Abstract
Matrix metalloproteinases (MMPs) have been identified as agents that disintegrate the collagen structures of dental hybrid layers, resulting in reduced restorative bond strength. Multiple MMP inhibitors (MMPIs) are known to counteract this degenerative mechanism, thereby preserving bond strength and promoting the longevity of resin-based restorations. Additionally, literature suggests that certain MMPI materials possess antimicrobial/anticariogenic properties, potentially reducing the risk of secondary caries development. Therefore, this review article aims to narrate on the integration of matrix metalloproteinase inhibitors into adhesive systems and their impact on bond strength.
Keywords: Matrix metalloproteinase inhibitors and bond strength, matrix metalloproteinases, matrix metalloproteinase inhibitors
INTRODUCTION
Dentistry, particularly restorative dentistry, has undergone multidimensional changes over the last 100 years. It has become apparent that the esthetic concerns within the patient community have increased significantly.[1,2] Tooth-colored restorations, coupled with modern adhesive strategies, have transfigured our perspectives on dental restorations. Introduction of acid-etching technique by Michael Buonocore in 1955 marked the embarkation of a new era of restorative dentistry. Subsequently, Masuhara et al. reported a major breakthrough in research. They discovered that when tri-n-butylborane was used along with methyl methacrylate, it produced a commendable bond in a collagen-added wet ivory model. Later, in 1969, the very first dental adhesive was made available in the commercial market by the name “direct bonding system,” which was used for orthodontic bonding procedures.[3]
Dental composites are among the most commonly used restorative materials for their biomimetic capacity. A resin adhesive is used to achieve adequate bonding of composite resins to the tooth structure.
Matrix metalloproteinases (MMPs), a set of closely related extracellular proteolytic enzymes, are crucial for diverse physiological functions such as embryonic development, tissue repair, and bone restructuring. In addition, they contribute significantly to pathological situations such as inflammation and arthritis.[4] Many cell types are known to produce MMPs. These include fibroblasts, endothelial cells, cementoblast-like cells, and epithelial cells.[5]
Ensuing the formation and mineralization of the collagen matrix during tooth development, MMPs enter an inactive state and become trapped within the calcified matrix. There is the potential for these dormant MMPs to be reactivated when exposed to an acidic environment.[6] This rise in acidity is attributed to the increased activity of caries causing bacteria or through the application of acidic agents during dental procedures. In case of etch-and-rinse adhesive, an acid etchant is utilized [Figure 1].[7] In Nakabayashi’s own words, who proposed the concept of the hybrid layer, “the dentinal peptides (including collagen) must not be denatured when the dentin is decalcified. Furthermore, if the acid is too aggressive, it may expose collagen below the hybrid layer leaving a zone of weak dentin that is susceptible to long-term degradation.”[8] Thus, this signifies the need to prevent collagen degradation to preserve adequate bond strength. Recent studies have pointed out that the degradation of collagen fibers within the hybrid layer and the consequential compromise of bond strength and integrity at the tooth–restoration interface are attributed to the activity of MMPs as a significant factor.[9] This suggests that the inhibition of MMPs is advantageous for the resin–hybrid layer complex, ultimately enhancing bond stability. Various materials have been shown to hinder or decrease the activity of MMPs.
Figure 1.
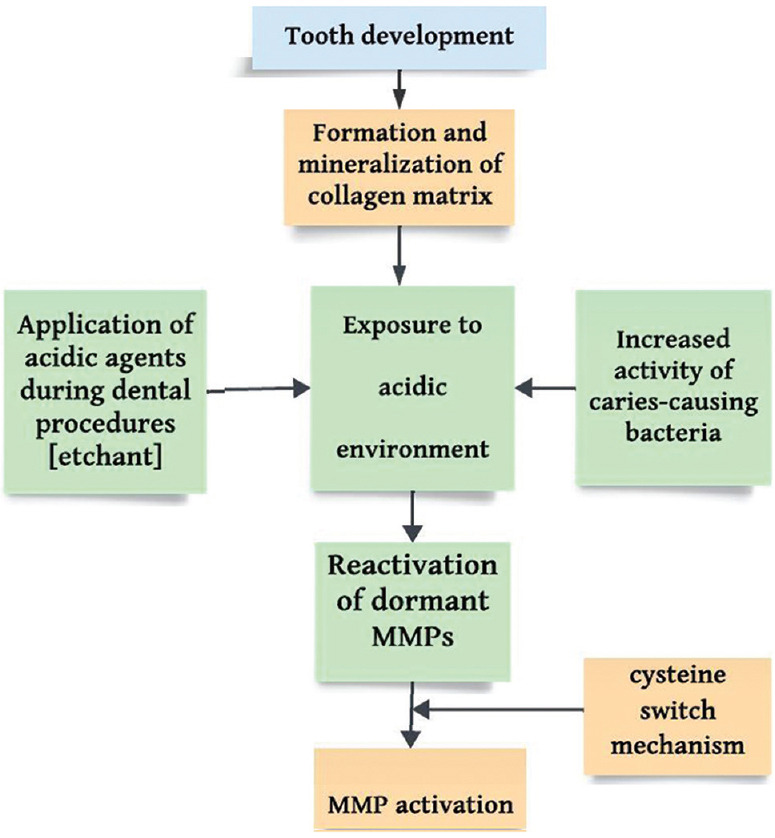
Mechanism of activation of matrix metalloproteinases in human tooth
Chlorhexidine (CHX) is one of the most commonly studied MMP inhibitors (MMPIs) in dentistry.[10] Other than the effects caused by the MMPs at the hybrid layer, it is also noteworthy to mention that MMPs play a key role in the progression of periodontal diseases. Numerous studies have been carried out so far to identify modalities to inhibit this pathological process.[11]
The aim of this article was to perform a literature review on the integration of MMPIs into adhesive systems and their impact on bond strength.
MATERIALS AND METHODS
The review article conducted a comprehensive evaluation of the existing literature concerning various MMPs and their intricate roles in dental adhesive bonding mechanisms. Scientific databases such as PubMed and Google Scholar were used to pool relevant articles. Terms such as “MMP,” “Matrix metalloproteinase,” “MMP inhibitor,” “MMP inhibitor and dental,” and “MMP inhibitor and bond strength” were used to retrieve articles from the databases. We included studies that addressed the mechanism of MMP and MMPIs, their uses in dental restorations, advantages, and limitations. Studies with insufficient data were excluded. Ultimately, this review focused on 63 articles concerning MMPIs and their applications in dentistry.
LITERATURE REVIEW
The discovery of MMPs can be traced back to the early 1960s. The initial breakthrough occurred when collagenase activity was observed in an enzyme found in amphibian species.[12] Subsequently, this group of collagenases was termed “Matrix Metalloproteinase” by Harris et al.[13] Since then, the scientific literature has been filled with a vast array of studies on MMPs and their inhibitors. It is now well established that MMPs are involved in a multitude of metabolic processes, including tissue repair, bone remodeling, cell proliferation, wound healing, programmed cell death, and gonadal tissue reproduction. MMPs are classified broadly into six major groups that include (a) collagenases, (b) gelatinases, (c) stromelysins, (d) matrilysins, (e) membrane-bound MMPs, and (f) other MMPs or miscellaneous MMPs [Figure 2].[14] The relevance of MMPs in dentistry is manifold. Numerous MMPs are identified to be of dental tissue origin. In this regard, odontoblasts are known to produce MMP-2, MMP-9 belonging to the gelatinases,[15] MMP-3 (stromelysin),[16] MMP-8 (collagenase),[17] and membrane type 1 MMP.[17] The exact role of MMPs in dental odontoblasts is well known to none, but it has been suggested that these MMPs might play a role in organization of the dentinal organic matrix ahead of the mineralization process.[18] As previously mentioned in the introduction section, MMPs undergo a process where they become inactive and are then trapped within the calcified matrix following tooth development. Caron et al.[19] pointed to the presence of MMP-2 in secretory odontoblastic cells which are involved in dentin formation during tooth development. Later, Martin De Las Heras et al.[20] discovered the presence of MMP-2, a gelatinase, in human dentin. Sulkala et al.[21] proved the presence of MMP-20 in the dentinal complex. Given this brief outlook on MMPs and its dental presence, it is crucial to understand its effects in dental restorations and the subsequent bond strength.
Figure 2.
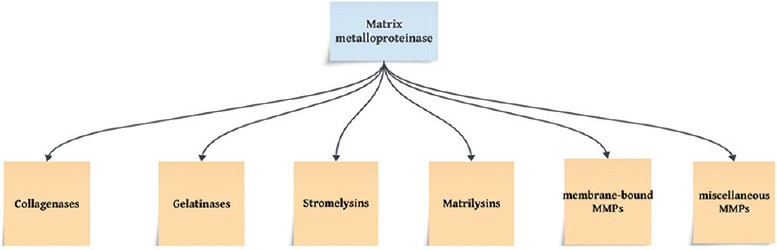
Classification of matrix metalloproteinases - the six major groups
All MMP gene family members have a common characteristic: they are initially synthesized in an inert (latent), inactive form. At the molecular level, all MMPs possess just two domains: the propeptide domain, containing an essential cysteine residue, and the catalytic domain, which encompasses the zinc-binding site. Earlier studies on the latency in human fibroblast collagenase (HFC) stem from the creation of an intramolecular complex. This complex forms between a cysteine residue within its propeptide domain and the vital zinc ion located in the catalytic domain, effectively obstructing the active site. The activation of latent HFC can be induced through a variety of methods, all of which lead to the separation of the cysteine residue from the complex. This process is commonly referred to as the “cysteine switch” mechanism.[22]
The activation of MMP occurs in an acidic, low pH environment by triggering the cysteine switch. It has also been observed that MMPs activated by acids released by bacteria play a critical role in dentin destruction in caries.[23] The activity of endogenous collagenolytic and gelatinolytic enzymes in phosphoric acid-treated dentin can be regained through the use of etch-and-rinse adhesives. Furthermore, it was observed that the amount of regaining proteolytic activity in the tooth was proportional to the extent of acidity offered by the adhesive systems.[7] Lehmann et al. demonstrated that self-etching adhesives stimulate the release of MMP-2 from odontoblast cells.[24]
It is of no surprise that the scientific fraternity was intrigued to inhibit this mechanism, thus favoring better bond strength. Substances or materials that inhibit MMPs are termed as MMPIs. MMPIs can be broadly classified into two categories: endogenous inhibitors and synthetic inhibitors. Endogenous inhibitors are further subdivided into specific endogenous inhibitors and nonspecific endogenous inhibitors.[25] These endogenous inhibitors provide the necessary physiological counter balance between the extracellular matrix and MMPs. Tissue inhibitors of metalloproteinase are examples for specific endogenous inhibitors. Certain proteins have been identified as nonspecific endogenous inhibitors such as α2-macroglobulin and membrane-bound β-amyloid precursor protein.
It is, therefore, apparent that compounds studied for their MMP-inhibiting properties from the perspective of adhesive dentistry belong to the category of synthetic inhibitors.
Chlorhexidine
CHX is the most widely studied material for its MMP inhibition properties. In general, MMPIs are incorporated into adhesive, etchant, or primer. The proposed inhibitory mechanism involves cation chelation of calcium and zinc ions found in MMPs.[26] The bonding between CHX and the dentinal surfaces occurs due to electrostatic bonding.[27]
Distilled water or artificial saliva is the preferred aging solution.[10] The addition of 2% CHX to acid-etched dentin or its incorporation into the phosphoric acid conditioner reduces collagen degradation,[4,28] thus enhancing immediate bond strength. Long-term preservation of bond strength is noted in multiple studies.[29,30]
Studies have also pointed out that when CHX was incorporated into the etchant, the decrease in microtensile bond strength (μTBS) was less significant compared to the control group.[29] Loguercio et al.[29] observed the persistence of CHX molecules within the hybrid layer, as revealed by Raman spectroscopy, even after a span of 5 years.
Gendron et al. have pointed out that CHX has direct inhibition effects on MMP-2, MMP-9, and MMP-8, of which the former two belong to the gelatinase group, while the latter is of collagenase group.[26]
A study evaluated the effect of CHX in the prevention of secondary caries in experimental models and concluded that pretreatment of dentin with CHX slowed down the development of secondary caries.[31]
A study asserted that the considerable water solubility of large CHX molecules facilitates their leaching, resulting in a reduction of bond strength between interfaces.[32]
Literature lacks sufficient information to arrive at a conclusive decision on minimum MMP inhibition concentration.
Benzalkonium chloride
Benzalkonium chloride (BAC) is categorized within the quaternary ammonium compound group. BAC is a cationic compound (positively charged) and thus exhibits affinity toward negative carboxylic groups in collagen.[33] According to Sabatini et al., specific experimental groups with BAC exhibited increased μTBS when compared to the control group at both the 24-h and 6-month time points. In addition, dentin matrices treated with BAC showed reduced mass loss and lower release of hydroxyproline (parameter used for the assessment of collagen solubilization) compared to the control.[31] Comba et al. conducted gelatin zymography assays and in situ zymography quantification analyses, revealing that formulations containing BAC led to a reduction in the expression of MMPs.[34]
Immediate micro-TBS (μTBS) values increased significantly at 24 h in an experimental BAC group,[35] whereas μTBS performance of 1% BAC experimental group was inferior than the control according to Comba et al.[34]
Sabatini et al. showed that BAC effectively deactivates dentin proteinases attached to the matrix in demineralized dentin structures, and this deactivation follows a dosage-dependent pattern. When BAC was applied at concentrations of 0.5% and 1.0% for a duration of 60 s, it resulted in a 31% and 54% reduction in total MMP activity, respectively.[36]
Adhesives containing BAC decrease endogenous enzymatic activity both immediately and over an extended period. Nevertheless, in certain experimental conditions with BAC, there was an observed rise in gelatinolytic activity over time, coupled with a decline in bond strength, irrespective of the adhesive employed.
Epigallocatechin-3-gallate (EGCG)
Epigallocatechin gallate (EGCG) is a polyphenolic compound abundantly found in tea leaves. It is also the major catechin found in green tea.[37] A previous research has noted that catechins found in green tea can impede the activities of MMPs and the activation of proMMP-2.[38] As per Gerhardt et al., the use of epigallocatechin gallate at a 2% concentration on dentin resulted in an enhancement of the bond strength of a self-etching adhesive to regular dentin.[39] Several research studies indicate that the addition of epigallocatechin gallate into the adhesive system resulted in consistent and stable bond strength over an extended period.[28,40,41] Yet, according to Amaral FL et al., there was a reduction in bond strength values after a 6-month duration, regardless of the dentin treatment.[42]
Proanthocyanidin
Proanthocyanidin (PA), a flavonoid classified among polyphenolic compounds, offers notable health advantages for humans. Typically derived from sources such as grape seeds and blueberries, this compound is commonly extracted.[43] PA is known to reduce oxidative stress.[44] Its antimicrobial potential is also widely studied.[45,46,47] The antimicrobial and anti-inflammatory properties of PA have attracted attention from the dental fraternity. Dias et al. ascertained the μTBS immediately and after a 12-month storage duration, along with the antimicrobial effectiveness of an adhesive containing various concentrations (%) of PA: 0%, 1%, 2%, 4.5%, and 6%. The incorporation of 2%, 4.5%, and 6% PA maintained the dentin μTBS even after a 12-month storage period, without affecting the solubility (Sp), sorption (Sl), or degree of conversion (DC%) of the adhesives.[48] Treating demineralized dentin with nanohydroxyapatite PA might serve as an alternative approach to enhance its strength by enhancing collagen stability and providing reinforcement.[49]
Zinc salts
Zinc ions act as competitive inhibitors for MMPs. This is due to the fact that the collagen contains four Zn binding sites located at the exact positions as the cleavage sites that collagenases target, thus preventing the action of collagenases and protects collagen degeneration.[50] Various salts of zinc were studied for its effects on dentin bonding, which includes zinc oxide (ZnO), Zn-methacrylate, ZnN3, and zinc chloride (ZnCl2). Of which, ZnCl2 demonstrated better inhibition properties.[51]
Barcellos et al. conducted a study about the lasting bond strength and cytotoxic impact of dentin adhesive infused with zinc over a 6-month period. The incorporation of zinc oxide nanoparticles (ZnO-n) effectively decreased the cytotoxicity associated with the adhesive. Furthermore, ZnO-n preserved the μTBS even after a 6-month storage period, preventing any decline.[52] These results align with the previous investigation conducted by Toledano et al.[53]
Incorporation of zinc ions either into experimental adhesive systems or commercially available adhesive systems had no notable distinctions in the tensile strength, flexural modulus, flexural strength compressive strength, and water sorption.[52]
The addition of 2 wt% of ZnCl2 was observed to inhibit both the reduction in resin–dentin bond and the increase in nanoleakage after 1 year of storage in water. According to Almeida et al., due to its high solubility, zinc chloride may undergo considerable leaching in the oral cavity, potentially influencing its efficacy over an extended period. It was further noted that additional studies would be required to investigate this issue.[51]
GM1489
GM1489 is a human-made synthetic inhibitor of MMPs, meticulously designed to target MMP 1, 2, 3, 8, and 9.[15] Our literature search revealed limited studies available in dental literature on GM1489. Adhesive systems incorporating GM1489 exhibited superior material properties and maintained bond strength stability, even under certain experimental conditions which involved storage of samples in water for 12 months.[30] da Silva et al. also proposed that GM1489 could function as an MMPI in etchant and adhesive systems, with the potential to preserve bonding stability over an extended duration.[30]
Sodium fluoride
The positive effects of sodium fluoride (NaF) in preserving and sustaining adhesive dentin interface bond strength have been demonstrated in studies. Kato et al. reported a reduction in dentin degradation by MMPs when NaF gel was incorporated into demineralized dental matrices.[54] Furthermore, investigations have explored NaF’s impact on the intrinsic activity of MMPs within dentin matrices.[55] An in vitro study by Alaghehmand et al., where fluoride was integrated into the adhesive system, concluded that fluoride-containing adhesive enhances the durability of resin–dentin bonds.[56]
Other matrix metalloproteinase inhibitors
Hesperidin (HPN), a citrus extract, is a natural flavonoid with various advantages, including antioxidant and anti-inflammatory properties, collagen cross-linking abilities, caries protection, remineralization, and antimicrobial effects.[57] Dental adhesive systems containing 0.5 wt% HPN demonstrated a favorable antibacterial property without adversely affecting adhesive properties. However, the μTBS was notably diminished after thermocycling.[57] In a research conducted by Islam et al., the introduction of HPN into the self-etching primer demonstrated a beneficial impact on both the immediate μTBS and the mechanical characteristics of the bonded interface.[58] Islam et al. noted that the inclusion of 2% HPN in the self-etching primer had a positive influence on both the immediate μTBS and the mechanical properties of the resin–dentin interfaces. In addition, in the 5% HPN group, the structure of collagen in the hybrid layer remained intact even after a storage period of 1 year in artificial saliva.[59]
Batimastat, a synthetic analog of the collagen substrate, functions as a zinc ion chelator. Initially, it was investigated for its ability to impede tumor progression and metastasis.[60] In a study by Almahdy et al., which infused two MMPIs (batimastat BB94 and galardin GM6001), it was found that the μTBSs of all adhesive systems remained unchanged compared to their respective control groups without MMPIs.[61]
Ilomastat, which is well known by its proprietary name galardin, is a broad-spectrum MMPI. In experimental models, galardin completely inhibited MMP-2 and MMP-9; however, incorporation of galardin as an added primer produced no effect on immediate bond strength. Nevertheless, it significantly decreased bond degradation after 1 year when stored in artificial saliva aging solution.[62] In a study conducted with galardin and its solvents in extracted third molars, significant results were observed, with no decrease in immediate bond strength.[63]
Limitations
The research studies found in literature search are limited to in vitro/experimental studies
In long-term studies, the study samples were primarily aged using distilled water or artificial saliva; thus, the oral environment could not be reproduced
The majority of the studies focused on noncarious dentin.
CONCLUSION
The incorporation of MMPIs yields a significant enhancement in dentin bond strength, with no discernible regression observed. This indicates promising prospects for advancing dental bonding procedures. However, further investigations are warranted to evaluate the biocompatibility of MMPI-incorporated dental adhesive systems and to conduct clinical trials to ascertain their ultimate effectiveness in clinical practice.
Clinical significance
Incorporation of MMPI at any step of the dental adhesive system reduces the rate of bond strength degradation. This enhancement contributes to the longevity of dental resin-based restorations. Some materials used as matrix metalloproteinase inhibitors also exhibited antimicrobial/anticariogenic properties. Thus, incorporation of matrix metalloproteinase inhibitors in adhesive systems could be a viable option, as the incidence of secondary caries development shall be reduced considerably. This underscores a compelling direction for the advancement of dental adhesive technologies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Afroz S, Rathi S, Rajput G, Rahman SA. Dental esthetics and its impact on psycho-social well-being and dental self confidence: A campus based survey of North Indian university students. J Indian Prosthodont Soc. 2013;13:455–60. doi: 10.1007/s13191-012-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pani SC, Saffan AA, AlHobail S, Bin Salem F, AlFuraih A, AlTamimi M. Esthetic Concerns and Acceptability of Treatment Modalities in Primary Teeth: A Comparison between Children and Their Parents. Int J Dent. 2016;2016:3163904. doi: 10.1155/2016/3163904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuhara E, Kojima K, Hirasawa T, Tanuni N, Kimura T. Studies on dental self-curing resins(3). Effect of alkylborone on the adhesive force to ivory and tooth surface. Rep Res Inst Dent Mater. 1963;2:457–465. [Google Scholar]
- 4.Page McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du M, Wang Y, Liu Z, Wang L, Cao Z, Zhang C, et al. Effects of IL-1β on MMP-9 expression in cementoblast-derived cell line and MMP-mediated degradation of type I collagen. Inflammation. 2019;42:413–25. doi: 10.1007/s10753-018-00951-6. [DOI] [PubMed] [Google Scholar]
- 6.DeVito Moraes AG, Francci C, Vidal CM, Scaffa PM, Nesadal D, Yamasaki LC, et al. Phosphoric acid concentration affects dentinal MMPs activity. J Dent. 2016;53:30–7. doi: 10.1016/j.jdent.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin bonding mechanism. J Esthet Dent. 1991;3:133–8. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazzoni A, Tjäderhane L, Checchi V, Di Lenarda R, Salo T, Tay FR, et al. Role of dentin MMPs in caries progression and bond stability. J Dent Res. 2015;94:241–51. doi: 10.1177/0022034514562833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghmoor RB, Jamal H, Abed H, Allan E, Ashley P, Young A. Incorporation of MMP inhibitors into dental adhesive systems and bond strength of coronal composite restorations: A systematic review and meta-analysis of in vitro studies. Jpn Dent Sci Rev. 2022;58:298–315. doi: 10.1016/j.jdsr.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Checchi V, Maravic T, Bellini P, Generali L, Consolo U, Breschi L, et al. The role of matrix metalloproteinases in periodontal disease. Int J Environ Res Public Health. 2020;17:4923. doi: 10.3390/ijerph17144923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkedal Hansen H. From tadpole collagenase to a family of matrix metalloproteinases. J Oral Pathol. 1988;17:445–51. doi: 10.1111/j.1600-0714.1988.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 13.Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9:1076. doi: 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakiyanov O, Kalousová M, Zima T, Tesař V. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kidney disease. Adv Clin Chem. 2021;105:141–212. doi: 10.1016/bs.acc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Heikinheimo K, Salo T. Expression of basement membrane type IV collagen and type IV collagenases (MMP-2 and MMP-9) in human fetal teeth. J Dent Res. 1995;74:1226–34. doi: 10.1177/00220345950740051301. [DOI] [PubMed] [Google Scholar]
- 16.Hall R, Septier D, Embery G, Goldberg M. Stromelysin-1 (MMP-3) in forming enamel and predentine in rat incisor-coordinated distribution with proteoglycans suggests a functional role. Histochem J. 1999;31:761–70. doi: 10.1023/a:1003945902473. [DOI] [PubMed] [Google Scholar]
- 17.Palosaari H, Wahlgren J, Larmas M, Rönkä H, Sorsa T, Salo T, et al. The expression of MMP-8 in human odontoblasts and dental pulp cells is down-regulated by TGF-beta1. J Dent Res. 2000;79:77–84. doi: 10.1177/00220345000790011401. [DOI] [PubMed] [Google Scholar]
- 18.Caron C, Xue J, Bartlett JD. Expression and localization of membrane type 1 matrix metalloproteinase in tooth tissues. Matrix Biol. 1998;17:501–11. doi: 10.1016/s0945-053x(98)90098-1. [DOI] [PubMed] [Google Scholar]
- 19.Caron C, Xue J, Sun X, Simmer JP, Bartlett JD. Gelatinase A (MMP-2) in developing tooth tissues and amelogenin hydrolysis. J Dent Res. 2001;80:1660–4. doi: 10.1177/00220345010800071201. [DOI] [PubMed] [Google Scholar]
- 20.Martin De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase Gelatinase a in human dentine. Arch Oral Biol. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 21.Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002;81:603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 22.Van Wart HE, Birkedal Hansen H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann N, Debret R, Roméas A, Magloire H, Degrange M, Bleicher F, et al. Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J Dent Res. 2009;88:77–82. doi: 10.1177/0022034508327925. [DOI] [PubMed] [Google Scholar]
- 25.Laronha H, Carpinteiro I, Portugal J, Azul A, Polido M, Petrova KT, et al. Challenges in matrix metalloproteinases inhibition. Biomolecules. 2020;10:717. doi: 10.3390/biom10050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca BM, Barcellos DC, Silva TM, Borges AL, Cavalcanti BD, Prakki A, et al. Mechanical-physicochemical properties and biocompatibility of catechin-incorporated adhesive resins. J Appl Oral Sci. 2019;27:e20180111. doi: 10.1590/1678-7757-2018-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loguercio AD, Hass V, Gutierrez MF, Luque Martinez IV, Szezs A, Stanislawczuk R, et al. Five-year effects of chlorhexidine on the in vitro durability of resin/dentin interfaces. J Adhes Dent. 2016;18:35–42. doi: 10.3290/j.jad.a35514. [DOI] [PubMed] [Google Scholar]
- 30.da Silva EM, de SáRodrigues CU, de Oliveira Matos MP, de Carvalho TR, dos Santos GB, Amaral CM. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors: Physicochemical characterization and resin-dentin bonding stability. J Dent. 2015;43:1491–7. doi: 10.1016/j.jdent.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Maske TT, Kuper NK, Cenci MS, Huysmans MD. Chlorhexidine, a matrix metalloproteinase inhibitor and the development of secondary caries wall lesions in a microcosm biofilm model. Caries Res. 2019;53:107–17. doi: 10.1159/000490195. [DOI] [PubMed] [Google Scholar]
- 32.Sabatini C, Kim JH, Ortiz Alias P. In vitro evaluation of benzalkonium chloride in the preservation of adhesive interfaces. Oper Dent. 2014;39:283–90. doi: 10.2341/13-131-LR. [DOI] [PubMed] [Google Scholar]
- 33.Tezvergil Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–40. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comba A, Maravic T, Valente L, Girlando M, Cunha SR, Checchi V, et al. Effect of benzalkonium chloride on dentin bond strength and endogenous enzymatic activity. J Dent. 2019;85:25–32. doi: 10.1016/j.jdent.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Tekçe N, Tuncer S, Demirci M, Balci S. Do matrix metalloproteinase inhibitors improve the bond durability of universal dental adhesives? Scanning. 2016;38:535–44. doi: 10.1002/sca.21293. [DOI] [PubMed] [Google Scholar]
- 36.Sabatini C, Pashley DH. Aging of adhesive interfaces treated with benzalkonium chloride and benzalkonium methacrylate. Eur J Oral Sci. 2015;123:102–7. doi: 10.1111/eos.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–55. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 39.Gerhardt KM, Oliveira CA, França FM, Basting RT, Turssi CP, Amaral FL. Effect of epigallocatechin gallate, green tea extract and chlorhexidine application on long-term bond strength of self-etch adhesive to dentin. Int J Adhes Adhes. 2016;71:23–7. [Google Scholar]
- 40.Du X, Huang X, Huang C, Wang Y, Zhang Y. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J Dent. 2012;40:485–92. doi: 10.1016/j.jdent.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Albuquerque N, Neri JR, Lemos M, Yamauti M, de Sousa F, Santiago SL. Effect of polymeric microparticles loaded with catechin on the physicochemical properties of an adhesive system. Oper Dent. 2019;44:E202–11. doi: 10.2341/18-112-L. [DOI] [PubMed] [Google Scholar]
- 42.Czech R, Oliveira CA, França FM, Basting RT, Turssi CP, Amaral FL. Incorporation of EGCG into an etch-and-rinse adhesive system: Mechanical properties and bond strength to caries affected dentin. J Adh Sci Technol. 2019;33:2430–42. [Google Scholar]
- 43.Rauf A, Imran M, Abu-Izneid T, Iahtisham-Ul-Haq, Patel S, Pan X, et al. Proanthocyanidins: A comprehensive review. Biomed Pharmacother. 2019;116:108999. doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 44.Haq IU, Butt MS, Shamshad A, Suleria HA. Heath benefits of anthocyanins in black carrot (Daucus carota) In: Goyal MR, Suleria HA, editors. Human Health Benefits of Plant Bioactive Compounds: Potentials and Prospects. New York, USA: Apple Academic Press; 2019. [Google Scholar]
- 45.Zang X, Shang M, Xu F, Liang J, Wang X, Mikage M, et al. A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities. Molecules. 2013;18:5172–89. doi: 10.3390/molecules18055172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bisha B, Weinsetel N, Brehm Stecher BF, Mendonca A. Antilisterial effects of gravinol-s grape seed extract at low levels in aqueous media and its potential application as a produce wash. J Food Prot. 2010;73:266–73. doi: 10.4315/0362-028x-73.2.266. [DOI] [PubMed] [Google Scholar]
- 47.Karioti A, Sokovic M, Ciric A, Koukoulitsa C, Bilia AR, Skaltsa H. Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: Evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J Agric Food Chem. 2011;59:6412–22. doi: 10.1021/jf2011535. [DOI] [PubMed] [Google Scholar]
- 48.Dias PG, da Silva EM, Carvalho CM, Miranda ME, Portela MB, Amaral CM. Characterization and antibacterial effect of an experimental adhesive containing different concentrations of proanthocyanidin. J Adhes Dent. 2020;22:139–47. doi: 10.3290/j.jad.a44280. [DOI] [PubMed] [Google Scholar]
- 49.Enrich Essvein T, Rodríguez Navarro AB, Álvarez Lloret P, Cifuentes Jiménez C, Bolaños Carmona MV, González López S. Proanthocyanidin-functionalized hydroxyapatite nanoparticles as dentin biomodifier. Dent Mater. 2021;37:1437–45. doi: 10.1016/j.dental.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Henn S, de Carvalho RV, Ogliari FA, de Souza AP, Line SR, da Silva AF, et al. Addition of zinc methacrylate in dental polymers: MMP-2 inhibition and ultimate tensile strength evaluation. Clin Oral Investig. 2012;16:531–6. doi: 10.1007/s00784-011-0551-x. [DOI] [PubMed] [Google Scholar]
- 51.Almeida GS, da Silva EM, Guimarães JGA, da Silva RNL, Dos Santos GB, Poskus LT. ZnCl2 Incorporated into Experimental Adhesives: Selected Physicochemical Properties and Resin-Dentin Bonding Stability. Biomed Res Int 2017. 2017:5940479. doi: 10.1155/2017/5940479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barcellos DC, Fonseca BM, Pucci CR, Cavalcanti BD, Persici Ede S, Gonçalves SE. Zn-doped etch-and-rinse model dentin adhesives: Dentin bond integrity, biocompatibility, and properties. Dent Mater. 2016;32:940–50. doi: 10.1016/j.dental.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Toledano M, Sauro S, Cabello I, Watson T, Osorio R. A Zn-doped etch-and-rinse adhesive may improve the mechanical properties and the integrity at the bonded-dentin interface. Dent Mater. 2013;29:e142–52. doi: 10.1016/j.dental.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Kato MT, Leite AL, Hannas AR, Calabria MP, Magalhães AC, Pereira JC, et al. Impact of protease inhibitors on dentin matrix degradation by collagenase. J Dent Res. 2012;91:1119–23. doi: 10.1177/0022034512455801. [DOI] [PubMed] [Google Scholar]
- 55.Brackett MG, Agee KA, Brackett WW, Key WO, Sabatini C, Kato MT, et al. Effect of sodium fluoride on the endogenous MMP activity of dentin matrices. J Nat Sci. 2015;1:e118. [PMC free article] [PubMed] [Google Scholar]
- 56.Samani Y, Alaghehmand H, Jafari Z, Khafri S, Tashakkorian H. Sodium fluoride addition to a two-step etch and rinse adhesive system: Effect on dentin microtensile bond strength and durability. Caspian J Dent Res. 2018;7:16–23. [Google Scholar]
- 57.Ghorab S, Ashraf I. Effect of hesperidin on antibacterial activity and adhesive properties of an etch-and-rinse adhesive system. Egypt Dent J. 2018;64:3801–12. [Google Scholar]
- 58.Islam S, Hiraishi N, Nassar M, Yiu C, Otsuki M, Tagami J. Effect of natural cross-linkers incorporation in a self-etching primer on dentine bond strength. J Dent. 2012;40:1052–9. doi: 10.1016/j.jdent.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Islam MS, Hiraishi N, Nassar M, Yiu C, Otsuki M, Tagami J. Effect of hesperidin incorporation into a self-etching primer on durability of dentin bond. Dent Mater. 2014;30:1205–12. doi: 10.1016/j.dental.2014.08.371. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res. 1994;54:4726–8. [PubMed] [Google Scholar]
- 61.Almahdy A, Koller G, Sauro S, Bartsch JW, Sherriff M, Watson TF, et al. Effects of MMP inhibitors incorporated within dental adhesives. J Dent Res. 2012;91:605–11. doi: 10.1177/0022034512446339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26:571–8. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaharom ME, Rahbar M, Golbaz S. Effect of galardin and its solvents on the microtensile bond strength of different adhesive systems to dentin. Pesqui Bras Odontopediatri Clín Integr. 2019;19:4438. [Google Scholar]


