Abstract
Aim:
The aim of the study was to evaluate the ability of cultivated odontoblast to form dentin-like tissue using fibroblast growth factor (FGF) and insulin-like growth factor (IGF).
Materials and Methods:
Dental pulp stem cells (DPSCs) were extracted from 10 human teeth. They were isolated and cultivated in vitro with the use of stem cell markers. The human DPSCs were characterized for trilineage differentiation. They were then differentiated into odontoblasts. The ability of cultivated odontoblasts to form dentin-like tissue was evaluated using FGF and IGF.
Results:
IGF showed superior ability to form dentin-like tissue as compared to FGF. The addition of FGF showed no significant difference in the formation of dentin-like tissue. A combination of FGF and IGF in odontoblast showed an enhanced ability to form dentin-like tissue.
Conclusion:
The use of growth factors IGF and FGF with dental stem cells showed a greater potential to form dentin-like tissue. This can profoundly alter the paradigms of conservative vital pulp therapy, which may eventually make it possible to treat dental diseases by regeneration of lost dentine.
Keywords: Dental pulp stem cells, dentin, fibroblast growth factor, insulin-like growth factor
INTRODUCTION
Oral health is compromised by tooth loss. Although a number of prosthetic techniques, including dental implants and artificial dentures are clinical treatments for tooth loss disorders, there are concerns about their safety and duration of use. The dentin and pulp often function as a unit and can experience reversible pulpal inflammation due to a variety of causes, including dental caries, trauma, periapical illnesses, and iatrogenic causes.[1] If left untreated, it may result in irreversible pulpitis and eventually pulp necrosis.
Endodontic treatment is suggested when the pulp is damaged irreversibly.[2] Despite the possibility that this treatment may be effective, the treated endodontium may again get infected, and/or the root could develop brittleness which could result in fracture and tooth loss.[3] Alternative therapies include surgery and implants which is both invasive and come at a considerable expense. No restorative material has been able to replicate every aspect of tooth tissue’s mechanical and physical characteristics. Furthermore, there is no conservative solution for some problems, such as irreversible pulpitis and an immature tooth with extensive coronal damage.
An emerging branch of tissue engineering and regenerative medicine, regenerative endodontics (REs) attempts to provide alternatives to established treatment modalities.[4] The objective of RE is to regenerate the endodontium in the root canal system by replacing the infected pulp with various agents that promote healing.[5] The essential components for RE can be broadly classified under stem cells, scaffolds, and growth factors.[4] Cell therapy is a crucial component of RE in this regard,[6,7] and several types of stem cells can be employed to generate the cells needed for endodontium regeneration.[8,9]
Transplanted cells should differentiate into a variety of lineages, including fibroblasts, nerve cells, endothelial cells, and odontoblasts, to restore nerve supply, blood supply, and endodontium, making it a challenge.[10,11] Postnatal stem cells can be found in the endodontium, periodontium, dental follicle, gingiva, bone, alveolar bone, and papilla, among other places in the oral cavity.[12] Among these, dental pulp stem cells (DPSCs) can be easily obtained and have a higher capacity for differentiation.[13] Furthermore, when not exposed to differentiating signaling molecules, postnatal stem cells could self-replicate for an extended period of time and retain their capacity for repeated differentiation.[11]
Growth factors are bioactive agents which are made of proteins. They can be grouped under translatory type (transforming growth factor [TGF]), insulin-like growth factors (IGFs), bone morphogenic proteins (BMPs), vascular endothelial type (vascular endothelial growth factor), and connective tissue type (connective tissue growth factor [CTGF]).[11] The fibroblast type (fibroblast growth factor [FGF]) comes under the CTGFs. The effects of GFs are multitargeting, overlapping, and cross-linking with antagonistic and synergistic actions. Among these, IGF and FGF have been shown to play a significant role in cellular proliferation and differentiation.[13]
This in vitro study was conducted to learn more about the capacity of cultured odontoblasts to produce dentin-like tissue under the influence of IGF and FGF.
MATERIALS AND METHODS
Collection of samples
Incisors, cuspids, and bicuspid teeth were collected from healthy donors with good oral hygiene (n = 10). Patients included in the study were in the age group of 18–25 years from both genders and intact healthy teeth indicated for extraction (premolars for orthodontic or periodontal reasons, impacted third molars). Medically compromised patients and patients unwilling to give written informed consent were excluded from the study. Approval from the institutional ethics committee and institutional stem cells committee was taken, and informed consent was obtained from the patient. Before extraction, the patients were asked to rinse with 0.2% chlorhexidine to reduce oral bacterial load. Using an air-rotor handpiece with a bur chuck attachment, access was gained to the pulp chamber. Sterile forceps were then used to carefully remove the pulp. This was immediately transported to the laboratory and submerged in tubes containing phosphate-buffered solution (PBS) containing antibiotic-antimycotic solution.
Isolation of dental pulp stem cells
Pulp tissue obtained from a tooth was chopped and put into 60 mm culture dishes as 1–2 mm-sized fragments. Fetal bovine serum (FBS) was applied to the tissues in enough quantity to thoroughly cover them. Incubation of the culture dishes was done at 37°C with 5% CO2 for a period of 24 h. Following incubation, explants were kept at 37°C in a humidified atmosphere containing 5% CO2 in minimal essential medium supplemented with 10% FBS and antibiotic antimycotic (AA) solution. The culture media were changed every 3 days, and cell outgrowth was monitored daily using an inverted phase-contrast microscope (Olympus CKX53, Japan). The outgrown cells were removed at 70%–80% confluence using a 0.25% Trypsin-EDTA solution. To test the proliferative potential of DPSCs, 1 × 104 cells were planted into culture plates with 12 wells at passage 4, and the count was obtained every other day for 9–10 days. Calculation of population doubling time (PDT) was then done for a period of 48 h using the following equation:
PDT = T log2= ðlogFCC × logICC
Where, ICC is the initial cell count, FCC is the final cell count, and T is the incubation time (in hours). The growth curve was plotted for 10 days simply using cell numbers [Graph 1].
Graph 1.

Growth curve
Colony-forming unit-fibroblast assay
A colony-forming unit-fibroblast (CFU-F) assay was used to assess cells’ ability to form colonies. In culture media (Dulbecco’s Modified Eagle’s medium [DMEM] with 10% FBS and 1% AA), cells were seeded into 6-well plates at an initial density of 5 × 102 per well. Cells were rinsed with PBS and stained with 0.3% crystal violet after 7 days of culture. Colonies with aggregates of 50 or more cells were scored as CFU-F.
Differentiation of adipogenic cells
The DPSCs were sown in a 24-well plate (2500/cm2) with full growth media for adipogenic differentiation. After 24 h, the cells were given adipogenic media twice a week for 3 weeks (DMEM supplemented with 10% FBS, 1% AA, 1 mM dexamethasone, 10 mM insulin, 200 mM indomethacin, and 0.5 mM isobutyl-methylxanthine). Adipocyte differentiation was confirmed by staining lipid droplets with 0.3% oil red O [Figure 1A].
Figure 1.

(A-C) Tri lineage differentiation of human dental pulp cells
Osteogenic differentiation
In a 24-well plate supplanted with growth media, osteogenic differentiation cells were sown at a density of 2500 cells per cm2. After 24 h, the cells were subjected to induction media which is composed of 50 mM ascorbate-2-phosphate, 10 mM b-glycerophosphate, 10% FBS, 1% AA, and 0.1 mM dexamethasone. The medium was changed twice a week. As a control, cells in basal media were used. After 21 days, the cells were frozen and stained with 2% alizarin red S to show the osteogenic differentiation and mineralization (pH 4.1–4.3) [Figure 1B].
Chondrogenic differentiation
The human dental pulp cells (hDPSCs) were centrifuged in a 15 mL polypropylene tube for 5 min at 1000 rpm before being rinsed with DMEM to stimulate chondrogenic differentiation. The cells were cultured in high-glucose DMEM till confluent. Confluent cells were treated with chondrogenic differentiation media containing 1X-ITS, 1 mM sodium pyruvate, 100 nM dexamethasone, 50 mg/mL ascorbate-2-phosphate, 40 mg/mL L-proline, and 10 ng/mL TGF-b3. These were then cultured for 4 weeks at 37°C in a 5% CO2 environment, with medium changes occurring every 2–3 days. After 28 days, the pellets were fixed with 4% paraformaldehyde overnight, and paraffin-embedded sections were stained with 0.1% safranin O to show the chondrogenic differentiation [Figure 1C].
Odontogenic differentiation
In a 24-well plate with full growth media, cells were planted at a density of 2500/cm2. The pyrrolidine dithiocarbamate cells were subjected to an induction medium (DMEM supplemented with 10% FBS, 1% AA, 0.1 mM dexamethasone, 50 mM ascorbate-2-phoshate, and 10 mM b-glycerophosphate) after 24 h. Two times per week, the medium was changed. Control cells were grown in basal media. 21 days later, the cells were frozen, and 2% alizarin red S (pH 4.1–4.3) was used for staining to confirm odontogenic differentiation and mineralization. Rosette shape morphology was observed after 20 days of induction [Figure 2].
Figure 2.
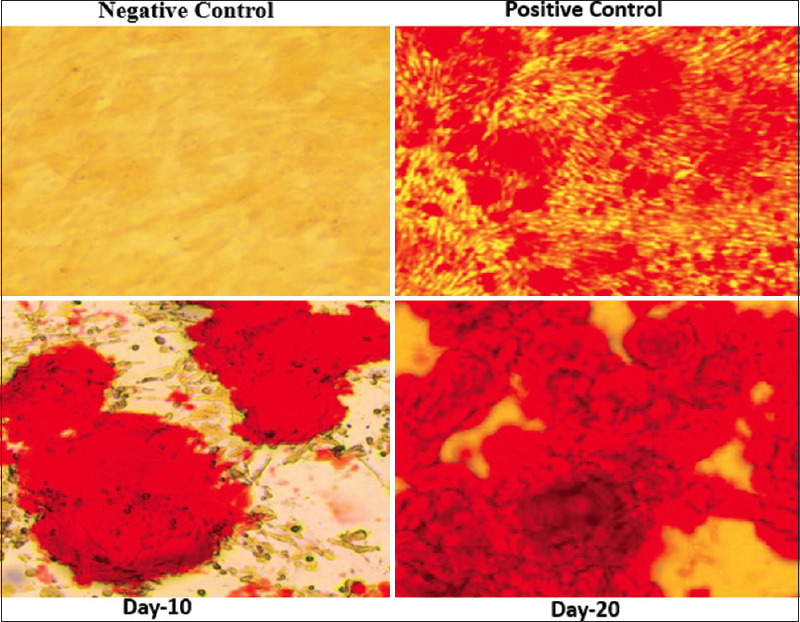
Odontogenic differentiation
Surface markers analysis
Washing of the cells was done with fluorescence-activated cell sorting (FACS) buffer, then fixed with 3.7% paraformaldehyde for a period of 10 min. Later washing was carried out and blocked with 10% human serum for 30 min (all steps were performed at 4°C). This was incubated for 45 min with fluorescent-conjugated antibodies and their respective isotype controls. After washing, cells were analyzed for CD 90, CD 105, CD 73, CD34, CD45, and HLA-DR using FACS Vantage (10,000 events) and Cell Quest Pro Software (BD Biosciences).
RESULTS
IGF showed a superior ability to form dentin-like tissue as compared to FGF. The addition of FGF showed no significant difference in the formation of dentin-like tissue. The combination of FGF and IGF in odontoblasts showed an enhanced ability to form dentin-like tissue [Graph 2].
Graph 2.

Cell differentiation with different growth factors
DISCUSSION
Ameloblasts and odontoblasts differentiate into specialized and highly mineralized tissues called enamel and dentin during development because of interconnections between cells of the inner enamel organ being epithelial in nature and cells of the dental papilla being mesenchymal in nature. These tissues do not remodel once they have been created, in contrast to bone which does so steadily throughout postnatal life. The dental pulp is protected by a reparative dentin barrier, a mineralized matrix that is poorly organized and develops following dentinal damage brought on by mechanical trauma, chemical exposure, or disease processes after tooth eruption. It is believed that progenitors are drawn from tooth pulp and used to create terminally differentiated odontoblasts and supporting connective tissue.[14] Tooth tissue regeneration facilitates the critical process of physiologic dentin deposition, which contributes to the structural integrity of the tooth and reduces issues such as interfacial failure and microleakage.
Extracellular matrix (ECM) interactions occur between all cells, and stem cells rely on the ECM to guide differentiation. The commitment of a stem cell lineage is influenced by the nanotopography, stiffness, stress protein composition, and strain inherent in any given ECM.[15] DPSCs have a great ability for differentiation and can restore a complex resembling dentin and pulp. The ability of DPSCs to differentiate in processes such as dentinogenesis, neurogenesis, adipogenesis, osteogenesis, chondrogenesis, and angiogenesis has been shown in numerous investigations. Growth factors and scaffolds have an impact on the molecular mechanisms and roles of the differentiation process in DPSCs. Basic FGF, TGF-beta, nerve growth factor, platelet-derived growth factor, and BMPs are a few examples of growth factors that influence the fate of DPSCs such as differentiation, cell proliferation, and wound healing.[15]
The proliferation and differentiation of dentin-forming cells depend on the effective binding of the IGF to the receptor-activated pathways like mitogen-activated protein kinase. FGFs, on the other hand, have a positive effect on the stimulation and differentiation of osteoblasts, chondrocytes, and periosteal cells, resulting in the formation of Type I collagen.[16,17]
In this study, a method for characterization of the grown DPSCs for stem cell markers to assess whether such a cell exists in dental pulp was employed. To identify the cell type and lineage, special proteins called markers are expressed on the cell surface. These cell markers can be classified into positive (CD90, CD44, CD29, CD105, CD73, and CD34) and negative types (CD45, CD31, and CD14).[16] Research showed that a small population with aggregates of 50 or more cells within adult human dental pulp is clonogenic. This was confirmed by analyzing for CD-positive and CD-negative markers such as CD 90, CD 105, CD 73, CD34, CD45, and HLA-DR.
A powerful modulator of cell motility, proliferation, and differentiation is FGF.[16] According to reports, the FGF locus on chromosome 4 has mRNAs that are 4.6 kb in length and an additional 2.2 kb in the hypothalamus.[17] Two of the most important properties of FGF are its high affinity for heparin/heparan sulfate and the natural translocation of glycosaminoglycans to the ECM. Endothelial and bone cell-derived FGF may have a role in the ECM.[17] Furthermore, the maintenance of numerous distinct target tissues is impacted by this strong affinity for heparin/heparin sulfate.
FGF has been discovered in DPSCs and following endodontic irrigation therapy.[18] In addition, it was shown that the basic FGF receptors (FGFR1 and FGFR2) are expressed in hDPSCs.[19] In addition, DPSCs were treated with FGF, which caused them to multiply and differentiate during osteogenesis and neurogenesis.[20,21] It has also been demonstrated that FGF treatment duration influences DPSCs’ ability to differentiate into osteoblasts. The literature has demonstrated that just 1 week of therapy increased osteogenic differentiation.[21] These results demonstrate that FGF stimulates proliferation and is associated with osteogenic, odontogenic, and neurogenic differentiation.
Another growth factor used in this study was IGF. Many diverse cell types and tissues use IGF-1 as a significant growth, differentiation, and apoptosis regulator. The IGF-1 receptor mediates IGF-1’s biological effects.[22] IGF-1 induces ameloblast and odontoblast differentiation, increases dentin regeneration, and creates complex structures that resemble enamel and dentin. IGF-1 has been shown to promote dental pulp cell proliferation and mineralization.[22] In addition, stem cells from the apical papilla and periodontal ligament that are odontogenic in nature can multiply and differentiate in response to IGF-1.[22] It has been hypothesized that IGF-1 may have an impact on hDPSC proliferation through ERK1/2 MAPK activation.[23] The osteo/odontogenic differentiation of hDPSCs was also accelerated by IGF-1, as demonstrated by increased matrix mineralization, increased alkaline phosphate activity, and increased expression of osteo/odontogenic markers in IGF-1-treated hDPSCs.[23]
Moreover, osteocalcin is a reparative molecule thought to be present in the tooth pulp and odontoblasts can express and is also found in the dentin matrix.[24] The dentin sialophosphoprotein (DSPP) gene and DSPP, which are possible indicators of odontogenic differentiation, are involved in the formation and control of the hydroxyapatite mineral phase during dentin calcification.[25] Recent investigations have demonstrated that DSPP has a favorable impact on the development of minerals, odontoblast-like differentiation, and DPSC maturation.[25] In odontoblasts, the expression of each of these noncollagenous proteins can be seen.
In the present study, FGF and IGF were used to evaluate the ability of cultivated odontoblasts to form dentin-like tissue. The study showed that both IGF and FGF can encourage the proliferation and odontogenic differentiation of hDPSC. IGF is a better alternative as compared to FGF and showed better differentiation and proliferation in comparison to FGF. When used together, FGF and IGF work in synergy to cultivate odontoblasts to form dentin-like tissue.
CONCLUSION
The tooth pulp physiology and its ability to heal after injury depends on stem cells. DPSCs can be prospective treatment targets in irreversible pulpitis. The use of FGF and IGF with these cells has the potential to alter the paradigms of conservative vital pulp therapy and endodontic treatments which may eventually lead to regeneration of diseased dental tissues. The ability of culturing odontoblasts opens up opportunities to form dentin-like tissue which has tremendous potential in deep carious lesion management and in the field of REs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shah N, Logani A. SealBio: A novel, non-obturation endodontic treatment based on concept of regeneration. J Conserv Dent. 2012;15:328–32. doi: 10.4103/0972-0707.101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan A, Saini A, Sharma S, Kumar V, Chawla A, Logani A. India's contribution to regenerative endodontics: A bibliometric analysis. J Conserv Dent. 2020;23:325–9. doi: 10.4103/JCD.JCD_178_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudagi KB, Rudagi B. One-step apexification in immature tooth using grey mineral trioxide aggregate as an apical barrier and autologus platelet rich fibrin membrane as an internal matrix. J Conserv Dent. 2012;15:196–9. doi: 10.4103/0972-0707.94582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmar A, Ansari NA, Parmar G, Krishnakumar A. Evaluation of cell viability of human dental pulp stem cells in two dimensional and three dimensional fibrin glue Scaffold. J Conserv Dent. 2020;23:479–83. doi: 10.4103/JCD.JCD_439_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galler KM, D'Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–41. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Brar GS, Toor RS. Dental stem cells: Dentinogenic, osteogenic, and neurogenic differentiation and its clinical cell based therapies. Indian J Dent Res. 2012;23:393–7. doi: 10.4103/0970-9290.102239. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez Lozano FJ, Insausti CL, Iniesta F, Blanquer M, Ramírez MD, Meseguer L, et al. Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal. 2012;17:e1062–7. doi: 10.4317/medoral.17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617–31. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Liu Z, Xie Y, Hu J, Wang H, Fan Z, et al. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 2015;6:249. doi: 10.1186/s13287-015-0244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Nangia D, Saini A, Sharma S, Kumar V, Chawla A, Perumal V, et al. Treatment outcome of regenerative endodontic procedures in mature permanent teeth compared to nonsurgical endodontic treatment: A systematic review and meta-analysis. J Conserv Dent. 2021;24:530–8. doi: 10.4103/jcd.jcd_535_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry –Part I: Stem cell sources. J Prosthodont Res. 2012;56:151–65. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Nuti N, Corallo C, Chan BM, Ferrari M, Gerami Naini B. Multipotent differentiation of human dental pulp stem cells: A literature review. Stem Cell Rev Rep. 2016;12:511–23. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 14.Smith AJ, Cassidy N, Perry H, Bègue Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. 1995;39:273–80. [PubMed] [Google Scholar]
- 15.Hadden WJ, Choi YS. The extracellular microscape governs mesenchymal stem cell fate. J Biol Eng. 2016;10:16. doi: 10.1186/s13036-016-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiba H, Fujita T, Doi N, Nakamura S, Nakanishi K, Takemoto T, et al. Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cell Physiol. 1998;174:194–205. doi: 10.1002/(SICI)1097-4652(199802)174:2<194::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Klagsbrun M. The fibroblast growth factor family: Structural and biological properties. Prog Growth Factor Res. 1989;1:207–35. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- 18.Kunimatsu R, Nakajima K, Awada T, Tsuka Y, Abe T, Ando K, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501:193–8. doi: 10.1016/j.bbrc.2018.04.213. [DOI] [PubMed] [Google Scholar]
- 19.Chang YC, Chang MC, Chen YJ, Liou JU, Chang HH, Huang WL, et al. Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. J Endod. 2017;43:936–42. doi: 10.1016/j.joen.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Lian M, Cao P, Bao G, Xu G, Sun Y, et al. Effects of nerve growth factor and basic fibroblast growth factor promote human dental pulp stem cells to neural differentiation. Neurochem Res. 2017;42:1015–25. doi: 10.1007/s11064-016-2134-3. [DOI] [PubMed] [Google Scholar]
- 21.Qian J, Jiayuan W, Wenkai J, Peina W, Ansheng Z, Shukai S, et al. Basic fibroblastic growth factor affects the osteogenic differentiation of dental pulp stem cells in a treatment-dependent manner. Int Endod J. 2015;48:690–700. doi: 10.1111/iej.12368. [DOI] [PubMed] [Google Scholar]
- 22.Rebouças EL, Costa JJ, Passos MJ, Silva AW, Rossi RO, van den Hurk R, et al. Expression levels of mRNA for insulin-like growth factors 1 and 2, IGF receptors and IGF binding proteins in in vivo and in vitro grown bovine follicles. Zygote. 2014;22:521–32. doi: 10.1017/S0967199413000166. [DOI] [PubMed] [Google Scholar]
- 23.Lv T, Wu Y, Mu C, Liu G, Yan M, Xu X, et al. Insulin-like growth factor 1 promotes the proliferation and committed differentiation of human dental pulp stem cells through MAPK pathways. Arch Oral Biol. 2016;72:116–23. doi: 10.1016/j.archoralbio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Gu S, Liang J, Wang J, Liu B. Histone acetylation regulates osteodifferentiation of human dental pulp stem cells via DSPP. Front Biosci (Landmark Ed) 2013;18:1072–9. doi: 10.2741/4163. [DOI] [PubMed] [Google Scholar]
- 25.He P, Zheng L, Zhou X. IGFs in dentin formation and regeneration: Progress and remaining challenges. Stem Cells Int. 2022:1–7. doi: 10.1155/2022/3737346. [DOI] [PMC free article] [PubMed] [Google Scholar]


