Abstract
Introduction:
Host immune responses may impact dengue severity in adults. Vitamin D has multiple immunomodulatory effects on innate and adaptive immunity.
Methods:
We evaluated the association between systemic 25-hydroxyvitamin D [25-(OH) D] and dengue disease severity in adults. We measured plasma for total 25-(OH) D levels with an electrochemiluminescence immunoassay using stored samples from participants with laboratory-confirmed dengue, who were prospectively enrolled in 2012–2016 at our institution.
Results:
A total of 80 participants (median age 43 years) were enrolled in the study. Six participants had severe dengue based on the World Health Organization (WHO) 1997 criteria (i.e. dengue haemorrhagic fever/dengue shock syndrome) and another six had severe dengue based on the WHO 2009 criteria. Median 25-(OH) D at the acute phase of dengue was 6.175 (interquartile range 3.82–8.21, range 3.00–15.29) mcg/L in all participants. The 25-(OH) D showed an inverse linear trend with severe dengue manifestations based on the WHO 2009 criteria (adjusted risk ratio 0.72, 95% confidence interval 0.57–0.91, P < 0.01) after adjustment for age, gender and ethnicity.
Conclusion:
Limited studies have evaluated the role of systemic 25-(OH) D on dengue severity. Our study found low systemic 25-(OH) D was associated with increased dengue disease severity, particularly for severe bleeding that was not explained by thrombocytopenia. Further studies investigating the underlying immune mechanisms and effects on the vascular endothelium are needed.
Keywords: 25-hydroxyvitamin D, dengue, severe dengue, vitamin D
INTRODUCTION
Dengue remains a globally important vector-borne infection, with the World Health Organization (WHO) estimates of 50 million annual dengue infections and approximately 2.5 billion individuals at risk in dengue-endemic areas.[1] A more recent estimate using cartographic approaches revealed an annual burden of 390 million (95% credible interval 284–528) dengue infections, of which 96 million (67–136) are symptomatic.[2] The risk of severe dengue (SD) in adults is associated with host comorbidities such as diabetes mellitus and other components of the host immune response.[3,4] The critical phase during dengue infection occurs during viral clearance, suggesting host immune responses may play an important role and could be targeted in approaches to mitigate SD infection. Various immunopathogenesis and virus–host interaction factors have been studied, including the role of proinflammatory cytokines (tumour necrosis factor-alpha [TNF-α], interferon-gamma [IFN-γ], interleukin [IL]-10), innate immunity, antibody-mediated enhancement and endothelial activation.[3,5,6]
There is now a licensed live attenuated dengue vaccine CYD TDV (Dengvaxia®), which may be used in certain patient subpopulations, and there are other candidate dengue vaccines in development.[7,8] However, at the time of writing, there is no licensed vaccine for use in older adults, and CYD TDV may not be appropriate for widespread implementation in all populations of risk. There is a need for further research to delineate the mechanisms of dengue pathogenesis in the context of rational development of therapeutic and immunomodulatory interventions to prevent dengue-related complications in adults.[9,10]
Recently, there has been escalating interest in the immunomodulatory actions of vitamin D and its association with susceptibility to certain infections.[11,12] Vitamin D has robust actions on the innate immune response, acting as a chemoattractant for monocytes, T cells and neutrophils. It triggers a shift to a Th2-type cytokine response (characterised by increased levels of IL-4, IL-5 and IL-10 and reduced levels of IL-2, IFN-γ and TNF-α, i.e., proinflammatory cytokines). 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], the active metabolite produced endogenously from 25-hydroxyvitamin D [25-(OH) D], inhibits IL-17 and IL-22 producing Th17 cells and increases CD4+/CD25+ regulatory T cells.[13,14] Vitamin D also has an influence on peripheral homing and the migration of T cells to the skin.[14]
Few studies have evaluated the association between vitamin D and dengue disease severity.[15,16,17,18,19,20,21,22] Several recent studies have suggested a dose–response relationship between exposure to vitamin D and dengue pathogenesis and severity.[15,18,20] In contrast, other studies have shown contrasting results, that is, higher 25-(OH) D associated with more SD (dengue haemorrhagic fever [DHF]/dengue shock syndrome [DSS]).[17,19] Importantly, the threshold of systemic 25-(OH) D for its actions on innate and adaptive immunity are not yet known and it may not be directly congruent to the levels relevant to skeletal health.[23,24] The specific actions of 1,25-(OH)2D3 on the vascular endothelium is unknown. Dengue disease course is dynamic, and the timing of 25-(OH) D assessment, extent of plasma leakage, patient’s prior 25-(OH) D status and comorbidities all play a role in studying this association.
We measured systemic 25-(OH) D in adult dengue patients with uncomplicated and severe disease who were prospectively enrolled at the largest tertiary teaching hospital for dengue management in Singapore. We hypothesised that low systemic 25-(OH) D would be associated with more SD clinical outcomes.
METHODS
We conducted a cohort study among adult patients (age ≥21 years) presenting with acute dengue infection to the Department of Infectious Diseases, Tan Tock Seng Hospital, a 1700-bed adult tertiary care public hospital in Singapore. The 25-(OH)D levels were measured in stored samples. The source population was identified from an ongoing prospective adult dengue cohort study active since 2009, henceforth referred to as ‘Study A’. Study A included individuals with acute dengue confirmed by either positive dengue polymerase chain reaction[25] or non-structural protein 1 antigen or serology (IgM and IgG) tests based on a single acute sample.[26,27] Study A included three study visits — first visit on presentation to the hospital (acute illness), second visit at day 14–28 of illness (early convalescence) and third visit at day 45–120 of illness (late convalescence). Each study visit involved clinical assessment and venepuncture. For the present study, we performed convenience sampling to obtain our study population from the source population with the following eligibility criteria: (a) individuals who had completed study visit during the acute illness phase, and (b) sufficient residual sample available at acute time point for testing of plasma 25-(OH) D. Age groups of individuals were also considered to ensure an adequate representation of various age groups in the final cohort. We excluded patients who did not give consent to be included in this study. This study was designed as a pilot exploratory study, and hence, formal a priori sample size calculation was not performed.
25-hydroxyvitamin D assessment
We utilised residual cryopreserved plasma samples after obtaining informed consent from the patients. The plasma was frozen in aliquots at -80°C immediately after processing of blood following collection and thawed only before the 25-(OH) D assay. Plasma total 25-(OH) D was measured on a Roche e601 immunoassay analyser (electrochemiluminescence immunoassay) using the Elecsys Vitamin D total II assay with manufacturer-supplied reagents and calibrators (Roche Diagnostics, Mannheim, Germany). The assay uses a vitamin D-binding protein to bind 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2. The mean cross-reactivity of 25-hydroxyvitamin D3 in the assay is 100%, while the cross reactivity of 25-hydroxyvitamin D2 is 93.7%. Cross-reactivity to 24,25-dihydroxyvitamin D is blocked by a specific monoclonal antibody. The method has been standardised using internal standards that are traceable to an isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) method, which is, in turn, traceable to the National Institute of Standards and Technology Standard Reference Material 2972.[28] The limit of blank was 2 mcg/L.
Severe dengue manifestations
Severe clinical presentation of dengue was classified as DHF or DSS according to the WHO 1997 criteria, and SD according to WHO 2009 criteria. The hospital and outpatient course for each dengue-infected patient was documented using a standardised dengue care path that recorded relevant clinical, laboratory and radiological data in a standardised manner. Clinical data were extracted by the trained study team from the first day of hospital presentation until discharge for inpatients or until follow-up for outpatients. We retrospectively classified the severity of patients’ illness based on the WHO dengue criteria.
The DHF cases (WHO, 1997) met these criteria: fever and all of the following — (a) haemorrhagic manifestations, (b) thrombocytopenia <100 × 109/L, and (c) plasma leakage evidenced by pleural effusion or ascites or change in haematocrit ≥20% or hypoproteinaemia. Dengue shock syndrome (WHO, 1997) was defined as the presence of tachycardia with narrow pulse pressure <20 mmHg or hypotension (systolic blood pressure <90 mmHg) in addition to DHF.[29] The SD cases (WHO, 2009) met the following criteria: (a) severe plasma leakage with respiratory distress or shock, (b) severe bleeding, defined as a minimum of WHO grade 2 bleeding scale or any bleeding requiring whole blood or packed red cell transfusion, and (c) severe organ involvement — acute liver injury with aspartate transaminase and alanine transaminase ≥1000 IU/L or acute kidney injury or myocarditis or encephalopathy.[1]
Data collection and statistical analysis
Data collection was performed independently by trained research assistants following standardised procedures. Systemic 25-(OH) D was analysed as a continuous variable using median value and interquartile ranges (IQR) for descriptive statistics. Chi-square test was used for bivariate inference method. We used univariable and multivariable Poisson regression with robust error variance[30] to estimate crude risk ratio and adjusted risk ratio (aRR) with 95% confidence interval (CI) assessing the association between serum 25-(OH) D concentration and SD manifestations, as well as each subcategory signifying severity, namely plasma leakage leading to shock, bleeding and organ involvement. In view of small sample size, we adjusted only demographic variables in the adjusted model. Statistical significance threshold was set at P value < 0.05. All analyses were carried out with Stata version 13.1 (StataCorp LP, College Station, TX, USA).
Ethics approval
The study was approved by the Domain Specific Review Board of the National Healthcare Group, Singapore (DSRB- 2016/01167). Informed consent was obtained via completed returned reply slips posted to invited participants. Study team followed up with phone call if no response was received at 2 weeks following mailing of a letter, and informed consent was obtained verbally and documented in patient’s medical record.
RESULTS
A total of 199 participants who had been admitted for dengue infection between 2012 and 2016 were screened for eligibility for enrolment [Figure 1]. Of the participants, 119 were not eligible either due to lack of informed consent or insufficient residual samples. Thus, 80 participants aged 21–69 years were enrolled, with male predominance. Six participants had SD based on WHO 1997 criteria (i.e. DHF/DSS) and another six participants had SD based on WHO 2009 criteria. Two participants had SD fulfilling both WHO 1997 and 2009 classifications. Seventy participants had uncomplicated dengue. Median day of illness at the time of acute visit for all participants was 5 (IQR 4–6) days. Table 1 shows the demographic, 25-(OH) D levels and clinical characteristics of the study population. Median 25-(OH) D was lower in the younger age group (4.50 mcg/L vs. 6.59 mcg/L vs. 6.87 mcg/L in 21–40 years, 41–60 years and 61–69 years, respectively, P = 0.042) and in non-Chinese patients (5.83 mcg/L in Malays and Indians vs. 6.76 mcg/L in Chinese, P = 0.009). Median 25-(OH) D was 4.42 (IQR 3.00–6.74) mcg/L in those with DHF/DSS based on WHO 1997 criteria compared to 6.39 (IQR 3.93–8.36) mcg/L in those without DHF/DSS (P = 0.115), and 5.41 (IQR 3.00–5.84) mcg/L in those with SD (WHO 2009 criteria) compared to 6.64 (IQR 3.82–8.36) mcg/L in those without SD (P = 0.101).
Figure 1.
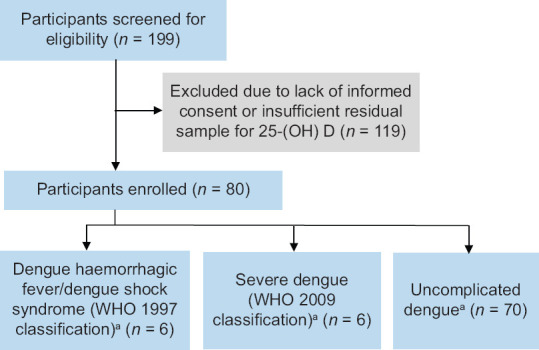
Study flow diagram. aThere were two patients who were classified as severe dengue based on both World Health Organization (WHO) 1997 and 2009 classifications.
Table 1.
Demographic and clinical characteristics of enrolled patients and serum 25(OH) D at the acute time point (n=80).
| Characteristic | n (%) | 25(OH) Da (mcg/L) | P | Characteristic | n (%) | 25(OH) Da (mcg/L) | P |
|---|---|---|---|---|---|---|---|
| Age (yr) | 0.042 | Aspirin | NS | ||||
|
| |||||||
| 21–40 | 36 (45.0) | 4.50 (3.06–7.75) | No | 77 (96.3) | 6.07 (3.81–8.21) | ||
|
| |||||||
| 41–60 | 32 (40.0) | 6.59 (4.74–8.76) | Yes | 3 (3.7) | 6.54 (5.11–9.30) | ||
|
| |||||||
| 61–69 | 12 (15.0) | 6.87 (5.95–10.17) | Thrombocytopeniab | ||||
|
| |||||||
| Gender | NS | WHO 1997 criteria | |||||
|
| |||||||
| Male | 56 (70.0) | 5.90 (3.96–8.15) | DHF/DSS | NS | |||
|
| |||||||
| Female | 24 (30.0) | 6.52 (3.08–8.44) | No | 74 (92.5) | 6.39 (3.93–8.36) | ||
|
| |||||||
| Ethnicity | 0.009 | Yes | 6 (7.5) | 4.42 (3.00–6.74) | |||
|
| |||||||
| Chinese | 63 (78.8) | 6.76 (4.46–8.68) | Plasma leakage | NS | |||
|
| |||||||
| Non-Chinese | 17 (21.2) | 5.83 (3.93–7.89) | No | 67 (83.8) | 6.28 (3.82–8.21) | ||
|
| |||||||
| CCI | NS | Yes | 13 (16.2) | 6.07 (3.81–7.08) | |||
|
| |||||||
| 0 | 75 (93.7) | 5.96 (3.66–8.21) | Haemorrhagic manifestations | NS | |||
|
| |||||||
| ≥1 | 5 (6.3) | 6.98 (6.54–9.30) | No | 57 (71.3) | 6.54 (3.82–8.67) | ||
|
| |||||||
| Hypertension | 0.049 | Yes | 23 (28.7) | 5.84 (3.49–7.08) | |||
|
| |||||||
| No | 66 (82.5) | 5.83 (3.25–8.09) | WHO 2009 criteria | ||||
|
| |||||||
| Yes | 14 (17.5) | 6.94 (6.07–9.20) | Severe dengue | NS | |||
|
| |||||||
| Hyperlipidaemia | NS | No | 74 (92.5) | 6.64 (3.82–8.36) | |||
|
| |||||||
| No | 68 (85.0) | 6.12 (3.58–8.21) | Yes | 6 (7.5) | 5.41 (3.00–5.84) | ||
|
| |||||||
| Yes | 12 (15.0) | 6.31 (5.29–7.85) | Severe plasma leakage leading to shock | NS | |||
|
| |||||||
| Past dengue infection | NS | No | 78 (97.7) | 6.39 (3.82–8.21) | |||
|
| |||||||
| No | 74 (92.5) | 5.90 (3.81–8.04) | Yes | 2 (2.3) | 4.54 (3.00–6.07) | ||
|
| |||||||
| Yes | 6 (7.5) | 8.38 (6.07–9.68) | Severe bleeding | NS | |||
|
| |||||||
| Antihypertensive drugs | NS | No | 74 (95.0) | 6.52 (3.88–8.29) | |||
|
| |||||||
| No | 70 (87.5) | 5.84 (3.49–8.09) | Yes | 6 (5.0) | 4.35 (3.00–5.77) | ||
|
| |||||||
| Yes | 10 (12.5) | 6.94 (6.54–9.2) | Epidemic yearc | NS | |||
|
| |||||||
| Antihyperlipidemic drugs | NS | 2013, 2014 (DENV1) | 9 (11.3) | 6.93 (5.11–9.48) | |||
|
| |||||||
| No | 74 (92.5) | 6.12 (3.66–8.21) | 2012, 2015, 2016 (DENV2) | 71 (88.7) | 5.96 (3.81–8.21) | ||
|
| |||||||
| Yes | 6 (7.5) | 6.31 (5.53–6.98) | |||||
|
| |||||||
| Antidiabetic drugs | NS | ||||||
|
| |||||||
| No | 78 (97.5) | 6.02 (3.81–8.21) | |||||
|
| |||||||
| Yes | 2 (2.5) | 6.76 (6.54–6.98) | |||||
Not significant (NS): P>0.05. aData presented as median (interquartile range). bThrombocytopenia was defined as the lowest platelet count during hospital stay <100×109/L. cEpidemic year was used as a surrogate index to estimate the circulating dengue serotype. 25(OH) D: 25hydroxyvitamin D, CCI: Charlson’s comorbidity index, DENV: dengue virus serotype, DHF: dengue haemorrhagic fever, DSS: dengue shock syndrome, WHO: World Health Organization
Multivariable analysis
WHO 1997 dengue classification
No statistically significant association was found between serum 25-(OH) D and (a) DHF/DSS (aRR 0.82, 95% CI 0.64–1.05, P = 0.113) or its severity indicators including (b) haemorrhagic manifestations (aRR 0.98, 95% CI 0.86–1.12, P = 0.801) and (c) plasma leakage (aRR 0.98, 95% CI 0.84–1.13, P = 0.749) based on the WHO 1997 dengue criteria [Table 2].
Table 2.
Risk ratio for association between plasma 25-(OH) D level and severe dengue manifestations based on the WHO 1997 and 2009 criteria.
| Severe dengue manifestation | n (%) | Crude | Adjusteda | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| RR | 95% CI | P | RR | 95% CI | P | ||
| WHO 1997 dengue classification | |||||||
|
| |||||||
| DHF/DSS | 6 (7.5) | 0.76 | 0.55–1.05 | NS | 0.82 | 0.64–1.05 | NS |
|
| |||||||
| Haemorrhagic manifestations | 23 (28.8) | 0.95 | 0.84–1.08 | NS | 0.98 | 0.86–1.12 | NS |
|
| |||||||
| Plasma leakage | 13 (16.3) | 0.94 | 0.79–1.12 | NS | 0.98 | 0.84–1.13 | NS |
|
| |||||||
| WHO 2009 dengue classification | |||||||
|
| |||||||
| Severe dengue | 6 (7.5) | 0.77 | 0.61–0.97 | 0.025 | 0.72 | 0.57–0.91 | 0.005 |
|
| |||||||
| Severe bleeding | 4 (5.0) | 0.69 | 0.46–1.02 | NS | 0.71 | 0.53–0.96 | 0.024 |
|
| |||||||
| Severe plasma leakage leading to shock | 2 (2.3) | 0.72 | 0.41–1.26 | NS | 0.73 | 0.48–1.114 | NS |
aAdjusted for age, gender and ethnicity. NS (non-significant): P>0.05. 25-(OH) D: 25-hydroxyvitamin D, CI: confidence interval, DHF: dengue haemorrhagic fever, DSS: dengue shock syndrome, RR: risk ratio, WHO: World Health Organization
WHO 2009 dengue classification
A significant inverse linear trend of association between serum 25-(OH) D and SD (aRR 0.72, 95% CI 0.57–0.91, P = 0.005) was observed after adjusting for age, gender and ethnicity, based on the WHO 2009 dengue criteria as shown in Table 2. Similarly, serum 25-(OH) D had statistically significant association with severe bleeding (aRR 0.71, 95% CI 0.53–0.96, P = 0.024). However, there was no significant association for severe plasma leakage leading to shock (aRR 0.73, 95% CI 0.48–1.114, P = 0.142). The association of low 25-(OH) D with severe bleeding does not appear to be mediated by thrombocytopenia as median 25-(OH) D levels were higher in patients with thrombocytopenia as shown in Table 1. Tables 3 and 4 show a more detailed clinical course of these patients who had SD.
Table 3.
Clinical characteristics of participants with severe dengue based on WHO 1997 dengue classification.
| Characteristic | Subject ID | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 041 | 044 | 047 | 050a | 001a | 061 | |
| Age (yr) | 36 | 44 | 21 | 46 | 31 | 34 |
|
| ||||||
| Gender | Female | Male | Female | Female | Female | Male |
|
| ||||||
| Ethnicity | Chinese | Chinese | Chinese | Chinese | Chinese | Others |
|
| ||||||
| Comorbidities | Nil | Nil | Nil | Hyperlipidaemia, hypothyroidism | Nil | Nil |
|
| ||||||
| Year of presentation | 2012 | 2012 | 2012 | 2012 | 2015 | 2016 |
|
| ||||||
| Day of fever at hospital presentation | 1 | 5 | 2 | 5 | 4 | 4 |
|
| ||||||
| WHO dengue 1997 classification | DHF, DSS | DHF, DSS | DHF | DHF | DHF | DHF |
|
| ||||||
| Haemorrhagic manifestations/ mucosal bleeding | Yes; petechiae | Yes; gum bleeding, petechiae | Yes; petechiae | Yes; menorrhagia, petechiae | Yes; haematemesis | Yes; gum bleeding |
|
| ||||||
| Severe plasma leakage | Yes; hypo-proteinaemia | Yes; hypo-proteinaemia | Yes; haemo-concentration | No | Yes; haemo-concentration | Yes; haemo-concentration |
|
| ||||||
| Key physical exam findings | Hypotension SBP <90 mmHg; No HSM | Hypotension SBP <90 mmHg; No HSM | Not hypotensive; No HSM | Hypotensive SBP <90 mmHg; HSM | Tachycardic >100; No hypotension or HSM | Tachycardic >100; No hypotension or HSM |
|
| ||||||
| Transaminitisb | Moderate | No | Moderate | Mild | Mild | Unknown |
|
| ||||||
| Lowest platelet count (×109/L) | 70 | 73 | 49 | 16 | 56 | 33 |
|
| ||||||
| Platelet transfusion | No | No | No | Yes | No | No |
|
| ||||||
| Length of inpatient stay (day) | 6 | 5 | 3 | 4 | 5 | 5 |
|
| ||||||
| Serum 25(OH) D (mcg/L) | 7.08 | 5.83 | 6.74 | 3.00 | 3.00 | 3.00 |
aSubjects 001 and 050 had severe dengue based on both World Health Organization (WHO) 1997 and 2009 definitions. bTransaminitis definition: ‘mild’ defined as transaminase elevation up to 2 times the upper limit of normal laboratory reference range, ‘moderate’ defined as between 2 and 5 times the upper limit of normal and ‘severe’ defined as more than 5 times the upper limit of normal. (Reference range for AST: 755 units/L, ALT: 848 units/L). 25(OH) D: 25hydroxyvitamin D, ALT: alanine transaminase, AST: aspartate transaminase, DHF: dengue haemorrhagic fever, DSS: dengue shock syndrome, HSM: hepatosplenomegaly, SBP: systolic blood pressure
Table 4.
Clinical characteristics of participants with severe dengue based on WHO 2009 dengue classification.
| Characteristic | Subject ID | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 050a | 023 | 001a | 054 | 008 | 032 | |
| Age (yr) | 46 | 63 | 31 | 50 | 63 | 31 |
|
| ||||||
| Gender | Female | Male | Female | Female | Female | Male |
|
| ||||||
| Ethnicity | Chinese | Chinese | Chinese | Chinese | Chinese | Chinese |
|
| ||||||
| Comorbidities | Hyperlipidaemia, hypothyroidism | Hypertension, hyperlipidaemia | Hyperlipidaemia | Chronic hepatitis B (normal transaminases at baseline) | Hyperlipidaemia, hyperthyroidism, psoriasis, osteoarthritis | Nil |
|
| ||||||
| Year of presentation | 2012 | 2013 | 2015 | 2015 | 2015 | 2016 |
|
| ||||||
| Day of fever at hospital presentation | 5 | 5 | 4 | 2 | 3 | 2 |
|
| ||||||
| WHO dengue 2009 classification | Severe dengue | Severe dengue | Severe dengue | Severe dengue | Severe dengue | Severe dengue |
|
| ||||||
| Haemorrhagic manifestations/mucosal bleeding | Yes; menorrhagia | No | Yes; haematemesis | Yes; rectal bleeding, gum bleeding | No | Yes; rectal bleeding |
|
| ||||||
| Severe plasma leakage | No | No | Yes; haemoconcentration | No | Yes; pleural effusion, radiologically diagnosed | No |
|
| ||||||
| Key physical exam findings | Hypotension SBP <90 mmHg and hepatosplenomegaly | Hypotension SBP <90 mmHg; No hepatosplenomegaly | Tachycardia, HR >100; No hepatosplenomegaly or hypotension | Tachycardia, HR >100; No hepatosplenomegaly or hypotension | Hypotension SBP <90 mmHg; No hepatosplenomegaly | None |
|
| ||||||
| Transaminitisb | Mild | Mild | Mild | Moderate | Moderate | No |
|
| ||||||
| Lowest platelet count (×109/L) | 16 | 40 | 56 | 12 | 12 | 140 |
|
| ||||||
| Platelet transfusion | Yes | No | No | Yes | No | No |
|
| ||||||
| Length of inpatient stay (day) | 4 | 5 | 5 | 3 | 3 | 2 |
|
| ||||||
| Serum 25-(OH) D (mcg/L) | 3.00 | 5.11 | 3.00 | 5.84 | 6.07 | 5.70 |
aSubjects 001 and 050 had severe dengue, both based on World Health Organization (WHO) 1997 and 2009 definitions. bTransaminitis definition: ‘mild’ defined as transaminase elevation up to 2 times the upper limit of normal laboratory reference range, ‘moderate’ defined as between 2 and 5 times the upper limit of normal and ‘severe’ defined as more than 5 times the upper limit of normal. Reference range for AST: 7–55 units/L and for ALT: 8–48 units/L). 25-(OH) D: 25-hydroxyvitamin D, ALT: alanine transaminase, AST: aspartate transaminase, HR: heart rate, SBP: systolic blood pressure
DISCUSSION
We report the association of low systemic 25-(OH) D with higher dengue severity (WHO, 2009), particularly for bleeding manifestations that are not explained by thrombocytopenia in our adult cohort study. The bleeding manifestations were mainly mucosal bleeding, and none of the patients required blood transfusions or intensive care unit care [Tables 3 and 4]. A small number of patients received platelet transfusions in the setting of bleeding. The strength of our study is that it is one of few clinical studies investigating the association between systemic 25-(OH) D and dengue disease severity outcomes based on WHO 1997 and 2009 criteria in adults in a cohort that includes older adults. The use of standardised dengue clinical care path that contains clinical and laboratory data for the course of dengue illness ensures systematic method of collection and minimises bias.
Examining the potential role of immunomodulators and modifiable factors, such as systemic 25-(OH) D, is an approach that may have translational potential to attenuate disease severity. Importantly, the 25-(OH) D threshold defining ‘deficiency’ is based on its role in bone health and thresholds defining immune-relevant actions are not known. The 25-(OH) D is the main systemically available form of vitamin D with a half-life of 2–3 weeks and is reflective of an individual’s vitamin D stores.[23,24] Of significance, biologically active form of vitamin D, that is, calcitriol or 1,25-(OH)2 D3, is also locally produced (CYP27B1, 1-alpha hydroxylase) in various immune cells from systemic 25-(OH) D. Vitamin D receptor (VDR) is expressed in many human tissues including cells from the innate and adaptive immune systems, and VDR binds systemically available and locally produced 1,25-(OH)2 D, leading to downstream tissue-specific intracrine and paracrine actions.[11,12]
Vitamin D deficiency is not uncommon in Singapore and other tropical dengue-endemic areas despite higher year-round exposure to ultraviolet rays.[31,32] Our study participants had overall low 25-(OH) D levels at the acute time point, and lower levels were observed in those of Malay and Indian ethnicity compared to Chinese, as has been reported in other studies.[31] The comparatively higher 25-(OH) D levels in older participants may have been from supplementation (non-prescription); however, this data were not available to the study team.
The immune mechanisms for the association of 25-(OH) D with dengue disease course and severity are not entirely elucidated. Few authors have evaluated this in more detail. Of interest, an in vitro study involving human myelomonocytic and hepatic cell lines exposed to various concentrations of 1,25-(OH)2 D3, which were subsequently infected with dengue virus serotype (DENV)-4, found significantly reduced percentage of infected cells and reduced production of TNF-α, IL-1B, IL-6 and IL-12p70, with a dose–response relationship observed with 1,25-(OH)2 D3.[15] The underlying immune mechanisms are not yet clear. Arboleda Alzate et al.[20] exposed monocyte-derived macrophages (from healthy volunteers) in vitro to varying concentrations of 1,25-(OH)2 D3 and subsequently infected them with DENV-2. The macrophages that differentiated in the presence of higher 1,25-(OH)2 D3 concentrations had decreased DENV-2 infectivity, potentially due to reduced expression of receptors required for DENV entry into macrophages, and also had reduced proinflammatory cytokine levels (specifically TNF α, IL-1β and IL-10) in response to DENV infection. Another in vitro study challenged monocyte-derived macrophages from participants enrolled in a vitamin D supplementation study with DENV-2. Macrophages from participants exposed to higher-dose (4000 IU/day) supplementation were not as susceptible to DENV-2 infection as compared to those that received lower-dose supplementation, thereby showing its a protective effect.[18] The TNF-α levels were lower while IL-10 and IL-8 were higher in the higher-dose supplementation group. However, serum 25-(OH) D levels were not quantified in this study. Interestingly, a recent in vitro study examining seven VDR agonists found five of the compounds significantly inhibited DENV-2 infection of HEK293T/17 cells with reduced virus production of up to 3 Log10.[33] There are many immunological postulations as to how vitamin D may be influencing the susceptibility to infection and inflammatory response; however, this still needs further study.[18]
There are a few limitations in our study that could be addressed in future studies. Since we invited previously enrolled participants to participate in this study, there is possibility of bias in recruitment, as some participants were not contactable for informed consent. The number of SD patients in this cohort was limited. We also did not have control groups of non-dengue febrile patients or well patients without any febrile illness. We did not perform a sample size calculation a priori as this was designed as a pilot study, hence our study was not sufficiently powered to examine the effects that 25-(OH) D might exert on different subgroups of patients and the severity indicators of dengue. Although multivariable models were used to control for the main confounding variables, residual confounding might persist. A commonly used immunoassay bench method was used for total 25-(OH) vitamin D measurement, rather than an ID-LC-MS/MS reference method. Such methods generally show poorer precision than reference methods and do not allow differentiation of vitamin D2 from vitamin D3. However, the assay was traceable to the reference method, and the lower accuracy and precision should not have affected the conclusions of this study.
In conclusion, further studies are needed in cohorts with a higher number of SD patients to validate our findings, and preferably, they should include control groups. Underlying immune and other mechanisms should also be studied, such as effects on vascular endothelium, certain markers of innate and adaptive immunity as well as cytokine responses, where appropriate. We note that few other clinical studies have shown higher 25-(OH) D is associated with higher probability of DHF/DSS,[17,19] which is contrary to findings from human monocyte studies.[18,20] Whether this is related to the timing of venepuncture, phase of dengue illness, population variability, performance of assay or other factors remains unclear.
An emerging concept in the understanding of the non-skeletal actions of 25-(OH) D is the ‘personal vitamin D response index’, which is thought to arise from a set of molecular and epigenetic variations in the vitamin D signalling pathway.[34] This may, in turn, explain variable ‘threshold’ of ‘sufficiency’ or vitamin D responsiveness for certain individuals and population groups, and in turn may potentially explain the conflicting results of vitamin D observational and supplementation studies as mentioned in the article. Ideally, well-designed human intervention studies with vitamin D supplementation or VDR agonists should include baseline 25-(OH) D, evaluate various dosing regimens while also stratifying based on the ‘vitamin D response index’ of the study population once this is better defined.
In summary, our study found low systemic 25-(OH) D was associated with increased dengue disease severity based on the WHO 2009 criteria, particularly for severe bleeding that was not explained by thrombocytopenia. Further studies are needed in cohorts with a higher number of SD patients. Studies on the impact of 25-(OH) D on the course of dengue infection in terms of underlying immune mechanisms and effects on the vascular endothelium are needed.
Financial support and sponsorship
We acknowledge funding support from NMRC Centre Grant (Ref. NMRC/CG/003/2013).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank all the study participants. Residual sera were received from National Medical Research Council (NMRC) Translational and Clinical Research STOP Dengue Programme (Ref. NMRC/TCR/005/2008).
REFERENCES
- 1.World Health Organization. Dengue: Guidelines for diagnosis, treatment, prevention and control 2009. [[Last accessed on 2019 Oct 01]]. Available from: https://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf . [PubMed]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Shachar R, Koelle K. Minimal within-host dengue models highlight the specific roles of the immune response in primary and secondary dengue infections. J R Soc Interface. 2015;12:20140886. doi: 10.1098/rsif.2014.0886. doi: 10.1098/rsif. 2014.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Htun NSN, Odermatt P, Eze IC, Boillat-Blanco N, D’Acremont V, Probst-Hensch N. Is diabetes a risk factor for a severe clinical presentation of dengue? --Review and meta-analysis. PLoS Negl Trop Dis. 2015;9:e0003741. doi: 10.1371/journal.pntd.0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee I-K, Hsieh C-J, Chen R-F, Yang Z-S, Wang L, Chen C-M, et al. Increased production of Interleukin-4, Interleukin-10, and granulocyte-macrophage colony-stimulating factor by type 2 diabetes'mononuclear cells infected with dengue virus, but not increased intracellular viral multiplication. BioMed Res Int. 2013;2013:965853. doi: 10.1155/2013/965853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivino L, Lim MQ. CD4+and CD8+T-cell immunity to Dengue –lessons for the study of Zika virus. Immunology. 2017;150:146–54. doi: 10.1111/imm.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. [[Last accessed on 2019 Oct 01]];Dengue vaccine: WHO position paper. 2018 93:457–476. Available from: https://www.who.int/wer/2018/wer9336/en/ [Google Scholar]
- 8.Biswal S, Reynales H, Saez-Llorens X, Lopez P, Borja-Tabora C, Kosalaraksa P, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med. 2019;381:2009–19. doi: 10.1056/NEJMoa1903869. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Liu J, Cheng G. Vaccines and immunization strategies for dengue prevention. Emerg Microbes Infect. 2016;5:1–6. doi: 10.1038/emi.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low JGH, Ooi EE, Vasudevan SG. Current status of dengue therapeutics research and development. J Infect Dis. 2017;215((Suppl 2)):S96–102. doi: 10.1093/infdis/jiw423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang PO, Aspinall R. Can We translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients. 2015;7:2044–60. doi: 10.3390/nu7032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh J-C, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 13.Sadarangani SP, Whitaker JA, Poland GA. “Let there be light”: The role of vitamin D in the immune response to vaccines. Expert Rev Vaccines. 2015;14:1427–40. doi: 10.1586/14760584.2015.1082426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puerta-Guardo H, De la Cruz Hernández SI, Rosales VH, Ludert JE, del Angel RM. The 1α,25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell lines and cytokine production in the infected monocytes. Antiviral Res. 2012;94:57–61. doi: 10.1016/j.antiviral.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Loke H, Bethell D, Phuong CXT, Day N, White N, Farrar J, et al. Susceptibility to dengue hemorrhagic fever in vietnam: Evidence of an association with variation in the vitamin D receptor and Fc gamma receptor IIa genes. Am J Trop Med Hyg. 2002;67:102–6. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 17.Alagarasu K, Bachal RV, Bhagat AB, Shah PS, Dayaraj C. Elevated levels of vitamin D and deficiency of mannose binding lectin in dengue hemorrhagic fever. Virol J. 2012;9:86. doi: 10.1186/1743-422X-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giraldo DM, Cardona A, Urcuqui-Inchima S. High-dose of vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: An exploratory study. Clin Chim Acta. 2018;478:140–51. doi: 10.1016/j.cca.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 19.Villamor E, Villar LA, Lozano A, Herrera VM, Herrán OF. Vitamin D serostatus and dengue fever progression to dengue hemorrhagic fever/dengue shock syndrome. Epidemiol Infect. 2017;145:2961–70. doi: 10.1017/S0950268817002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arboleda Alzate JF, Rodenhuis-Zybert IA, Hernández JC, Smit JM, Urcuqui-Inchima S. Human macrophages differentiated in the presence of vitamin D3 restrict dengue virus infection and innate responses by downregulating mannose receptor expression. PLoS Negl Trop Dis. 2017;11:e0005904. doi: 10.1371/journal.pntd.0005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voge NV, Perera R, Mahapatra S, Gresh L, Balmaseda A, Loroño-Pino MA, et al. Metabolomics-based discovery of small molecule biomarkers in serum associated with dengue virus infections and disease outcomes. PLoS Negl Trop Dis. 2016;10:e0004449. doi: 10.1371/journal.pntd.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langerman SD, Ververs M. Micronutrient supplementation and clinical outcomes in patients with dengue fever. Am J Trop Med Hyg. 2021;104:45–51. doi: 10.4269/ajtmh.20-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine (IOM) Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington, DC: The National Academies Press; 2011. 1132 p. [PubMed] [Google Scholar]
- 25.Barkham TM, Chung YK, Tang KF, Ooi EE. The performance of RT-PCR compared with a rapid serological assay for acute dengue fever in a diagnostic laboratory. Trans R Soc Trop Med Hyg. 2006;100:142–8. doi: 10.1016/j.trstmh.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gan VC, Tan L-K, Lye DC, Pok K-Y, Mok S-Q, Chua RC-R, et al. Diagnosing dengue at the point-of-care: Utility of a rapid combined diagnostic kit in Singapore. PLoS One. 2014;9:e90037. doi: 10.1371/journal.pone.0090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blacksell SD, Newton PN, Bell D, Kelley J, Mammen MP, Jr, Vaughn DW, et al. The comparative accuracy of 8 commercial rapid immunochromatographic assays for the diagnosis of acute dengue virus infection. Clin Infect Dis. 2006;42:1127–34. doi: 10.1086/501358. [DOI] [PubMed] [Google Scholar]
- 28.Carter GD. 25-Hydroxyvitamin D: A difficult analyte. Clin Chem. 2012;58:486–8. doi: 10.1373/clinchem.2011.180562. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 30.Zou G. A Modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Man REK, Li L-J, Cheng C-Y, Wong TY, Lamoureux E, Sabanayagam C. Prevalence and determinants of suboptimal vitamin D levels in a multiethnic Asian population. Nutrients. 2017;9:313. doi: 10.3390/nu9030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi X, Tey SL, Leong C, Quek R, Henry CJ. Prevalence of vitamin D deficiency in Singapore: Its implications to cardiovascular risk factors. PLoS One. 2016;11:e0147616. doi: 10.1371/journal.pone.0147616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaratsittisin J, Xu B, Sornjai W, Weng Z, Kuadkitkan A, Li F, et al. Activity of vitamin D receptor agonists against dengue virus. Sci Rep. 2020;10:10835. doi: 10.1038/s41598-020-67783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol. 2018;175:12–7. doi: 10.1016/j.jsbmb.2016.12.011. [DOI] [PubMed] [Google Scholar]


