Abstract
After the transition from the acute to the chronic phase of human immunodeficiency virus (HIV) infection, complement mediates long-term storage of virions in germinal centers (GC) of lymphoid tissue. The contribution of particular complement receptors (CRs) to virus trapping in GC was studied on tonsillar specimens from HIV-infected individuals. CR2 (CD21) was identified as the main binding site for HIV in GC. Monoclonal antibodies (MAb) blocking the CR2-C3d interaction were shown to detach 62 to 77% of HIV type 1 from tonsillar cells of an individual in the presymptomatic stage. Although they did so at a lower efficiency, these antibodies were able to remove HIV from tonsillar cells of patients under highly active antiretroviral therapy, suggesting that the C3d-CR2 interaction remains a primary entrapment mechanism in treated patients as well. In contrast, removal of HIV was not observed with MAb blocking CR1 or CR3. Thus, targeting CR2 may facilitate new approaches toward a reduction of residual virus in GC.
Follicular dendritic cells (FDC) are thought to play a pivotal role in the initiation and maintenance of secondary antibody responses. FDC operate in germinal centers (GC) of lymphoid follicles, where they form a three-dimensional network. Antigens complexed with immunoglobulins and complement fragments are trapped on the surface of FDC in form of immune-complex-coated bodies or “iccosomes” that contribute to B-cell maturation and B-cell memory (24). FDC express substantial quantities of complement receptor 1 (CR1; CD35) and CR2, in addition to lesser amounts of CR3 (CD11b/CD18) (21). This unique pattern of CR expression allows FDC to interact with all complement C3 fragments: with C3b through CR1, with iC3b through CR2 or CR3, and with C3d via CR2.
In the course of human immunodeficiency virus type 1 (HIV-1) infection, most of the virus is produced by CD4+ T lymphocytes or macrophages and is subsequently stored on the surface of FDC (1, 25). This attachment process was shown to be complement dependent in vitro (16, 23). Quantitative image analysis demonstrated that, after transition to the chronic phase, more than 98% of the viral RNA in lymphoid tissue (LT) is found in virions extracellularly bound to FDC (9, 10, 19). Early histopathological alterations in HIV infection include follicular hyperplasia, destruction of FDC, and lysis of the lymphoid follicles. In the late stage, FDC are destroyed and viral RNA decreases in GC, whereas the level of infectious virions in plasma increases. As FDC are diminished, lymphoid follicles involute and generalized immunosuppression occurs. It was proposed previously that at least part of these effects are mediated by virions trapped in the FDC network (3).
After highly active antiretroviral therapy (HAART) is initiated, the amount of HIV trapped on FDC decreases very quickly and plasma HIV-1 RNA becomes undetectable in most cases (4, 27). After 6 months of HAART, a >2,500-fold reduction in the FDC pool of virions was observed, but HIV RNA on FDC was still detectable in some GC (4). This residual virus probably represents a source of HIV in the case of drug resistance or if therapy is discontinued (9).
Several mechanisms are considered to contribute to the trapping of HIV by FDC (3): (i) virions form immune complexes coated with complement C3 fragments and subsequently attach to CR or Fc receptors on FDC, (ii) gp120 molecules on the viral envelope may specifically interact with immunoglobulins of the VH3 family and thereby constitute immune-complexed virions that bind to FDC, and (iii) the viral envelope acquires adhesion molecules during budding from the host cell which then may interact with their cellular counterparts. In this report, tonsillar tissue from HIV-infected individuals was studied to assess the relative contributions of particular CRs to virus trapping in GC and the possibilities of detaching HIV from its reservoir.
MATERIALS AND METHODS
Antibodies.
Mouse monoclonal antibodies (MAb) used in this study were 3D9 and 1B4, anti-CR1 blocking the C3b-binding site (18); E11, nonblocking anti-CR1 (Pharmingen, San Diego, Calif.); HB5, nonblocking anti-CR2, obtained from the American Type Culture Collection (ATCC; Manassas, Va.); 4C2, directed against the C3d fragment of complement C3 (17); and AB2 and BB5 (both anti-C3d) and FE8, anti-CR2, developed in our laboratory. FE8 (W. M. Prodinger, M. G. Schwendinger, and M. P. Dierich, 25 March 2000, European Patent Office) is currently the only available MAb that blocks both C3d binding and Epstein-Barr virus binding to CR2 (20). TMG6-5 (26) and TS1/18 (obtained from the ATCC) are directed against human CD11b and CD18, respectively. TMG6-5 blocks the binding of iC3b to CR3 (5). TIB191 (immunoglobulin G1 [IgG1]) and TIB109 (IgG2a), both from the ATCC, were used as isotype controls. A single-chain fragment variable (scFv) MAb, scFv-FE8, was generated from the FE8 hybridoma using the Recombinant Phage Antibody System (Pharmacia Biotech, Uppsala, Sweden).
Stripping of C3d-coated beads from Raji cells.
B-lymphoblastoid, CR2+ Raji cells (ATCC) were cultured in complete medium. Fluorescein isothiocyanate (FITC)-labeled, C3d-coated agarose microbeads were prepared as described previously (20). For detachment of complement-coated beads from CR2, 5 × 105 Raji cells were washed with Hanks' balanced salt solution (HBSS) and incubated for 30 min at room temperature with predetermined saturating concentrations of beads. Thereafter, cells were washed with HBSS and the pellet was resuspended in 50 μl of HBSS containing MAb. Samples were incubated for 30 min at 37°C, washed twice with HBSS, and analyzed using a FACScan (Becton Dickinson, Hialeah, Fla.). Nonspecific binding was determined using Raji cells incubated with FITC-microbeads lacking C3 fragments.
Tonsillar tissue.
Surgical specimens of palatine tonsils were obtained from HIV-positive individuals by tonsillectomy done for medical reasons unrelated to this study (Table 1). Tonsils were collected in 0.9% saline and processed immediately. One half of the tissue was cut into 5-mm blocks, snap frozen in liquid nitrogen, and used for cryosections. The other half was homogenized to obtain a suspension of tonsillar cells (TC).
TABLE 1.
Profiles of tonsillectomized HIV-positive individuals
| Patient | Date of first positive HIV test result | Agea (yr) | Stage of infectiona | CD4+ T-cell counta (no. of cells/μl) | Plasma HIV RNAa (no. of copies/ml) | Antiretroviral drugsb | No. of mo receiving HAARTa |
|---|---|---|---|---|---|---|---|
| 1 | March 1995 | 28 | Presymptomatic | 378 | 8,912 | None | None |
| 2 | November 1993 | 35 | Late | 499 | <400 | d4T, 3TC, IDV, RTV | 19 |
| 3 | November 1997 | 33 | Late | 465 | <400 | AZT, 3TC, IDV | 21 |
At time of tonsillectomy.
d4T, stavudine; 3TC, lamivudine; AZT, zidovudine; IDV, indinavir; RTV, ritonavir.
Preparation of TC.
Tonsil tissue was cut into small pieces when immersed in X-VIVO 10 medium (Bio-Whittaker, Verviers, Belgium) and incubated with 1 mg of collagenase type IV (Sigma, St. Louis, Mo.) per ml for 60 min at 37°C. After 30 min, released TC were collected and fresh enzyme solution was added to nondigested tissue. TC were pooled, washed twice with complete medium (RPMI 1640 with 10% fetal calf serum [FCS]), and passed through a 100-μm-pore-size cell strainer (Becton Dickinson) to remove cell clumps and nondigested tissue. Finally, TC were frozen in RPMI 1640 with 12.5% dimethyl sulfoxide and 20% FCS and stored at −70°C until further use.
Stripping of HIV from TC.
Frozen aliquots of TC were thawed and washed with complete medium before stripping. TC (2 × 105 cells in 100 μl) were seeded into 96-well V-bottomed microplates and incubated in the presence or absence of MAb for 1 h at 37°C. To determine the size of the intracellular viral pool, TC were incubated in the presence of different concentrations of proteinase K under the same conditions. After centrifugation, cell pellets were washed three times with complete medium, lysed with 1% NP-40 in RPMI medium, and used for quantification of HIV-1 p24 antigen. Supernatants from stripping experiments were stored at −70°C until determination of either p24 or HIV-1 RNA.
p24 capture ELISA.
HIV-1 p24 antigen was quantified in TC lysates and for some experiments also in supernatants from stripping experiments by p24 capture enzyme-linked immunosorbent assay (ELISA) as described elsewhere (2).
Stripping from tonsil cryosections.
Six-micrometer-thick sections were cut at different levels from frozen tissue blocks and stored at −70°C until use. Thawed sections were air dried quickly, and nonspecific binding was blocked by incubation with 10% FCS in HBSS for 30 min at room temperature. The sections were allowed to react for 1 h at 37°C with 1 μg of MAb per ml in 10% FCS–HBSS. Samples were subsequently washed with HBSS and fixed in methanol-acetone (4:1).
Immunohistochemistry.
Sections were blocked in 10% FCS in phosphate-buffered saline and incubated with anti-mouse IgG-FITC conjugate (DAKO, Glostrup, Denmark) to stain the stripping MAb. Thereafter, sections were incubated with biotinylated anti-p24 MAb (clone 37G12, kindly provided by H. Katinger, Institute for Applied Microbiology, Vienna, Austria) followed by extravidin-alkaline phosphatase conjugate (Sigma). The sections were developed using FastRed substrate (Sigma), mounted with DAKO fluorescent mounting medium, and examined with a confocal laser microscope (LSM-510; Carl Zeiss, Jena, Germany).
HIV-1 RNA quantification.
HIV-1 virions in supernatants of TC samples were quantified using the AMPLICOR HIV-1 MONITOR test version 1.5 (Roche Diagnostics, Branchburg, N.J.). An ultrasensitive procedure was used according to the manufacturer's instructions. Linearity for RPMI-FCS culture supernatant specimens was reached between 200 and 50,000 copies per ml (detection limit, 200 copies per ml), as determined in preliminary experiments.
RESULTS
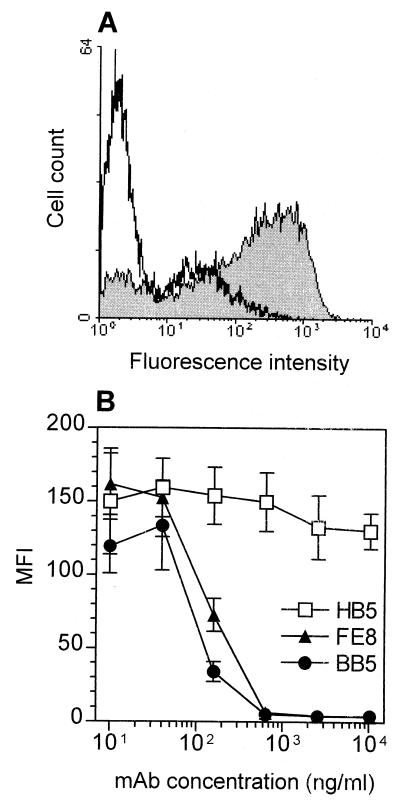
We have recently established a MAb, FE8, directed against CR2 that blocks the binding of C3d-coated ligands (20). Here we demonstrate that FE8 is able to dissociate already-bound C3d-opsonized particles from CR2. Using CR2+ Raji B cells as a model system, more than 97% of C3d-beads could be removed after 1 h of incubation in the presence of ≥500 ng of FE8 per ml (Fig. 1A). BB5, a MAb directed against the CR2-binding site in C3d, showed similar effects (Fig. 1B). Therefore, MAb FE8 and BB5 are pertinent tools to assess the contribution of the CR2-C3d interaction to HIV entrapment in LT.
FIG. 1.
Detachment of fluorescent agarose microbeads opsonized with C3d from CR2 of Raji B cells by MAb against CR2 or C3d. (A) Fluorescence intensity of Raji cells loaded with predetermined saturating concentrations of beads and subsequently stripped with 1 μg of isotype control antibody (grey histogram) per ml or 1 μg of MAb FE8 (white histogram) per ml. (B) Median fluorescence intensities (MFI) of Raji cells incubated in the presence of anti-CR2 MAb HB5 or FE8 and anti-C3d MAb BB5. Blocking antibodies are shown with closed symbols; nonblocking MAb are shown with open symbols. Values are means from two experiments ± standard errors of the means.
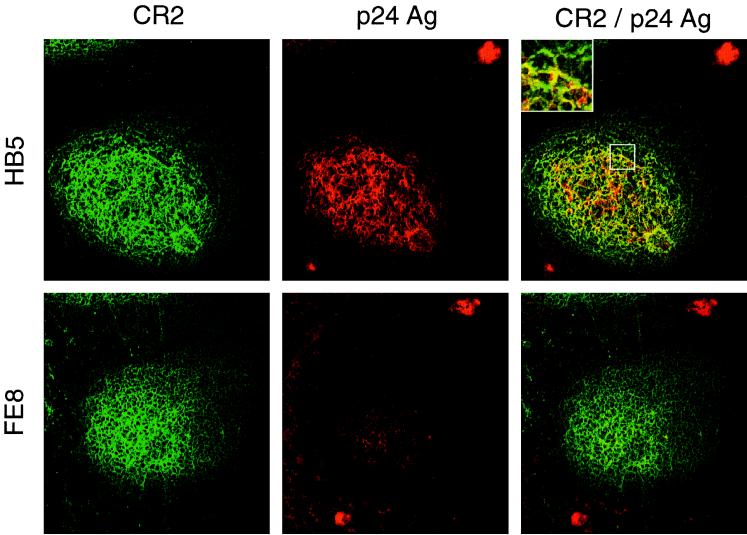
To this end, we performed experiments on tonsillar tissue specimens obtained from three HIV-positive individuals (for patient characteristics, see Table 1). To detach extracellularly bound HIV-1, tonsil cryosections and isolated TC of patient 1 were incubated in the presence of blocking MAb. In sections, most of the virus was localized in the FDC network, whereas virus-replicating cells were found in the T-cell areas of the follicle (Fig. 2). Treatment of cryosections with MAb FE8 led to the disappearance of HIV-1 p24 antigen from GC, while p24 within productively infected cells was not affected. In contrast, nonblocking anti-CR2 MAb HB5 did not affect HIV detachment. These results indicated that blocking the CR2-C3d interaction allows detachment of HIV-1 from GC.
FIG. 2.
Double staining for HIV-1 p24 antigen (Ag) and for CR2 of serial cryosections from tonsil tissue of patient 1. Unfixed sections were treated with MAb HB5 (nonblocking anti-CR2; upper panels) or MAb FE8 (blocking anti-CR2; lower panels), subsequently fixed, stained and photographed. Each section shows a typical GC: CR2+ cells forming a network typical for FDC are stained green, and p24 antigen trapped in GC or found in virus-replicating cells (upper right and lower left, respectively) is stained red. p24 mainly colocalizes (yellow color) with the FDC network within GC (see inset). Overlays were generated by digital superimposition of single color images.
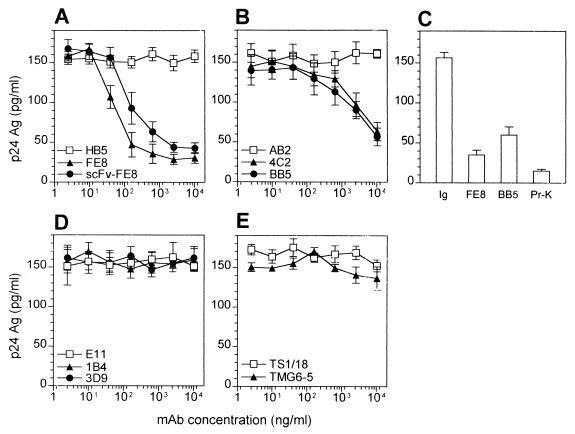
To quantify the removal of trapped virus, TC were treated with blocking MAb against CR2, C3d, CR1, or CR3, and cell-associated p24 levels were determined subsequently by capture ELISA. In our hands, blocking with anti-CR2 MAb FE8, as well as blocking with anti-C3d MAb BB5 and 4C2, was able to remove substantial amounts of virus from TC, whereas anti-CR1 and anti-CR3 MAb had no effect (Fig. 3). Furthermore, an FE8-derived scFv antibody, although requiring a fivefold-higher concentration on a molar basis, was shown to be as effective as FE8 IgG. Stripping of TC with FE8 and BB5 demonstrated that (≤22.5 ± 3.7)% and (≤38.3 ± 6.7)% of p24, respectively, remained cell associated, whereas proteinase K-resistant p24 represented less than 10% (Fig. 3C). This suggests that >90% of p24 is found within virions trapped on the surface of TC and only a very small part is localized intracellularly, which is in good agreement with previous reports (9).
FIG. 3.
(A, B, D, and E) Stripping of HIV from TC from an untreated, presymptomatic HIV-positive individual (patient 1) by MAb or derived scFv antibodies directed against CR2 (A), C3d (B), CR1 (D), or CR3 (E). Closed symbols represent treatment with blocking antibodies, and open symbols represent treatment with nonblocking MAb. HIV-1 p24 was quantified by capture ELISA in lysates of TC after treatment with MAb or derived scFv antibody. (C) p24 release from TC after treatment with 10 μg of isotype control, FE8, or BB5 (values taken from panels A and B) per ml compared with proteinase K (Pr-K) digestion (2.5 μg/ml). p24 was measured in TC lysates by capture ELISA. Data represent means ± standard errors of the means from experiments done in triplicate. Ag, antigen.
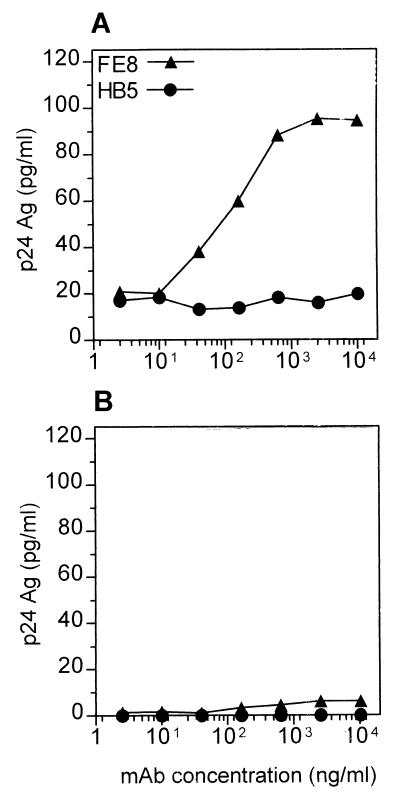
To further distinguish whether liberated p24 was virion associated or virus free, the amounts of p24 in supernatants of TC after detachment were measured in the presence or absence of 1% NP-40 (Fig. 4). The major part of p24 became detectable only after addition of detergent and lysis of virions. Throughout all MAb concentrations used in this experiment, only (5.7 ± 1.2)% of total stripped p24 was virus free. In parallel experiments, release of HIV-1 RNA from TC was determined using quantitative reverse transcription-PCR with similar results (data not shown), underlining that most of the released p24 was localized within virions.
FIG. 4.
Discrimination between virion-associated and virus-free p24 detached from TC. Total amounts of p24 (A) were measured by capture ELISA in TC supernatants after stripping with FE8 (triangles) or HB5 control MAb (circles) and addition of 1% NP-40 (final concentration). Virus-free p24 (B) was determined in the same way in the absence of detergent. Data represent means ± standard errors of the means of a representative experiment done in triplicate. Ag, antigen.
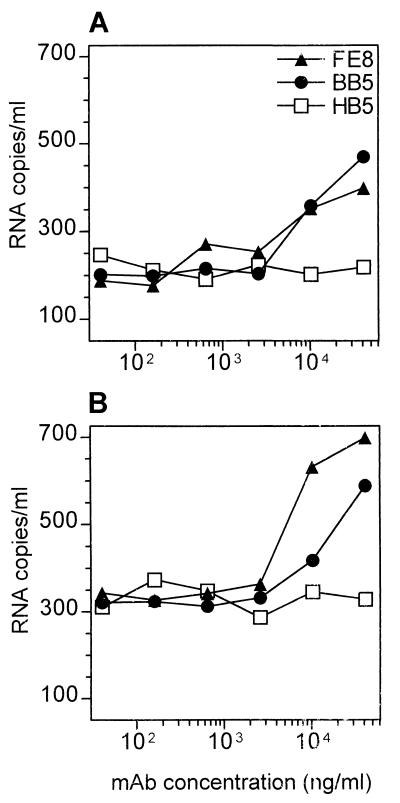
Further tonsil specimens were obtained from patients 2 and 3 undergoing HAART, whose plasma HIV-1 RNA levels had remained below the detection limit (i.e., <400 copies/ml) for more than 1 year. p24 levels in lysates of 107 TC from these two patients were 3.7 ± 0.3 and 5.6 ± 0.8 pg/ml, respectively, i.e., >1,000-fold less than those in the presymptomatic patient 1 (7,750 ± 250 pg/ml). Quantitative reverse transcription-PCR demonstrated that HIV-1 RNA was removed from TC by targeting CR2 or C3d (Fig. 5), although >10-fold-higher amounts of MAb were required than those for tissue from patient 1.
FIG. 5.
Stripping of HIV from TC of patient 2 (A) and patient 3 (B) undergoing long-term, effective HAART. Residual amounts of HIV-1 RNA released into the supernatants after stripping of 107 cells in 100 μl with different concentrations of nonblocking (open symbols) or blocking (closed symbols) MAb are given (see Fig. 3 legend for MAb specificities). Values represent means from two separate experiments.
DISCUSSION
HIV-1 trapped on the surface of FDC constitutes by far the largest viral reservoir in LT, once the infection has reached the chronic phase (9, 10, 19). Both HIV-specific antibody and complement were shown to be indispensable for virus entrapment on FDC in vitro (16), although recent reports suggest that HIV starts to accumulate in this pool already in the acute phase (22). Furthermore, virus trapped on FDC was shown to remain infectious for T cells migrating through GC even after being coated with neutralizing antibody (11, 14).
Concerning this interplay of HIV and GC, we addressed the following issues in this report. With all CRs being expressed in FDC, what are their relative contributions to HIV entrapment? Do virions change their anchoring sites in the course of infection or during HAART? If FDC are the major sanctuary for HIV, can targeting of CR reduce this viral pool?
Incubation of tonsillar tissue cryosections and of suspended TC with blocking MAb directed against CR2 or C3d detached HIV-1 p24 from GC, whereas anti-CR1 or anti-CR3 had no effect. From these results, we assume that the interaction of in vivo opsonized HIV within GC is predominantly mediated through binding of the final C3 breakdown product C3d to CR2. This is even more likely, because C3d rather than C3b or iC3b can be expected to be present on the virion after a period of several days (13).
In addition to FDC, activated B cells in GC express CR1 and CR2 and therefore can potentially bind opsonized HIV. However, immunohistochemical staining has shown that HIV preferentially associates with the FDC network (4, 9). Since FDC express more CR1 and CR2 than do B cells (21), the density and/or surface distribution of CR seems to be critical for trapping of HIV on FDC. On the other hand, B cells could be rather important for disseminating infectious virus throughout the body (15).
Treatment of TC with proteinase K did remove 15 to 20% more extracellular HIV-1 than did either FE8 or BB5. This implies either that not all virions attached to CR2 can be removed by blocking MAb or that other virus-trapping molecules do exist on FDC. In this regard, Fcγ receptors (21) or adhesion molecules like intercellular adhesion molecule 1 (ICAM-1) and LFA-1 (8) have been suggested to contribute to virus trapping, but their relative contributions have not been assessed. Further experiments are under way to clarify this issue.
To investigate whether the C3d-CR2 interaction remains the main trapping mechanism throughout advanced stages of HIV infection, we tested tonsillar tissue from two individuals under HAART. HIV-1 RNA could still be removed from TC by targeting C3d or CR2, although at much lower efficiency than that for TC of the presymptomatic individual. A recent mathematical analysis which assumes that virions are trapped in LT exclusively via C3d-CR2 binding points out that during HAART most virions will dissociate from FDC within days or weeks, whereas those coated abundantly with C3d may persist for years (13). Therefore, such extensively opsonized virions can be expected in a larger quantity in LT of patients under HAART. Indeed, our data imply that the effectiveness of HIV detachment through CR2 blockade will be much lower in LT of treated patients than in LT of presymptomatic individuals.
So far, the infectivity of viral particles released from FDC has not been investigated in detail. Our data show that naturally opsonized virions become available for experimentation. In preliminary experiments, virus capture assays (7) revealed that C3d and IgG are the most abundant proteins detected in the viral envelope of such virions, whereas gp120 and gp41 were hardly accessible for capture antibodies (data not shown). Further studies will have to prove if complement fragments on the surface of HIV acquired in vivo exert significant effects on viral infectivity and tropism.
The data presented here suggest that reduction of the viral reservoir in LT through a blockade of the CR2-C3d interaction is a possible therapeutic approach, albeit it has to take into account several caveats. First, other trapping mechanisms could eventually substitute for CR2. Second, it is unclear at present which time point in the course of HIV infection would be suitable for such an intervention. From the data presented here, release of extracellularly trapped HIV from LT would appear to be more effective in the presymptomatic stage. On the other hand, direct targeting of nonreplicating virus trapped on FDC in advanced stages of disease and during HAART would be highly desirable. However, in later stages more virions may be coated extensively with C3d and therefore may resist a CR blockade (13). Furthermore, not only HIV but all other immune complexes will be removed from or prohibited from binding to FDC. In this regard, in vivo experiments in mice have shown that blocking CR2 negatively affected, although it did not completely abrogate, primary and secondary humoral immune responses (6, 12). These unknowns have to be addressed by forthcoming experimentation.
ACKNOWLEDGMENTS
We thank Brigitte Müllauer and M. Wurm for excellent technical assistance; Heidrun Recheis for help with confocal laser microscopy; R. Zangerle and O. Moling for helpful discussions and support; Malgorzata Krych, R. Taylor, V. Koistinen, G. D. Ross, and I. Ando for providing MAb; and B. Sterlacci for reading of the manuscript.
This work was supported by the Austrian Science Fund (grant F202), the Ludwig Boltzmann Society, and the Austrian Ministry of Science (grant GZ 70.028/2-Pr/4/98).
REFERENCES
- 1.Armstrong J A, Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984;ii:370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- 2.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 3.Burton G F, Masuda A, Heath S L, Smith B A, Tew J G, Szakal A K. Follicular dendritic cells (FDC) in retroviral infection: host/pathogen perspectives. Immunol Rev. 1997;156:185–197. doi: 10.1111/j.1600-065x.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 4.Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z Q, Mills R, McDade H, Schuwirth C M, Goudsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 5.Diamond M S, Garcia-Aguilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang Y, Xu C, Fu Y X, Holers V M, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 7.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger H W, Dierich M P. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara M, Tsunoda R, Shigeta S, Yokota T, Baba M. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J Virol. 1999;73:3603–3607. doi: 10.1128/jvi.73.5.3603-3607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase A T. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 10.Haase A T, Henry K, Zupancic M, Sedgewick G, Faust R A, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang Z Q, Dailey P J, Balfour H H, Erice A, Perelson A S. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 11.Heath S L, Tew J G, Szakal A K, Burton G F. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 12.Heyman B, Wiersma E J, Kinoshita T. In vivo inhibition of the antibody response by a complement receptor-specific monoclonal antibody. J Exp Med. 1990;172:665–668. doi: 10.1084/jem.172.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hlavacek W S, Wofsy C, Perelson A S. Dissociation of HIV-1 from follicular dendritic cells during HAART: mathematical analysis. Proc Natl Acad Sci USA. 1999;96:14681–14686. doi: 10.1073/pnas.96.26.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hufert F T, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, Racz P, von Laer D. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997;11:849–857. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Jakubik J J, Saifuddin M, Takefman D M, Spear G T. B lymphocytes in lymph nodes and peripheral blood are important for binding immune containing HIV-1. Immunology. 1999;96:612–619. doi: 10.1046/j.1365-2567.1999.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joling P, Bakker L J, Van Strijp J A, Meerloo T, de Graaf L, Dekker M E, Goudsmit J, Verhoef J, Schuurman H J. Binding of human immunodeficiency virus type-1 to follicular dendritic cells in vitro is complement dependent. J Immunol. 1993;150:1065–1073. [PubMed] [Google Scholar]
- 17.Koistinen V, Wessberg S, Leikola J. Common binding region of complement factors B, H and CR1 on C3b revealed by monoclonal anti-C3d. Complement Inflamm. 1989;6:270–280. doi: 10.1159/000463102. [DOI] [PubMed] [Google Scholar]
- 18.Krych M, Hauhart R, Atkinson J P. Structure-function analysis of the active sites of complement receptor type 1. J Biol Chem. 1998;273:8623–8629. doi: 10.1074/jbc.273.15.8623. [DOI] [PubMed] [Google Scholar]
- 19.Pantaleo G, Cohen O J, Schacker T, Vaccarezza M, Graziosi C, Rizzardi G P, Kahn J, Fox C H, Schnittman S M, Schwartz D H, Corey L, Fauci A S. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nat Med. 1998;4:341–345. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]
- 20.Prodinger W M, Schwendinger M G, Schoch J, Köchle M, Larcher C, Dierich M P. Characterization of C3dg binding to a recess formed between short consensus repeats 1 and 2 of complement receptor type 2 (CR2; CD21) J Immunol. 1998;161:4604–4610. [PubMed] [Google Scholar]
- 21.Reynes M, Aubert J P, Cohen J H, Audouin J, Tricottet V, Diebold J, Kazatchkine M D. Human follicular dendritic cells express CR1, CR2, and CR3 complement receptor antigens. J Immunol. 1985;135:2687–2694. [PubMed] [Google Scholar]
- 22.Schacker T, Little S, Connick E, Gebhard-Mitchell K, Zhang Z-Q, Krieger J, Pryor J, Havlir D, Wong J K, Richman D, Corey L, Haase A T. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181:354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 23.Stoiber H, Clivio A, Dierich M P. Role of complement in HIV infection. Annu Rev Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- 24.Szakal A K, Kosco M H, Tew J G. Microanatomy of lymphoid tissue during the induction and maintenance of humoral immune responses: structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- 25.Tenner Racz K, Racz P, Bofill M, Schulz Meyer A, Dietrich M, Kern P, Weber J, Pinching A J, Veronese Dimarzo F, Popovic M, et al. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986;123:9–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Uciechowski P, Schmidt R E. Cluster report: CD11. In: Knapp W, Dörken B, Gilks W R, Rieber E P, Schmidt R E, Stein H, von dem Borne A E G, editors. Leukocyte typing IV. White cell differentiation antigens. Oxford, United Kingdom: Oxford University Press; 1989. pp. 543–551. [Google Scholar]
- 27.Zhang L, Ramratnam B, Tenner Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]