Abstract
Background:
Malignant pleural mesothelioma (MPM) is a disease for which there remains an unmet need for better therapeutic options. Nintedanib is an oral multikinase inhibitor impacting VEGF, FGF, PDGFR, and other kinase activity such as TGFß signaling pathways. We conducted a phase II trial of nintedanib in patients with recurrent MPM.
Methods:
Patients with MPM previously treated with platinum-based chemotherapy, performance status (PS) 0–1, adequate organ function, and no contraindications to anti-angiogenic therapy were eligible and were treated with nintedanib 200 mg twice per day until disease progression or unacceptable toxicity. The primary endpoint was 4-month progression-free survival (PFS).
Results:
Twenty patients were enrolled. The median age was 70 years (range 32–81), 90% were male, and 80% were PS 1. The histology was 70% epithelioid, 5% sarcomatoid, 10% biphasic, and 15% unknown. 15% had prior bevacizumab. The median follow-up was 4.1 mo. There were no responses but 40% had stable disease at 8 weeks. The median PFS was 1.8 mo. (95% CI: 1.68, 3.55) and the 4-month PFS rate was 13%. The median OS was 4.2 mo. (95% CI: 2.53, 8.74) and the 4-month OS rate was 55%. Toxicities were primarily grade 1–2 and included diarrhea, fatigue, edema, transaminase elevation, anorexia, nausea, vomiting and dyspnea.
Conclusions:
The activity of nintedanib in previously treated MPM patients was. modest. The trial did not meet its primary PFS endpoint. Even though 2 patients had prolonged stable disease for >4 months, the efficacy of nintedanib remains unproven.
Keywords: mesothelioma, nintedanib, angiogenesis, tyrosine kinase inhibitor
MicroAbstract:
This is a Phase II trial assessing the efficacy of nintedanib, an oral multi-targeted kinase inhibitor, in patients with recurrent malignant pleural mesothelioma. The drug was well tolerated but the activity was modest with no responses and 40% stable disease at 8 weeks. The trial did not meet its endpoint but there was a subset of patients who had prolonged stable disease.
Introduction
Most patients diagnosed with malignant pleural mesothelioma (MPM) will succumb to their disease due to its inherent resistance to therapeutic interventions. Most patients have disease that is not amenable to local treatments such as surgery and/or radiotherapy because of the advanced stage at diagnosis.1 Chemotherapy had been the standard of care for primary treatment of MPM based on a phase III study of the combination of pemetrexed plus cisplatin versus cisplatin alone resulting in both a superior response rate (41.3% vs. 16.7%) and median survival (12.2 vs. 9.3 months) favoring the combination.2 Recently, immunotherapy has demonstrated benefit in MPM, but there is considerable room for improvement and the development of agents targeting critical pathways in the malignant cell has afforded this opportunity.
Vascular endothelial growth factor (VEGF) has a crucial role in mesothelioma pathogenesis.3 In the phase III MAPS trial bevacizumab, an inhibitor of angiogenesis, was combined with chemotherapy and compared to the control arm of chemotherapy alone. This resulted in a significant improvement in overall survival, 18.8 vs. 16.1 months.4 Cediranib (AZD2171) is a tyrosine kinase inhibitor (TKI) targeting VEGFR 1–3, c-Kit, and PDGF-β that was evaluated in a Phase II trial in previously treated MPM patients and demonstrated a disease control rate of 42% and 2 patients were exquisitely sensitive to the drug.5 Nintedanib (BIBF 1120) is a potent, orally available TKI targeting VEGFR 1–3, PDFR α/β, FGFR 1–3, and Src-family members.6 The drug is approved for use in the treatment of idiopathic pulmonary fibrosis and in previously treated non-small cell lung in combination with docetaxel in the European Union (LUME-Lung 1)7 The simultaneous abrogation of multiple pathways results in effective growth inhibition of both endothelial cells and PDGF- and FGF-receptors of perivascular cells, which may be more effective than inhibition of endothelial cell growth via the VEGF pathway alone. VEGF, FGF-1, FGF-2, and TGFβ immunoreactivity is present in mesothelioma.8 Combined immunohistochemistry levels of these markers have been associated with increased intra-tumoral microvascular density and poor prognosis. Nintedanib, being an inhibitor of both VEGFR and FGFR, was felt to be an ideal candidate for evaluation in MPM. Please note that this trial was conceived and completed before the studies that evaluated cediranib and nintedanib with chemotherapy as well as the immunotherapy trials in MPM.
Methods
Patients were eligible for the trial if they had a histologically confirmed diagnosis of unresectable MPM. Patients were unresectable by local guidelines. Patients must have had prior platinum-based chemotherapy and no more than 2 prior systemic therapeutic regimens. Prior bevacizumab was allowed. Prior surgery and/or radiation was allowed if 28 days had elapsed since treatment. Patients had to have an ECOG performance status (PS) of 0 or 1, adequate hematologic, renal, and hepatic function, and no > grade 2 proteinuria. Disease could be measurable or non-measurable as documented by CT scan. No evidence of cavitary or necrotic tumors or centrally located disease with radiographic evidence of local invasion of major blood vessels was allowed. No therapeutic anticoagulation was allowed, and patients could not have a pathologic condition that had a high risk of bleeding or significant cardiovascular disease. Patients of child-bearing age were required to use effective birth control. The study was approved by the Institutional Review Board and all patients were required to give written informed consent in accordance with institutional and federal guidelines.
Nintedanib 200mg was administered orally twice per day. One treatment cycle was 4 weeks. Patients continued treatment until disease progression or unacceptable toxicity. Disease was evaluated for response every 2 cycles. Response was assessed via RECIST and modified RECIST for pleural tumors. Treatment beyond progression was not allowed.
Descriptive statistics were performed for baseline characteristics. All time-to-event endpoints were estimated using Kaplan Meier methods, from which the median and rates at 4 months were calculated. All patients registered were used in the analysis of PFS and OS. Frequency estimates of the Grade 3–4 toxicity rate was computed for each type of toxicity encountered, using all toxicity-evaluable patients. The primary goal of this study was to evaluate the 4-month progression-free survival (PFS) in patients with MPM treated with nintedanib. Based on the results from a previous Southwest Oncology Group study in this patient population5 and the rates published in Francart at al9, it is assumed that if the 4-month PFS was 30% or less than nintedanib would not warrant further investigation in this setting. A two-stage design was used with 20 patients enrolled in the first stage. If 5 or more patients were alive and progression-free at 4 months than an additional 35 patients would be enrolled in stage 2. This design had a significance level of 0.04 and a power of 0.91. Secondary study endpoints include median PFS, overall survival (OS), and toxicity.
Results
Twenty patients were enrolled from 3/2016 until 4/2018. Patient characteristics are listed in Table 1. The median age was 70 years (range 32–81). The majority of patients were male (90%), had epithelioid histology (70%), and a PS of 1 (60%). All patients received prior platinum and pemetrexed chemotherapy. Three patients had prior bevacizumab and 4 patients received prior immunotherapy.
Table 1.
Patient Characteristics
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 18 (90) |
| Female | 2 (10) |
| Histology | |
| Epithelioid | 14 (70) |
| Sarcomatoid | 1 (5) |
| Biphasic | 2 (10) |
| Unknown | 3 (15) |
| PS | |
| 0 | 4 (20) |
| 1 | 16 (80) |
| Prior Therapy | |
| Surgery | 7 (35) |
| Platinum/Pemetrexed | 20 (100) |
| Bevacizumab | 3 (15) |
| Immunotherapy | 4 (20) |
| Other | 3 (15) |
| Prior Lines of Treatment | |
| 1 | 12 (60) |
| 2 | 8 (40) |
| Age | Median 70 yr |
| Range 32–81 yr |
There were no responses to treatment. Eight patients had stable disease at 8 weeks for a disease control rate of 40%. Two patients had prolonged control of their disease at 15 and 18 months. Seventeen (85%) discontinued treatment secondary to progressive disease, 2 patients withdrew from the study, and one stopped treatment because of toxicity.
The median follow-up was 4.1 months (range 0.7–45.6). The 4-month PFS rate was 12% (95% CI: 0%, 28%) and the median PFS was 1.8 months (95% CI: 1.6, 3.0) (Figure 1). The median OS was 4.2 months (95% CI: 2.5, 8.7) and the OS rate at 4 months was 55% (95% CI: 31%,79%) (Figure 2). The 6 and 12-month OS are 31% (95% CI: 8%, 54%) and 22% (95% CI: 2%, 43%), respectively. The trial did not meet its endpoint, so it did not proceed to the second stage.
Figure 1.
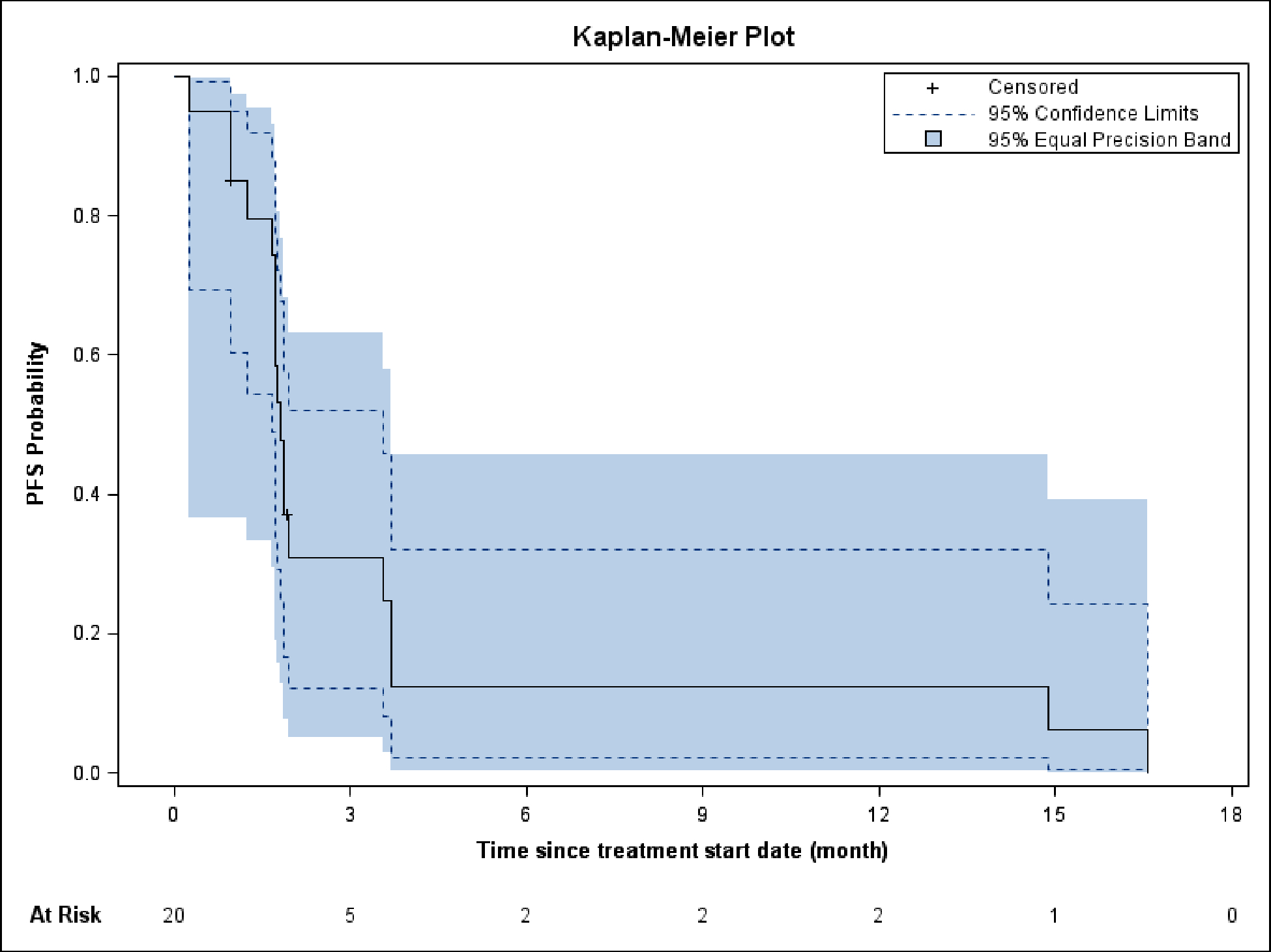
Progression-free survival
Figure 2.
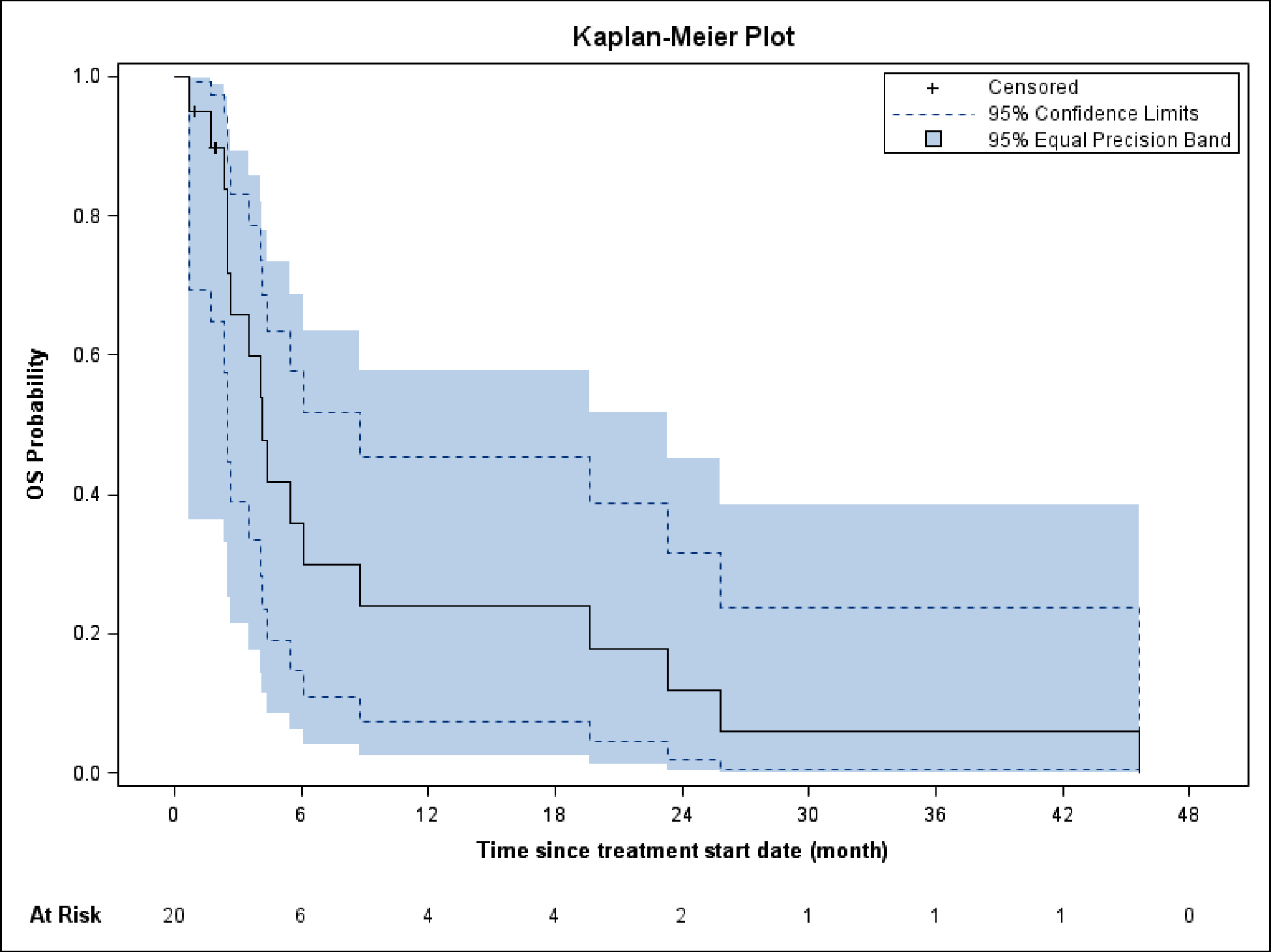
Overall survival
The median number of cycles delivered was 2 (range 1–18). The most common treatment-related adverse events were Grades 1 and 2 and consisted of gastrointestinal side effects (diarrhea, nausea, vomiting), transaminase elevation, constitutional symptoms (anorexia, fatigue), edema, and dyspnea (Table 2). There were 4 episodes of grade 3 toxicity that were possibly related to treatment including arrythmia, ascites, syncope, and pulmonary embolus. There were no related grade 4 or 5 toxicities. There were no significant incidences of hypertension, proteinuria, or hemorrhage.
Table 2.
Toxicities
| Toxicities | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Gastrointestinal | 15 | 11 | 1 (ascites) | - |
| Metabolic | 11 | 2 | - | - |
| Constitutional | 5 | 2 | - | - |
| Respiratory | 2 | 1 | - | - |
| Pain | 3 | - | - | - |
| Hematologic | 2 | - | - | - |
| Edema | 1 | 3 | - | - |
| Arrythmia | 1 | - | 1 | - |
| Thrombosis | - | - | 1 | - |
| Rash | 1 | - | - | - |
| Syncope | - | - | 1 | - |
Discussion
This phase II trial of nintedanib in relapsed MPM did not meet its primary endpoint based on PFS. There were 2 patients with prolonged disease stabilization, but it is uncertain as to whether this represents a hint of benefit because of treatment. A phase II trial of pazopanib in MPM patients with 0–1 prior treatments did show some activity but did not meet its primary endpoint and was poorly tolerated.10 It is possible that angiogenesis inhibitors need to be combined with other systemic treatment such as chemotherapy in order to establish their efficacy. The MAPS trial did demonstrate a modest but significant survival benefit when bevacizumab was combined with chemotherapy in the first-line treatment of MPM.4 The treatment benefit was most notable in the sarcomatoid population. This combination is included in treatment guidelines but is not FDA approved. A randomized phase II trial (RAMES) evaluated gemcitabine with or without ramucirumab in relapsed MPM.11 There was a survival advantage for the combination (13.8 vs. 7.5 months) but this has not led to its use in common practice. Both TKIs, cediranib and nintedanib, have been evaluated in combination with chemotherapy in larger trials. SWOG S0905 assigned patients to receive cediranib or placebo with chemotherapy.12 There was increased toxicity with only a small increment in PFS for the combination. LUME-Meso was a phase II/III randomized trial designed to assess the efficacy of nintedanib plus chemotherapy as first-line treatment of MPM. The phase II portion of the trial reported an improvement in PFS (HR 0.54) and OS (HR 0.77) particularly in epithelioid histology favoring nintedanib, however in the phase III part of the trial the primary PFS (HR 1.01) endpoint was not met and the phase II findings were not confirmed.13,14 Toxicities were similar between the 2 phases of the trial. It should be mentioned that sarcomatoid patients were excluded from the trial which may have had an influence on the outcome. Nintedanib has also been evaluated as switch maintenance therapy after chemotherapy in the EORTC 08112 NEMO trial (NCT02863055). The study is fully accrued, and results are awaited.
Immune checkpoint inhibitors have become the standard of care in the treatment of many solid tumors. In the setting of relapsed MPM, the phase III PROMISE-Meso trial compared pembrolizumab to vinorelbine or gemcitabine resulting in no survival benefit for immunotherapy over single agent chemotherapy.15 Subsequently, the CONFIRM trial showed a survival benefit (10.2 months vs. 6.9 months) for nivolumab over placebo.16 A meta-analysis of the use of single agent PD-1/PD-L1 inhibitors in the treatment of relapsed MPM revealed an 18% RR (27% in PD-1 positive) and a disease control rate of 55%.17 The results of CheckMate 743 established a role for immunotherapy for the treatment of MPM in the first-line setting.18 Patients were randomized to receive either ipilimumab/nivolumab or chemotherapy. There was a significant improvement in OS (18.1 vs. 14.1 months) for the patients receiving immunotherapy, particularly for those with sarcomatoid histology.
There has been an interest in combining immunotherapy with angiogenesis inhibition. There is some rationale for this in that normalization of tumor vasculature can transform an immunosuppressed tumor microenvironment into an immune rich environment.19 The BEAT-meso study is an ongoing clinical trial investigating carboplatin/pemetrexed/bevacizumab ± atezolizumab in MPM treatment (NCT03762018).
Both the inhibition of angiogenesis and immunotherapy play a role in the treatment of MPM. Despite the improvement in survival demonstrated in recent clinical trials, most patients still do not have adequate control of their disease. The Phase 1b PEMBIB trial treated 30 MPM patients with a combination of nintedanib and pembrolizumab. Associated correlative studies suggest that the presence of proinflammatory cytokines, IL-6 and CXCL8, resulted in higher tumor secretion of VEGF and enrichment of regulatory T cells.20 One of the goals of further investigations should be to identify biomarkers that will help direct the appropriate therapy for these patients. The future of nintedanib and other agents are dependent on defining predictors of potential clinical benefit. Continued participation in clinical trials is also necessary to discover novel efficacious treatment.
Clinical Practice Points:
Novel therapies are needed for the treatment of malignant pleural mesothelioma (MPM).
Agents targeting angiogenesis such as bevacizumab have a role in the treatment of MPM.
Nintedanib had modest activity involving prolonged stable disease in a small group of patients.
Immunotherapy is now important in the treatment of MPM but angiogenesis inhibitors in combination with immunotherapy and/or chemotherapy may still have a role.
It is very important to discover biomarkers that can help with patient selection to individualize treatment.
Participation in clinical trials evaluating novel agents remains very important in advancing treatment strategies.
Acknowledgements:
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). This was an independent, investigator-initiated study supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). BIPI had no role in the design, analysis, or interpretation of the results of this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Footnotes
Disclosures:
Antoinette J. Wozniak – Consulting: Genentech, Daiichi, Beigene, Incyte, Janssen; Research funding: GlaxoSmithKline, Boehringer Ingelheim, Janssen
Bryan Schneider – None
Gregory P. Kalemkerian – Research funding: Abbvie, Blueprint, Bristol Myers Squibb, Cullinan, Daiichi, Merck, Takeda
Bobby Daly – Consulting: Varian Medical Systems; Equity: Roche, Eli Lilly (family member)
Wei Chen – None
Jaclyn Ventimiglia – None
Misako Nagasaka – Consulting: AstraZeneca, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Janssen, Pfizer, Eli Lilly, Genentech, Caris Life Sciences, Blueprint; Travel support: AnHeart Therapeutics
Marjorie G. Zauderer – Consulting: Curis, Ikena, Takeda, GlaxoSmithKline, AldeyraTherapeutics, Novocure; Honoraria (CME): PER, Medscape, Research to Practice, Medical Learning Institute, OncLive; Research funding (MSKCC): Department of Defense, NIH, Precog, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium/Takeda, Curis, Atara; Chair of the Board of Directors of the Mesothelioma Applied Research Foundation (uncompensated)
References
- 1.Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg. 2001;25(2):210–7. 10.1007/s002680020021. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44. 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Strizzi L, Catalano A, Vianale G, Orecchia S, Casalini A, Gianfranco T, Puntoni R, Mutti L, Procopio A. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 4.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, Riviere F, Janicot H, Gervais R, Locher C, Milleron B, Tran Q, Lebitasy M-P, Morin F, Creveuil C, Parienti J-J, Scherpereel A. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothellioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14. 10.1016/S0140-6736(15)01238.6. [DOI] [PubMed] [Google Scholar]
- 5.Garland LL, Chansky K, Wozniak AJ, Tsao AS, Gadgeel SM, Verschraegen CF, DaSilva MA, Redman M, Gandara DR. Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thorac Oncol. 2011;6:1938–45. 10.1097/JTO.0b013e318229586e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–82. 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 7.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S: LUME-Lung 1 Study Group. Lancet Oncol. 2014;15(2):143–55. 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar-Singh S, Weyler J, Martin MJ, Vermeulen PB, Van Marck E. Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and −2 and TGF beta expression. J Pathol. 1999;189(1):72–8. . [DOI] [PubMed] [Google Scholar]
- 9.Francart J, Legrand C, Sylvester R, Van Glabbeke M, van Meerbeeck JP, Robert A. Progression-free survival rate as primary end point for phase II cancer clinical trials: application to mesothelioma—The EORTC Lung Cancer Group. J Clin Oncol. 2006;24(19):3007–12. 10.1200/JCO.2005.05.1359. [DOI] [PubMed] [Google Scholar]
- 10.Parikh K, Mandrekar SJ, Allen-Ziegler K, Esplin B, Tan AD, Marchello B, Adjei AA, Molina JR . A phase II study of pazopanib in patients with malignant pleural mesothelioma: NCCTG No623 (Alliance). Oncologist. 2020;25(6):523–31. 10.1634/theoncologist.2019-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto C, Zucali PA, Pagano M, Grosso F, Pasello G, Garassino MC, Tiseo M, Parra HS, Grossi F, Cappuzzo F, de Marinis F, Pedrazzoli P, Bonomi M, Gianoncelli L, Perrrino M, Santoro A, Zanelli F, Bonelli C, Maconi A, Frega S, Gervasi E, Boni L, Ceresoli GL. Gemcitabine with or without ramucirumab as second-line treatment of malignant pleural mesothelioma (RAMES): a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2021;22(10):1438–47. 10.1016/S1470-2045(21)00404-6. [DOI] [PubMed] [Google Scholar]
- 12.Tsao AS, Miao J, Wistuba II, Vogelzang NJ, Heymach JV, Fossella FV, Lu C, Velasco MR, Box-Noriega B, Hueftle JG, Gadgeel S, Redman MW, Gandara DR, Kelly K. Phase II trial of cediranib in combination with cisplatin and pemetrexed in chemotherapy-naive patients with unresectable malignant pleural mesothelioma (SWOG S0905). J Clin Oncol. 2019;37(28):2537–47. 10.1200/JCO.19.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosso F, Steele N, Novello S, Nowak AK, Popat S, Greillier L, John T, Leighl NB, Reck M, Taylor P, Planchard D, Sorensen JB, Socinski MA, von Wangenheim U, Loembe AB, Barrueco J, Morsli M, Scagliotti G. Nintedanib plus pemetrexed/cisplatin in patients with malignant pleural mesothelioma: phase II results from the randomized placebo-controlled LUME-Meso trial. J Clin Oncol 2017;35(31):3591–3600. 10.1200/JCO.2017.72.9012. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Gaafar R, Nowak AK, Nakano T, van Meerbeeck J, Popat S, Vogelzang NJ, Grosso F, Aboelhassan R, Jakopovic M, Ceresoli GL, Taylor P, Orlandi F, Fennell DA, Novello S, Scherpereel A, Kuribayashi K, Cedres S, Sørensen JB, Pavlakis N, Reck M, Velema D, von Wangenheim U, Kim M, Barrueco J, Tsao AS. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7(7):569–580. 10.1016/S2213-2600(19)30139-0. [DOI] [PubMed] [Google Scholar]
- 15.Popat S, Curioni-Fontecedro A, Dafni U, Shah R, O’Brien M, Pope A, Fisher P, Spicer J, Roy A, Gilligan D, Gautschi O, Nadal E, Janthur WD, López Castro R, García Campelo R, Rusakiewicz S, Letovanec I, Polydoropoulou V, Roschitzki-Voser H, Ruepp B, Gasca-Ruchti A, Peters S, Stahel RA. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9–15) PROMISE-meso trial. Ann Oncol. 2020;31(12):1734–1745. 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, Danson S, Steele N, Nye M, Johnson L, Lord J, Middleton C, Szlosarek P, Chan S, Gaba A, Darlison L, Wells-Jordan P, Richards C, Poile C, Lester JF, Griffiths G; CONFIRM trial investigators. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22(11):1530–40. 10.1016/S1470-2045(21)00471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tagliamento M, Bironzo P, Curcio H, De Luca E, Pignataro D, Rapetti SG, Audisio M, Bertaglia V, Paratore C, Bungaro M, Olmetto E, Artusio E, Reale ML, Zichi C, Capelletto E, Carnio S, Buffoni L, Passiglia F, Novello S, Scagliotti GV, Di Maio M. A systematic review and meta-analysis of trials assessing PD-1/PD-L1 immune checkpoint inhibitors activity in pre-treated advanced stage malignant mesothelioma. Crit Rev Oncol Hematol. 2022;172:103639. 10.1016/j.critrevonc.2022.103639. [DOI] [PubMed] [Google Scholar]
- 18.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, Oulkhouir Y, Bautista Y, Cornelissen R, Greillier L, Grossi F, Kowalski D, Rodríguez-Cid J, Aanur P, Oukessou A, Baudelet C, Zalcman G. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–86. 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 19.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–40. 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danlos F-X, Texier M, Job B, Mouraud S, Cassard L, Baldini C, Varga A, Yurchenko AA, Rabeau A, Champiat S, Letourneur D, Bredel D, Susini S, Blum U, Parpaleix A, Parlavecchio C, Tselikas L, Fahrner J-E, Goubet A-G, Rouanne M, Rafie S, Abbassi A, Kasraoui I, Breckler M, Farhane S, Ammari S, Laghouati S, Gazzah A, Lacroix L, Besse B, Droin N, Deloger M, Cotteret S, Adam J, Zitvogel L, Nikolaev SI, Chaput N, Massard C, Soria J-C, Gomez-Roca C, Zalcman G, Planchard D, Marabelle A. Genomic instability and pro-tumoral inflammation are associated with primary resistance to anti-PD1 + anti-angiogenesis in malignant pleural mesothelioma. Cancer Discov. 2023;Jan 20;CD-22–0886. 10.1158/2159-8290.CD-22-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]


