Vaccines are hailed as one of the most important public health achievements of the 20th century.1 In the next five to 15 years, new vaccines and new vaccine delivery technology will fundamentally change how clinicians prevent and treat disease, with a substantial impact on public health. This review describes recent developments in the basic science underpinning the development of new vaccines and summarises the potential of these vaccines to treat and prevent a wide range of infectious and non-infectious diseases.2–5 In addition, research is being carried out on much needed vaccines for the developing world for diseases such as malaria, hookworm, dengue, enterotoxigenic Escherichia coli, shigella, and tuberculosis, but these are beyond the scope of this brief review.
Summary points
New prophylactic and therapeutic vaccines will prevent and potentially cure disease by providing people with the necessary immunological tools
Advances in current vaccines such as conjugated pneumococcal vaccines for adults, nasal spray vaccines for influenza, and adult acellular pertussis vaccines will provide an efficient way to produce longlasting protective immunity
Development of vaccines against non-infectious diseases (such as cancer, diabetes, and Alzheimer's disease) and nicotine and cocaine dependence will provide alternative treatments
Vaccines against biological weapons will be possible by advances in DNA vaccines
New vaccine delivery technology will provide easier delivery routes (such as transcutaneous, depot, nasal, and oral delivery) without compromising efficacy
Methods
We searched PubMed and Medline databases (1995-2001), as well as our own libraries, for articles of relevance to this brief review.
New vaccines against infectious diseases
Development of DNA vaccines
One approach generating great interest is that of inducing protective immune responses by injecting engineered DNA sequences from infectious organisms against which protection is desired. If an antigen can be identified it is possible to insert the DNA sequence coding for the protein antigen into a carrier genome (such as several of the poxviruses or alphaviruses). Once delivered into the host, the organism (and hence the inserted DNA) undergoes limited replication, the protein of interest is produced, and the host develops an immune response against the protein.
In a related strategy, so called naked DNA is injected directly into the host to produce an immune response (fig 1). Naked DNA is simply sequences of DNA inserted into bacterial plasmids (simple, extrachromosomal rings of DNA found in bacterial cells) and injected into the host. These have been effective in animal models, but intramuscularly injected DNA in humans has failed to generate vigorous immune responses, although transdermal or intradermal delivery of DNA has been more encouraging. A clinical trial of transdermally delivered microscopic gold beads coated with DNA coding for hepatitis B surface antigen generated protective levels of antibodies to the antigen.6 This vaccine has also generated CD8 cytotoxic lymphocytes.6 Although efforts have been successful in animal models of vaccines against several pathogens, progress in humans has been much slower. To date, only DNA vaccines against hepatitis B6 and malaria7 have induced immune responses thought to be protective in humans.
Figure 1.
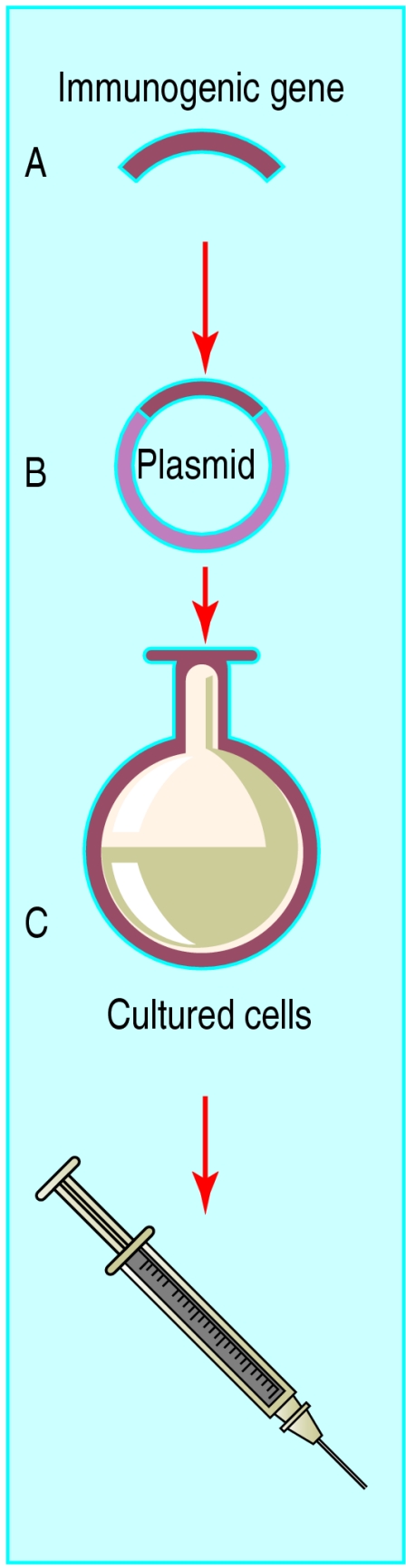
Principle of DNA vaccination. An immunogenic gene is inserted into an expression plasmid (A), which is inserted into cultured cells (B). The cells are screened for expression of the gene protein and then cultured. The plasmid DNA is then extracted from the cells and purified before being used to immunise a host (C)
Development of therapeutic vaccines
Traditional vaccination is the prevention of a specific infectious disease by delivering an immunogenic antigen derived from the surface of the infectious agent, resulting in immunity against the foreign organism replicating and establishing an infection. A therapeutic vaccine, however, can limit or eradicate an already present and established infectious agent or condition. The development of therapeutic vaccines has depended in part on the ability of DNA vaccination to induce both humoral and cell mediated immune responses by inoculation of plasmid DNA containing sequences for transcription and translation, resulting in the in vivo synthesis of an immunogenic peptide or protein.
Attempts are being made to develop a therapeutic vaccine against HIV that will induce virus-specific cytotoxic T lymphocytes against HIV, with the goal of having activated T cells destroy latently infected cells. Other efforts include developing therapeutic vaccines against Helicobacter pylori, mucosal candidiasis, herpes viruses, and human papillomavirus. DNA vaccination for hepatitis B virus has shown great promise. The delivery of viral DNA sequences can induce longlasting humoral and cell mediated immunity in mice infected with hepatitis B virus.8,9 In transgenic mice, at least, there is a decrease in or clearance of the hepatitis B surface antigen, with evidence of induction of antibodies and proliferation of CD4 T cells.10 Clearly, the capabilities of the immune system to eliminate an infectious agent even after an infection or disease is established could substantially improve human health.
Other important examples of therapeutic vaccine development include the development of vaccines against certain cancers,11 which is discussed later.
Advances in current vaccines
The bacterium Streptococcus pneumoniae and influenza viruses account for considerable morbidity and mortality worldwide. Now approved in several Western countries, S pneumoniae conjugate vaccines should help reduce the number of cases of invasive S pneumoniae disease (bacteraemia, meningitis, and sepsis) in infants and young children. A live, attenuated influenza virus vaccine is nearing approval in the United States. This vaccine, administered as an intranasal spray, should stimulate both systemic and mucosal immunity, while decreasing reliance on the use of parenteral injections (see box for a list of potential vaccines).
Potential vaccination in the 21st century (adapted with permission from Plotkin (2001)5)
New maternal vaccines—Group B streptococcus, respiratory syncytial virus
New vaccines for neonates—Respiratory syncytial virus, hepatitis B
New vaccines for infants aged 2-6 months—Paediatric combinations (acellular pertussis (DtacP), Haemophilus influenzae type b (Hib), hepatitis B, pneumococcal, meningococcal, hepatitis A, etc), otitis (non-typable Haemophilus influenzae, Branhamella catarrhalis), rotavirus (new), meningococcal conjugate
New vaccines for the developing world—Enterotoxigenic Escherichia coli, shigella, malaria, dengue, tuberculosis
Vaccines for children aged 1-2 years—Measles-mumps-rubella-varicella (MMRV), influenza (intranasal)
Vaccines for children aged 4-6 years—MMRV booster, paediatric combination booster, Streptococcus mutans (oral) (anti-caries), Lyme disease and tick-borne encephalitis (endemic areas)
Vaccines for children aged 11-13 years—HIV, human papillomavirus, herpes simplex virus 2, Neisseria gonorrhoeae, cytomegalovirus, parvovirus, Epstein-Barr virus
Vaccines for young adults—Tetanus and diphtheria toxoids, acellular pertussis, Helicobacter pylori (anti-ulcer), Chlamydia pneumoniae (anti-atherosclerosis)
Travel vaccines—Therapeutic vaccines against diabetes, multiple sclerosis, meningococcal conjugate
Vaccines for people aged ⩾50 years—Influenza (subcutaneous and intranasal), pneumonococcus (protein and polysaccharide), herpes zoster, cancer (prophylactic and therapeutic vaccines)
Streptococcus pneumoniae
Multivalent polysaccharide vaccines for S pneumoniae have been available in the United States since 1977, but they produce a poor or inconsistent immune response in children, especially those less than 2 years old. Polysaccharide vaccines induce antibodies primarily by mechanisms independent of the T cells and are not long lasting and do not induce an immune memory response. For these reasons, a protein carrier conjugated to a polysaccharide antigen of S pneumoniae has now been developed, which causes the immune response to be T cell dependent, allowing infants and children to respond better to the vaccine. The US licensed heptavalent S pneumoniae conjugated polysaccharide vaccine contains the seven serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) most commonly associated with invasive disease among infants and young children. The new vaccine is also expected to have the benefit of reducing nasopharyngeal carriage of these seven S pneumoniae serotypes.
Influenza virus
The only influenza vaccines currently licensed in the United States are parenteral inactivated influenza virus vaccines prepared in chick embryos. Because of changes in the influenza viruses circulating each year (antigenic drift), protection of high risk individuals requires annual vaccination.
A live attenuated influenza virus vaccine being proposed for US approval contains recombinant cold-adapted strains of influenza A and B and is given by intranasal spray. Several studies have examined the use of live attenuated influenza vaccines in children and adults.12–14 In seronegative children more than 15 months old antibody responses to the influenza A and B components after a single dose of vaccine indicated an overall efficacy of 93%.12 Use of a live attenuated trivalent vaccine in adults significantly reduced the occurrence of illness, visits to healthcare providers, and days of work lost.14
New vaccines against non-infectious diseases
When correctly targeted, an immune response can be used to eliminate cells with aberrant behaviour (dysplasia) or aberrant genomic function (malignancy) or to reduce the amount of inflammation affecting a specific organ (such as in diabetes).15,16 This raises the possibility of developing vaccines against diseases not known to be related to infectious agents. Two of the most exciting and promising areas in this regard are vaccines against cancer and autoimmune diseases.
Cancer
The identification of specific tumour antigens (tumour associated antigens) that are present only in cancer cells—such as those found in leukaemia, breast cancer, melanoma, prostate cancer, and colon cancer—provide immune targets for which immunogenic vaccines may conceivably be designed. For example, the expression of protein GPI-B7-1 transferred onto membranes from a murine thymoma tumour cell protects mice against this kind of tumour.17 In humans it is possible to stimulate T cell responses using isolated membranes surgically removed from human tumour tissues that express major histocompatibility complex (MHC) class II molecules, suggesting the possibility of establishing an immune response that could specifically target and eliminate tumour cells.18
Other efforts include therapeutic vaccines against melanoma, colorectal cancer, leukaemia, and other cancers.19,20 The ability of DNA vaccines to deliver precise and specific nucleotide sequences representing target genes—such as the ALVAC gp100 gene for melanoma and the ALVAC CEA-B7.1 gene for colorectal cancer—and specific protein fragments such as the HER2/Neu peptide found in breast cancer cells21,22 have been studied as a potential means with which to induce an immune response.19,23
Autoimmune diseases
Diseases related to pathological immune activation, such as autoimmune diseases and allergies, might be treatable or preventable with vaccines. Efforts are being made to develop vaccines against rheumatoid arthritis, multiple sclerosis, myasthenia gravis, food allergies, and especially type 1 diabetes because of its associated substantial morbidity and mortality.
In the case of type 1 diabetes, lymphocytes infiltrate the pancreatic islets and selectively destroy the insulin secreting β cells. One strategy for vaccine development is to reduce the pathological lymphocytic infiltration by tolerisation.15,16,24 Tolerisation involves the administration of small amounts of the same antigens that are the target of the aberrant immune response, which, in the absence of cytokine costimulation, fuels the activation of T cells, which reduce inflammation.
In disorders such as Alzheimer's disease, it may be possible to target the β amyloid protein that is responsible for the neurodegenerative plaques observed in this disorder. In murine models vaccines have been shown to reduce and prevent plaque formation, with some improvement in cognitive function.25 Other examples of potential vaccine development include vaccines to prevent cocaine and nicotine addiction. With the use of immunopharmacotherapy, antibodies can be designed to neutralise a drug rather than target the receptors in the brain. Efforts are also being made to develop vaccines against atherosclerosis and to prevent conception.26–28
Vaccines against biological weapons of mass destruction
Interest has increased in biological weapons of mass destruction as terrorists look for methods with which to inflict harm on the greatest number of people, with the lowest possible cost and technology needs, while creating mass panic. While vaccines have been licensed against smallpox, plague, anthrax, and others, only limited amounts of anthrax vaccine are being produced in the United States for specific risk groups. Limited and ageing stockpiles of smallpox and plague vaccine are available but are insufficient for large numbers of people.
Because of the ability of biological weapons to infect and kill large numbers of people, and the risk of person-to-person transmission, vaccines are likely to be the only practical means of protection.29,30 Second generation vaccines against anthrax, smallpox, and plague are being developed, and vaccines against other agents of bioterrorism such as the haemorrhagic fever viruses and others are also in development. However, major obstacles in producing such vaccines for public use include the need for a financially viable market, the impossibility of conducting human efficacy trials, the intangible risk:benefit ratio at the public health level, and governments' reluctance to face the reality of bioterrorism.
New vaccine delivery technology
Virtually all recommended immunisations require parenteral administration, and many require a series of injections. To be effective, vaccines for some diseases will need to enhance mucosal immunity as well as systemic immunity. For these reasons, new vaccine delivery methods, specifically alternatives to injections, are being sought. Topically applied (transcutaneous) vaccines, transgenic edible plants that contain genes for human vaccine antigens, and controlled delivery depot systems with vaccine antigens encapsulated in biodegradable polymers are possibilities currently under study. Such new delivery methods could decrease reliance on repeated injections, the need for trained healthcare workers, and perhaps the need for a stringent cold chain for vaccine storage.
Transcutaneous immunisation
Animal studies have shown the production of both systemic and mucosal antibodies after topical vaccine application. Agents such as cholera toxin and the heat labile enterotoxin of Escherichia coli, in combination with a vaccine antigen such as tetanus toxoid, act as an adjuvant and produce protective antibodies after being applied to the skin of animals.31 Non-toxic mutants or subunits of cholera toxin and E coli enterotoxin would be needed, however, for any application on to human mucosal surfaces. Various other adjuvants besides cholera toxin and E coli enterotoxin (including bacterial ADP-ribosylating exotoxins, interleukin –1β fragment, interleukin 2, and tumour necrosis factor -α) have also been shown to produce an immune response after topical application.32
Additional educational resources
Centers for Disease Control and Prevention (www.cdc.gov/)
World Health Organization (www.who.int/home-page/)
Merck Vaccines (www.merckvaccines.com/)
National Vaccine Information Center (www.909shot.com/)
DNAvaccine.com (www.dnavaccine.com/) a global platform for vaccine research
Food and Drug Administration (www.fda.gov/default.htm)
National Foundation for Infectious Diseases (www.nfid.org/)
American Society for Microbiology (www.asm.org/)
Infectious Diseases Society of America (www.idsociety.org/)
Transgenic edible plants to deliver vaccines
The development of plants capable of expressing vaccine antigens is a novel and promising strategy (fig 2). Such genetically engineered plants would produce vaccine antigens in their edible parts and would, like subunit vaccine preparations, contain no genes capable of replicating a whole infectious organism.33 Because food plants can be regenerated rapidly, it may be possible that crops containing vaccine antigens could be produced indefinitely and on a local basis. Potato and tomato plants have synthesised antigens from Norwalk virus, enterotoxigenic E coli, Vibrio cholerae, and hepatitis B virus. A recently completed human study has shown that a recombinant bacterial antigen, subunit B of heat labile enterotoxin, produced in a potato and eaten resulted in production of both serum antibodies (IgG and IgA) and mucosal antibodies (sIgA) to the antigen.34 Other plants, such as bananas, and other vaccine antigens, including tetanus and diphtheria toxoids, may be included in future studies.
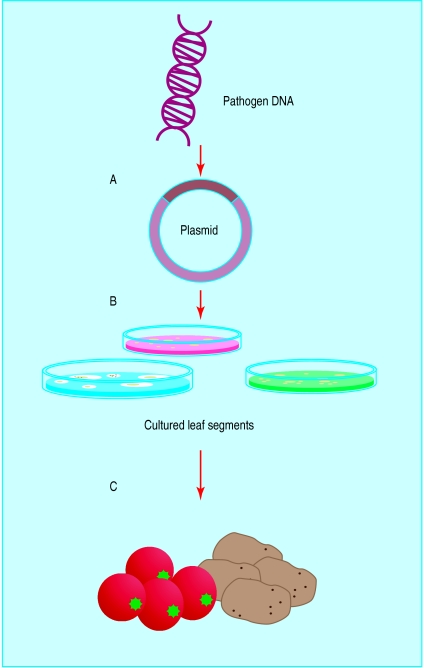
Figure 2.
Principle of delivering vaccines in edible plants. A gene from a human pathogen is inserted into a bacterium that infects plants (A). The bacterium then infects cultured leaf segments of the selected food plant (B), which sprout into whole plants containing the human pathogen gene (C). Once the plant is eaten, it triggers an immune response to the pathogen
Controlled delivery depot systems
The use of controlled delivery of vaccine antigen, or depot vaccine technology, reduces the number of parenteral injections while potentially mimicking natural infection. Various vaccine antigens have been encapsulated in microspheres composed of biodegradable polymers such as poly (lactic/glycolic) acid (PLGA), which can be targeted to various cells in the immune system or can form a depot at the injection site, allowing slow release of the antigen over time.35 The release profile of vaccine antigen depends on the particle size of the delivery vehicle, and a combination of large and small microspheres can create a pattern that mimics the antigen concentration profile in conventional immunisation, combining both primary and booster injections. A recent study in animals found that encapsulated tetanus toxoid or Haemophilus influenzae type b polysaccharide elicited high antibody levels that persisted for months.36
Conclusions
The future of vaccinology provides tremendous promise for controlling diseases. Vaccines will be delivered orally, by nasal spray, or transcutaneously by a minimally trained layperson and in a manner that does not require expensive equipment. However, despite rapid advances in the development of new vaccines, concerns about vaccine safety and a rise in anti-vaccine sentiment adversely affect immunisation coverage, the willingness of manufacturers to develop new vaccines, and the willingness of individuals and healthcare workers to use them.37,38 As advanced vaccines and vaccine technologies become available, massive public education efforts will be required to alleviate these concerns. This is particularly true for DNA vaccines, combination vaccines, vectored vaccines, and vaccines administered in a parenteral depot fashion. The more distant potential for person-specific vaccines based on individual genotyping (vaccines against a specific malignancy in a specific individual) will also raise serious concerns. None the less, the prospect of both preventing and treating many serious diseases by the use of vaccines portends an exciting era in public health and vaccinology.
Acknowledgments
We thank Kim Zabel for her excellent editorial assistance in the development of this review. DM's current address is Pediatric Infectious Diseases, Medical College of Georgia, Augusta, GA, USA.
Footnotes
Funding: GAP and this work was supported in part by a grant from the Centers for Disease Control and Prevention (AVA 001) and grants from the National Institutes of Health (AI 33144 and AI 48793).
Competing interests: None declared.
References
- 1.Centers for Disease Control. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48(12):241–243. [PubMed] [Google Scholar]
- 2.Poland GA. Current paradoxes and changing paradigms in vaccinology. Vaccine. 1999;17:1605–1611. doi: 10.1016/s0264-410x(98)00417-4. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman N, Bonilla R, O'Kane D, Poland GA. Gene expression microarrays: a 21st century tool for directed vaccine design. Vaccine. 2001;20:22–30. doi: 10.1016/s0264-410x(01)00319-x. [DOI] [PubMed] [Google Scholar]
- 4.Poland GA, Ovsyannikova IG, Johnson KL, Naylor S. The role of mass spectrometry in vaccine development. Vaccine. 2001;19:2692–2700. doi: 10.1016/s0264-410x(00)00505-3. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin SA. Vaccines in the 21st century. Infect Dis Clin North Am. 2001;15:307–327. doi: 10.1016/s0891-5520(05)70280-4. [DOI] [PubMed] [Google Scholar]
- 6. Poland GA, Rottinghaus ST, Jacobson RM, Roy M. A phase 1C study of a DNA hepatitis B vaccine in healthy patients nonresponsive to licensed hepatitis B vaccines: preliminary results [abstract]. The fourth annual conference on vaccine research, Arlington, VA, April 23-25 2001;S37:57 ( www.nfid.org/conferences/vaccine01/abstracts/abss37-40.pdf).
- 7.Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 8.Oka Y, Fazle Akbar SM, Horiike N, Joko K, Onji M. Mechanism and therapeutic potential of DNA-based immunization against the envelope proteins of hepatitis B virus in normal and transgenic mice. Immunology. 2001;103:90–97. doi: 10.1046/j.1365-2567.2001.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancini M, Hadchouel M, Davis HL, Whalen RG, Tiollais P, Michel ML. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci USA. 1996;93:12496–12501. doi: 10.1073/pnas.93.22.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 11.Moingeon P. Cancer vaccines. Vaccine. 2001;19:1305–1326. doi: 10.1016/s0264-410x(00)00372-8. [DOI] [PubMed] [Google Scholar]
- 12.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 13.King JC, Jr, Lagos R, Bernstein DI, Piedra PA, Kotloff K, Bryant M, et al. Safety and immunogenicity of low and high doses of trivalent live cold-adapted influenza vaccine administered intranasally as drops or spray to healthy children. J Infect Dis. 1998;177:1394–1397. doi: 10.1086/517822. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 15.Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104:189–194. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Herrath MG, Whitton JL. DNA vaccination to treat autoimmune diabetes. Ann Med. 2000;32:285–292. doi: 10.3109/07853890008995930. [DOI] [PubMed] [Google Scholar]
- 17.McHugh RS, Nagarajan S, Wang YC, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer Res. 1999;59:2433–2437. [PubMed] [Google Scholar]
- 18.Poloso NJ, Nagarajan S, Bumgarner GW, Selvaraj P. Development of therapeutic vaccines by direct modification of cell membranes from surgically removed human tumor tissue with immunostimulatory molecules. Vaccine. 2001;19:2029–2038. doi: 10.1016/s0264-410x(00)00424-2. [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia J, Bonnet M, Berinstein N, Barber B, Klein M, Moingeon P. Therapeutic vaccines against melanoma and colorectal cancer. Vaccine. 2001;19:2571–2575. doi: 10.1016/s0264-410x(00)00491-6. [DOI] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Molldrem JJ. Leukemia vaccines. Curr Oncol Rep. 2001;3:193–200. doi: 10.1007/s11912-001-0050-3. [DOI] [PubMed] [Google Scholar]
- 21.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Emtage P, Zhu Q, Foley R, Muller W, Hitt M, et al. Induction of ErbB-2/neu-specific protective and therapeutic antitumor immunity using genetically modified dendritic cells: enhanced efficacy by cotransduction of gene encoding IL-12. Gene Ther. 2001;8:316–323. doi: 10.1038/sj.gt.3301396. [DOI] [PubMed] [Google Scholar]
- 23.Sivanandham M, Shaw P, Bernik SF, Paoletti E, Wallack MK. Colon cancer cell vaccine prepared with replication-deficient vaccinia viruses encoding B7.1 and interleukin-2 induce antitumor response in syngeneic mice. Cancer Immunol Immunother. 1998;46:261–267. doi: 10.1007/s002620050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simone EA, Wegmann DR, Eisenbarth GS. Immunologic “vaccination” for the prevention of autoimmune diabetes (type 1A) Diabetes Care. 1999;22:7–15. [PubMed] [Google Scholar]
- 25.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 26.Naz RK, Zhu X, Kadam AL. Cloning and sequencing of cDNA encoding for a novel human testis-specific contraceptive vaccinogen: role in immunocontraception. Mol Reprod Dev. 2001;60:116–127. doi: 10.1002/mrd.1068. [DOI] [PubMed] [Google Scholar]
- 27.Santhanam R, Naz RK. Novel human testis-specific cDNA: molecular cloning, expression and immunobiological effects of the recombinant protein. Mol Reprod Dev. 2001;60:1–12. doi: 10.1002/mrd.1055. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa A, Hamada Y, Shigeta M, Koyama K. Contraceptive potential of synthetic peptides of zona pellucida protein (ZPA) J Reprod Immunol. 2002;53:91–98. doi: 10.1016/s0165-0378(01)00084-5. [DOI] [PubMed] [Google Scholar]
- 29.Lillibridge SR, Bell AJ, Roman RS. Centers for disease control and prevention bioterrorism preparedness and response. Am J Infect Control. 1999;27:463–464. doi: 10.1016/s0196-6553(99)70020-9. [DOI] [PubMed] [Google Scholar]
- 30.Cieslak TJ, Christopher GW, Kortepeter MG, Rowe JR, Pavlin JA, Culpepper RC, et al. Immunization against potential biological warfare agents. Clin Infect Dis. 2000;30:843–850. doi: 10.1086/313812. [DOI] [PubMed] [Google Scholar]
- 31.Scharton-Kersten T, Glenn GM, Vassell R, Yu J, Walwender D, Alving CR. Principles of transcutaneous immunization using cholera toxin as an adjuvant. Vaccine. 1999;17:S37–S43. doi: 10.1016/s0264-410x(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 32.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999;67:1100–1106. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langridge WH. Edible vaccines. Sci Am. 2000;283:66–71. doi: 10.1038/scientificamerican0900-66. [DOI] [PubMed] [Google Scholar]
- 34.Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Z, Leong KW. Controlled delivery of antigens and adjuvants in vaccine development. J Pharm Sci. 1996;85:1261–1270. doi: 10.1021/js9602812. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev Biol Stand. 1998;92:63–78. [PubMed] [Google Scholar]
- 37.Poland GA, Jacobson RM. Vaccine safety: injecting a dose of common sense. Mayo Clin Proc. 2000;75:135–139. doi: 10.4065/75.2.135. [DOI] [PubMed] [Google Scholar]
- 38.Poland GA, Jacobson RM. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine. 2001;19:2440–2445. doi: 10.1016/s0264-410x(00)00469-2. [DOI] [PubMed] [Google Scholar]