Abstract
PURPOSE:
To assess central and peripheral retinal and choroidal diseases using ultra-widefield (UWF) fundus imaging in combination with navigated central and peripheral cross-sectional and three-dimensional (3D) swept source optical coherence tomography (SS-OCT) scans.
METHODS:
Retrospective study involving 332 consecutive patients, with a nearly equal distribution of males and females. The mean age of patients was 52 years (range 18–92 years). Average refractive error was −3.80 D (range +7.75 to −20.75 D).
RESULTS:
The observations in this study demonstrate the efficacy of peripheral navigated SS-OCT in assessing various ocular conditions. The technology provides high-quality images of the peripheral vitreous, vitreoretinal interface, retina, and choroid, enabling visualization of vitreous floaters and opacities, retinal holes and tears, pigmented lesions, and peripheral retinal degenerations. 3D OCT scans enhance the visualization of these abnormalities and improve diagnostic and therapeutic decisions.
CONCLUSION:
Navigated central and peripheral cross-sectional and 3D SS-OCT scans offer significant complementary benefits in the assessment and management of retinal diseases. Their addition to UWF imaging provides a comprehensive view of central and peripheral ocular structures, aiding in early detection, precise anatomical measurements, and objective monitoring of disease progression. In addition, this technology serves as a valuable tool for patient education, a teaching tool for trainees, and documentation for medico-legal purposes.
Keywords: Branch vein occlusion, diabetic retinopathy, inherited retinal diseases, lattice degeneration, navigated swept source optical coherence tomography, ora serrata, pigmented lesion, retinal detachment, retinal hole, retinal tear, retinal vasculopathies, retinitis pigmentosa, retinoschisis, sickle cell retinopathy, swept source optical coherence tomography, three-dimensional navigated optical coherence tomography, ultra widefield imaging, vitreoretinal tuft, vitreous floaters and opacities, vitreous, widefield imaging
INTRODUCTION
Retinal imaging has undergone dramatic improvements since the invention of the first ophthalmoscope by Hermann von Helmholtz in 1851.[1,2] Recent developments in ultra-widefield (UWF) angiographic imaging with simultaneous navigated central and peripheral optical coherence tomography (OCT) have led to a better understanding of vitreoretinal and choroidal diseases.[3]
The introduction of a broader spectrum of wavelengths in UWF imaging modalities, including red-green (RG), fundus autofluorescence (FAF), fundus fluorescein angiography (FFA), and indocyanine green (ICG) angiography has enabled not only more precise diagnoses but also prognoses for numerous conditions.[3,4,5] The use of these multimodal UWF imaging techniques has demonstrated to be essential in the daily clinical practice, especially in detecting retinal vasculopathies such as diabetic retinopathy (DR),[3,6,7,8,9,10,11] vein occlusions,[3,12,13,14] and sickle cell retinopathy (SCR),[3,15,16,17,18,19,20] among others.[1,21,22]
The dawn of OCT has revolutionized diagnostics in ophthalmology since David Huang demonstrated this for the first time in 1991.[23] Over the years, OCT has undergone numerous improvements in speed, resolution, and depth of image[24] and has now become one of the most important clinical imaging tools for the evaluation of the vitreous, vitreoretinal interface (VRI), retina, and choroid.[25]
However, while conventional OCT systems play a key role in diagnosing posterior pole diseases, they are not able to image the peripheral retina.[26] This limitation can be overcome by devices that allow for tilting of the scanner head, such as the spectral-domain OCT based Spectralis device (Heidelberg Engineering, Germany), or UWF devices with incorporated OCT such as the Silverstone device (Optos PLC, Dunfermline, UK), which uses swept source OCT (SS-OCT) technology.[5]
Furthermore, standard OCT evaluation of ocular structures is often limited due to the two-dimensional nature of the OCT scans,[26] whereas three-dimensional (3D) images potentially allow for a more comprehensive assessment of the vitreous, VRI, retina, and choroid, which are inherently 3D structures.[26,27,28,29] Stacks of cross-sectional SS-OCT images yield 3D images, thus providing a noninvasive technique for obtaining spatial and volumetric information about these structures. Such 3D OCT scans also allow measurement and objective monitoring of shape and volume of both normal and pathological ocular tissues.[26,27,28,29]
The evaluation of the peripheral retina using UWF devices has gained significant interest, being particularly useful in patients with poor pupil dilation or with media opacity where standard ophthalmoscopy may be challenging to carry out.[1,3]
The purpose of this study was to obtain high quality steering-based navigated SS-OCT images of the vitreous, VRI, retina, and choroid to visualize and identify peripheral pathologies either using cross-sectional and/or 3D volume rendering scans.
METHODS
A total of 332 consecutive adult patients (664 eyes) aged 18 years and above were scanned by three experienced operators (SEFS, UIR, and FJVB) at The Retina Clinic London between July 2022 and July 2023. Subjects selected for this study signed a full consent form permitting their clinical data and imaging results to be retrospectively analysed and reported. The inclusion criteria were age >18 years. We used the following exclusion criteria: high media opacities, previous history of advanced ocular disorders causing the complete disruption of retinal anatomy posterior vitreoretinal alterations (e.g., familiar exudative retinopathies and uveitis), previous history of systemic disorders potentially affecting the interpretation of the findings (e.g., uncontrolled diabetes and uncontrolled arterial hypertension), or any ocular surgery performed in the 6 months before the inclusion in the study.
The procedures used conformed to the tenets of the Declaration of Helsinki. This retrospective study was performed in accordance with local data protection regulations.
Vitreoretinal examination and assessment
Each patient underwent a complete ophthalmic examination as part of their standard care, including best-corrected visual acuity in logMAR, slit-lamp biomicroscopy (Topcon Sl-D70, Topcon Corp.), indirect ophthalmoscopy (Pan Retinal® 2.2 hand-held lens, Volk Optical, OH, USA) with scleral indentation (hand-held scleral depressor, E5106, Storz, St. Louis, USA), and UWF and navigated SS-OCT scans using the Optos Silverstone (Optos PLC, Dunfermline, Scotland, United Kingdom).
Imaging technique
A custom dilation and imaging protocol was used. Patients were dilated with Tropicamide (1%) and Phenylephrine (2.5%) before UWF imaging and WF SS-OCT scans. No additional lenses or refractive correction were required.
All patients were scanned using the Optos Silverstone (Optos PLC, Dunfermline, Scotland, United Kingdom) which produces a 200° single capture RG, FAF, FFA, or ICGA image. In addition, navigated peripheral WF SS-OCT images were taken with a scanning speed of 100,000 A-scans per second using a central laser wavelength of 1050 nm.[1] Only high-quality images, assessed by manufacturer quality indexes, were included. Poor quality data were re-acquired or discarded.
Prior to selecting the area to perform the SS-OCT scans, RG images were taken with the eye first in primary position and then steered toward superior, temporal, inferior, and nasal directions. Further central 200° single-capture scans were taken in the FAF, FFA or ICGA modes. In addition, a raster scan centred over the foveal depression with an area of 14 mm × 9 mm was obtained. Navigated standard volume scans of 6 mm × 6 mm or high-definition volume scans of 6 mm × 3.5 mm centred onto a specific area that corresponded with a vitreo-retinal or chorio-retinal abnormality were performed.
Three-dimensional image processing
The OptosAdvance™ software (Optos PLC, Dunfermline, Scotland, United Kingdom) allows to create 3D images in both the central and peripheral retina. These 3D OCT volume scans provide accurate spatial localization of vitreoretinal and chorioretinal features observed on the en face and cross-sectional images. Together with the UWF images obtained by the Optos Silverstone (RG, FAF, FFA and ICGA), it was possible to reliably diagnose retinal abnormalities and monitor morphological changes over time. The study aimed to assess central and peripheral vitreoretinal alterations in a large population. For this reason, a statistical analysis is not required.
RESULTS
Of the 332 consecutive patients, 172 were male and 160 were female. The mean age was 52 years (range 18–92 years) and the average refractive error was −3.80 D (range: +7.75 to −20.75 D).
High-quality and medically meaningful images of the peripheral retina and VRI were obtained in 657 eyes (98.95%). UWF imaging and SS-OCT revealed several vitreoretinal abnormalities, including vitreous floaters and opacities (VFO), posterior vitreous detachment (PVD), retinal holes and tears, pigmented lesions, and peripheral retinal degenerations.
3D central and peripheral SS-OCT enhanced the visualization of these findings. The ability to rotate volume scans provided different viewing angles, aiding image interpretation and improving the diagnostic and therapeutic options.
Ora serrata
The visualization of ora serrata and pars plana cysts was achievable in eyes with large mydriasis and good patient collaboration, when steering the UWF images and navigating the WF SS-OCT scans.
Pars plana cysts which appear as smooth, bullous elevations between the nonpigmented and pigmented ciliary epithelium, were effectively identified using peripheral SS-OCT [Figure 1]. In addition, a denser vitreous with tight attachment to the retina was also observed in the same region [Figure 1].
Figure 1.

(a) Sectorial ultra-widefield (UWF) red-green (RG) image (Optos Silverstone) of a healthy 34-year-old female demonstrates a clear representation of the ora serrata and vitreous base (green arrow). (b) A cross-sectional scan using navigated peripheral swept source optical coherence tomography (OCT) (Optos Silverstone) shows a cystic cavity with underlying retinal atrophy corresponding to a pars plana cyst (green arrow). (c) Sectorial UWF RG image of a 58- year-old female suffering from vitreous floaters and opacities. (d) A hyperreflective vitreous cortex condensation corresponding to a Weiss ring (red arrow) can be seen using a central cross-sectional OCT scan. (e) This peripheral three-dimensional (3D) OCT scan highlights the vitreous anatomy and its characteristic cobweb structure (yellow arrow). (f) This central 3D OCT scan clearly visualizes the posterior cortical vitreous (red arrows), allowing a better assessment of an on-going partial posterior vitreous detachment
Furthermore, far peripheral yellow dots were frequently detected in UWF RG scans in schitic areas of myopic and highly myopic eyes. These pinpoint lesions corresponded to hyperreflective dots within the retina layers, as revealed by cross-sectional OCT scans.
Vitreous
The use of central and peripheral cross-sectional SS-OCT showed a detailed view of the vitreous structure and the status of the VRI. This technology enabled the visualization of VFO, as well as fluid-filled spaces within the vitreous, including cisterns. It was possible to observe these features from any angle in the 3D reconstructed images of the vitreous [Figure 1].
Both cross-sectional SS-OCT and 3D volume scans were essential in assessing the vitreous status, specifically in determining the presence or absence of a PVD [Figure 1]. For example, a vitreous attachment along the posterior pole, with vitreous, however, detached above the temporal vascular arcades, was a common finding in our cohort, thus underlining how UWF multimodal imaging may reveal more information compared to OCT scans taken only in the posterior pole area. The ability to rotate the image in 3D view also offered a comprehensive understanding of the vitreous anatomy, its characteristic “cobweb” structure and the area of vitreous detachment [Figure 1].
Furthermore, the use of central and peripheral 3D scans facilitated the detection of inflammatory cells and blood cells.
Retinal holes and tears
A total of 56 retinal holes and tears were identified by RG UWF scans. These were further and better characterized using the complementary navigated SS-OCT. Laser retinopexy was performed in 30 eyes presenting with retinal holes/tears (53.57%) based and guided on interpretation of the cross-sectional and 3D scans.
Navigated OCT provided more reliable information about the severity and extent of peripheral retinal diseases compared to UWF imaging alone. For example, the navigated SS-OCT over a suspicious area detected in UWF scans accurately identified retinal holes and tears with or without vitreous attachment, enabling a precise treatment plan with laser retinopexy and appropriate monitoring of the area [Figure 2].
Figure 2.

Retinal Holes and Retinal Tears. (a) Sectorial ultra-widefield (UWF) red-green (RG) image demonstrates a superonasal retinal hole. (b) Cross-sectional peripheral swept source optical coherence tomography (SS-OCT) of A allows to assess the retinal hole (red arrow) and reveals absence of subretinal fluid or vitreoretinal traction. The three-dimensional scan is useful to evaluate the extent of the retinal damage (white arrow). (c) Sectorial UWF RG image demonstrates an inferotemporal retinal hole (red arrow). (d) The three-dimensional (3D) SS-OCT scan of the same eye not only reveals the extent of the damage on the retinal surface (white arrow) but also shows the piece of retinal tissue floating within the vitreous cavity (orange arrow). (e) An extremely peripheral superonasal retinal tear (blue arrow) can be shown using an UWF RG scan. (f) The peripheral 3D SS-OCT scan in the same eye highlights the area of the retina affected (white arrow) and shows vitreoretinal traction on the edge of the tear (yellow arrow)
The visualization of vitreous traction attached to the edge of the hole or apex of the tear flap was identified using either cross-sectional or 3D SS-OCT scans. The 3D volume scans accurately depicted the extent of the vitreous attachment to the retina in these delicate areas.
In addition, the presence of subretinal fluid (SRF) detected by peripheral cross-sectional SS-OCT scans beneath retinal holes and tears was essential in the decision-making of treatment of such abnormalities. OCT was a valuable complementary tool to establish personalized follow-up timing and to monitor SRF over time after laser treatment, confirming the efficiency of the performed retinopexy.
A potential consequence of a peripheral retinal tear retinopexy is the development of a peripheral epiretinal membrane (pERM) as was observed by UWF RG and FFA images and confirmed by peripheral SS-OCT [Figure 3]. This development has a higher prevalence in patients suffering from inflammatory disease [Figure 3].
Figure 3.
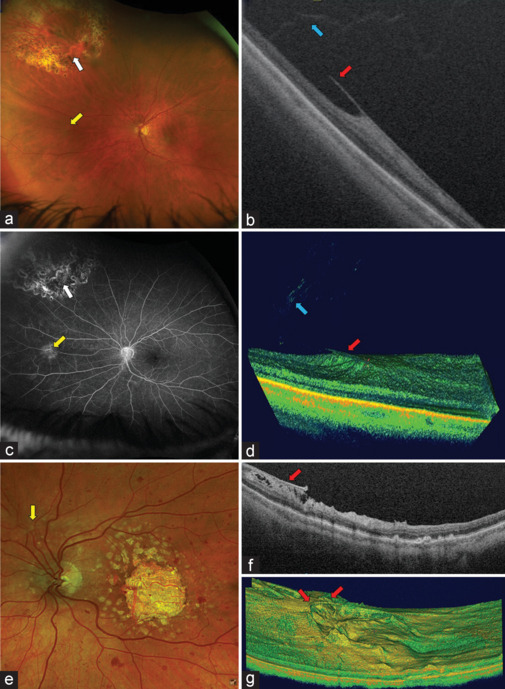
Peripheral Epiretinal Membrane (pERM). (a) Ultra-widefield (UWF) red-green (RG) image demonstrates a previously treated peripheral retinal tear (white arrow). A retinal area with tortuosity of a retinal blood vessel is observed below the tear (yellow arrow). (b) Cross-sectional swept source optical coherence tomography (SS-OCT) of the same area shows a pERM (red arrow) and the attachment of peripheral vitreous (blue arrow). (c) The UWF FFA scan of the same eye as in image A highlights a scar in a retinal tear area (white arrow) and demonstrates a hyperfluorescent area (yellow arrow) that corresponds to the pERM in the previously treated retinal tear area. (d) Peripheral navigated three-dimensional (3D) SS-OCT scans allow to assess the extension and volume of the pERM (red arrow) of images A to C as well as the vitreous status in the same area (blue arrow). (e) Sectorial UWF image of a patient with advanced geographic atrophy and retinal haemorrhages in all four quadrants possibly due to an inflammatory process. An epiretinal tissue that modifies the direction of a blood vessel (yellow arrow) is shown. (f) Navigated cross-sectional SS-OCT demonstrates a pERM (red arrow) in the same eye as in E. (g) 3D rendering allows to assess the extent and elevation of the pERM (red arrows) of the same eye, providing additional information
Peripheral retinoschisis and retinal detachments
The combined use of UWF imaging and SS-OCT significantly contributed to the precise diagnosis of retinoschisis versus retinal detachment (RD), thereby facilitating the planning of either observation or treatment. Navigated peripheral SS-OCT allowed to identify the presence of schisis with outer or inner retinal breaks, particularly in challenging cases, by demonstrating the layer of splitting within the neurosensory retina. The 3D scans enabled the assessment of the extend of schisis over time.
A total of ten rhegmatogenous retinal detachments (RRD) and one tractional RD were detected and assessed using this imaging technique. In addition, one extreme peripheral subclinical chronic RRD was identified. Peripheral SS-OCT confirmed the neurosensorial detachment from the retinal pigment epithelium and revealed the signs of chronicity such as intraretinal cysts. Despite the chronicity, the subclinical RD was “walled off” with laser retinopexy to prevent further progression, possible surgical intervention and vision loss [Figure 4].
Figure 4.

Retinal detachment (RD). (a, c and e) Sectorial ultra-widefield (UWF) red-green (RG) images of a RD. (b) Navigated cross-sectional swept source optical coherence tomography (SS-OCT) showing one of the multiple retinal tears that lead to the RD. The high-quality scans allow to visualize the vitreous attached to the retina (green arrows) and an on-going retinal tear (red arrow). (d) In this SS-OCT scan, the vitreous is still attached in some areas (green arrow) while it is pulling away from the retina in other areas causing traction resulting in the RD (yellow arrows). (f) Posterior pole SS-OCT scan demonstrating the RD (red arrow) with an incomplete macular hole (green arrow) and the morphologic changes in the photoreceptor layers (orange arrows). Hyperreflective dots within the vitreous cavity can be observed (white circle). (g) UWF RG image showing a chronic and inferior RD with proliferative vitreoretinopathy (PVR). (h) Peripheral cross-sectional SS-OCT scan of the same eye allows the visualization of PVR with epiretinal (red arrow), intraretinal (orange arrow) and subretinal membranes (blue arrow). (i) UWF RG image demonstrating an asymptomatic chronic RD with retinal tear (red arrow) and recent laser retinopexy spots (white arrow) right next to the chronic RD area. (j) Extreme peripheral SS-OCT scans of the same eye show the morphology of the RD (red arrow) highlighting additional schitic changes within the retina (yellow arrow), which is useful in understanding the chronicity of the RD
Lattice degeneration
The classic features of lattice degeneration (LD), such as thinning of the neurosensory retina and presence of a vitreo-retinal attachment, were clearly visible using navigated SS-OCT. Furthermore, peripheral OCT aided in detecting the absence or presence of related SRF and in identifying partial or full-thickness atrophic retinal holes and tears associated with LD.
Vitreoretinal tufts
Peripheral and navigated OCT scans allowed to identify vitreoretinal tufts and differentiate them from retinal tears.
Retinal vascular disease
The use of UWF imaging such as RG and FFA, in tandem with cross-sectional and 3D volume SS-OCT scans, enabled us to assess in-depth mid and far peripheral areas of neovascularization and ischemia.
The peripheral navigated SS-OCT scans identified neovascularization growing toward the vitreous, particularly evident in those regions with hyperfluorescent leakage visualized on UWF FFA images. The detection of abnormal blood vessels was considerably enhanced using 3D volume scans compared to cross-sectional OCT scans [Figure 5].
Figure 5.

Proliferative diabetic retinopathy (PDR). (a) Ultra-widefield (UWF) red-green (RG) image allows to identify retinal haemorrhages (green arrow) in a mid-aged female suffering from PDR. (b) The UWF FFA scan of the same eye as in A demonstrates a vascular pin-point leak (yellow arrow). (c) Cross-sectional swept source optical coherence tomography (SS-OCT) scan showing two blood-vessels growing towards the vitreous cavity (red arrows). (d and e) Peripheral 3D SS-OCT scans of the same eye as in C more accurately allow to identify these two additional new blood vessels (red arrows). (f-h) Clinical features of PDR such as a cotton-wool spot (blue arrow) can be visualized and monitored using a UWF RG image (f), a cross-sectional SS-OCT scan (g) and three-dimensional volume rendering (h)
Cross-sectional scans were also useful to monitor cotton wool spots and retinal hemorrhages in DR patients, with nerve fiber layers thickened and appearing hyperreflective [Figure 5].
SS-OCT, especially when used in 3D mode, played a critical role in assessing response to targeted retinal photocoagulation procedures in 21 eyes suffering from proliferative diabetic retinopathy (PDR) or branch vein occlusion. Posttreatment OCT scans identified vascular regression and fibrosis following therapy with either anti-vascular endothelial growth factor or laser photocoagulation.
Moreover, central and peripheral SS-OCT was also useful to assess vitreoretinal tractions in PDR patients and the typical “sea fan” neovascularization with traction in SCR.
Oncology and pigmented lesions
UWF RG imaging allowed the detection of several pigmented lesions. Peripheral SS-OCT was performed in 19 subjects. Of these, 14 patients were diagnosed with choroidal nevi, 4 with congenital hypertrophy of retinal pigment epithelium, and 1 with choroidal melanoma.
UWF multi-wavelength imaging showed the presence or absence of orange pigment, and UWF FFA was performed to rule out associated choroidal neovascularization when SRF was present. The complementary use of cross-sectional SS-OCT was essential to assess the presence or absence of SRF, intraretinal fluid (IRF), and drusen. 3D volume scans enabled to measure the dimensions of the lesion supplementing clinical examination, UWF, and ultrasound (US) scans.
Inherited retinal diseases: Sectorial retinitis pigmentosa
Simultaneous UWF RG or UWF FAF scans with SS-OCT clearly detected IRD and related complications, such as sectorial retinitis pigmentosa (RP) with vitreomacular attachment and secondary cystoid macular edema. Notably, UWF FAF highlighted the typical sectorial RP pigment dispersion along the inferior hemiretina. Navigated UWF and peripheral SS-OCT in the infero-temporal mid-and far-periphery identified intraretinal pigment dispersion in the cross-sectional and 3D images [Figure 6].
Figure 6.

Sectorial Retinitis Pigmentosa. (a) Ultra-widefield (UWF) red-green (RG) image demonstrating inferior hemiretinal sectorial RP with the distinctive bone spicules. (b) Vitreoretinal traction (green arrow) with central macular oedema can be observed using central cross-sectional optical coherence tomography (OCT) scans. (c) Maximum intensity projection showing the vitreoretinal traction (green arrow). (d) UWF FAF scan highlights the typical sectorial RP pigment dispersion along the inferior hemiretina. (e) Navigated UWF and peripheral cross-sectional SS-OCT around the inferotemporal mid- and far- periphery demonstrating intraretinal pigment dispersion (red arrow). (f) The pigment dispersion is more visible in detail (red arrow) using the peripheral three-dimensional swept source-OCT scan modality
DISCUSSION
The adequate visualization of the peripheral retina is of importance in ocular disease surveillance, especially in detecting clinically silent pathologies originating from a peripheral area and are generally not visualized with standard imaging.[30]
The advent of UWF imaging has further enhanced these capabilities, offering a more comprehensive view of the retina.[3] Peripheral and navigated SS-OCT has emerged as a pivotal tool in the detection of peripheral retinal lesions, providing clinicians a noninvasive, fast scanning, high-resolution imaging modality that can visualize the retina layers.[3,26]
The results of this study underscore the significant role of peripheral OCT in enhancing our understanding of various retinal conditions and improving patient outcomes. The ability to obtain high-quality images of the peripheral retina and VRI in our patients is a testament to the potential of this technology in the clinical practice. Our study has also demonstrated that the routine use of these tools enhances clinical decision-making.
Ora serrata
The ora serrata, an anatomical landmark often referred to as the most anterior edge of the retina,[31,32] can be clearly visualized using these advanced technologies. The anatomy of the ora serrata seems to play a role in the pathogenesis of peripheral retinal tears or RDs as the vitreous base close to the ora serrata bears the strongest attachment capability of the vitreous to the retina.[33,34] Visualization of the ora serrata is important as associations of pars plana cysts with microcystoid degeneration, chorioretinal scars and retinoschisis have been reported in the literature.[35]
Although UWF devices do not have the ability yet to assess the entirety of the retina, we were able to visualize these extreme peripheral areas by indirect ophthalmoscopy with or without scleral indentation. A well-trained imaging technician and the patient’s collaboration were necessary to capture meaningful peripheral SS-OCT scans of these extreme peripheral areas.
We believe that imaging the ora serrata and the far peripheral retina will equip eye care specialists with a deeper understanding of the pathophysiology of ocular and systemic diseases. In addition, its visualization may also potentially help vitreoretinal surgeons to more accurately plan laser or surgical procedures.
Vitreous
The assessment of the vitreous and VRI with respect to the absence or presence of VFO bears an increasing importance among eye care specialists.[36,37] There is no doubt about the significance of the vitreous status and its relationship with both central and peripheral VRI abnormalities such as retinal holes, retinal tears or RD.[36]
We demonstrated that assessment of the peripheral vitreous with guided OCT is essential as persistent vitreous adhesion either during or before PVD can lead to retinal traction, specifically in sites of strong vitreoretinal adhesion such as the vitreous base.
SS-OCT imaging also confirmed its usefulness to assess and monitor the important role of the vitreous in inflammatory and vascular diseases and to track the clearance of vitreous hemorrhages.
Retinal holes and tears
The progressive risk associated with a retinal hole is generally considered low in asymptomatic patients.[38,39] Atrophic holes are thought to be unrelated to vitreoretinal traction, hence they are rarely treated.[37] However, recent articles suggest that certain types such as operculated holes which form through increased vitreoretinal traction, can be accompanied by SRF and thus can be linked to an increased risk of RD.[40,41]
In our study, peripheral SS-OCT scans were useful not only to detect holes and tears but also to detect abnormalities that can influence management, such as partial vitreous detachment and SRF. The use of cross-sectional and 3D volume scans was essential in decision-making and enabled us to treat 53.57% of eyes with retinal holes/tears with laser retinopexy. This imaging technology significantly aids in monitoring the laser’s efficacy preventing progression of SRF.
Moreover, we have shown that some potential laser treatment adverse events such as the formation of pERM, can be effectively diagnosed using a multimodal approach.
Peripheral retinoschisis and retinal detachments
Differentiating between peripheral retinoschisis (PR) and RD is essential to plan therapeutic options. However, making this distinction based on clinical biomicroscopic examination or indirect ophthalmoscopy alone can be challenging for the nonretina specialist.[42] Imaging techniques such as WF infrared reflectance have been useful to differentiate between these entities.[43]
Nonetheless, UWF fundus imaging may not always provide sufficient accuracy to assess all cases. We have demonstrated that peripheral SS-OCT allows a more precise assessment. When compared to indirect ophthalmoscopy and UWF imaging, peripheral SS-OCT provided a more detailed account of the chronicity and extent of PR or RD, including features such as neurosensory detachment and SRF in RD and IRF as a sign of chronicity.
This new imaging technology may yield novel insights into the identification of lesions predisposing to RD and its progression. Moreover, both SS-OCT and 3D volume scans may facilitate a better approach to RD surgery thanks to detecting epiretinal, intraretinal, or subretinal fibrous tissue in complex RD with proliferative vitreoretinopathy (PVR).
Given that RDs are a sight-threatening condition, early diagnosis and prompt identification of the cause of RD are crucial to guide appropriate treatment. In our clinic, we also used cross sectional and 3D volume SS-OCT to assess vitreous attachment status, capture the morphology of retinal elevation, identify multiple retinal tears, and observe the presence of PVR with epiretinal, intraretinal or subretinal scar membranes in these patients [Figure 4]. This helped to provide better information to the patient about the chronicity of the RD and the prognosis after surgery, as well as to plan the procedure.
Lattice degeneration
LD poses a higher risk of retinal breaks and RD.[38] However, prophylactic treatment of LD is still under debate and only suggested when peripheral detachments are progressive or symptomatic.[38] We have demonstrated the characteristics of LD using peripheral SS-OCT scans.
Cross-sectional OCT was indispensable in detecting associated SRF as well as the presence of associated retinal holes or tears. In addition, 3D volume scans offered superior visualization of the VRI in the affected retinal area.
By enabling the early detection of complications, navigated OCT may cater for prophylactic treatments and thus potentially avert vision loss, illustrating the crucial role of this advanced imaging technology in managing LD.
Retinal tufts
Retinal tufts are a developmental peripheral vitreoretinal abnormality in which the retina is stretched by the attached vitreous. Persistent vitreous traction may lead to retinal tears and detachment.[44,45] The combination of UWF imaging with OCT allows to better assess these lesions and establish the risk factors for lesions to progress to become vision-threatening complications thus enabling necessary treatments while avoiding unnecessary ones.
Retinal vascular diseases
Visualization of the mid and peripheral retina is essential in the management of retinal vascular diseases.[3,46,47,48,49,50]
Multimodal UWF imaging has been shown to be particularly useful in detecting peripheral retinal lesions such as neovascularization and ischemia, as well as in grading the severity and monitoring retinal vasculopathies.[49,51,52]
We observed that the integration of UWF imaging with peripheral SS-OCT enhanced our understanding and diagnosis in these diseases. We have demonstrated that both cross-sectional and 3D SS-OCT not only enhance the accuracy of diagnoses but also provide valuable insights into retinal vasculopathies such as DR, retinal vein occlusion, SCR or vasculitis, and its treatment. Therefore, enhanced and high-quality care was provided to our patients.
These techniques may help to identify novel peripheral OCT biomarkers, thereby refining the decision-making process in managing these conditions, reinforcing the vital role of advanced imaging technologies in retinal disease management. Furthermore, the use of UWF FA and ICG has proven to be extremely useful in evaluating retinal and choroidal peripheral ischemia, neovascularization, or inflammatory changes which led to better comprehension and monitoring of disease.
Oncology and pigmented lesions
The advent of multimodal UWF imaging and peripheral OCT has also changed the way clinicians approach, diagnose, and manage pigmented ocular lesions.[3,53] UWF fundus photography has aided in monitoring the dimensions of peripheral lesions which were in some cases previously impossible to reach.
As peripheral SS-OCT allows for cross-sectional and 3D imaging of the retina, the device provides detailed insights into the cross-sectional structure of the lesions. For instance, peripheral SS-OCT can detect SRF, IRF, and drusen associated with pigmented lesions. In addition, the software of the Optos Silverstone SS-OCT has the ability to provide measurements of the lesion’s size and volume, aiding in monitoring the changes over time and thus contributing to therapeutic decision-making.
Inherited retinal diseases: Sectorial retinitis pigmentosa
IRD are a diverse group of disorders characterized by progressive retinal degeneration, leading to visual impairment. Among them, RP represents a considerable diagnostic and management challenge due to its variable phenotype, including sectorial RP with the degeneration affecting only a portion of the retina.
The feasibility of using UWF imaging during investigations in infants and in patients with poor fixation has been demonstrated.[54]
We here propose to assess IRD using navigated SS-OCT. The high resolution of OCT scans allows to visualize intraretinal pigment dispersion, a key pathological change in RP, beyond the posterior pole. The detailed visualization of structural changes in the retina due to RP as well as its related adverse events allow for a comprehensive assessment of disease progression and potentially guide therapeutic interventions. The wider and more detailed visualization of the retina can lead to better monitoring of the disease progression and a more personalized approach to patient care.
Advantages and limitations of peripheral navigated swept source optical coherence tomography
Advantages
As we demonstrated above, the multi-modal Optos Silverstone device with peripheral navigated SS-OCT technology offers several benefits compared to the conventional posterior pole OCT.
Advanced technology allows for a visual representation of the ocular structures, which can be instrumental in educating patients about their diseases. By visualizing the changes in the retina, patients can better understand their condition, which can improve their cooperation with the proposed treatment plan.
In addition to patient education, peripheral OCT can serve as a valuable tool for clinicians to validate their clinical findings. It can provide objective and quantifiable data to support the clinical examination, thereby enhancing diagnostic accuracy. This can lead to a better understanding of disease pathogenesis, progression, and response to treatment.
Moreover, peripheral OCT allows to reveal subtle changes in the retina that may not be visible through traditional clinical examination, which can lead to the early detection of systemic and retinal diseases, potentially improving patient outcomes.
Limitations
Nevertheless, this technology also carries certain limitations. One of the main challenges is its inability to image the full extent of large lesions or areas of pathology, in particular lesions in the far periphery. This is particularly problematic for large and highly elevated lesions.
Inversion artifacts, or mirroring due to the eye’s curvature, imaging shadows caused by elevated lesions, poor registration between the fundus image and the OCT scan in very peripheral lesions, and reduced image quality are also limitations to consider.
The quality of peripheral OCT scans can also be affected by several factors such as poor patient cooperation, ocular media opacity, or technical characteristics of the OCT device itself. Poor scan quality can limit the interpretability of the images, potentially leading to misdiagnosis.
Another limitation is the requirement for a trained and skilled operator, ideally familiar with fundus abnormalities, to acquire accurate images which could potentially impact the accessibility and widespread use of this technology.
Future directions
Looking ahead, there are several potential directions for the development and application of the peripheral SS-OCT technology.
Continuous technological advancements could mitigate current limitations and provide better imaging resolution, larger scanning areas, deeper imaging windows, improved registration when imaging the extreme retinal periphery, as well as improved image quality even for highly elevated lesions. These improvements would enhance the diagnostic accuracy and clinical utility of SS-OCT.
Furthermore, integration of artificial intelligence and machine learning algorithms with SS-OCT could automate the process of image analysis. This could lead to a more efficient and accurate detection of retinal abnormalities, as well as better predictive models for disease progression and response to treatment. SS-OCT may also become a central tool in teleophthalmology.
UWF imaging and peripheral SS-OCT can open a valuable window to better understand the pathogenesis of various retinal and systemic diseases, leading potentially to developing new therapeutic targets and interventions.
CONCLUSION
The integration of UWF imaging with SS-OCT presents a significant advancement in ophthalmic imaging, offering a more comprehensive evaluation of retinal and systemic diseases to ophthalmic personnel and the retina specialist.
This multimodal approach has proven invaluable in detecting subtle changes in the retina, often undetectable through standard clinical examination, enabling early intervention and potentially improving patient outcomes.
This new imaging technology also serves as a valuable tool for patient education, teaching trainees, and documentation for medicolegal purposes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Quinn N, Csincsik L, Flynn E, Curcio CA, Kiss S, Sadda SR, et al. The clinical relevance of visualising the peripheral retina. Prog Retin Eye Res. 2019;68:83–109. doi: 10.1016/j.preteyeres.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Panwar N, Huang P, Lee J, Keane PA, Chuan TS, Richhariya A, et al. Fundus photography in the 21st century – A review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016;22:198–208. doi: 10.1089/tmj.2015.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo FJ, Ayliffe W, Stanga SF, Reinstein UI, Moxham R, Tariq Z, et al. New imaging technology for simultaneous multiwavelength-UWF fundus fluorescein angiography and indocyanine green angiography with navigated central and peripheral SS-OCT. Ophthalmic Surg Lasers Imaging Retina. 2023;54:401–10. doi: 10.3928/23258160-20230607-01. [DOI] [PubMed] [Google Scholar]
- 4.Jones NP, Sala-Puigdollers A, Stanga PE. Ultra-widefield fundus fluorescein angiography in the diagnosis and management of retinal vasculitis. Eye (Lond) 2017;31:1546–9. doi: 10.1038/eye.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodhi SK, Golding J, Trimboli C, Choudhry N. Feasibility of peripheral OCT imaging using a novel integrated SLO ultra-widefield imaging swept-source OCT device. Int Ophthalmol. 2021;41:2805–15. doi: 10.1007/s10792-021-01837-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid H, Schwartz R, Nicholson L, Huemer J, El-Bradey MH, Sim DA, et al. Widefield optical coherence tomography angiography for early detection and objective evaluation of proliferative diabetic retinopathy. Br J Ophthalmol. 2021;105:118–23. doi: 10.1136/bjophthalmol-2019-315365. [DOI] [PubMed] [Google Scholar]
- 7.Couturier A, Rey PA, Erginay A, Lavia C, Bonnin S, Dupas B, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126:1685–94. doi: 10.1016/j.ophtha.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Pichi F, Smith SD, Abboud EB, Neri P, Woodstock E, Hay S, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1901–9. doi: 10.1007/s00417-020-04773-x. [DOI] [PubMed] [Google Scholar]
- 9.Kiss S, Berenberg TL. Ultra widefield fundus imaging for diabetic retinopathy. Curr Diab Rep. 2014;14:514. doi: 10.1007/s11892-014-0514-0. [DOI] [PubMed] [Google Scholar]
- 10.Hussain N, Edraki M, Tahhan R, Sanalkumar N, Kenz S, Akasha NK, et al. Telemedicine for diabetic retinopathy screening using an ultra-widefield fundus camera. Clin Ophthalmol. 2017;11:1477–82. doi: 10.2147/OPTH.S135287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus DM, Silva PS, Liu D, Aiello LP, Antoszyk A, Elman M, et al. Association of predominantly peripheral lesions on ultra-widefield imaging and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140:946–54. doi: 10.1001/jamaophthalmol.2022.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka S, Tanaka Y, Inoue T, Nagura K, Arasaki R, Okawa K, et al. Retinal haemorrhages on ultra-widefield red channel images and perfusion status in central retinal vein occlusion. Eye (Lond) 2023;37:2305–9. doi: 10.1038/s41433-022-02337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan CS, Li KZ, Sadda SR. Wide-field angiography in retinal vein occlusions. Int J Retina Vitreous. 2019;5:18. doi: 10.1186/s40942-019-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato A, Asaoka R, Tanaka S, Nagura K, Tanaka Y, Arasaki R, et al. Using ultra-widefield red channel images to improve the detection of ischemic central retinal vein occlusion. PLoS One. 2021;16:e0260383. doi: 10.1371/journal.pone.0260383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bistour A, Mehanna CJ, Chuttarsing B, Colantuono D, Amoroso F, Beaumont W, et al. Widefield oct-angiography-based classification of sickle cell retinopathy. Graefes Arch Clin Exp Ophthalmol. 2023;261:2805–12. doi: 10.1007/s00417-023-06115-z. [DOI] [PubMed] [Google Scholar]
- 16.Linz MO, Scott AW. Wide-field imaging of sickle retinopathy. Int J Retina Vitreous. 2019;5:27. doi: 10.1186/s40942-019-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han IC, Zhang AY, Liu TY, Linz MO, Scott AW. Utility of ultra-widefield retinal imaging for the staging and management of sickle cell retinopathy. Retina. 2019;39:836–43. doi: 10.1097/IAE.0000000000002057. [DOI] [PubMed] [Google Scholar]
- 18.Cho M, Kiss S. Detection and monitoring of sickle cell retinopathy using ultra wide-field color photography and fluorescein angiography. Retina. 2011;31:738–47. doi: 10.1097/IAE.0b013e3181f049ec. [DOI] [PubMed] [Google Scholar]
- 19.Cai S, Parker F, Urias MG, Goldberg MF, Hager GD, Scott AW. Deep learning detection of sea fan neovascularization from ultra-widefield color fundus photographs of patients with sickle cell hemoglobinopathy. JAMA Ophthalmol. 2021;139:206–13. doi: 10.1001/jamaophthalmol.2020.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han IC, Linz MO, Liu TY, Zhang AY, Tian J, Scott AW. Correlation of ultra-widefield fluorescein angiography and OCT angiography in sickle cell retinopathy. Ophthalmol Retina. 2018;2:599–605. doi: 10.1016/j.oret.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Shoughy SS, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015;63:575–81. doi: 10.4103/0301-4738.167122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midena E, Zennaro L, Lapo C, Torresin T, Midena G, Frizziero L. Comparison of 50 handheld fundus camera versus ultra-widefield table-top fundus camera for diabetic retinopathy detection and grading. Eye (Lond) 2023;37:2994–9. doi: 10.1038/s41433-023-02458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanga PE, Sala-Puigdollers A, Caputo S, Jaberansari H, Cien M, Gray J, et al. In vivo imaging of cortical vitreous using 1050-nm swept-source deep range imaging optical coherence tomography. Am J Ophthalmol. 2014;157:397–404.e2. doi: 10.1016/j.ajo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Pi S, Hormel TT, Wang B, Bailey ST, Hwang TS, Huang D, et al. Volume-based, layer-independent, disease-agnostic detection of abnormal retinal reflectivity, nonperfusion, and neovascularization using structural and angiographic OCT. Biomed Opt Express. 2022;13:4889–906. doi: 10.1364/BOE.469308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanga PE, Pastor-Idoate S, Reinstein U, Vatas P, Patel U, Dubovy S, et al. Navigated single-capture 3D and cross-sectional wide-field OCT of the mid and peripheral retina and vitreoretinal interface. Eur J Ophthalmol. 2022;32:1642–51. doi: 10.1177/11206721211026100. [DOI] [PubMed] [Google Scholar]
- 27.Aaker GD, Gracia L, Myung JS, Borcherding V, Banfelder JR, D’Amico DJ, et al. Volumetric three-dimensional reconstruction and segmentation of spectral-domain OCT. Ophthalmic Surg Lasers Imaging. 2011;42(Suppl):S116–20. doi: 10.3928/15428877-20110627-11. [DOI] [PubMed] [Google Scholar]
- 28.Ahdi A, Rabbani H, Vard A. A hybrid method for 3D mosaicing of OCT images of macula and optic nerve head. Comput Biol Med. 2017;91:277–90. doi: 10.1016/j.compbiomed.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Arthur E, Papay JA, Haggerty BP, Clark CA, Elsner AE. Subtle changes in diabetic retinas localised in 3D using OCT. Ophthalmic Physiol Opt. 2018;38:477–91. doi: 10.1111/opo.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni S, Nguyen TP, Ng R, Woodward M, Ostmo S, Jia Y, et al. Panretinal optical coherence tomography. IEEE Trans Med Imaging. 2023;42:3219–28. doi: 10.1109/TMI.2023.3278269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straatsma BR, Landers MB, Kreiger AE. The ora serrata in the adult human eye. Arch Ophthalmol. 1968;80:3–20. doi: 10.1001/archopht.1968.00980050005002. [DOI] [PubMed] [Google Scholar]
- 32.Gallogly P, Smiddy WE, Feuer WJ. The effect of myopia on the position of the ora serrata. Ophthalmic Surg Lasers Imaging. 2007;38:518–9. doi: 10.3928/15428877-20071101-16. [DOI] [PubMed] [Google Scholar]
- 33.Schepens CL, Bahn GC. Examination of the ora serrata. Its importance in retinal detachment. AMA Arch Ophthalmol. 1950;44:677–90. doi: 10.1001/archopht.1950.00910020689006. [DOI] [PubMed] [Google Scholar]
- 34.Joussen F, Spitznas M. The fine structure of the human retina at the ora serrata. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;185:177–88. doi: 10.1007/BF00417613. [DOI] [PubMed] [Google Scholar]
- 35.Choudhry N, Golding J. Optical coherence tomography of an ora serrata pearl. JAMA Ophthalmol. 2015;133:e145362. doi: 10.1001/jamaophthalmol.2014.5362. [DOI] [PubMed] [Google Scholar]
- 36.Stanga PE, Valentin Bravo FJ, Reinstein UI, Stanga SF, Marshall J, Archer TJ, et al. New terminology and methodology for the assessment of the vitreous, its floaters and opacities, and their effect on vision: Standardized and kinetic anatomical and functional testing of vitreous floaters and opacities (SK VFO Test) Ophthalmic Surg Lasers Imaging Retina. 2023;54:306–15. doi: 10.3928/23258160-20230412-02. [DOI] [PubMed] [Google Scholar]
- 37.Sebag J. Vitreous and vision degrading myodesopsia. Prog Retin Eye Res. 2020;79:100847. doi: 10.1016/j.preteyeres.2020.100847. [DOI] [PubMed] [Google Scholar]
- 38.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration preferred practice pattern®. Ophthalmology. 2020;127:P146–81. doi: 10.1016/j.ophtha.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson CP. Interventions for asymptomatic retinal breaks and lattice degeneration for preventing retinal detachment. Cochrane Database Syst Rev. 2014;2014:CD003170. doi: 10.1002/14651858.CD003170.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhry N, Golding J, Manry MW, Rao RC. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123:1368–74. doi: 10.1016/j.ophtha.2016.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casswell EJ, Abou Ltaif S, Carr T, Keane PA, Charteris DG, Wickham L. Widefield spectral-domain optical coherence tomography imaging of peripheral round retinal holes with or without retinal detachment. Retina. 2019;39:1047–53. doi: 10.1097/IAE.0000000000002133. [DOI] [PubMed] [Google Scholar]
- 42.Jalalizadeh RA, Smith BT. Characterization and diagnosis of retinoschisis and schisis detachments using spectral domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2023;261:375–80. doi: 10.1007/s00417-022-05801-8. [DOI] [PubMed] [Google Scholar]
- 43.Banda HK, Shah A, Shah GK. Application of wide-field infrared reflectance imaging in retinoschisis, retinal detachments, and schisis detachments. Int J Retina Vitreous. 2019;5:42. doi: 10.1186/s40942-019-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Chen L, Wu M, Zheng B. Spectral-domain optical coherence tomography characteristics of cystic retinal tuft. BMC Ophthalmol. 2022;22:412. doi: 10.1186/s12886-022-02636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byer NE. Cystic retinal tufts and their relationship to retinal detachment. Arch Ophthalmol. 1981;99:1788–90. doi: 10.1001/archopht.1981.03930020662007. [DOI] [PubMed] [Google Scholar]
- 46.Lucente A, Taloni A, Scorcia V, Giannaccare G. Widefield and ultra-widefield retinal imaging: A geometrical analysis. Life (Basel) 2023;13:202. doi: 10.3390/life13010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao Y, Huang Z, Yuan Q, Du X, Li Z, Nie X, et al. Comparison of quantitative assessment and efficiency of diabetic retinopathy diagnosis using ETDRS seven-field imaging and two ultra-widefield imaging. Eye (Lond) 2023;37:3558–64. doi: 10.1038/s41433-023-02549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antropoli A, Arrigo A, La Franca L, Bianco L, Barlocci E, Fusi E, et al. Peripheral and central capillary non-perfusion in diabetic retinopathy: An updated overview. Front Med (Lausanne) 2023;10:1–12. doi: 10.3389/fmed.2023.1125062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan TE, Ibrahim F, Chandrasekaran PR, Teo KY. Clinical utility of ultra-widefield fluorescein angiography and optical coherence tomography angiography for retinal vein occlusions. Front Med (Lausanne) 2023;10:1–12. doi: 10.3389/fmed.2023.1110166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan W, Fleming A, Hemert JV, Wykoff CC, Brown DM, Robertson G, et al. Retinal vascular bed area in eyes with retinal vein occlusion on ultra-widefield fluorescein angiography: Wave study. Retina. 2022;42:1883–8. doi: 10.1097/IAE.0000000000003549. [DOI] [PubMed] [Google Scholar]
- 51.Young BK, Bommakanti N, Yu G, Patel TP, Azzouz L, Powell C, et al. Retinal neovascularization as self-organized criticality on ultra-widefield fluorescein angiography imaging of diabetic retinopathy. Eye (Lond) 2023;37:2795–800. doi: 10.1038/s41433-023-02422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giocanti-Aurégan A, Fajnkuchen F. Proliferative sickle cell retinopathy in the retinal periphery detected by ultra-widefield imaging: A case report. Case Rep Ophthalmol. 2023;14:159–64. doi: 10.1159/000529479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu A, Chen C. Clinical application of ultra-widefield fundus autofluorescence. Int Ophthalmol. 2021;41:727–41. doi: 10.1007/s10792-020-01609-9. [DOI] [PubMed] [Google Scholar]
- 54.Cicinelli MV, Marchese A, Bordato A, Manitto MP, Bandello F, Battaglia Parodi M. Reviewing the role of ultra-widefield imaging in inherited retinal dystrophies. Ophthalmol Ther. 2020;9:249–63. doi: 10.1007/s40123-020-00241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


