Summary:
Pediatric hand burns are difficult to treat, with thin tissue with critical structures close to the skin and the small scale of the anatomy of children’s hands. Additionally, pediatric burns can be challenging due to the concern for donor-site morbidity and the paucity of donor sites when reconstructing these wounds. In this report, we discuss the successful application of a piscine-derived acellular dermal matrix in a 13-month-old child with deep partial thickness and full-thickness burns to the right upper extremity. She had excellent long term cosmetic results and function by 3 years postoperatively, including full extension and flexion of all digits in her right hand.
Grease burns in children tend to be sustained on their face, neck, chest, and upper extremities.1 These burns are often full thickness and prone to infection, necessitating thorough removal of nonviable tissue. As critical structures are in close proximity to the skin in hand and upper extremity burns, balancing the need for debridement with the risk of exposing critical structures can be challenging.2,3 These injuries are also associated with the risk of compartment syndrome and the potential for future burn contractures, which can be functionally debilitating.4 These patients require targeted interventions, including meticulous scar management, aggressive postoperative occupational and physical therapy, and ongoing surveillance for contracture as the patient grows.
Weighing donor-site morbidity is an important consideration in burn reconstruction, especially in children. For children, there is limited surface area where full-thickness skin grafts can be obtained. Split-thickness skin grafts have a greater risk for long-term dyspigmentation. Using synthetic skin substitutes such as acellular dermal matrices (ADMs) has become of particular interest to burn, reconstructive, and hand surgeons. Kerecis Omega3 Wound (Kerecis, Isafjordur, Iceland) is a piscine-derived ADM described in the treatment of burns.5,6 This ADM is minimally processed, shelf stable, and has been shown to have antiinflammatory and antimicrobial properties which facilitate wound healing.6
In this report, we describe the use of a piscine-derived ADM for reconstruction of a hand burn in a 13-month-old child. To our knowledge, this is the first description of its use in a pediatric burn, and the second description of the ADM’s use in the pediatric population.7
CASE PRESENTATION
A 13-month-old female child was admitted to the neonatal intensive care unit for 3% total body surface area deep partial and full-thickness burns to her right hand and forearm after reaching into a deep fryer. Medical history was significant for meconium aspiration, renal failure, and hepatic failure, necessitating the use of extracorporeal membrane oxygenation and dialysis, from which she recovered with no long-term sequelae. Upon arrival to the burn unit, the child underwent emergent escharotomies.
On postburn day 3, she underwent tangential excision of the burn wounds with placement of cadaveric allograft, per facility protocol. Five days postoperatively, the allograft was removed, and the patient had piscine ADM (Kerecis Omega3 Wound) applied to the burns on her fingers (Figs. 1 and 2). No allograft was used in this case. The patient’s hand was then dressed with interfacing and wet Kling soaked in 5% sulfamylon, followed by dry burn pads. The patient’s right hand was splinted in extension and secured with Ace wrap.
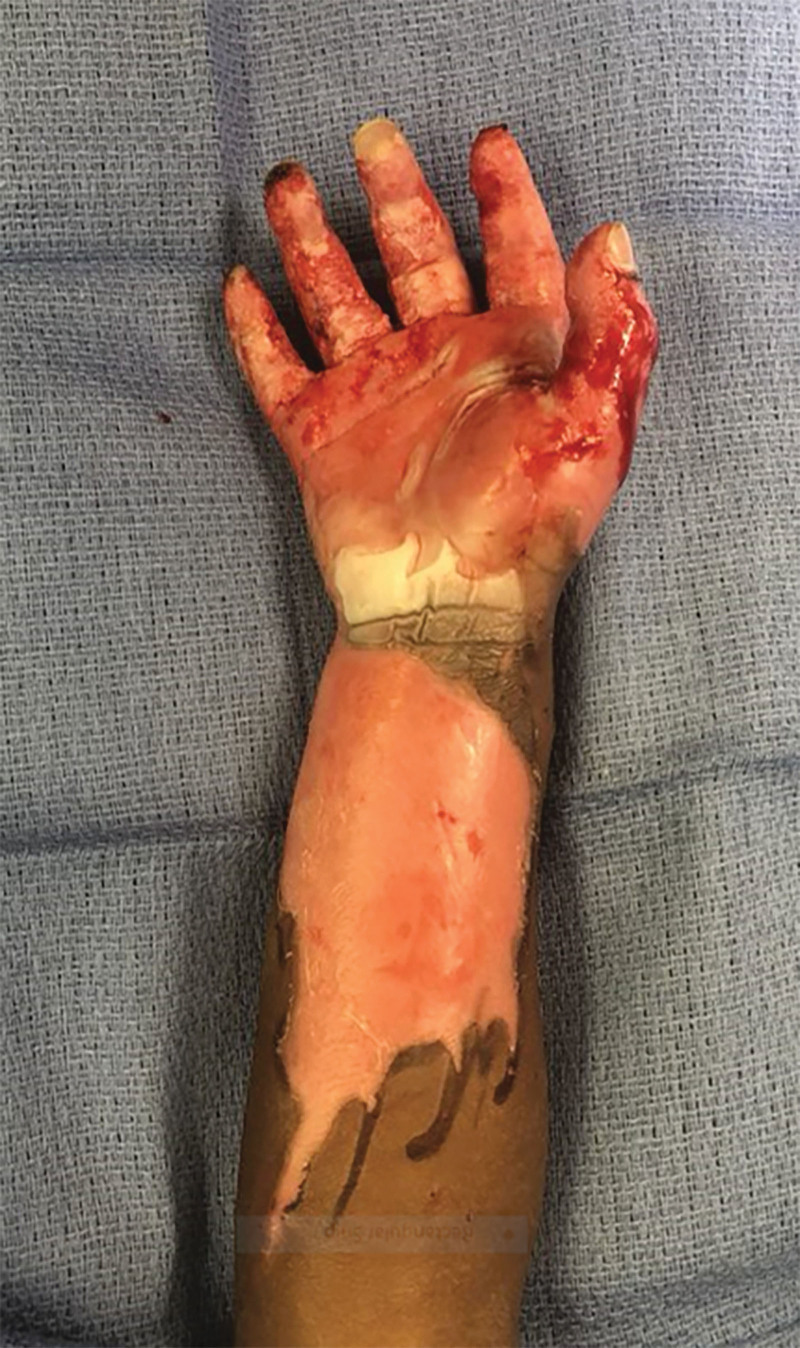
Fig. 1.
Preoperative view. The patient’s right upper extremity preoperatively with mixed superficial and deep partial thickness burns to the volar surface of the hand and volar forearm, in addition to full-thickness burns on the index, middle, ring, and small fingers.
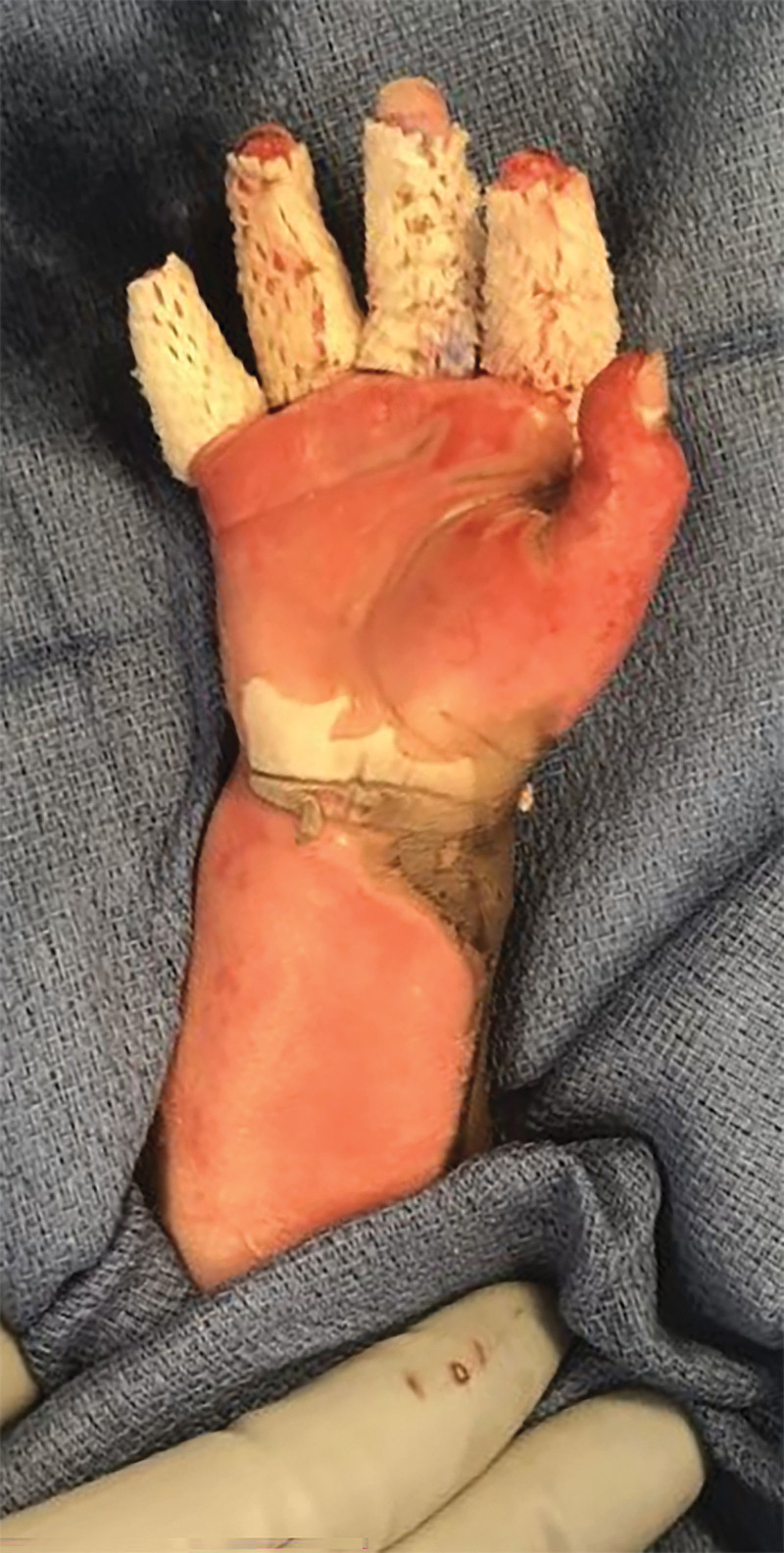
Fig. 2.
Intraoperative view. The patient’s right upper extremity after application of the piscine acellular dermal matrix to the index, middle, ring, and small fingers.
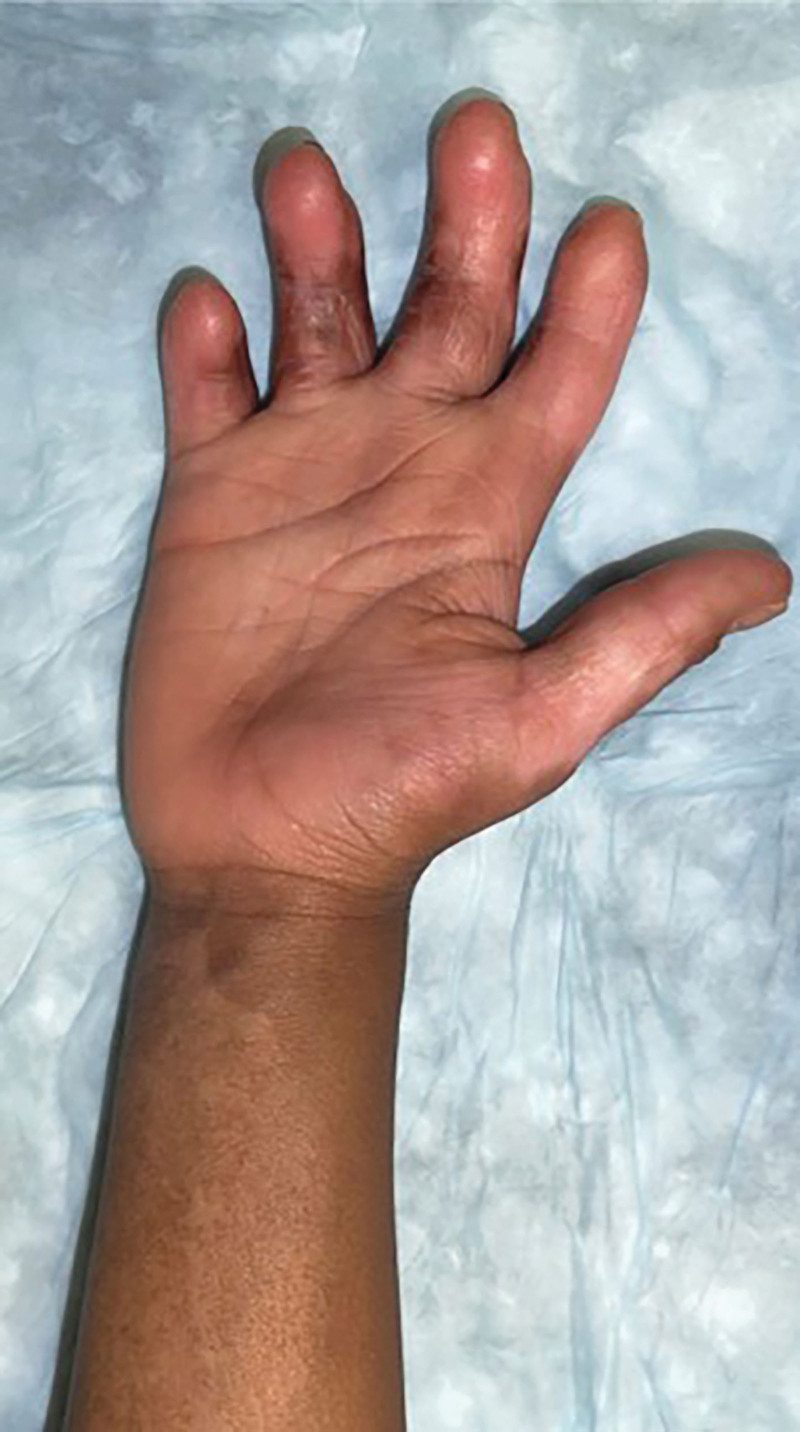
The ADM incorporated after 10 days. She had aggressive physical therapy and occupational therapy while inpatient and immediately after discharge. Six months postoperatively, the patient underwent fractionated CO2 laser therapy and Kenalog injection for dyspigmentation and scarring. She is now being followed up annually by a hand surgeon. Her most recent appointment marks 3 years since the ADM placement, and the patient was noted to have full use of her right hand with complete flexion and extension, flat hand profile on tabletop test, and a good cosmetic result with no apparent functional deficits (Fig. 3). She has required no further interventions.
Fig. 3.
The patient’s right upper extremity at 2 years postoperative.
DISCUSSION
Burns are a common form of household injury that can be a particularly devastating form of trauma. The mortality due to burns is highest in patients between the ages of 0 and 2.8 In the United States, the most common form of burn injury in children are scald burns, followed by contact and flame burns.8 Burns involving hot grease or oil are associated with full-thickness burns and infection; however, they result in decreased mortality when compared with other pediatric burns.1
The standard treatment for burns usually involves infection prevention and the promotion of a moist wound environment to prevent wound conversion.9 In children, concern for donor site morbidity and the effect of time (ie, growth) impact the managing surgeon’s algorithm as we reconstruct these wounds.
The Kerecis Omega3 Wound may serve as an effective method of treating pediatric burn wounds and avoiding the disadvantages of a traditional autologous skin graft. The microporous structure of Kerecis provides a scaffolding for granulation tissue and angiogenesis, leading to enhanced healing.6 This is advantageous for poorly vascularized wound beds, such as the overexposed structures of the hands, which require a robust foundation of granulation tissue for definitive skin grafting.10 Omega3, specifically, has been shown to regulate inflammation and have antibacterial properties.6 Kerecis is decellularized and is incorporated into the body along with intrinsic structures of the piscine dermis as a scaffolding. We have found this to be useful in complex upper and lower extremity wounds, including this case.10,11
The use of skin substitutes in the treatment of pediatric burn wounds is a viable alternative to allografts, as they are effective in promoting wound healing and reducing infection risk while avoiding the problems typically associated with allografts, including cost.12 The use of ADM eliminates donor-site morbidity, which is helpful in pediatric patients, who require conservation of body surface area. By using quickly incorporating ADM, such as Kerecis, we have been able to quickly rehabilitate patients, obviating the need for autologous grafting and minimizing the risk of contracture.
CONCLUSIONS
In our experience, we found application of Kerecis to be a simple and effective option for wound coverage. The ADM incorporated within 10 days, obviating the need for autologous grafting in this pediatric patient with full-thickness burns to her fingers. With the help of intralesional steroid injection and laser therapy after complete closure of the wounds, the patient’s hand had excellent cosmesis with full function, including full flexion and extension of all digits. We found ADM can be safely and effectively applied to a pediatric hand burn wound. Further prospective studies are needed to investigate the long term functional outcomes of piscine ADM placement in pediatric hand burn patients.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
Consent was obtained (or waived) by all participants in this study. The institutional review board of the, University of New Mexico Health Sciences Center issued approval for this study (#22-133).
Footnotes
Published online 9 July 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Murphy JT, Purdue GF, Hunt JL. Pediatric grease burn injury. Arch Surg. 1995;130:478–482. [DOI] [PubMed] [Google Scholar]
- 2.Stamatas GN, Nikolovski J, Luedtke MA, et al. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27:125–131. [DOI] [PubMed] [Google Scholar]
- 3.Birchenough SA, Gampper TJ, Morgan RF. Special considerations in the management of pediatric upper extremity and hand burns. J Craniofac Surg. 2008;19:933–941. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Sittah GS, El Khatib AM, Dibo SA. Thermal injury to the hand: review of the literature. Ann Burns Fire Disasters. 2011;24:175–185. [PMC free article] [PubMed] [Google Scholar]
- 5.Stone R, Saathoff EC, Larson DA, et al. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int J Mol Sci. 2021;22:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam K, Jeffery SLA. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: a case series. Mil Med. 2019;184(Supplement_1):16–20. [DOI] [PubMed] [Google Scholar]
- 7.Ahn KH, Park ES. A rare case report of neonatal calcinosis cutis induced by distant and delayed extravasation of intravenous calcium gluconate. Arch Plast Surg. 2021;48:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmieri TL. Pediatric burn resuscitation. Crit Care Clin. 2016;32:547–559. [DOI] [PubMed] [Google Scholar]
- 9.Vloemans AF, Hermans MH, van der Wal MB, et al. Optimal treatment of partial thickness burns in children: a systematic review. Burns. 2014;40:177–190. [DOI] [PubMed] [Google Scholar]
- 10.Shahriari SR, Ederle AC, Whisonant C, et al. Successful upper extremity limb salvage using cellular- and tissue-based products in a patient with uncontrolled diabetes. Wounds. 2022;34:E104–E107. [PubMed] [Google Scholar]
- 11.Shahriari S, Ensign E, Huang S, et al. Successful treatment of wounds from nonuremic calciphylaxis with acellular piscine dermis. Plast Reconstr Surg Glob Open. 2023;11:e5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Mohapatra DP, Chittoria RK, et al. Human skin allograft: is it a viable option in management of burn patients? J Cutan Aesthet Surg. 2019;12:132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]