based on the presence of clinical findings, rather than a diagnosis of exclusion.[1] SLD is an overarching term that includes metabolic dysfunction-associated SLD (MASLD) meant to specifically replace NAFLD. SLD also includes other subclassifications, namely MASLD and increased alcohol intake (MetALD), alcohol-associated liver disease (ALD), and etiology-specific/cryptogenic SLD.
These new classification criteria have been endorsed by leading liver societies, including the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Latin American Association for the Study of the Liver (ALEH), and are anticipated to be broadly applied—more than 1 billion people globally across clinical care, research, and clinical trials could be potentially affected.[1,2] An understanding of how this change in disease classification affects disease burden estimates and patient phenotypes is imperative to inform clinicians, researchers, and those involved in drug development for SLD.
While a recent study[2] provided prevalence estimates for SLD, that study used criteria from an interim proposal, which differs from new consensus criteria. First, among several differences, the interim proposal (vs. new consensus criteria) required a higher quantity of metabolic risk factors, had a higher threshold for waist circumference, and also classified patients based on hypersensitive C-reaction protein levels.[1,2] Second, that study’s data availability was limited to 2017–2018 only—additional survey years to add to sample size may provide more generalizable and precise prevalence estimates. Moreover, the interim proposal did not yet define MetALD[1,2]; given the rising coexistence of metabolic risk factors and alcohol use in the US population and across the world, it is a key sub-classification of interest.[1,3] Further characterization and prevalence estimates for MetALD are critical to inform clinical trials targeted toward this subpopulation and to guide public health interventions.
Additional key knowledge gaps remain. We hypothesized that the new nomenclature could affect inclusion for clinical trials in other specific ways. First, the new nomenclature requires the presence of steatosis, but steatosis can become absent with advanced disease and has not been an absolute criterion for the diagnosis[4] of NAFLD and for inclusion in recent NAFLD clinical trials[5] (ie, thresholds by NAFLD activity score can be met with no steatosis), which had typically relied on histopathology. Second, the threshold of average daily alcohol use ≥ 20/30 g (women/men) for MetALD is higher than the National Institute on Alcohol Abuse and Alcoholism (NIAAA) threshold for heavy drinking[6] and may exclude patients among whom alcohol use is an important contributor to liver disease, yet below the threshold of 20/30 g/d.
Thus, to inform these knowledge gaps, we sought to (1) assess the national landscape of SLD and its subclassifications using new consensus nomenclature and (2) to provide exploratory analyses regarding the potential contribution of metabolic risk factors and alcohol use to liver disease in patients who do not meet these specific criteria.
METHODS
Data source and study population
The National Health and Nutrition Examination Survey (NHANES) is a continuous, multistage, nationally representative survey of the noninstitutionalized US civilian population, collected in 2-year stages. We used data from January 2017 to March 2020, which are currently the only available years with vibration-controlled transient elastography measures for quantifying liver steatosis and fibrosis. Data collection for the 2019–2020 cycle was suspended in March 2020 due to the COVID-19 pandemic; this incomplete survey cycle was combined with the 2017–2018 cycle to generate a nationally representative prepandemic file.[7] The overall response rate during the study period for the interview component was 51.0% and 46.9% for the examination component.[7] We included participants 18 years of age or above and with available controlled attenuation parameter (CAP) and liver stiffness measures (LSMs) by vibration-controlled transient elastography. We excluded participants with unknown alcohol use in the past 12 months.
SLD was defined according to new consensus nomenclature, with differences from a prior study using the interim proposal summarized in Supplemental Table S1, http://links.lww.com/HEP/I12.[1,2] The subclassifications were defined as mutually exclusive subgroups as follows:
MASLD = steatosis (CAP of ≥ 288 dB) + ≥ 1 metabolic risk factor. In a sensitivity analysis, we repeated analyses defining steatosis with a lower CAP threshold ( ≥ 248 dB). CAP thresholds were consistent with the other study.[2]
MetALD = MASLD + average daily alcohol intake 20–50 g (women)/ 30–60 g (men) in the past 12 months. We further stratified this group into 2 average daily quantities [(20/30 g)-39 g and, 40 g-(50 g/60 g] g (women/men)] based on cutoffs provided in the new consensus nomenclature.[1]
ALD =steatosis + average daily alcohol intake > 50 g (women)/> 60 g (men) + ≥ 1 metabolic risk factor or steatosis + average daily alcohol intake ≥ 20 g (women)/ ≥ 30 g (men) + no metabolic risk factors.
Specific etiology or cryptogenic SLD = steatosis + no metabolic risk factors + average daily alcohol intake <20 g (women)/ < 30 g (men). Specific etiologies include DILI, monogenic diseases (eg, Wilson disease, inborn errors of metabolism), and miscellaneous causes (eg, HCV and celiac disease), while cryptogenic reflects no identifiable cause.
Metabolic risk factors were defined according to new consensus nomenclature as follows: systolic/diastolic blood pressure of ≥ 130/85 mm Hg or taking hypertension medications; body mass index ≥ 25 (≥ 23 if Asian) or waist circumference > 94 cm in men or >80 cm in women; fasting plasma glucose level of ≥ 100 mg/dL or hemoglobin A1c ≥ 5.7% or diabetes mellitus medications; serum level of triglycerides of ≥ 150 mg/dL or lipid-lowering agent; HDL cholesterol level of <40 mg/dL in men or <50 mg/dL in women or lipid-lowering agent.
Age, sex, race, medical conditions, and alcohol use were collected during in-home interviews. Race was included because of known race differences in risk of SLDs and liver fibrosis.[8,9] aspartate aminotransferase, alanine transaminase, and metabolic risk factors were collected during examinations with standard protocols.
Given that steatosis may be absent in the setting of disease progression, we performed a sensitivity analysis assuming advanced liver fibrosis (LSM ≥ 11.7 kPa) as a surrogate for steatosis.
Key subgroups
The study cohort was stratified into key subgroups: age (< 45 vs. ≥ 45), sex (men vs. women), race (self-reported non-Hispanic White, non-Hispanic Black, Asian, Hispanic, and Other), and presence of advanced fibrosis (LSM ≥ 11.7 kPa vs. LSM <11.7 kPa). The age cut point of 45 was based on prior literature showing the rise in ALD-related mortality driven by young adults.[8] The advanced fibrosis LSM cutoff was the same used by the other study[2] assessing the interim proposal to define advanced liver fibrosis, and separately validated[10] among patients with NAFLD to have an area under the receiver operating characteristic of 0.88.
Assessing the overlap between MASLD and NAFLD
To evaluate the overlap between MASLD and NAFLD, we additionally identified those with NAFLD as any patient with steatosis (CAP ≥ 288 dB) with <20/30 g (women/men) of average daily alcohol use and without viral hepatitis. NAFLD has no requirement for metabolic risk factor. We categorized the NAFLD/MASLD population as those meeting NAFLD criteria only, those meeting both NAFLD and MASLD criteria, and those meeting MASLD criteria only. The weighted percentages were calculated to reflect the collective proportion of NAFLD/MASLD subpopulation within each category.
Exploratory analyses to assess the potential effect of metabolic risk factors and alcohol use among participants not meeting specified criteria
We performed 2 exploratory analyses:
Among participants without SLD, we quantified metabolic risk factors and alcohol use among participants with versus without advanced liver fibrosis (LSM ≥ 11.7 vs. <11.7 kPa). Then, among participants with advanced liver fibrosis, we repeated these analyses among participants with versus without SLD.
Among patients with MASLD with alcohol use in the past 12 months [all participants with MASLD have <20/30 g (women/men) average daily alcohol use], we assessed: (1) the quantity of alcohol use among alcohol users in this subgroup, stratified by sex; and (2) the association of alcohol use with advanced fibrosis as the outcome, stratified by sex. In this exploratory analysis only, we specifically chose to exclude participants with no alcohol use in the past 12 months, as those with strict abstinence often appear to have worse outcomes in unadjusted analyses in other epidemiologic studies, likely due to confounding.[11]
Statistical analysis
All analyses used survey procedures to account for the complex, cluster-stratified design of NHANES. Exam sampling weights provided by NHANES for the January 2017 to March 2020 prepandemic period were used to generate nationally representative estimates. Variance was estimated by Taylor series linearization to derive accurate SEs and CI.
The weighted means and percentages for demographic factors were estimated. Prevalence of SLD and its subclassifications was estimated as weighted percentages overall and within key subgroups (age, sex, race, and advanced fibrosis), though ALD and specific etiology/cryptogenic SLD prevalence were not reported by subgroup due to small sample sizes. In exploratory analyses, differences by group were assessed using the t test and Rao-Scott chi-squared test. For the second exploratory analysis among participants with MASLD and consuming alcohol, the relationship between advanced fibrosis and alcohol use was (1) visualized using locally weighted regression (Stata lowess) and (2) quantified with OR estimated from unadjusted logistic regression; both analyses were stratified by sex.
Two-sided p-values with a significance level of 0.05 were used for all tests. Analyses were conducted using SAS version 9.4 and StataMP version 17.4.
RESULTS
Among 7367 participants, 2549 met the new definition for SLD (mean age 51 years, 57.7% male, 63.2% non-Hispanic White; Table 1). The estimated prevalence of SLD was 34.2% (95% CI 31.9%–36.5%): MASLD 31.3% (95% CI 29.2%–33.4%), MetALD 2.2% (95%CI 1.6–2.9%), ALD 0.7% (95%CI 0.5–0.9%) and etiology-specific or cryptogenic SLD 0.03% (95% CI 0.01%–0.08%) (Figure 1A). Prevalence estimates were similar in a sensitivity analysis assuming advanced liver fibrosis as a surrogate for steatosis: SLD 35.0% (95% CI 32.6–37.5%), MASLD 31.9% (95% CI 29.8%–34.1%), MetALD 2.3% (95% CI 1.8–3.1), ALD 0.7% (95% CI 0.05–0.10), and etiology-specific or cryptogenic SLD 0.07% (95% CI 0.02–0.19). The prevalence of ALD with 0 metabolic risk factors was 0.08% (95% CI 0.04–0.22). Prevalence estimates increased in a sensitivity analysis in which the CAP threshold for steatosis was reduced to ≥ 248 dB (Figure 1B). The estimated prevalence of SLD and subclassifications among age, sex, race, and advanced fibrosis subgroups are summarized in Figure 2A–D.
TABLE 1.
Characteristics of 7367 US adults by SLD in NHANES from 2017 to March 2020
| SLD |
MASLD |
MetALD |
Non-SLD |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | Prevalence (95% CI) | No. | Prevalence (95% CI) | No. | Prevalence (95% CI) | No. | Prevalence (95% CI) |
| Age, mean (95% CI) | 2549 | 50.8 (49.3–52.3) | 2365 | 51.1 (49.6–52.7) | 120 | 48.2 (43.4–52.9) | 4818 | 45.3 (44.1–46.5) |
| Male sex | 1446 | 57.7 (54.4–61.0) | 1305 | 56.3 (52.7–59.9) | 85 | 66.4 (47.8–81.1) | 2253 | 45.7 (43.7–47.6) |
| Race | ||||||||
| Non-Hispanic White | 951 | 63.2 (57.1–68.9) | 868 | 62.6 (56.3–68.5) | 60 | 73.9 (60.4–84.0) | 1616 | 63.2 (57.9–68.2) |
| Non-Hispanic Black | 511 | 8.0 (6.0–10.5) | 471 | 8.0 (6.0–10.7) | 26 | 7.2 (4.3–12.0) | 1417 | 12.5 (9.6–16.1) |
| Asian | 259 | 4.8 (3.4–6.9) | 254 | 5.2 (3.7–7.3) | 4 | 1.1 (0.3–3.7) | 579 | 5.7 (4.0–8.0) |
| Hispanic | 704 | 19.7 (15.6–24.5) | 657 | 20.0 (15.8–25.0) | 24 | 10.1 (6.9–14.7) | 956 | 14.6 (12.0–17.7) |
| Other | 124 | 4.3 (3.1–6.0) | 115 | 4.0 (3.0–5.4) | 6 | 7.6 (2.3–22.6) | 250 | 3.9 (3.1–5.0) |
| Advanced fibrosis | 184 | 7.6 (6.0–9.5) | 174 | 7.6 (6.0–9.5) | 5 | 5.9 (1.8–17.4) | 89 | 1.3 (0.8–2.2) |
Note: ALD and etiology-specific or cryptogenic SLD are not presented due to small sample sizes.
Abbreviations: ALD, alcohol-associated liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease and increased alcohol intake; NHANES, National Health and Nutrition Examination Survey; SLD, steatotic liver disease.
FIGURE 1.
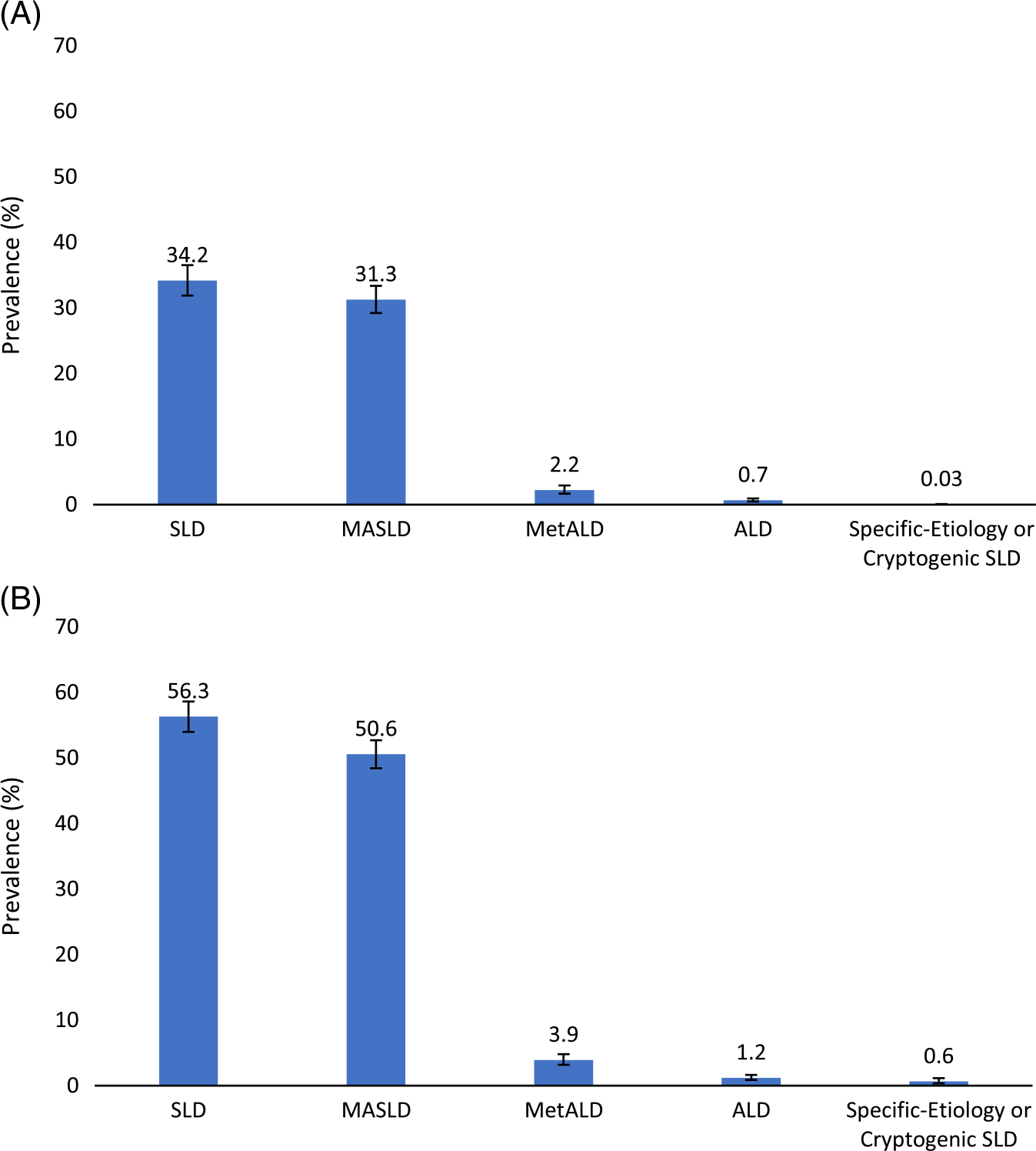
Estimated National Prevalence of Steatotic Liver Disease and subclassifications. Estimated national prevalence of SLD, MASLD, MetALD, ALD, and specific etiology or cryptogenic SLD with 95% CIs using CAP of ≥ 288 dB (A) or ≥ 248 dB (B) to define presence of liver steatosis. Abbreviations: ALD, alcohol-associated liver disease; CAP, controlled attenuation parameter; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease and increased alcohol intake; SLD, steatotic liver disease.
FIGURE 2.
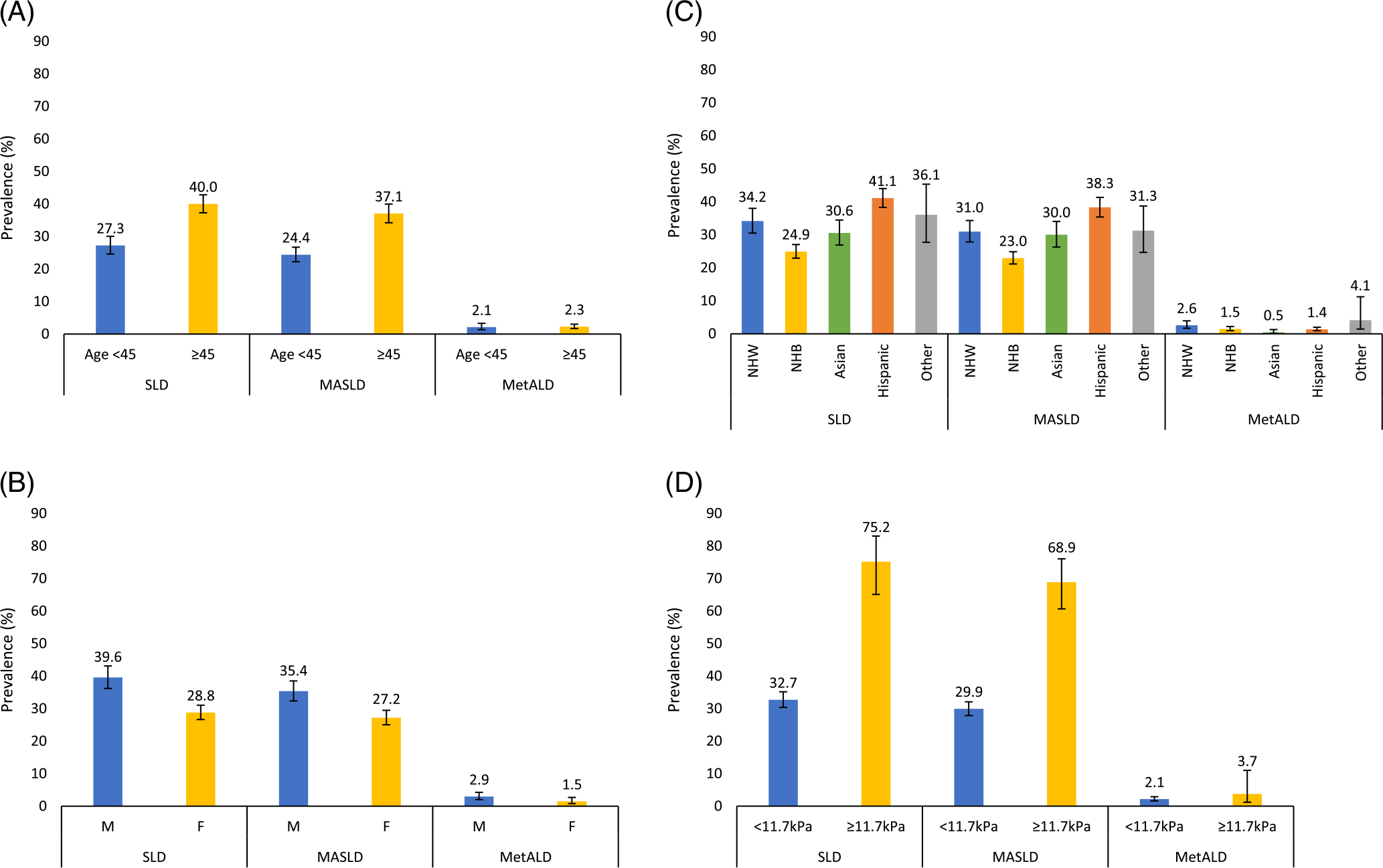
Estimated National Prevalence of Steatotic Liver Disease and subclassifications by key subgroups. Estimated national prevalence of SLD, MASLD, and MetALD with 95% CIs, among adults with age below 45 vs. 45 or above (A), men versus women (B), by race/ethnicity (non-Hispanic White, non-Hispanic Black, Asian, Hispanic, and Other) (C), without versus with advanced liver fibrosis as determined by vibration-controlled transient elastography (LSM <11.7 vs. ≥ 11.7 kPa) (D). ALD and specific etiology or cryptogenic SLD are not presented by key subgroups due to small sample sizes. Abbreviations: LSM, liver stiffness measurement; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction-associated steatotic liver disease and increased alcohol intake; SLD, steatotic liver disease.
Among 120 participants with MetALD, the proportion with average daily alcohol use of [20 g/30 g]–39 g and 40g–[50 g/60 g] [women/men] was 48.9% and 51.1%, respectively.
Overlap between NAFLD and MASLD
Among those with NAFLD or MASLD, 99.0% (95% CI 97.6%–99.6%) met the criteria for both NAFLD and MASLD. The MASLD criteria were not met 0.1% (95% CI 0.05%–0.28%) reflecting a small subgroup with NAFLD but lacking metabolic risk factors. Another 0.9% (95% CI 0.3%–2.3%) only met the MASLD criteria reflecting a small subgroup with viral hepatitis not captured by NAFLD. These results are summarized in Figure 3.
FIGURE 3.
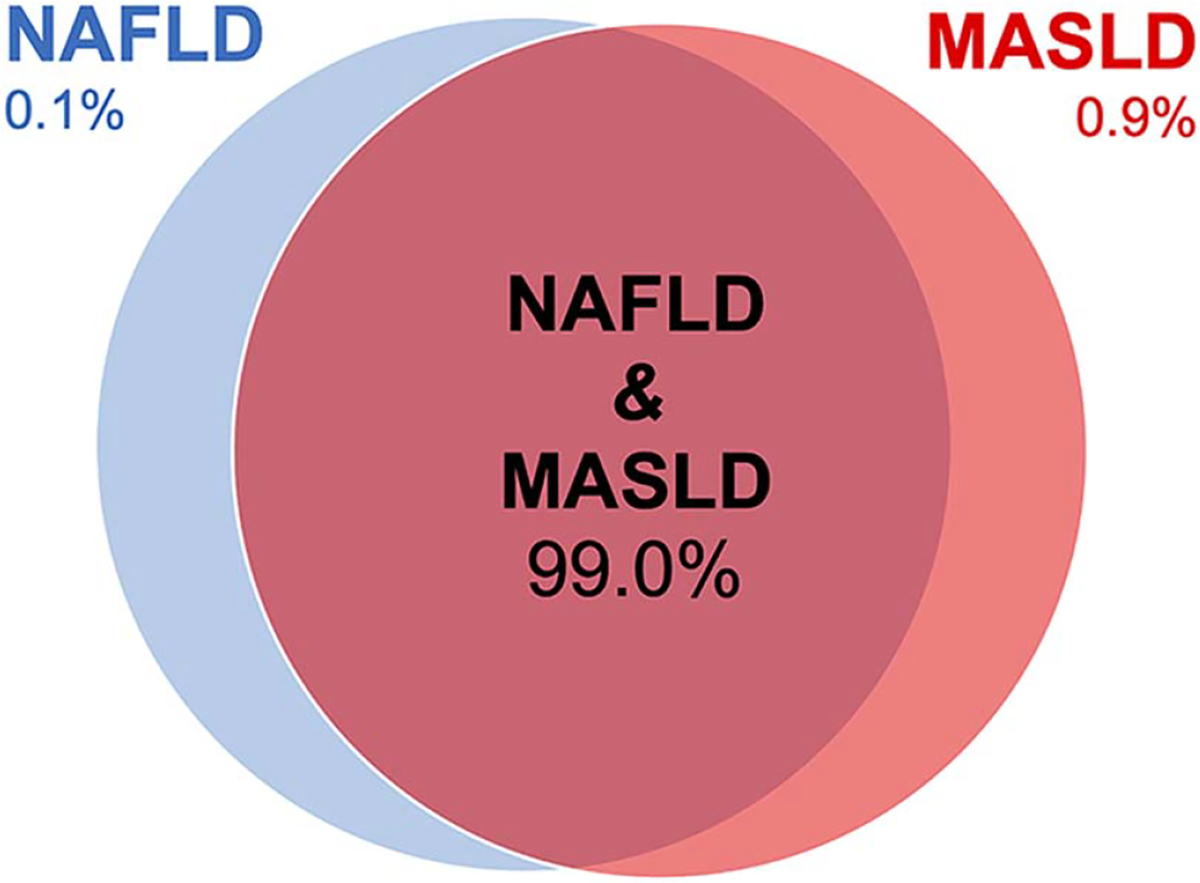
Overlap between NAFLD and MASLD. Among those with NAFLD or MASLD, 99.0% (95% CI 97.6%–99.6%) met the criteria for both NAFLD and MASLD. The MASLD criteria were not met 0.1% (95% CI 0.05%–0.28%) reflecting a small subgroup with NAFLD but lacking metabolic risk factors. Another 0.9% (95% CI 0.3%–2.3%) only met the MASLD criteria reflecting a small subgroup with viral hepatitis not captured by NAFLD. Abbreviation: MASLD, metabolic dysfunction-associated steatotic liver disease.
Exploratory analysis #1
A total of 4818 participants were classified as non-SLD, of which 89 had advanced fibrosis. Participants with (vs. without) advanced fibrosis had a higher mean count of metabolic risk factors [2.7 (95% CI 2.3–3.1) vs. 2.0 (95% CI 1.9–2.0) metabolic risk factors, p=0.001] and higher proportion with average daily alcohol intake ≥20 g (women)/≥30 g (men) [20.9% (95% CI 6.2%–51.3%) vs. 7.2% (6.1%–8.4%), p=0.07], although the CIs overlap for the latter.
A total of 273 participants had advanced liver fibrosis according to LSM, of which 184 were with SLD. Participants with (vs. without) SLD had a higher mean count of metabolic risk factors [3.3 (95% CI 3.1–3.6) vs. 2.7 (95% CI 2.3–3.1) metabolic risk factors, p=0.005] but lower proportion with average daily alcohol intake ≥20 g (women)/≥30 g (men) [8.4% (95% CI 3.7%–17.6%) vs. 20.9% (95% CI 6.2%–51.3%), p=0.17], although the CIs overlap for the latter.
Exploratory analysis #2
Among 2365 participants with MASLD, 1580 (920 men, 660 women) reported some alcohol use in the past 12 months. For MASLD participants reporting alcohol use in the past 12 months, the mean average alcohol use among men was 6.1 g/d (95% CI 5.2–7.0 g/d) and among women was 2.7 g/d (95% CI 2.2–3.2 g/d). The association of advanced fibrosis with alcohol use (per daily increase of 1 g) was OR 0.93 (95%CI 0.78–1.11) among women, but nonlinear among men. Based on the lowess curve, average daily alcohol intake was categorized as <5 g/d, 5–10 g/d, and > 10 g/d in men with OR of 4.57 (95% CI 1.24–16.84) for intake > 10 g/d and OR of 3.81 (95% CI 1.26–11.57) for intake <5 g/d, compared to 5–10 g/d.
DISCUSSION
In this national study, we estimate the prevalence of SLD to be 34.2% (ie, > 90 million US adults), with MASLD accounting for the majority (31.3%), followed by MetALD (2.2%), ALD (0.7%) and etiology-specific/cryptogenic SLD <0.1% of the population, using new consensus nomenclature. The overall SLD estimate is slightly higher than reported[2] using the interim proposal, but the combined MetALD and ALD estimates are lower than the interim definition for “alcohol-associated SLD.” Indeed, the new consensus nomenclature is less restrictive in identifying SLD with a single metabolic risk factor, and we calculated average daily and weekly alcohol use over the past 12 months rather than a single-week report, resulting in a more restrictive methodology to classify MetALD and ALD. Importantly, we found that SLD is present in over 75% of participants with advanced liver fibrosis. This high proportion reflects the encompassing nature of the new nomenclature, which allows for the coexistence of other forms of liver disease with MASLD, including alcohol and viral hepatitis. These estimates provide more clarity to clinicians, researchers, and industry members regarding the potential effects of the new consensus nomenclature to patients with liver disease from metabolic risk factors, alcohol use, and other factors.
Our study highlights several benefits to the new consensus nomenclature. First, we show that ALD with 0 metabolic risk factors, even accounting for potential underestimation, represents a minimal proportion of US adults—these findings highlight that alcohol use resulting in liver disease is almost always present with coexisting metabolic risk factors, and that the separation of NAFLD and ALD as previously done did not reflect real-world practice. The new nomenclature addresses this issue and provides guidance to classify these patients with concomitant daily alcohol use of 20–50 g/30–60 g (women/men) and any metabolic risk factor as MetALD, and those with > 50/60 g (women/men) as ALD, which can help improve clinical care pathways and streamline research in this subpopulation.
Second, we show that more than 1 in 3 US adults has SLD—the nomenclature allows for consistent criteria to identify SLD without histopathology, which has clear benefits since liver biopsy is not practical for widespread use with such a high prevalence disease.
Our exploratory analyses highlight special considerations that clinicians and researchers should recognize when applying the new consensus nomenclature. First, we found that many with advanced fibrosis classified as non-SLD had metabolic risk factors almost in comparable quantities to those with advanced fibrosis classified as SLD, but with potentially higher alcohol use, although not statistically significant. In parallel, among participants with non-SLD, those with advanced fibrosis (vs. without advanced fibrosis) had a higher number of metabolic risk factors and potentially higher alcohol use. Taken together, these analyses suggest that participants with advanced fibrosis classified as non-SLD, may in fact have had metabolic risk factors and alcohol use in the past as potential contributors to advanced fibrosis. We suggest the study of patients with advanced liver fibrosis without steatosis, but with a history of metabolic risk factors and/or alcohol use, as an area for future targeted research. Second, we found that the new SLD definition may be allowing those with potentially clinically significant alcohol consumption to be categorized as MASLD, thus under-representing ALD and MetALD. Specifically, the average daily threshold of ≥20–50 g/30–60 g (women/men) of alcohol consumption for “moderate” drinking may be too high: (i) This threshold is similar/higher than the NIAAA definition[6] for “heavy drinking” ≥ 14/21 g/d (women/men); (2) among patients with MASLD, intended to represent patients with SLD without significant contribution from alcohol use, increasing levels of alcohol use beyond 5–10 g/d was associated with increased odds of advanced liver disease among men, but not women, as measured by vibration-controlled transient elastography. We caution, however, that causality cannot be confirmed, especially given limitations including cross-sectional design, unadjusted confounders, and lack of histopathology. Indeed, very low alcohol use <5 g/d (vs. 5–10 g/d) was associated with increased odds of advanced liver disease, which may be related to the fact that general ill health is associated with nondrinking in the general population.[12,13] In parallel, prior observational studies[14–16] studying the effect of low to moderate alcohol use in NAFLD have produced conflicting results. Our findings reinforce the need for future prospective studies with accurate and sensitive markers of alcohol use,[17] and among different subpopulations according to etiology and stage of fibrosis, to better define thresholds for “safe” levels of alcohol use among different SLD populations.
This study had limitations. First, the diagnosis of SLD subclassifications and advanced fibrosis was made through self-report of alcohol use in the past year and noninvasive testing, rather than longitudinal high-sensitivity alcohol metabolites[17] and confirmation of liver disease and fibrosis through liver biopsy, respectively, so misclassification is possible. NHANES collects self-reported alcohol consumption in a standardized way, by asking respondents about their quantity and frequency of alcohol consumption in the past 12 months, and explaining how to quantify a drink accurately. It is possible that alcohol consumption is underestimated for reasons such as inaccurate recall or lack of candor. We suspect that this study design thus underestimates the prevalence of liver disease attributable to alcohol (ie, MetALD and ALD subgroups), but does likely reflect how clinical trials in SLD may apply the consensus nomenclature criteria, as current NAFLD trials typically design alcohol-associated exclusion criteria based on recent alcohol use, average per day. Second, metabolic risk factors relied on self-reported diagnosis and/or single-occasion laboratory results, without secondary confirmatory results. NHANES cannot address whether metabolic risk factors are well controlled. Third, the sample sizes for SLD and subgroups including those with advanced fibrosis were relatively limited, so interpretation of results should carefully consider confidence intervals. While not feasible due to sample size limitations in our current study, potential interactions with cofactors of liver disease progression (eg, viral hepatitis, which accounts for <3% of the contemporary NHANES population with liver disease)[2] beyond metabolic risk factors and alcohol should be considered in future larger studies in SLD. Fourth, response rates for NHANES have declined over time for unclear reasons, and nonresponse bias is possible. Finally, these data are based on serial cross-sectional data, and causal inference is thus limited with residual confounding possible.
In conclusion, we provide prevalence estimates regarding the national landscape of SLD and its subclassifications using new consensus nomenclature. Our findings highlight the utility of this new consensus nomenclature to address deficiencies that were present with old nomenclature, and identified areas that would potentially benefit from future research to further refine classifications of SLD.
Supplementary Material
FUNDING INFORMATION
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number K23AA029752 and R01AA030960 (Brian P. Lee), the USC Research Center for Liver Diseases (P30DK048522). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ALD
alcohol-associated liver disease
- ALEH
Latin American Association for the Study of the Liver
- CAP
controlled attenuation parameter
- EASL
European Association for the Study of the Liver
- LSM
liver stiffness measurement
- MASLD
metabolic dysfunction-associated steatotic liver disease
- MetALD
metabolic dysfunction-associated steatotic liver disease and increased alcohol intake
- NHANES
National Health and Nutrition Examination Survey
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- SLD
steatotic liver disease
Footnotes
CONFLICTS OF INTEREST
Brian P. Lee advises Durect, GlaxoSmithKline, and HepaTx. He received grants from Siemens Healthineers. Norah A. Terrault received grants from Durect, Eiger, Genentech-Roche, Gilead, GlaxoSmithKline, Helio Health, Madrigal, and the NIH. She has other interests with Clinical Care Options and Simply Speaking. The remaining author has no conflicts to report.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
REFERENCES
- 1.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023. doi: 10.1097/HEP.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 2.Ng CH, Chan KE, Muthiah M, Tan C, Tay P, Lim WH, et al. Examining the interim proposal for name change to steatotic liver disease in the US population. Hepatology. 2023;77:1712–21. [DOI] [PubMed] [Google Scholar]
- 3.Lee BP, Dodge JL, Mack WJ, Leventhal AM, Terrault NA. National trends in alcohol use, metabolic syndrome, and liver disease from 1999 to 2018. Ann Intern Med. 2023;176:879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, Network NCR. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology. 2011;53:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drinking Levels Defined. Accessed March 6, 2023. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- 7.Akinbami L, Chen T, Davy O, Ogden C, Fink S, Clark J. National Health and Nutrition Examination Survey, 2017–March 2020 prepandemic file: Sample design, estimation, and analytic guidelines. Vital Health Stat. 2022;2:1–25. [PubMed] [Google Scholar]
- 8.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May FP, Rolston VS, Tapper EB, Lakshmanan A, Saab S, Sundaram V. The impact of race and ethnicity on mortality and healthcare utilization in alcoholic hepatitis: A cross-sectional study. BMC Gastroenterol. 2016;16:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016; 150:626–37. [DOI] [PubMed] [Google Scholar]
- 11.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng Fat L, Cable N, Marmot M, Shelton N. Persistent longstanding illness and non-drinking over time, implications for the use of lifetime abstainers as a control group. J Epidemiol Community Health. 2014;68:71–7. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Stockwell T, Naimi T, Churchill S, Clay J, Sherk A. Association between daily alcohol intake and risk of all-cause mortality: A systematic review and meta-analyses. JAMA Netw Open. 2023;6:e236185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sookoian S, Pirola CJ. How safe is moderate alcohol consumption in overweight and obese individuals? Gastroenterology. 2016;150:1698–703. e2. [DOI] [PubMed] [Google Scholar]
- 15.Ajmera V, Belt P, Wilson LA, Gill RM, Loomba R, Kleiner DE, et al. Among patients with nonalcoholic fatty liver disease, modest alcohol use is associated with less improvement in histologic steatosis and steatohepatitis. Clin Gastroenterol Hepatol. 2018;16:1511–20. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.VanWagner LB, Ning H, Allen NB, Ajmera V, Lewis CE, Carr JJ, et al. Alcohol use and cardiovascular disease risk in patients with nonalcoholic fatty liver disease. Gastroenterology. 2017;153:1260–72. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staufer K, Huber-Schönauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer TM, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed nonalcoholic fatty liver disease. J Hepatol. 2022;77:918–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


