Supplemental Digital Content is Available in the Text.
Key Words: LC-MS/MS, CDK inhibitors, letrozole, human plasma, TDM
Abstract
Background:
Therapeutic drug monitoring (TDM) using cyclin-dependent kinase inhibitors (CDK4/6is) is a novel approach for optimizing treatment outcomes. Currently, palbociclib, ribociclib, and abemaciclib are the available CDK4/6is and are primarily coadministered with letrozole. This study aimed to develop and validate an LC-MS/MS method for the simultaneous analysis of CDK4/6is, 2 active metabolites of abemaciclib (M2 and M20), and letrozole in human plasma for use in TDM studies.
Methods:
Sample pretreatment comprised protein precipitation with methanol and dilution of the supernatant with an aqueous mobile phase. Chromatographic separation was achieved using a reversed-phase XBridge BEH C18 column (2.5 μm, 3.0 × 75 mm XP), with methanol serving as the organic mobile phase and pyrrolidine–pyrrolidinium formate (0.005:0.005 mol/L) buffer (pH 11.3) as the aqueous mobile phase. A triple quadrupole mass spectrometer was used for the detection, with the ESI source switched from negative to positive ionization mode and the acquisition performed in multiple reaction monitoring mode.
Results:
The complete validation procedure was successfully performed in accordance with the latest regulatory guidelines. The following analytical ranges (ng/mL) were established for the tested compounds: 6–300, palbociclib and letrozole; 120–6000, ribociclib; 40–800, abemaciclib; and 20–400, M2 and M20. All results met the acceptance criteria for linearity, accuracy, precision, selectivity, sensitivity, matrix effects, and carryover. A total of 85 patient samples were analyzed, and all measured concentrations were within the validated ranges. The percent difference for the reanalyzed samples ranged from −11.2% to 7.0%.
Conclusions:
A simple and robust LC-MS/MS method was successfully validated for the simultaneous quantification of CDK4/6is, M2, M20, and letrozole in human plasma. The assay was found to be suitable for measuring steady-state trough concentrations of the analytes in patient samples.
INTRODUCTION
Palbociclib,1 ribociclib,2 and abemaciclib3 are oral targeted drugs that act as selective cyclin-dependent kinase (4/6) inhibitors (CDK4/6is). These drugs have been approved for the treatment of hormone receptor (HR)–positive, human epidermal growth factor receptor 2 (HER2)–negative locally advanced or metastatic breast cancer.4
These drugs are administered either in combination with an aromatase inhibitor (letrozole/anastrozole/exemestane) or the selective estrogen receptor degrader (SERD), fulvestrant.5–10
The oral administration of CDK4/6is offers clear advantages, such as enabling outpatient treatment and demedicalization of the therapeutic pathway. However, these treatments are associated with certain limitations. For example, adherence to the treatment plans is solely the responsibility of the patient.11
Nonadherence to therapy is due to several reasons, including a lack of understanding of the treatment intent and disease characteristics, side effects, complexity of the treatment regimen along with concomitant medications, and the patient's age. Nonadherence to therapy is a significant contributing factor to treatment failure.12
CDK4/6is are characterized by substantial interindividual variability in pharmacokinetics,12–14 and the one-size-fits-all strategy (currently applied) does not seem to be the optimal approach. Even slight variations in patient blood concentrations can jeopardize treatment efficacy or expose patients to adverse events.15,16 In fact, all 3 drugs have an exposure–toxicity relationship, which leads to neutropenia.17–20 A relationship between QTc prolongation and ribociclib exposure has also been established.19 Regarding the exposure–response relationship, the analysis was considered inconclusive for ribociclib,19 no association was found for palbociclib,21 and a positive relationship was found between exposure and progression-free survival (PFS) and tumor shrinkage for abemaciclib.20
The use of therapeutic drug monitoring (TDM) during CDK4/6i therapy has been suggested.11,16,17 TDM directly determines whether the actual drug concentration in blood (exposure) falls within the therapeutic target range. TDM also helps identify potential treatment nonadherence and unexpected drug–drug and/or herb–drug interactions.
A recent study revealed the feasibility of pharmacokinetically guided dose optimization of orally administered targeted therapies, such as palbociclib, in clinical practice.22 Indeed, pharmacokinetically guided dosing resulted in a 39.0% reduction (95% confidence interval, 28.0%–49.0%) in the proportion of underexposed patients compared with historical data.22
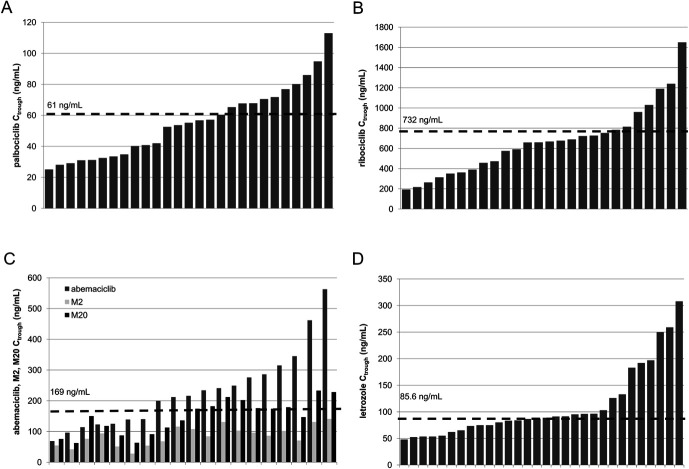
To date, no TDM target has been established for CDK4/6i. Nonetheless, Verheijen et al23 suggested that in the absence of evidence-based TDM target exposure, the average population exposure to the approved effective dose could serve as a proxy.11 Thus, for palbociclib, concentrations can be compared with the population mean Ctrough (CV %, reflecting interindividual variability) of 61 (42%) ng/mL,18 whereas for ribociclib, a mean Ctrough of 732 ng/mL (91%) can be used as a reference19 (Table 1). The proposed target of abemaciclib was 169 ng/mL24 (Table 1). Of note, abemaciclib has 2 active and significantly abundant metabolites, N-desethyl and hydroxy abemaciclib (also known as M2 and M20, respectively),3,17 which should be considered for TDM. For letrozole, a plasma concentration (Ctrough) ≥85.6 ng/mL is associated with a longer time to progression (TTP)25 and represents a potential TDM target for this drug.11 Table 1 shows the evidence and proposed TDM targets for these drugs.
TABLE 1.
Proposed TDM Target for CDK4/6i and Letrozole
| Drug | Exposure–response Relationship | Exposure–toxicity Relationship | Proposed Ctrough Target, ng/mL |
| Palbociclib | No | Yes (neutropenia) | 61 |
| Ribociclib | Inconclusive | Yes (neutropenia, QTc prolongation) | 732 |
| Abemaciclib | Yes (tumor shrinkage, PFS) | Yes (neutropenia) | 169 |
| Letrozole | Yes (TTP) | Not reported | 85.6 |
A prerequisite for implementing TDM of CDK4/6i is the availability of an analytical assay to determine its concentration in human plasma. To the best of our knowledge, only one analytical method has been developed for the simultaneous quantification of CDK4/6i and letrozole; however, this method does not enable the quantification of abemaciclib metabolites.26
More recently, 2 LC-MS/MS methods were developed for the quantification of CDK4/6i plus M227 or CDK4/6i plus the M2 and M20 metabolites28; however, both methods were not validated to quantify letrozole. Therefore, the aim of this study was to develop an LC-MS/MS method for the simultaneous quantification of palbociclib, ribociclib, abemaciclib, the major metabolites of abemaciclib, M2 and M20, and letrozole in human plasma. This method is intended to facilitate the use of TDM for these drugs in clinical practice and future studies to clearly define the exposure–efficacy relationship (if this is lacking) and their TDM target.
MATERIALS AND METHODS
Chemicals and Reagents
Abemaciclib (purity ≥99.7%) and the stable isotopically labeled internal standard (IS), D8-abemaciclib (2H purity 98.7%), were purchased from Clearsynth (Maharashtra, India). Palbociclib (purity 100%), ribociclib hydrochloride (purity ≥99.5%), and the ISs, D6-ribociclib (2H purity 99%), D8-palbociclib (2H purity 98.3%), and 13C2,15N2-letrozole (13C purity 99.6%, 15N purity 99.6%) were synthesized by Alsachim (Illkirch Graffenstaden, France). The M2 (purity 99.4%) and M20 (purity 98.2%) metabolites and letrozole (purity 100%) standards were supplied by MedChemExpress (Monmouth Junction, NJ).
Methanol (MeOH) for LC-MS was supplied by Carlo Erba (Milan, Italy). Ultrapure water was produced through double distillation using a Milli-Q system (Merck, Milan, Italy). Formic acid and dimethyl sulfoxide (DMSO) for HPLC were obtained from Merck-Sigma (Milan, Italy). Hydrochloric acid (HCl) 6N and pyrrolidine (purity 99%) were purchased from ThermoFisher Scientific (Rodano, Italy).
K2EDTA blank (drug-free) human plasma from healthy volunteers was used to prepare the calibration curves. Quality control samples (QCs) were collected at the Transfusion Unit of our Institution.
Preparation of the Standard Solutions
Stock solutions of each analyte and IS were prepared by dissolving a precise amount of the analytical standards in the appropriate solvent to obtain the following final concentrations: 0.2 mg/mL for palbociclib (in DMSO), 2 mg/mL for ribociclib and 1 mg/mL for letrozole (in MeOH), 0.5 mg/mL for abemaciclib and M20 (in DMSO), and 0.5 mg/mL for M2 (in HCl 0.01 M). The concentration of the IS stock solution was 1 mg/mL for each compound (D8-palbociclib and D8-abemaciclib in DMSO and D6-ribociclib and 13C2,15N2-letrozole in MeOH). Stock solutions of the analytes were prepared in duplicate to obtain (WSs) for both the calibrators and QCs. The stock solutions were mixed and diluted with MeOH to obtain the WSs for both the calibration curve (8 points, from A to H) and QC (H-high, M-medium, L-low), with the concentrations shown in Supplemental Digital Content 1 (see Table S1, http://links.lww.com/TDM/A715). The stock solutions of the ISs were mixed and diluted with MeOH to obtain a solution with the following concentrations: 62.5 ng/mL for D8-palbociclib and 13C2,15N2-letrozole, 225 ng/mL for D6-ribociclib, and 100 ng/mL for D8-abemaciclib. This solution was used directly for protein precipitation of plasma samples (ISWS).
Preparation of Calibration Curve, QCS, and Patient Sample
Calibrators and QCs were prepared as described in a previously published method.29 In brief, WS was spiked into the plasma at a ratio of 1:20. The concentration ranges of the calibration curves were as follows: 6–300 ng/mL for palbociclib and letrozole, 120–6000 ng/mL for ribociclib, 40–800 ng/mL for abemaciclib, and 20–400 ng/mL for M2 and M20. The calibrator and QC (L, M, and H) concentrations are listed in Supplemental Digital Content 1 (see Table S2, http://links.lww.com/TDM/A715). After 10 seconds of vortexing, 50 µL of spiked plasma was transferred to a 1.5 mL tube and 150 µL of the ISWS, was added for protein precipitation. The samples were then vortexed and centrifuged (16,200 g for 10 minutes at 4°C). Finally, 60 µL of the supernatant was diluted with 140 µL of aqueous mobile phase (MPA), vortexed, and centrifuged (16,200g 10 minutes at 4°C). One hundred and fifty µL of the resulting solution was transferred to a polypropylene vial and stored at 15°C in an LC autosampler until analysis. The patient sample (50 µL) was prepared as described for the calibrators and QCs by protein precipitation (150 µL of IS WS) and dilution (60 µL of supernatant plus 140 µL MPA).
Chromatographic and Mass Spectrometric Conditions
A SIL-20AC XR autosampler coupled with LC-20AD UFLC Prominence XR pumps (Shimadzu, Tokyo, Japan) was used for chromatographic separation. The MPA consisted of a pyrrolidine–pyrrolidinium formate buffer (0.005:0.005 mol/L, pH 11.3), while the organic mobile phase (MPB) consisted of MeOH. Reversed phase chromatography was performed using an XBridge BEH C18 (2.5 μm 3.0 × 75 mm XP) column and a Security Guard Cartridge (XBridge BEH C18, 2.5 µm, 2.1 × 5 mm) from Waters (Milford, MA). The following chromatographic gradient was applied: 30% MPB (MeOH) for 0.5 minutes (initial conditions), increased to 90% MPB in 7 minutes, 90% MPB maintained for 1.5 minutes (washing), decreased to 30% MPB for 0.5 minutes, and 30% MPB maintained for the re-equilibration step (6 minutes). The oven temperature was 45°C, and the total run time was 15.5 minutes. Analytes were ionized and detected using a triple quadrupole API 4000 (AB SCIEX, Framingham Massachusetts) equipped with a TurboIonSpray source. To optimize the source and compound-dependent parameters, 100 ng/mL of each analyte was continuously injected at a flow rate of 20 µL/min. Data processing and quantification of the analytes were performed using Analyst software (version 1.6.3). The source-dependent parameters were optimized based on the abemaciclib metabolites, M2 and M20, which had a lower signal than the other compounds. The source temperature was set at 500°C, the nebulizer gas pressure was set at 30 psi, and the heater gas pressure was set at 60 psi. Nitrogen was used as the curtain gas (30 psi) and collision gas (CAD) at medium intensity. The ion spray voltage was set at −1500 V to detect letrozole and letrozole IS and 5000 V to detect abemaciclib, M2, M20, palbociclib, ribociclib, and their ISs. Acquisition was performed in scheduled MRM mode, and each analyte was monitored using 2 product ions: The ion with the highest signal was used as the quantifier, and the other ion was used as the qualifier ion (to confirm the identity of the analyte) (see Table S3, Supplemental Digital Content 3, http://links.lww.com/TDM/A715). Quantification was performed using the following transitions: m/z 284 > 242 for LETRO; m/z 288 > 246 for 13C2,15N2-letrozole; m/z 507 > 393 for abemaciclib; m/z 523 > 409 for M20; m/z 479 > 393 for M2; m/z 515 > 393 for D8-abemaciclib; m/z 448 > 380 for palbociclib; m/z 456 > 388 for D8-palbociclib; m/z 435 > 322 for ribociclib; and m/z 441 > 373 for D6-ribociclib. Metabolites M2 and M20 were quantified using D8-abemaciclib as an IS.
Method Validation
A full validation study was performed according to the FDA and EMA guidelines to validate the bioanalytical methods,30,31 as previously reported by Poetto et al29
Extraction Recovery and Matrix Effect
Recovery from the matrix was assessed as the mean area ratio between the QC samples produced by spiking the matrix and those spiked after matrix extraction at all levels (H-high, M-medium, and L-low), which were prepared in quintuplicate.
An exploratory evaluation of matrix effect (ME) was performed using a postcolumn infusion experiment, and a solution of each analyte at a concentration of 200 ng/mL was infused using a syringe pump during the chromatographic run an extracted blank pooled plasma sample. Quantitative evaluation of ME was performed by calculating the ratio between the peak area of the analyte in the presence of the matrix (blank plasma spiked with the analyte after extraction) and the peak area in the absence of the matrix (analyte in MeOH). To prepare blank plasma samples spiked with analytes after extraction, samples from healthy donors (male patients and female patients) and plasma with mild hemolysis were used. Samples containing analytes in MeOH were prepared in triplicate. Both series (with and without matrix) were prepared low and high analyte concentrations (QCL and H). IS-normalized matrix factor (MF) was calculated as the ratio between the analyte ME and the IS ME. The coefficient of variation (CV %) of the IS-normalized MF should not exceed 15%.
Linearity, Sensitivity, and Selectivity
Calibration curves were generated using 8 nonzero calibration standards prepared as described in Section 2.3 (concentrations are given in Table S2, Supplemental Digital Content, http://links.lww.com/TDM/A715). Linearity was evaluated using 2 different sets of 8 calibrators in 4 analytical runs (on 4 different days). A linear regression model with a weighting factor of 1/χ2 was applied. Details regarding the definition of the best weighting factor are provided in the Supplementary Material (linearity assessment, see Table S4, Supplemental Digital Content, http://links.lww.com/TDM/A715). Accuracy, obtained as back-calculated concentrations, of each calibrator should be between 85% and 115% (80%–120% for the lower limit of quantification, LLOQ). The precision, calculated as CV %, should be ≤ 15%.
The sensitivity of the method was defined by the LLOQ, which is the lowest nonzero standard in the calibration curve (see Table S2, Supplemental Digital Content, http://links.lww.com/TDM/A715). The LLOQ response should be 5 times higher than that of the zero samples and have a signal-to-noise (S/N) ratio of ≥5. Sensitivity was evaluated in 3 runs using LLOQ samples prepared in quintuplicate: Precision should be ≤ 20% while accuracy should be between 80% and 120% for at least 67% of the samples.
Selectivity was assessed by analyzing blank plasma samples from 6 donors and a plasma sample with mild hemolysis (the same sample used for ME evaluation). The method was considered free of nonspecific interference if the response was <20% of the LLOQ for the analyte and <5% of the IS at the retention times of the analytes.
Carryover
Previously published methods32–34 highlighted the presence of carryover effects for both palbociclib and ribociclib. This phenomenon was evaluated as the percentage of the peak area of the blank sample injected after the upper limit of quantification (ULOQ) relative to the peak area of the LLOQ for each analyte. The carryover should not exceed 20% of the LLOQ.
Intraday and Interday Precision and Accuracy
Intraday and interday precision and accuracy were determined by analyzing the LLOQ, QCL, M, and H samples prepared in quintuplicate. Intraday precision and accuracy were assessed on a single working day, and the test was repeated 3 times (ie, 3 analytical runs) on 3 different days; the interday precision and accuracy were determined as the mean values of the 3 runs. The measured concentrations should be within ±15% of the nominal value (accuracy between 85% and 115%) with precision ≤15%. For the LLOQ samples, the accuracy should be between 80% and 120% with a precision of ≤20%.
Stability
The stability of the analytes was evaluated under different conditions using 3 QCL and QCH samples analyzed against a freshly prepared curve. The following stability tests were performed: short-term stability at room temperature, freeze-thaw stability, autosampler stability (15°C) of the sample extracts, and long-term stability of the plasma samples at −80°C. Stability under each condition was confirmed when the back-calculated concentrations had a deviation of less than ±15% of the nominal value. The only phase II metabolite reported for abemaciclib is the sulfate conjugate of M20.35,36 Owing to a lack of information on its concentration, its presence was classified as negligible in this study. Therefore, we assumed that the reconversion of the sulfate conjugate to M20 (if it occurred) during storage of the patient samples (−80°C) would not affect the quantification of M20.
Application of the Method to Clinical Samples
The proposed method was applied to quantify samples collected from patients treated with CDK4/6i in a clinical trial (protocol ID: CRO 2022-14, approval date: April 12, 2022; Parere-CEUR-2022-Os-65) at the IRCCS National Cancer Institute CRO Aviano (Italy). This study was approved by the local ethics committee (Comitato Etico Unico Regionale del Friuli Venezia Giulia—CEUR) and was conducted in accordance with the principles of the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants. When possible, sampling was performed at the Ctrough (minimum drug concentration at steady state).
Incurred Sample Reanalysis
A subset of samples for each CDK4/6i was analyzed twice in 2 runs to provide an additional assessment of the robustness and reproducibility of the method according to the EMA and FDA guidelines (incurred sample reanalysis (ISR). The 2 analyses were considered equivalent if the absolute percent difference (%diff) between the 2 measured concentrations, calculated using the formula, %diff = [(repeat-original) × 100/mean] was <20%.
RESULTS AND DISCUSSION
LC-MS/MS Method
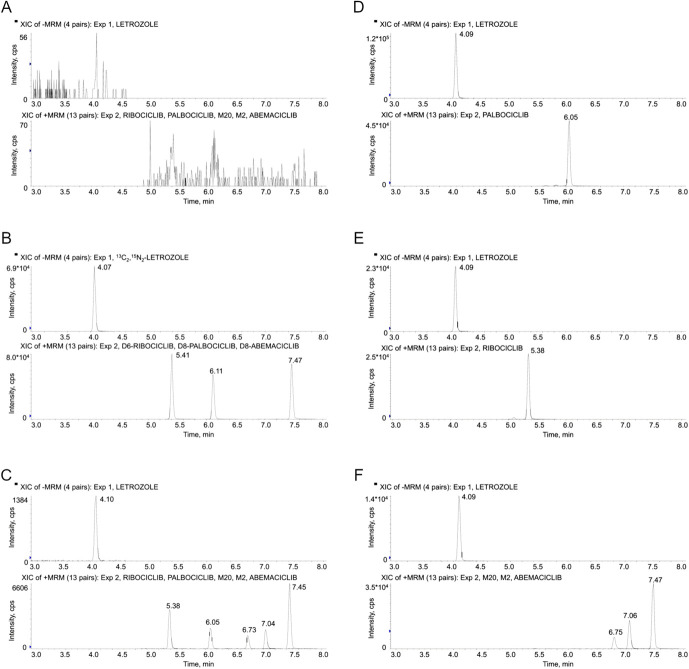
Adequate separation of analytes (resolution Rs > 2) and peak symmetry (tailing factor TF ≤ 1.4) were achieved.37 The following retention times were obtained for the analytes: 4.09 minutes, letrozole; 5.38 minutes, ribociclib; 6.05 minutes, palbociclib; 6.75 minutes, M2; 7.04 minutes, M20; and 7.45, abemaciclib (Fig. 1, panels C–F). The tailing phenomenon (TF) previously observed with abemaciclib and its metabolites (TF ∼2)29 was not observed with the optimized chromatographic parameters (especially the MPA pH of 11.3 and column type), which allowed to obtain a TF lower than 1.4 for all analytes.
FIGURE 1.
Representative SRM chromatograms of a human blank plasma sample (panel A), a human blank plasma sample containing the ISs (panel B), an LLOQ sample (panel C), and plasma samples from patients treated with palbociclib (panel D), ribociclib (panel E), and abemaciclib (panel F). The measured concentrations were 90 ng/mL for palbociclib and 270 ng/mL for letrozole (panel D), 727 ng/mL for ribociclib and 55 ng/mL for letrozole (panel E), and 140 ng/mL for abemaciclib, 54 ng/mL for M2, 91 ng/mL for M20, and 46 ng/mL for letrozole (panel F). The S/N ratio of the LLOQ sample (panel C) was 143 for letrozole, 92 for ribociclib, 141 for palbociclib, 35 for M2, 125 for M20, and 404 for abemaciclib.
Method Validation
Extraction Recovery and Matrix Effect
Protein precipitation revealed nearly complete recovery of analytes from the plasma matrix, with the percentage of recovery approaching 100% (see Table S5, Supplemental Digital Content, http://links.lww.com/TDM/A715). The obtained values were highly reproducible at different concentrations (QCL, M, and H), with an SD ≤ 5.8 and CV% ≤ 7.1%. Among the analytes, the lowest recovery was observed for abemaciclib, M20, and M2, with recoveries ranging from 81.3% to 92.5%. Quantitative evaluation of the matrix effect revealed only a slight increase in the signals of abemaciclib and its IS (ME of 111%–115% and 117%, respectively), with CV% ≤ 4.2% (see Table S5, Supplemental Digital Content, http://links.lww.com/TDM/A715). Nevertheless, the use of D8-abemaciclib as an IS of M20 and M2 seemed appropriate because the IS-normalized MF for these 2 analytes ranged from 0.9 to 1.1, with CV% ≤5.4% (see Table S5, Supplemental Digital Content, http://links.lww.com/TDM/A715). In general, the influence of the individual matrices on the quantification of CDK4/6i and letrozole was negligible. This finding was further confirmed by the “Post Column Infusion Test,” which revealed the absence of ion suppression and/or the enhancement phenomena during the retention time of the analytes.
Linearity, Sensitivity, and Selectivity
Good linearity was demonstrated by the correlation coefficients obtained for each analyte, which were ≥0.997. This result was also confirmed by the accuracy and precision results of the calibration curves: Accuracy ranged from 96.2% to 104.9% for all compounds, while precision was ≤8.7% (see Table S6, Supplemental Digital Content, http://links.lww.com/TDM/A715Table S6). Further details on the linearity assessment are provided in the Supplementary Material (linearity assessment) (see Table S4 and Fig. S1, Supplemental Digital Content, http://links.lww.com/TDM/A715 and http://links.lww.com/TDM/A712).
The LLOQ signal showed an S/N ≥ 30 (Fig. 1, panel C), with a precision of ≤7.7% and an accuracy between 103.4% and 108.3% (see Table S7, Supplemental Digital Content, http://links.lww.com/TDM/A715); these values were largely within the acceptance criteria.
The method proved to be selective because no interference was observed in any of the matrices tested (6 plasma samples from different donors and one plasma sample with mild hemolysis, see Fig. S2, Supplemental Digital Content, http://links.lww.com/TDM/A713).
Carryover
Analysis of the first blank sample injected after the ULOQ sample revealed no quantifiable peaks for any analyte (including ISs) (S/N ≤ 5) (Fig. 1, panel A), indicating no carryover.
Intraday and Interday Precision and Accuracy
The accuracy and precision were satisfactory for the intraday and interday tests. For all compounds, intraday accuracy ranged from 90.9% to 114.4% while precision was ≤9.1% (see Table S8, Supplemental Digital Content, http://links.lww.com/TDM/A713). The interday precision and accuracy data are presented in Table 2. Considering all the analytes, interday accuracy ranged from 97.4% to 108.3% while precision was ≤7.8%.
TABLE 2.
Interday Precision and Accuracy Data for Letrozole, Abemaciclib, M2, M20, Palbociclib, and Ribociclib
| Analyte | Nom Conc., ng/mL | Interd | ||
| Mean ± SD, ng/mL | Acc% | CV% | ||
| Letrozole | 6 | 6.20 ± 0.4 | 103.7 | 6.1 |
| 16.1 | 16.2 ± 0.7 | 100.4 | 4.6 | |
| 92 | 92.1 ± 4.6 | 100.1 | 5.0 | |
| 230 | 226.3 ± 8.5 | 98.4 | 3.8 | |
| Abemaciclib | 40 | 42.7 ± 1.9 | 106.7 | 4.4 |
| 93 | 97.7 ± 4.4 | 105.0 | 4.5 | |
| 248 | 260.6 ± 14.4 | 105.1 | 5.5 | |
| 620 | 652.8 ± 36.7 | 105.3 | 5.6 | |
| M2 | 20 | 20.9 ± 1.6 | 104.6 | 7.7 |
| 46.5 | 45.3 ± 3.5 | 97.4 | 7.8 | |
| 124 | 126.4 ± 8.5 | 102.0 | 6.8 | |
| 310 | 318.4 ± 19.4 | 102.7 | 6.1 | |
| M20 | 20 | 21.7 ± 1.1 | 108.3 | 5.0 |
| 46.5 | 48.7 ± 2.9 | 104.8 | 5.9 | |
| 124 | 126.6 ± 5.8 | 102.1 | 4.5 | |
| 310 | 315.6 ± 17.1 | 101.8 | 5.4 | |
| Palbociclib | 6 | 6.2 ± 0.3 | 103.4 | 5.6 |
| 16.1 | 16.4 ± 0.8 | 101.7 | 4.9 | |
| 92 | 94.6 ± 4.9 | 102.9 | 5.2 | |
| 230 | 235.8 ± 10.7 | 102.5 | 4.6 | |
| Ribociclib | 120 | 124.8 ± 6.6 | 104.0 | 5.3 |
| 315 | 321.1 ± 14.3 | 101.9 | 4.5 | |
| 1800 | 1854.6 ± 75.7 | 103.0 | 4.1 | |
| 4500 | 4437.0 ± 198.7 | 98.6 | 4.5 | |
Stability
Short-term stability of the compounds at room temperature was verified after 4.5 hours, with accuracy between 98.1% and 110.0% and precision ≤4.9% (see Table S9, Supplemental Digital Content, http://links.lww.com/TDM/A713). The analytes were stable after protein precipitation and storage in an autosampler (dark condition, 15°C) for 13 days, with accuracy ranging from 96.7% to 107.8% and precision ≤3.3% (see Table S9, Supplemental Digital Content, http://links.lww.com/TDM/A713) and after 3 freeze-thaw cycles, with accuracy ranging from 93.2% to 101.8% and precision ≤5.9% (Table 10). Analytes were stable in plasma matrix stored at −80°C for up to 19 months, with accuracy ranging from 88.7% to 97.6% and precision ≤7.4% (see Table S10, Supplemental Digital Content, http://links.lww.com/TDM/A713).
Application of the Method to Clinical Samples
The method was used to quantify 27 plasma samples from 27 patients treated with palbociclib (14 were also treated with letrozole), 31 plasma samples from 26 patients treated with ribociclib (20 in combination with letrozole), and 27 plasma samples from 23 patients treated with abemaciclib (18 in combination with letrozole).
Figure 1 shows the 3 chromatograms obtained from the analysis of samples obtained from patients treated with abemaciclib (panel D), palbociclib (panel E), and ribociclib (panel F). Table 3 presents the patient characteristics. Whenever possible, blood samples were collected at the time of Ctrough (the minimum steady-state drug concentration). For palbociclib, ribociclib, and letrozole, the Ctrough was reached 24 hours after the last drug intake (administered orally once daily), whereas for abemaciclib, the Ctrough was reached after 12 hours (administered twice daily). As indicated in Table 3, palbociclib samples were collected 24 hours after the last dose and ranged from 21.5 to 26.5 hours. The measured mean Ctrough of palbociclib was 53.5 ± 24.6 ng/mL (46% CV). For ribociclib, most samples for the Ctrough assessment were collected correctly (approximately 24 hours after the last pill intake), except 2 samples that were collected 12.5 and 17 hours after the last drug intake. Excluding these 2 samples, the mean Ctrough of ribociclib was 692 ± 340 ng/mL (49% CV). For abemaciclib, the mean Ctrough (excluding 5 samples that were not collected 12 hours after the last tablet intake) was 246.5 ± 127.9 ng/mL (52% CV). Figure 2 shows the variability in Ctrough between patients and the deviation of the concentrations found in patients from the TDM target value, which corresponds to the mean population exposure to each drug (61 ng/mL for palbociclib,11,22,23 732 ng/mL for ribociclib,11,23 and 169 ng/mL for abemaciclib23,24; Table 1). Abemaciclib is extensively metabolized by CYP3A4, producing 2 active metabolites, M2 and M20, whose areas under the plasma concentration–time curve (AUCs) represent 39% and 77% of that of the parent compound.3 The analyzed samples had a mean metabolic ratio of 39 ± 17% for M2 and 66 ± 23% for M20. Panel C of Figure 2 shows the concentrations of abemaciclib and its metabolites in each sample. These percentages are consistent with the data on AUC values3 and the mean metabolic ratio obtained in our previous study (44% (range 31%–55%) for M2 and 70% (range 48–94) for M20).29 Our data revealed high variability in the relative abundance of M2 and M20, highlighting the need for monitoring active metabolites together with abemaciclib, which is considered equipotent to the parent drug.3 A total of 52 patients treated with a CDK4/6i in combination with letrozole were enrolled in this clinical trial, and 57 plasma samples were collected. Of these, 30 samples could be evaluated for letrozole Ctrough because these samples were collected on average 24 hours (range: 20.5–26.5 hours) after the last administration. The measured mean Ctrough was 111.5 ± 67.3 ng/mL (60% CV), and panel D of Figure 2 shows all values in relation to the TDM target currently proposed for this drug (85.6 ng/mL).25
TABLE 3.
Patient Characteristics
| Palbociclib | Ribociclib | Abemaciclib | |
| Number of patients (100% female) | 27 | 26 | 23 |
| Number of patients treated with CDK4/6i plus letrozole | 14 | 20 | 18 |
| Mean age (range) | 63 (36–80) | 58 (34–81) | 59 (35–81) |
| CDK4/6i setting | Adjuvant: 0 First line: 19 Second line: 8 |
Adjuvant: 1 First line: 23 Second line: 2 |
Adjuvant: 6 First line: 15 Second line: 1 |
| Hormonal status | Premenopausal: 6 Menopausal: 21 |
Premenopausal: 7 Menopausal: 19 |
Premenopausal: 5 Menopausal: 18 |
| Dose (mg/d) (number of patients)* | 75 (7); 100 (6); 125 (14) | 300 (1); 400 (5*); 600 (21) | 100 (2); 200 (2); 300 (19) |
| Time (h) after last administration ±SD (range) | 24 ± 1 (21.5–26.5) | 23.5 ± 2.5 (12.5–26.5) | 13 ± 4.5 (4.0–24.5) |
The dose of ribociclib was reduced from 600 to 400 mg/mL for 1 patient.
FIGURE 2.
Plasma concentrations of palbociclib (panel A), ribociclib (panel B), abemaciclib (panels C and E), M2 (panel E), M20 (panel E), and letrozole (panel D) in patients. Multiple samples were collected from some patients: Patients 11, 15, 16, and 22 of ribociclib and 3 and 22 of abemaciclib; empty circles correspond to concentrations not reaching Ctrough.
A high variability in both CDK4/6i and letrozole exposure (expressed as the Ctrough) was observed in the patients enrolled in this study. Moreover, a high percentage of patients did not reach the proposed Ctrough value, underscoring the potential of TDM for oral anticancer drugs. Preliminary data from this study revealed that a large proportion of patients may have been underdosed. For abemaciclib and letrozole, the Ctrough levels were below the proposed TDM target in 63% and 57% of patients, respectively, whereas this percentage markedly decreased to 37% and 31% in patients treated with palbociclib and ribociclib, respectively. Among the potential benefits of TDM is its ability to detect treatment nonadherence and monitor drug–drug interactions, which are of particular interest for patients with cancer who often receive multiple therapies. In this context, the metabolic ratio variability observed in abemaciclib-treated patients may, in some cases, be used to detect the presence of CYP3A4-inhibiting or -inducing concomitant medications. This aspect should be considered in future analyses of abemaciclib metabolic ratios.
Incurred Sample Reanalysis
The reproducibility of the method was further evaluated by reanalyzing a subset of samples for each CDK4/6i (22 samples for letrozole, 12 for palbociclib and ribociclib, and 7 for abemaciclib). The calculated percent difference (%diff) was always (100% of the samples tested) within the acceptance criteria (±20%) and ranged from 13.5% to −7.0%, as shown in Supplemental Digital Content (see Figure S3, http://links.lww.com/TDM/A714).
CONCLUSIONS
The first LC-MS/MS method for the simultaneous quantification of CDK4/6is (palbociclib, ribociclib, and abemaciclib), the M2 and M20 metabolites of abemaciclib, and letrozole was developed and validated according to the EMA and FDA guidelines. The method demonstrated excellent linearity for all analytes within the concentration range (r ≥ 0.997): 6–300 ng/mL for palbociclib and letrozole, 40–800 ng/mL for abemaciclib, 20–400 ng/mL for M2 and M20, and 120–6000 ng/mL for ribociclib.
All concentrations, mainly at Ctrough, measured in the 85 plasma samples collected from 76 patients with breast cancer who participated in the clinical study (protocol ID: CRO 2022-14) were within the established concentration ranges, confirming the suitability of the method for TDM application.
The method features a straightforward sample treatment (protein precipitation), showcasing a high level of accuracy and precision for all analytes (interday accuracy ranged from 97.4% to 108.3%, with precision ≤7.8%). The method demonstrated robustness and reproducibility, with a percentage difference within the range of −7.0% to 13.5% for 100% of the reanalyzed samples. The effects of various anticoagulants on analyte quantification were not tested in this study, thereby serving as a limitation. When this method is applied to samples collected with other anticoagulants, the test should be re-evaluated.
Preliminary data on Ctrough values measured in patients for each drug revealed a high degree of interindividual variability in drug exposure, with CV% ranging from 46% to 60%. Further studies should be conducted to elucidate the role of TDM in personalized CDK4/6i therapy. The proposed method could prove useful for this purpose. The favorable features of this method, such as its robustness, reproducibility, and simplicity, render it suitable for use in routine clinical practice.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the Italian Ministry of Health, Ricerca Corrente, the Transfusion Unit, the IRCCS CRO Aviano nurses, and all patients that participated in the clinical study.
Footnotes
F. Puglisi has received honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Celgene, Eisai, Eli Lilly, Exact Sciences, Gilead, Ipsen, Menarini, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris. F. Puglisi is currently receiving research grants from AstraZeneca, EISAI, and Roche. L. Gerratana has received honoraria from AstraZeneca, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Incyte, Novartis, Pfizer, Menarini Stemmline, and AbbVie. L. Gerratana is currently receiving research grants from Menarini Silicon Biosystems. S. Corsetti has received a travel grant from AstraZeneca. The remaining authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
Contributor Information
Martina Zanchetta, Email: martina.zanchetta@cro.it.
Marco Orleni, Email: marco.orleni@cro.it.
Sara Gagno, Email: sgagno@cro.it.
Marcella Montico, Email: marcella.montico@cro.it.
Elena Peruzzi, Email: elena.peruzzi@cro.it.
Rossana Roncato, Email: rroncato@cro.it.
Lorenzo Gerratana, Email: lorenzo.gerratana@cro.it.
Serena Corsetti, Email: serena.corsetti@cro.it.
Fabio Puglisi, Email: fabio.puglisi@cro.it.
Giuseppe Toffoli, Email: gtoffoli@cro.it.
REFERENCES
- 1.European Medicine Agency (EMA). Ibrance (Palbociclib): EPAR—Product Information. Available at: https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf. Accessed June 26, 2023. [Google Scholar]
- 2.European Medicine Agency (EMA). Kisqali (Ribociclib): EPAR—Product Information. Available at:https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf. Published March 31, 2023. Accessed June 26, 2023. [Google Scholar]
- 3.European Medicine Agency (EMA). Verzenios (Abemaciclib): EPAR—Product Information. Available at: https://www.ema.europa.eu/en/documents/product-information/verzenios-epar-product-information_en.pdf. Accessed June 26, 2023. [Google Scholar]
- 4.Schettini F, Giudici F, Giuliano M, et al. Overall survival of CDK4/6-inhibitor–based treatments in clinically relevant subgroups of metastatic breast cancer: systematic review and meta-analysis. JNCI J Natl Cancer Inst. 2020;112:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2019;30:1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. [DOI] [PubMed] [Google Scholar]
- 8.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP, Toi M, Campone M, et al. Monarch 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 10.Sledge GW, Toi M, Neven P, et al. Monarch 2: abemaciclib in combination with fulvestrant in women with HR+/HER2—advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 11.Mueller-Schoell A, Groenland SL, Scherf-Clavel O, et al. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. 2021;77:441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gougis P, Palmieri LJ, Funck-Brentano C, et al. Major pitfalls of protein kinase inhibitors prescription: a review of their clinical pharmacology for daily use. Crit Rev Oncol Hematol. 2019;141:112–124. [DOI] [PubMed] [Google Scholar]
- 13.Roncato R, Gerratana L, Palmero L, et al. An integrated pharmacological counselling approach to guide decision-making in the treatment with CDK4/6 inhibitors for metastatic breast cancer. Front Pharmacol. 2022;13:897951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncato R, Peruzzi E, Gerratana L, et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed Pharmacother. 2023;164:114906. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso E, Guidi M, Blanchet B, et al. Therapeutic drug monitoring of targeted anticancer protein kinase inhibitors in routine clinical use: a critical review. Ther Drug Monit. 2020;42:33. [DOI] [PubMed] [Google Scholar]
- 16.Roušarová J, Šíma M, Slanař O. Therapeutic drug monitoring of protein kinase inhibitors in breast cancer patients. Prague Med Rep. 2021;122:243–256. [DOI] [PubMed] [Google Scholar]
- 17.Groenland SL, Martínez-Chávez A, van Dongen MGJ, et al. Clinical pharmacokinetics and pharmacodynamics of the cyclin-dependent kinase 4 and 6 inhibitors palbociclib, ribociclib, and abemaciclib. Clin Pharmacokinet. 2020;59:1501–1520. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Ibrance (Palbociclib). Clinical Pharmacology and Biopharmaceutics Review(s). Application Number: 207103Orig1s000. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103orig1s000clinpharmr.pdf. Accessed June 26, 2023. [Google Scholar]
- 19.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Kisqali (Ribociclib). Multi-Discipline Review. Application Number: 209092Orig1s000. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209092orig1s000multidiscipliner.pdf. Accessed June 26, 2023. [Google Scholar]
- 20.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Verzenio (Abemaciclib). Multi-Discipline Review. Application Number: 208855Orig1s000. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208855Orig1s000MultidisciplineR.pdf. Accessed June 27, 2023. [Google Scholar]
- 21.van der Kleij MBA, Guchelaar NAD, Mathijssen RHJ, et al. Therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacokinet. 2023;62:1333–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenland SL, van Eerden RaG, Westerdijk K, et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: a prospective multicenter study. Ann Oncol Off J Eur Soc Med Oncol. 2022;33:1071–1082. [DOI] [PubMed] [Google Scholar]
- 23.Verheijen RB, Yu H, Schellens JHM, et al. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6:740–753. [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Letrozole—Clinical Pharmacology and Biopharmaceutics Review—Application Number: Nda 20-726. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/20726_FEMARA%202.5MG_BIOPHARMR.PDF. Accessed May 5, 2022. [Google Scholar]
- 26.Sato Y, Shigeta K, Hirasawa T, et al. Establishment of an analytical method for simultaneous quantitation of CDK4/6 inhibitors, aromatase inhibitors, and an estrogen receptor antagonist in human plasma using LC-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1173:122655. [DOI] [PubMed] [Google Scholar]
- 27.Burke SM, Kamal M, Goey AKL. Development and validation of a quantitative LC-MS/MS method for CDK4/6 inhibitors palbociclib, ribociclib, abemaciclib, and abemaciclib-M2 in human plasma. Ther Drug Monit. 2023;45:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habler K, Kalla AS, Rychlik M, et al. Therapeutic drug monitoring in breast cancer therapy - LC-MS/MS method for quantification of the CDK4/6 inhibitors abemaciclib, palbociclib, ribociclib, and major metabolites abemaciclib M20 and M2 in human serum. J Pharm Biomed Anal. 2023;225:115211. [DOI] [PubMed] [Google Scholar]
- 29.Soledad Poetto A, Posocco B, Zanchetta M, et al. A new LC-MS/MS method for the simultaneous quantification of abemaciclib, its main active metabolites M2 and M20, and letrozole for therapeutic drug monitoring. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1207:123403. [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration (FDA). Bioanalytical Method Validation Guidance for Industry—Biopharmaceutics Contains Nonbinding Recommendations. 2018. Accessed June 26, 2023. [Google Scholar]
- 31.European Medicine Agency, EMA. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis Step5. Available at: https://www.ema.europa.eu/en/bioanalytical-method-validation-scientific-guideline. Accessed June 26, 2023. [Google Scholar]
- 32.Kala A, Patel YT, Davis A, et al. Development and validation of LC-MS/MS methods for the measurement of ribociclib, a CDK4/6 inhibitor, in mouse plasma and Ringer's solution and its application to a cerebral microdialysis study. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1057:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Chávez A, Rosing H, Hillebrand M, et al. Development and validation of a bioanalytical method for the quantification of the CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib in human and mouse matrices using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2019;411:5331–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posocco B, Buzzo M, Poetto AS, et al. Simultaneous quantification of palbociclib, ribociclib and letrozole in human plasma by a new LC-MS/MS method for clinical application. PLoS One. 2020;15:e0228822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Medicine Agency EMA. Assessment Report-Verzenios. Available at: https://www.ema.europa.eu/en/documents/assessment-report/verzenios-epar-public-assessment-report_en.pdf. Accessed June 26, 2023. [Google Scholar]
- 36.Thakkar D, Kate AS. Update on metabolism of abemaciclib: in silico, in vitro, and in vivo metabolite identification and characterization using high resolution mass spectrometry. Drug Test Anal. 2020;12:331–342. [DOI] [PubMed] [Google Scholar]
- 37.Kirkland JJ, Dolan JW, Snyder LR. Introduction to Modern Liquid Chromatography. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.