Abstract
Background:
Prior studies suggested that air pollution exposure may increase the risk of Parkinson’s Disease (PD). We investigated the long-term impacts of traffic-related and multiple sources of particulate air pollution on PD in central California.
Methods:
Our case-control analysis included 761 PD patients and 910 population controls. We assessed exposure at residential and occupational locations from 1981–2016, estimating annual average carbon monoxide (CO) concentrations - a traffic pollution marker - based on the California Line Source Dispersion Model, version 4. Additionally, particulate matter (PM2.5) concentrations were based on a nationwide geospatial chemical transport model. Exposures were assessed as 10-year averages with a 5-year lag time prior to a PD diagnosis for cases and an interview date for controls, subsequently categorized into tertiles. Logistic regression models were used, adjusting for various factors.
Results:
Traffic-related CO was associated with an increased odds ratio for PD at residences (OR for T3 vs. T1: 1.58; 95% CI: 1.20, 2.10; p-trend = 0.02) and workplaces (OR for T3 vs. T1: 1.91; 95% CI: 1.22, 3.00; p-trend < 0.01). PM2.5 was also positively associated with PD at residences (OR for T3 vs. T1: 1.62; 95% CI: 1.22, 2.15; p-trend < 0.01) and workplaces (OR for T3 vs. T1: 1.85; 95% CI: 1.21, 2.85; p-trend < 0.01). Associations remained robust after additional adjustments for smoking status and pesticide exposure and were consistent across different exposure periods.
Conclusion:
We found that long-term modeled exposure to local traffic-related air pollution (CO) and fine particulates from multiple sources (PM2.5) at homes and workplaces in central California was associated with an increased risk of PD.
Keywords: Parkinson’s disease, Air pollution, Long-term exposure, Case-control study
INTRODUCTION
Air pollution is a major public health concern as it has been consistently linked to adverse health outcomes and shortened life expectancy (Newby et al., 2015; U.S. Census Bureau, 2021). Motor vehicles are now a considerable source of air pollution, accounting for more than 50% of carbon monoxide (CO) emissions nationwide and approximately 10% of fine particulate matter (PM) emissions (Ernst, Corless and Greene-Roesel, 2003). The impact of air pollution, including traffic-related pollution, on the brain is being recognized, but its influence on Parkinson’s disease (PD) incidence, prevalence, and risk has not yet been adequately studied. PD is the fastest-growing of all neurodegenerative diseases and a leading cause of disability worldwide (GBD 2016 Parkinson’s Disease Collaborators, 2018). Given an aging population and increasing longevity globally, PD is expected to impose a great medical and economic burden in the future (GBD 2016 Parkinson’s Disease Collaborators, 2018).
Research suggests that ambient air pollutants may contribute to PD risk through systemic inflammation, oxidative stress, and direct toxicity after entry into the brain (Jankowska-Kieltyka, Roman and Nalepa, 2021; Wang et al., 2021). Small particulate pollutants might travel to the brain through the olfactory system, which provides a direct anatomical connection from the nose to the brain (Oberdorster et al., 2004). These small particles may also reach the brain via the autonomic nervous system or through the bloodstream (Eren and Ozturk, 2022). Toxicological and pathological studies conducted in highly polluted urban areas in Mexico observed oxidative damage in the olfactory bulb of feral dogs and children (Calderon-Garciduenas et al., 2010; Calderon-Garciduenas et al., 2008), as well as abnormal amyloid-β, hyperphosphorylated tau, and α-synuclein protein accumulations in postmortem human brain tissues (Calderon-Garciduenas et al., 2012; Calderon-Garciduenas et al., 2008). Additionally, particulates can make their way into the gastrointestinal tract, leading to changes in gut mucosal physiology, which can contribute to the development of α-synuclein pathology and alterations in the microbiome (Murata, Barnhill and Bronstein, 2022).
Despite plausible pathological mechanisms connecting air pollutants to PD, epidemiological studies examining the association between air pollution and PD have shown conflicting results, including studies of traffic-related pollution. Prior studies in Taiwan and Denmark reported an increased risk of PD with long-term traffic-related air pollution represented by modeled CO (Lee et al., 2016; Ritz et al., 2016), while other studies reported no association for measured CO (Chen et al., 2017; Jo et al., 2021). Similarly, while some studies linked PM2.5 to a higher risk of developing PD (Kirrane et al., 2015; Lee et al., 2022; Liu et al., 2016; Salimi et al., 2020; Shin et al., 2018; Yu et al., 2021; Yuchi et al., 2020), others found no associations (Cerza et al., 2018; Jo et al., 2021; Palacios et al., 2017; Palacios et al., 2014; Rumrich et al., 2023; Toro et al., 2019).
The conflicting results can partly be attributed to the complexities involved in studying the impact of air pollution on PD risk. One challenge arises from the extended lag time between disease initiation and diagnosis based on the clinical symptoms of motor dysfunction characteristic of PD (Berg et al., 2021; Braak et al., 2003). During this period, PD patients experience a prolonged prodromal phase characterized by non-motor symptoms such as constipation, sleep disorders, and depression. Ideally, researchers would aim to estimate the effects of air pollution even before this possibly decade-long prodromal phase. Additionally, distinguishing idiopathic PD from other parkinsonisms is difficult and can affect data quality in large administrative record-based PD studies that are otherwise well-suited for air pollution research.
Given the inconsistent results in epidemiological studies with challenges in long-term exposure and valid outcome assessment, we aim to estimate the effect of long-term exposure to CO as a marker of traffic pollution and PM2.5 on PD risk in a population-based case-control study. All PD patients were evaluated by movement disorder specialists from the University of California, Los Angeles (UCLA), with most patients being followed up and re-evaluated over time. Our study was conducted in central California, known for its persistently high air pollution levels. Mountains surround this region on three sides, trapping pollutants from heavy traffic along transportation routes connecting metropolitan areas and extensive agricultural activities, especially under meteorologic inversion conditions (U.S. Census Bureau, 2021). We employed a validated traffic-related pollution model for CO and a global three-dimensional atmospheric chemistry transport model for PM2.5 to estimate long-term air pollution exposures at residential and occupational addresses based on the life-long address histories we collected for both locations.
METHODS
Study population
Participants were drawn from the Parkinson’s Environmental and Genes (PEG) study, a population-based case-control study of environmental risk factors for PD in three counties (Kern, Fresno, and Tulare) in central California. Participants were recruited in two waves: PEG1 between 2001–2007 from local neurologist offices, PD support groups, local newspapers, and public radio announcements, and PEG2 between 2011–2017 from a population-based PD registry pilot program in California as well as the sources previously employed in PEG1. The study design and recruitment details are provided in prior publications (Kang et al., 2005; Wang et al., 2011). Briefly, eligible patients were early in disease at enrollment (<5 years, on average 3 years, SD = 2.6), with PD status confirmed by UCLA movement disorder specialists, resided in California for at least five years prior to PD diagnosis, did not have other neurological conditions or terminal illnesses, and agreed to participate in the study.
We recruited population controls from the same counties using mail or telephone contact and home visits after randomly selecting them from Medicare lists and property tax assessor lists. Two sampling strategies were utilized to achieve representativeness of the control population and increase enrollment success. In PEG1, we mailed letters to randomly selected residential parcels (or units) and enrolled controls through mail or telephone (Costello et al., 2009). In PEG2, we randomly selected clusters of five neighboring households and dispatched study staff to conduct home visits and enroll controls in person at their doorstep (Ritz, Paul and Bronstein, 2016). Controls fulfilled the same criteria as cases for eligibility in the study.
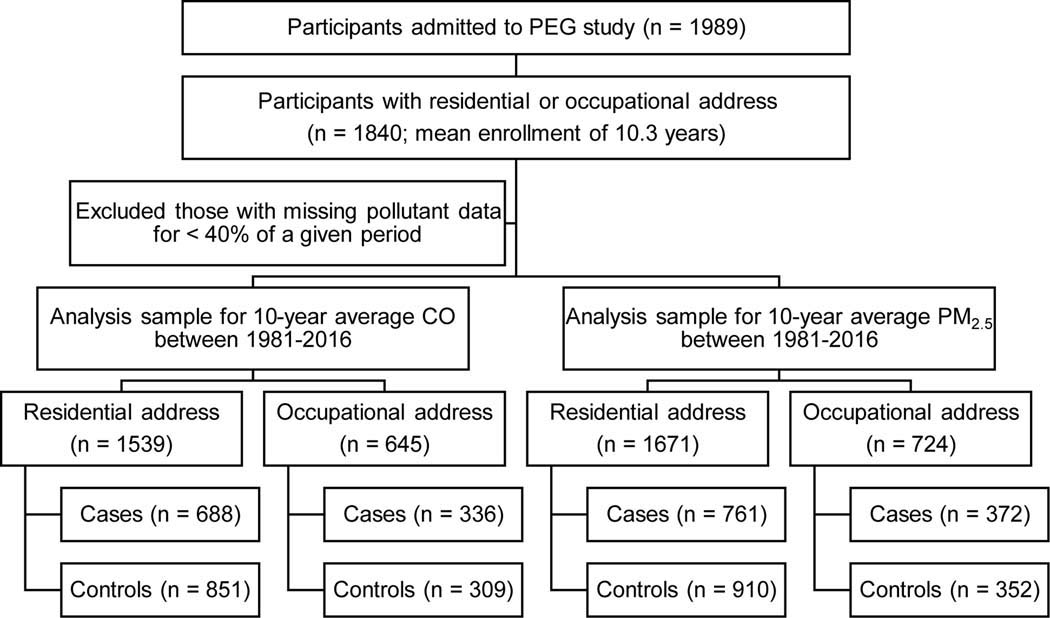
In total, 761 PD patients (337 from PEG1 and 424 from PEG2) and 910 controls (371 from PEG1 and 539 from PEG2) were available for our analyses (Fig. 1). Demographic information, including age, sex, race, years of education, and smoking status, was collected in interviews. To address potential confounding, long-term pesticide exposure information was incorporated, derived from a geospatial model that relied on the California pesticide use reporting system initiated in 1974 (for details, see Supplemental Methods). This study was approved by the UCLA Institutional Review Board. Informed written consent was obtained from all study participants.
Fig. 1.
Flow chart of study population, Parkinson’s Environmental and Gene (PEG) study.
Air pollution exposure assessment
We used established air pollution exposure models to estimate air pollutant concentrations at residential and occupational locations reported by participants for each year of life. For the mix of traffic-related pollutants, we employed the California Line Source Dispersion Model, version 4 (CALINE4), to estimate yearly concentrations of CO as a traffic tracer between 1981–2016 (Benson, 1989). This line-source Gaussian plume dispersion model predicts CO levels from sources within 1500 m of a geocoded location - the pre-determined receptor position - based on traffic information for highways and major roadways using emission rates, traffic fleet composition, and meteorological inputs (Flachsbart and Ott, 2019).
For PM2.5, we utilized a fine-resolution geospatially-derived model to obtain yearly concentration between 1981–2016 (Meng et al., 2019). This model provides validated and publicly available PM2.5 outputs at a 1 km resolution over North America by statistically fusing chemical transport modeling (GEOS-Chem) outputs and satellite observations of aerosol optical depth with ground-based PM2.5 observations using geographically weighted regression.
We evaluated long-term air pollution exposure by aggregating yearly concentrations into 10-year averages for CO and PM2.5 prior to PD diagnosis for cases and interview year for controls. We applied a 5-year lag time (i.e., a 10-year exposure period starts 15 years before the index year) to maximize the exposure duration available for all participants and include a sufficient lag time between the end of exposure and PD diagnosis (Fig. S1). We refer to this main exposure period as “10-year exposure” for brevity. For those with missing pollutant data for <40% of the averaging period, we filled in the missing data by taking the average concentration of previous and subsequent years to include the largest possible number of participants. We briefly describe our method but provide more detail in the Supplemental Methods.
Statistical analysis
We used unconditional logistic regression models separately for residential and occupational addresses to obtain odds ratios (ORs) and 95% confidence intervals (CIs). Air pollutant exposures were assessed as continuous (per interquartile range, IQR) and categorical variables (by tertile). Since the distributions of air pollution concentrations are skewed, we used tertile statistics to report their effects on the risk of PD. For linear trend tests, we used the midpoint value of each tertile as a continuous variable to minimize the influence of outliers. Two models were used to adjust effect estimates: model 1 adjusted for age (years), sex, race (white vs. non-white), education (years), and study wave (PEG1 vs. PEG2); model 2 was adjusted additionally for smoking status (pack-years) and pesticide exposure (a total count of all pesticides applied, within the same exposure window as the air pollution, either to the residential or workplace address). Occupational exposures were initially explored but were not included in the final models because they did not substantially change the reported results.
Sensitivity analyses were conducted. First, we examined alternative exposure periods: 1) a 5-year average exposure period lagged by five years prior to diagnosis/interview time and 2) a 15-year average exposure period prior to diagnosis/interview time without a lag (Fig. S1). Second, the effects of air pollutants were assessed in participants with complete information on both residential and occupational addresses only. Third, we conducted co-pollutant analyses to investigate potential confounding by the other pollutant. Lastly, the shape of the exposure-response relationship between air pollutants and PD was explored using splines with three knots.
RESULTS
The PD patients included in this study were on average 68 years of age, mostly of European ancestry, never smokers, and well-educated. Patients and controls were mostly similar with regard to these characteristics (Table 1). Most PD patients were male (64% male and 36% female), while more controls were female (47% male and 53% female), reflecting the gender distribution of the source population in this age range. The annual mean modeled CO concentrations were higher in the workplace area (mean:17.40 ppb) compared to the residential area (mean: 10.21 ppb), likely due to the influence of proximity to transportation corridors (Table S1). On the other hand, PM2.5 concentrations were similar at both locations, as expected for a pollutant that is more homogenously distributed and has multiple sources, including agricultural operations. The correlation between CO at residence and workplace was weakly positive (r = 0.32, p < 0.01), while PM2.5 at home and workplace had a strong positive correlation (r = 0.72, p < 0.01; Fig. S2).
Table 1.
Characteristics of the study population.
| Case (n=761) | Control (n=910) | |
|---|---|---|
|
| ||
| Age (years) | ||
| Mean (SD) | 67.7 (10.6) | 65.8 (11.6) |
| Sex | ||
| Male | 486 (63.9%) | 430 (47.3%) |
| Female | 275 (36.1%) | 480 (52.7%) |
| Race/Ethnicity | ||
| White | 587 (77.1%) | 607 (66.7%) |
| Latino | 125 (16.4%) | 197 (21.6%) |
| Asian | 18 (2.4%) | 28 (3.1%) |
| African American | 4 (0.5%) | 31 (3.4%) |
| Others | 27 (3.5%) | 47 (5.2%) |
| Education (years) | ||
| Mean (SD) | 13.6 (4.4) | 13.6 (4.3) |
| Smoking status | ||
| Never | 413 (54.3%) | 438 (48.1%) |
| Former | 321 (42.2%) | 360 (39.6%) |
| Current | 27 (3.5%) | 112 (12.3%) |
| study wave | ||
| PEG1 | 337 (44.3%) | 371 (40.8%) |
| PEG2 | 424 (55.7%) | 539 (59.2%) |
For the traffic marker CO, 10-year exposure was strongly associated with an increased risk of PD (Table 2). This association persisted even after adjusting for smoking status and pesticide exposure (Table 2, adjusted model 2). Exposure at the occupational address showed an even stronger association with PD compared to residential exposure. The OR for T3 vs. T1 was 1.58 (95% CI: 1.20, 2.10; p-trend = 0.02) for residential and 1.91 (95% CI: 1.22, 3.00; p-trend < 0.01) for occupational location exposure averages.
Table 2.
Association between 10-year average CO exposure with a 5-year lag time and Parkinson’s disease.
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
|
|
||||
| Residence | Cases/controls | Unadjusted | Model1a | Model2b |
|
| ||||
| Continuous CO (ppb)c | 688/851 | 1.07 (1.01, 1.13) | 1.08 (1.02, 1.14) | 1.09 (1.03, 1.16) |
| T1 (0.01–2.87) | 242/343 | Reference | Reference | Reference |
| T2 (2.88–9.06) | 242/292 | 1.17 (0.93, 1.49) | 1.24 (0.97, 1.59) | 1.38 (1.07, 1.77) |
| T3 (9.07–368.00) | 204/216 | 1.34 (1.04, 1.72) | 1.40 (1.07, 1.83) | 1.58 (1.20, 2.10) |
| p-trendd | 0.06 | 0.05 | 0.02 | |
|
| ||||
| Workplace | ||||
|
| ||||
| Continuous CO (ppb)c | 336/309 | 1.02 (0.97, 1.08) | 1.05 (0.99, 1.12) | 1.06 (1.00, 1.13) |
| T1 (0.01–2.87) | 70/73 | Reference | Reference | Reference |
| T2 (2.88–9.06) | 97/97 | 1.04 (0.68, 1.61) | 1.21 (0.76, 1.91) | 1.27 (0.79, 2.04) |
| T3 (9.07–368.00) | 169/139 | 1.27 (0.85, 1.89) | 1.72 (1.12, 2.65) | 1.91 (1.22, 3.00) |
| p-trendd | 0.18 | 0.01 | <0.01 | |
Adjusted for age, race, sex, education, and study wave.
Adjusted as in model 1 plus smoking status and pesticide exposure.
Change per interquartile range (IQR) of 10.27 ppb.
Based on linear model through the tertile midpoints.
Similarly, 10-year PM2.5 exposure was positively associated with the risk of PD (Table 3), and this association was slightly stronger after adjusting for smoking status and pesticide exposure. For occupational location, PM2.5 exposure was also more strongly associated with PD risk compared to residential exposure. The OR for T3 vs. T1 was 1.62 (95% CI: 1.22, 2.15; p-trend < 0.01) for residential location-based exposure and 1.85 (95% CI: 1.21, 2.85; p-trend <0.01) for occupational location. Spline models with three knots showed an overall increase in risk with increasing PM2.5 exposure at workplace and home (Fig. S3). However, for PM2.5 at the residence, associations with PD were not observed for exposures less than 16 μg/m3, which was close to our lowest exposure tertile boundary.
Table 3.
Association between 10-year average PM2.5 exposure with a 5-year lag time and Parkinson’s disease.
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
|
|
||||
| Residence | Cases/controls | Unadjusted | Model1a | Model2b |
|
| ||||
| Continuous PM2.5 (μg/m3)c | 761/910 | 1.29 (1.14, 1.47) | 1.39 (1.21, 1.61) | 1.49 (1.28, 1.73) |
| T1 (0.88–14.50) | 245/319 | Reference | Reference | Reference |
| T2 (14.51–17.80) | 242/316 | 1.00 (0.79, 1.26) | 1.05 (0.82, 1.34) | 1.15 (0.90, 1.49) |
| T3 (17.81–29.40) | 274/275 | 1.30 (1.02, 1.64) | 1.39 (1.06, 1.81) | 1.62 (1.22, 2.15) |
| p-trendd | 0.04 | 0.02 | <0.01 | |
|
| ||||
| Workplace | ||||
|
| ||||
| Continuous PM2.5(μg/m3)c | 372/352 | 1.24 (1.03, 1.48) | 1.50 (1.22, 1.86) | 1.59 (1.28, 1.99) |
| T1 (0.88–14.50) | 110/125 | Reference | Reference | Reference |
| T2 (14.51–17.80) | 133/107 | 1.41 (0.98, 2.03) | 1.76 (1.21, 2.59) | 2.03 (1.36, 3.04) |
| T3 (17.81–29.40) | 129/120 | 1.22 (0.86, 1.75) | 1.59 (1.06, 2.38) | 1.85 (1.21, 2.85) |
| p-trendd | 0.26 | 0.02 | <0.01 | |
Adjusted for age, race, sex, education, and study wave.
Adjusted as in model 1 plus smoking status and pesticide exposure.
Change per interquartile range (IQR) of 5.46 μg/m3.
Based on linear model using the tertile midpoints.
Sensitivity analyses using alternative exposure periods (5- and 15-year average exposure with/out lagging), restricted to participants only who reported both residential and occupational addresses, and co-pollutant models did not materially change associations with PD for the highest exposure levels of CO or PM2.5 (Table S2-9). Generally, the estimated effects of air pollution based on the sensitivity analyses were slightly weaker than those from the main analyses; however, they still showed the same trends and directions as the main results.
DISCUSSION
In this large population-based case-control study conducted in central California, we found that long-term average exposure to traffic-related air pollution, represented by CO modeled based on traffic emissions, and fine particulates from multiple sources are associated with an increased risk of developing PD. The estimates were largest for exposure modeled at occupational locations that also had much higher traffic exposures on average than residences. Specific pollutants or complex mixtures in traffic emissions, such as those found in diesel exhaust, could contribute to this effect. Additionally, the presence of other occupational exposures, including pesticides, heavy metals, or industrial chemicals, might interact with air pollutants or amplify PD risk. Our findings remained consistent after adjustment for smoking status and pesticide exposure, as well as in sensitivity analyses that employed alternative exposure averaging periods and exposure lagging.
We found a positive association between long-term exposure to traffic emissions (represented by CO) and the risk of PD. Among the studies that evaluated the risk of PD based on traffic mixture exposure, some showed positive associations (Lee et al., 2016; Ritz et al., 2016), while others showed no associations (Chen et al., 2017; Jo et al., 2021). The studies showing no associations had assessed a relatively short exposure duration prior to PD diagnosis (5 years or less). In contrast, the studies showing positive correlations, conducted in Denmark and Taiwan, examined long exposure periods (11 years or more). Therefore, the heterogeneity between these studies may reflect differences in the lengths of the exposure durations or lagging of exposures as well as differences in modeling approaches for assessing traffic mixtures that are here represented by CO.
We also observed a positive association between long-term exposure to fine particulates from multiple sources (modeled as PM2.5) and PD. In line with our study, previous studies reported positive associations between exposure to fine particulates from various sources and PD (Kirrane et al., 2015; Lee et al., 2022; Liu et al., 2016; Salimi et al., 2020; Shin et al., 2018; Yu et al., 2021; Yuchi et al., 2020). Among these studies, some were conducted in regions with higher PM2.5 levels, such as China and Korea, where mean levels were 38.2 μg/m3 and 30.2 μg/m3, respectively (Lee et al., 2022; Yu et al., 2021). In contrast, studies from regions like the United States (North Carolina and Iowa), Canada, and Australia reported average exposure levels of approximately 10 μg/m3, slightly lower than ours (Kirrane et al., 2015; Liu et al., 2016; Salimi et al., 2020; Shin et al., 2018; Yuchi et al., 2020). The differences in particle composition due to varying pollution sources in different regions, such as heavy industry and traffic sources in China, can impact the effect estimates of PM2.5 exposure on PD risk. The chemical composition of PM2.5, which varies according to different pollution sources and metrological conditions, could contribute to these inconsistent results, along with other methodologic differences, such as differences in study population characteristics, exposure assessment methods, adjustment for potential confounders, and statistical approaches used for analysis.
Our findings corroborate the mechanisms suggested by previous clinical and laboratory studies. Traffic-related air pollution reduces oxygen delivery, leading to a prooxidant environment in aerobic cells and affecting reactive oxygen species-related signaling pathways (Piantadosi, 2008). Fine particulates can directly enter the brain via the bloodstream or olfactory system, causing inflammation in the central nervous system (Oberdorster et al., 2004; Park et al., 2018). Exposure to traffic-related air pollution weakens the blood-brain barrier (BBB) (Oppenheim et al., 2013), increasing its permeability to circulating toxicants. Some of the components of diesel exhaust are neurotoxic by causing dysfunction in autophagy and disrupting proteostasis (Barnhill et al., 2020; Ha et al., 2022). Moreover, air pollution indirectly affects brain health through systemic mechanisms, releasing inflammatory cytokines from the lungs, which can weaken the BBB and lead to neuronal injury (Kempuraj et al., 2017; Wong, Magun and Wood, 2016). Elevated pro-inflammatory serum cytokines are associated with an increased risk and faster progression of PD (Qin et al., 2016; Williams-Gray et al., 2016), and systemic inflammation, especially in combination with elevated α-synuclein levels, can lead to neuroinflammation and loss of dopaminergic neurons (Gao et al., 2011). Additionally, air pollution affects the gastrointestinal tract, promoting α-synuclein pathology and altering the microbiome (Anselmi et al., 2018; Braak et al., 2006; Pan-Montojo et al., 2012; Shannon et al., 2012), which are implicated in PD development (Rani and Mondal, 2021).
The strength of this study is that we assessed air pollution exposures at both residential and occupational addresses, which sets it apart from most epidemiologic studies. Moreover, we were able to investigate longer-term exposure with lag time, unlike previous studies that covered relatively short exposure periods (Chen et al., 2017; Jo et al., 2021). We also included various exposure duration and lag analyses to account for the long prodromal stage of PD. Additionally, we accounted for potential confounding factors such as smoking and pesticide exposure. Lastly, our PD patients were confirmed by movement disorder specialists, minimizing outcome misclassification and enhancing the validity of our outcome assessment.
Our study has a few limitations, for example, we only modeled outdoor ambient air pollution, which may not reflect indoor concentrations. However, outdoor measurements of air pollutants are used in epidemiologic studies to assess risk from sources of air pollution that can be reduced through regulations. In addition, we did not have available data on other gaseous pollutants such as nitrogen dioxide (NO2) or ozone (O3). However, NO2 and CO may exhibit a high correlation due to shared emission sources. O3 differs as it is a secondary air pollutant primarily formed through photochemical reactions rather than direct emissions, but we do not have access to model for O3 historically as far back as CO. The associations we assessed were likely due to mixtures from sources rather than a single pollutant. Finally, air pollution data before 1981 were unavailable, limiting our ability to incorporate lag times longer than five years. While a few studies have used exposure periods over ten years, they either applied no lag or a shorter lag of 2 years (Lee et al., 2016; Palacios et al., 2017; Ritz et al., 2016). Our sensitivity analyses applying varying exposure periods and lags suggested that our results were fairly consistent across different exposure windows.
In summary, our large population-based case-control study suggests that the long-term impacts of traffic-related and other sources of PM air pollution are associated with an increased PD risk in central California, known for its extensive agriculture and food production but also the worst air pollution in the United States (American Lung Association, 2023). Due to its unique geography, the region’s heavy reliance on cattle and crops leads to fine particulate accumulation in the valley, forming a pollution-trapping basin. Truck traffic, oil field emissions, and forest fires worsen air quality, with nitric acid and ammonia emissions from cow manure and tailpipe reactions further contributing to PM2.5 pollution. While efforts have been made to reduce pollution, challenges in balancing regulation with economic interests persist.
Supplementary Material
Acknowledgments
We would like to thank all patients with Parkinson’s disease and their household members as well as all other study participants for their time and support.
Funding
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (grant numbers: R01 ES031106, R01 ES010544, U54-ES012078, P01-ES016732, P50-NS038367), and initial pilot funding from P30-ES07048, and the American Parkinson’s Disease Association (grant number 20161386) and the Parkinson Alliance as well as Levine Foundation contributing to Dr. Bronstein’s research.
Footnotes
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- American Lung Association, Most Polluted Cities: State of the Air. American Lung Association, American Lung Association, 2023. [Google Scholar]
- Anselmi L, et al. , 2018. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinsons Dis. 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhill LM, et al. , 2020. Diesel Exhaust Extract Exposure Induces Neuronal Toxicity by Disrupting Autophagy. Toxicol Sci. 176, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson PE, CALINE4 -- A Dispersion Model for Predicting Air Pollutant Concentrations near Roadways, Report No. FHWA/CA/TL-84/15. Office of Transportation Laboratory, California Department of Transportation, Sacramento, CA, Office of Transportation Laboratory, California Department of Transportation, Sacramento, CA, 1989. [Google Scholar]
- Berg D, et al. , 2021. Prodromal Parkinson disease subtypes - key to understanding heterogeneity. Nat Rev Neurol. 17, 349–361. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. , 2006. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 396, 67–72. [DOI] [PubMed] [Google Scholar]
- Braak H, et al. , 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2010. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 62, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2012. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis. 28, 93–107. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2008. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 68, 117–27. [DOI] [PubMed] [Google Scholar]
- Cerza F, et al. , 2018. Residential exposure to air pollution and incidence of Parkinson’s disease in a large metropolitan cohort. Environmental Epidemiology. 2, e023. [Google Scholar]
- Chen CY, et al. , 2017. Long-term exposure to air pollution and the incidence of Parkinson’s disease: A nested case-control study. PLoS One. 12, e0182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, et al. , 2009. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 169, 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren F, Ozturk S, 2022. Evaluation of the Effect of Air Pollution on Cognitive Functions, Cognitive Decline, and Dementia. Ann Indian Acad Neurol. 25, S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Corless J, Greene-Roesel R, Clearing the Air. A Report of the Surface Transportation Policy Project. Surface Transportation Policy Partnership, 2003. [Google Scholar]
- Flachsbart P, Ott W, 2019. Trends in passenger exposure to carbon monoxide inside a vehicle on an arterial highway of the San Francisco Peninsula over 30 years: A longitudinal study. J Air Waste Manag Assoc. 69, 459–477. [DOI] [PubMed] [Google Scholar]
- Gao HM, et al. , 2011. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 119, 807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Parkinson’s Disease Collaborators, 2018. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SM, et al. , 2022. Neurotoxicity of diesel exhaust extracts in zebrafish and its implications for neurodegenerative disease. Sci Rep. 12, 19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska-Kieltyka M, Roman A, Nalepa I, 2021. The Air We Breathe: Air Pollution as a Prevalent Proinflammatory Stimulus Contributing to Neurodegeneration. Frontiers in Cellular Neuroscience. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, et al. , 2021. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol. 78, 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang GA, et al. , 2005. Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov Disord. 20, 1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, et al. , 2017. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci. 11, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirrane EF, et al. , 2015. Associations of Ozone and PM2.5 Concentrations With Parkinson’s Disease Among Participants in the Agricultural Health Study. Journal of Occupational and Environmental Medicine. 57, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, et al. , 2022. Long-term exposure to particulate air pollution and incidence of Parkinson’s disease: A nationwide population-based cohort study in South Korea. Environ Res. 212, 113165. [DOI] [PubMed] [Google Scholar]
- Lee PC, et al. , 2016. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environment International. 96, 75–81. [DOI] [PubMed] [Google Scholar]
- Liew Z, et al. , 2014. Job Exposure Matrix (JEM)-Derived Estimates of Lifetime Occupational Pesticide Exposure and the Risk of Parkinson’s Disease. Archives of Environmental & Occupational Health. 69, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, et al. , 2016. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ Health Perspect. 124, 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, et al. , 2019. Estimated Long-Term (1981–2016) Concentrations of Ambient Fine Particulate Matter across North America from Chemical Transport Modeling, Satellite Remote Sensing, and Ground-Based Measurements. Environ Sci Technol. 53, 5071–5079. [DOI] [PubMed] [Google Scholar]
- Murata H, Barnhill LM, Bronstein JM, 2022. Air Pollution and the Risk of Parkinson’s Disease: A Review. Mov Disord. 37, 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby DE, et al. , 2015. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 36, 83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, et al. , 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 16, 437–45. [DOI] [PubMed] [Google Scholar]
- Oppenheim HA, et al. , 2013. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 10, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, et al. , 2017. Air Pollution and Risk of Parkinson’s Disease in a Large Prospective Study of Men. Environ Health Perspect. 125, 087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, et al. , 2014. Particulate matter and risk of Parkinson disease in a large prospective study of women. Environ Health. 13, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo F, et al. , 2012. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep. 2, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, et al. , 2018. Differential toxicities of fine particulate matters from various sources. Scientific Reports. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, 2008. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med. 45, 562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, et al. , 2016. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 73, 1316–1324. [DOI] [PubMed] [Google Scholar]
- Rani L, Mondal AC, 2021. Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci Res. 168, 100–112. [DOI] [PubMed] [Google Scholar]
- Ritz B, et al. , 2016. Traffic-Related Air Pollution and Parkinson’s Disease in Denmark: A Case-Control Study. Environ Health Perspect. 124, 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Paul KC, Bronstein JM, 2016. Of Pesticides and Men: a California Story of Genes and Environment in Parkinson’s Disease. Curr Environ Health Rep. 3, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumrich IK, et al. , 2023. Long-term exposure to low-level particulate air pollution and Parkinson’s disease diagnosis - A Finnish register-based study. Environ Res. 229, 115944. [DOI] [PubMed] [Google Scholar]
- Salimi F, et al. , 2020. Associations between long-term exposure to ambient air pollution and Parkinson’s disease prevalence: A cross-sectional study. Neurochem Int. 133, 104615. [DOI] [PubMed] [Google Scholar]
- Shannon KM, et al. , 2012. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s Disease? Evidence from 3 cases. Movement Disorders. 27, 716–719. [DOI] [PubMed] [Google Scholar]
- Shin S, et al. , 2018. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: a population-based cohort study. International Journal of Epidemiology. 47, 2038–2048. [DOI] [PubMed] [Google Scholar]
- Toro R, et al. , 2019. Parkinson’s disease and long-term exposure to outdoor air pollution: A matched case-control study in the Netherlands. Environ Int. 129, 28–34. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, Kern, Fresno, and Tulare Population. 2021.
- Wang A, et al. , 2011. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 26, 547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. , 2021. The Impact of Air Pollution on Neurodegenerative Diseases. Ther Drug Monit. 43, 69–78. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, et al. , 2016. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord. 31, 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Magun BE, Wood LJ, 2016. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. 11, 1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, et al. , 2021. Air pollution, surrounding green, road proximity and Parkinson’s disease: A prospective cohort study. Environ Res. 197, 111170. [DOI] [PubMed] [Google Scholar]
- Yuchi W, et al. , 2020. Road proximity, air pollution, noise, green space and neurologic disease incidence: a population-based cohort study. Environ Health. 19, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.