Abstract
Xenopus have been powerful model organisms for understanding vertebrate development and disease for over 100 years. Here, a rapid blood perfusion protocol in Xenopus, aimed at a consistent and drastic reduction of blood within all tissues, is defined. Perfusion is carried out by inserting a needle directly into the ventricle of the heart and pumping heparinized phosphate-buffered saline (PBS) through the vascular system. The procedure can be completed in approximately 10 min per animal. The blood is dominated by a few highly abundant proteins and cell types, creating numerous issues as these proteins mask most other molecules and cell types of interest. The reproducible characterization of adult Xenopus tissues with quantitative proteomics and single-cell transcriptomics will benefit from applying this protocol prior to organ sampling. The protocols for tissue sampling are defined in companion papers. These procedures are aimed at the standardization of practices across Xenopus of different sex, age, and health status, specifically X. laevis and X. tropicalis.
Introduction
The whole body perfusion of amphibians is routinely completed for the purposes of preservation and fixation1 , 2 , 3 , 4 , 5 , 6 . However, these procedures occur at a rate that limits the number of fresh samples that can be taken per animal. The goal of this work is to develop an effective blood perfusion protocol in Xenopus, prioritizing the speed of the technique. The protocol takes less than 10 min per animal for X. tropicalis and less than 15 min per X. laevis animal. The secondary priorities are the ease of replication and the use of easily acquired equipment so that high-quality samples can be shared widely between Xenopus labs.
Xenopus frogs are used widely in biomedical research to study fundamental biological and pathological processes conserved across species. This tetrapod has a closer evolutionary relationship with mammals than other aquatic models, having lungs, a three-chambered heart, and limbs with digits. The international community effectively uses Xenopus to gain a deeper understanding of human disease through in-depth disease modeling and molecular analysis of disease-related gene function. The numerous advantages of Xenopus as an animal model make them invaluable tools to study the molecular basis of human development and disease; these advantages include: large oocyte and embryo size, high fecundity, ease of housing, rapid external development, and ease of genomic manipulation. Xenopus have been estimated to share ~80% of the identified human disease genes7.
Compared to popular mammalian models, Xenopus is a rapid, cost-effective model, with the ease of morpholino knock-down and availability of efficient transgenics and targeted gene mutations using CRISPR8. Quantitative mass spectrometry and single-cell transcriptomics have been successfully applied to Xenopus embryos9 , 10 , but a recent cell atlas from Xenopus laevis shows that the composition of most tissues is dominated by blood cell types11. By developing a technique that exsanguinates tissue at a rapid rate and using chilled media, the sample freshness is minimally affected by perfusion. This is particularly important for applications where the goal is to profile physiologically unperturbed mRNA or protein expression.
Protocol
All experiments were performed in accordance with the rules and regulations of the Harvard Medical School IACUC (Institutional Animal Care and Use Committee) (IS 00001365_3).
NOTE: Though the primary method of euthanasia described is deemed an acceptable technique for euthanasia by the American Veterinary Medical Association12, it has not been found to lead to the cessation of a heartbeat13. Even the frequently used secondary method of double pithing does not prevent this, nor does removing the heart from the animal. Exsanguination of anesthetized animals is considered a humane and effective method for successful euthanasia12. As maintaining fresh tissues through euthanasia is the goal of this protocol, it is beneficial that the heart continues to beat through primary euthanasia with MS-222, and that perfusion is itself a secondary euthanasia method through exsanguination.
1. Preparation
Ensure that the research institution has approved the euthanasia and perfusion technique described in this protocol.
Prepare a solution of 5 g/L MS-222 (tricaine methanesulfonate) and 5 g/L sodium bicarbonate. The volume should be greater than the volume required to completely cover the animals being euthanized. Check the pH to ensure that it is ≥7.
Prepare 500 μL of 180 U/mL heparin in phosphate-buffered saline (PBS) per X. laevis, or 200 μL per X. tropicalis.
Perform primary euthanasia by placing the Xenopus in this solution (from step 1.2); the animal will remain submerged for a total of 1 h.
Confirm that the Xenopus has lost its pain response by pinching the foot 15 min into euthanasia. If the animal is reactive, return it to the euthanasia solution until this response is lost.
Weigh the Xenopus and take any additional measurements required before sampling.
Using a 31 G needle, inject X. laevis with 250 μL and X. tropicalis with 100 μL of 180 U/mL heparin in PBS (from step 1.3) into the musculature of each forelimb.
Prepare a solution of 1 mL/g of the animal weight of 54 U/mL heparin in PBS perfusate. Individuals more experienced with this protocol may find that less media is required to complete perfusion.
-
Use a 22 G hypodermic needle to perfuse X. laevis, and a 25 G hypodermic needle for X. tropicalis. Blunt the perfusion needle by trimming off the tip with wire cutters (Figure 1)14.
NOTE: This reduces the likelihood of the needle perforating through the ventricle if shifted. In addition to blunting, the needle may be ground down slightly with a sharpening stone or file, but still remaining sharp enough to pierce the ventricle.
Prepare the pump by attaching the trimmed needle and circulating 54 U/mL heparinized PBS perfusate (from step 1.8). Ensure to purge all air bubbles from the tubing to eliminate the possibility of air embolism, which leads to decreased perfusion efficiency or failure (see Table 1). Keep the perfusion media on ice for the duration of the procedure.
If the pump is not programmable, with the needle in place, measure the pumped volume of media under the different settings to determine which settings are closest to 5 mL/min and 10 mL/min. These flow rates will be used regardless of the species. If the perfusion pump is programmable, calibrate it with the needle in place, following the manufacturer’s instructions.
Place the dissection surface (tray or foam sheet) at an incline within a secondary container, or arrange it to facilitate blood drainage.
Once the frog has been in the solution for 1 h, primary euthanasia has been completed. Remove the frog and recheck the loss of pain response by performing a foot pinch.
Place the frog on its back and pin down each limb (Figure 2). If preserving the tissue of the limbs is required, thin pins may be placed through the digits or U-shaped staples around the limbs.
Using dissection scissors, cut through the skin, up the midline, and then laterally, making two flaps. (Figure 2)
Use forceps to grasp the linea alba and pull it away from the coelomic cavity (Figure 3). Carefully use scissors to cut up through the musculature. Make two flaps out of the cavity wall and cut or pin all the flaps out of the way.
Use dissection scissors to cut up through the coracoid bones and cut away excess tissue to gain better access to the heart (Figure 3).
The heart should still be beating. If the heart has stopped beating prior to perfusion, note that the sample freshness has been compromised.
Figure 1: Untrimmed and trimmed needles14.
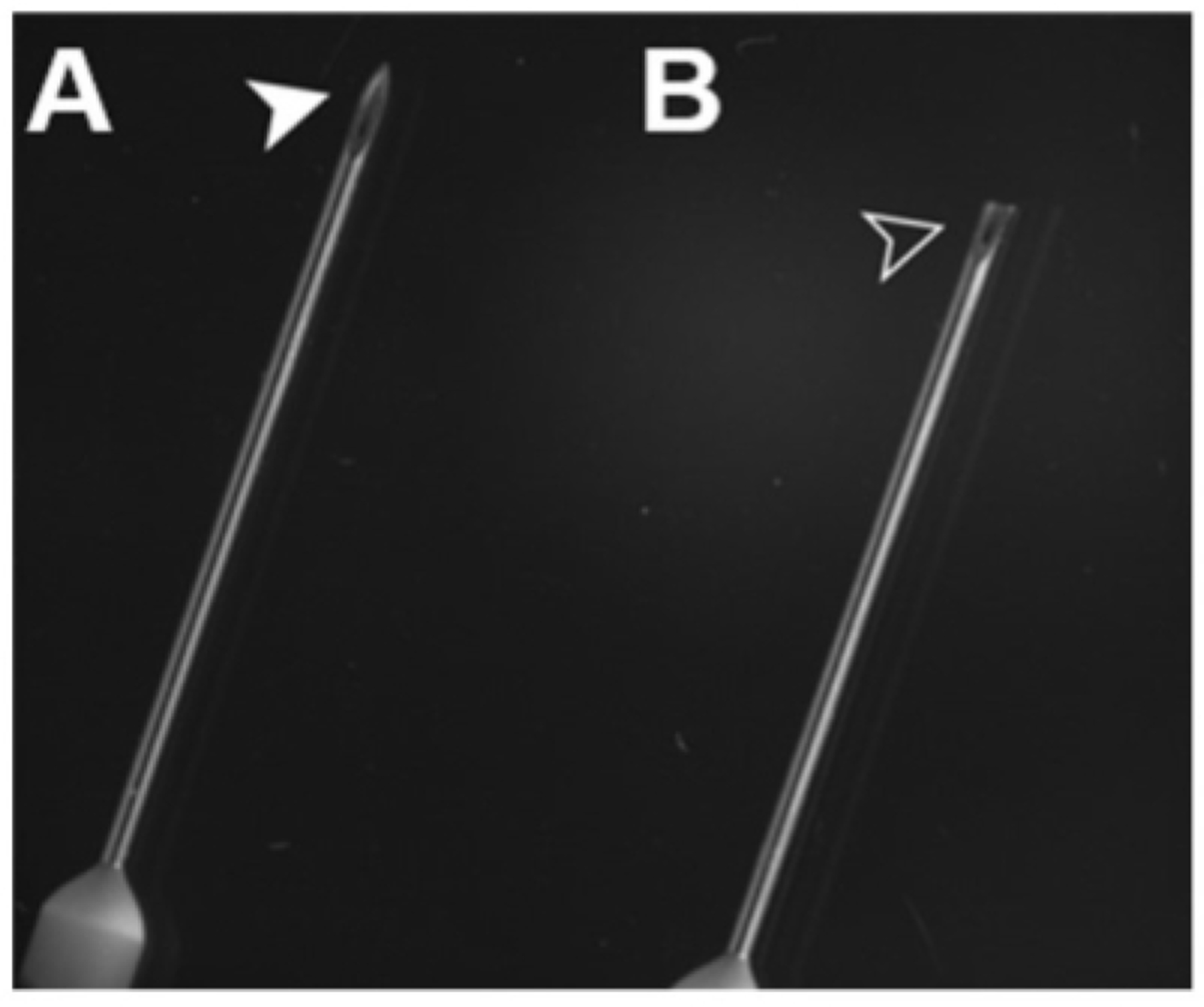
Using wire clippers, blunt the needle by cutting off its tip. It will be sharp enough to pierce the heart, yet perforating the ventricle will be less likely in the event of human error.
Table 1: Troubleshooting table.
Several typical problems, their possible causes, and suggested remedial actions are provided.
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| 5X Magnifying Glass with LED Light and Stand | amazon.com | B08QJ6J8P1 | light must not produce heat |
| Disposable Transfer Pipets | VWR | 414004-036 | |
| Dissecting Fine-Pointed Forceps | Fisher Scinetific | 08-875 | |
| Dissecting scissors sharp piont, straight 6.5” | VWR | 76457-374 | |
| Dissection Tray | Fisher Scinetific | 14-370-284 | styrofoam sheets are an acceptable alternative |
| Fine Dissection Pins | Living Systems Instrumentation | PIN-#3 | |
| General Use Hypodermic Needles, 22 gauge | Fisher Scientific | 14-826-5A | |
| General Use Hypodermic Needles, 25 gauge | Fisher Scientific | 14-826AA | |
| Heparin, Porcine Intestinal Mucosa | MilliporeSigma | 37-505-410MG | |
| Iridectomy Scissors 6” | vwr | 470018-938 | iris scissors are an acceptable alternative |
| Luer-to-Barb Adapter Male Luer with Lock Ring | amazon.com | B09PTX6M2Z | size will be dependant on the hosing of the pump used |
| Mayo-Hegar Needle Holder | Fisher Scinetific | 08-966 | mosquito forceps are an acceptable alternative |
| MS-222: Syncaine (formerly tricaine) | Pentair AES | TRS1 | |
| PBS x 1 | Corning | 21-040-CV | |
| Peristaltic Liquid Pump Dosing Pump 5-100mL/min | amazon.com | B07PWY4SM6 | any peristaltic pump capable of pumping 5-10mL/min is acceptable |
| Sodium Bicarbonate, Powder, USP | Fisher Scientific | 18-606-333 | |
| Specimen Forceps, Serrated | VWR | 82027-442 | |
| T-Pins for Dissecting | Fisher Scinetific | S99385 | |
| Ultra-Fine Short Insulin Syringes, 31 gauge | VWR | BD328438 | |
| Wire Flush Cutters, 6-inch Ultra Sharp & Powerful Side Cutter Clippers | amazon.com | B087P191LP |
Figure 2: Mature female X. tropicalis pinned through each limb.
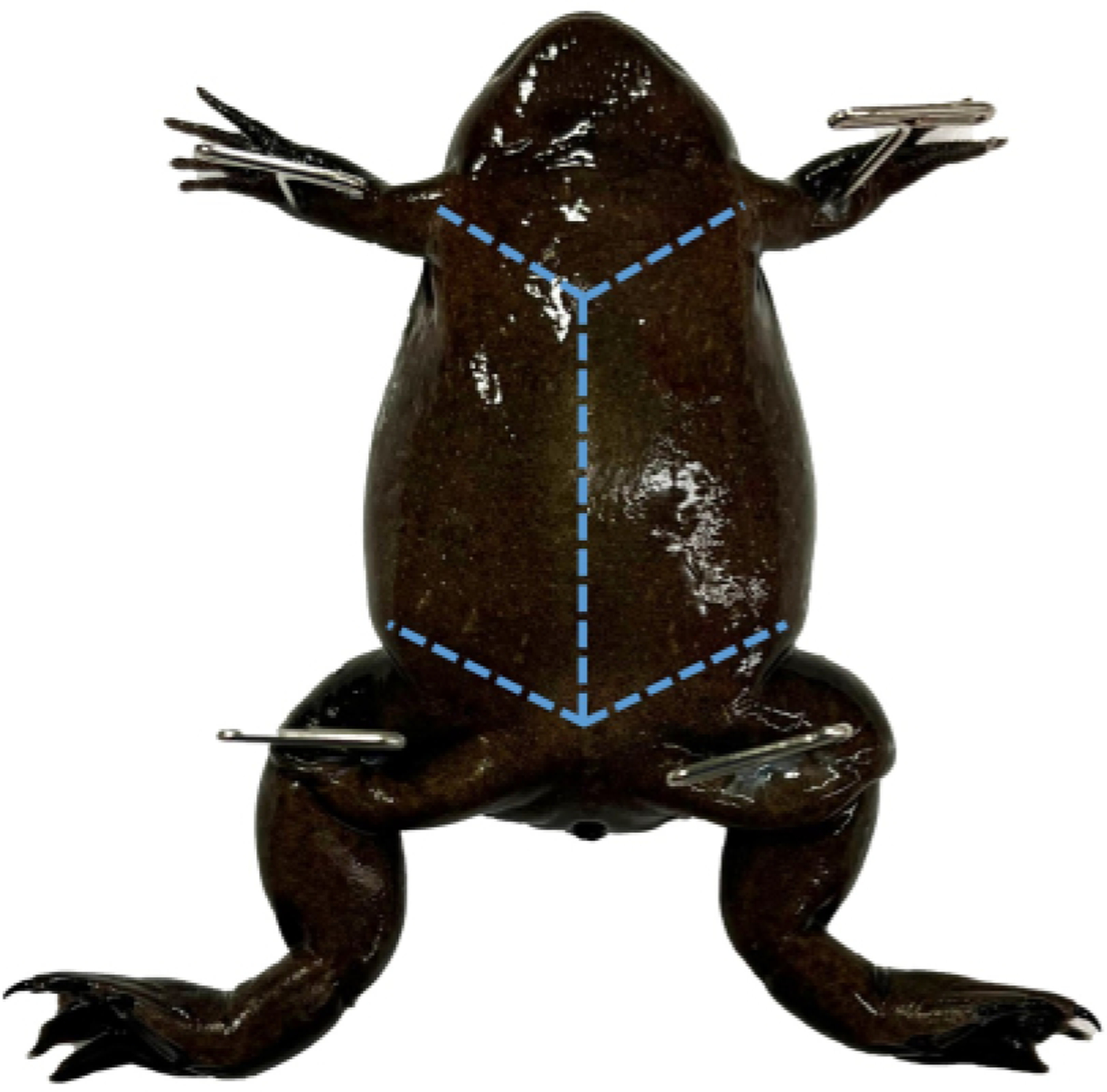
Use toothed dissecting forceps to pull the skin near the cloaca taught to perforate it with dissection scissors and create two flaps.
Figure 3: Muscular wall.
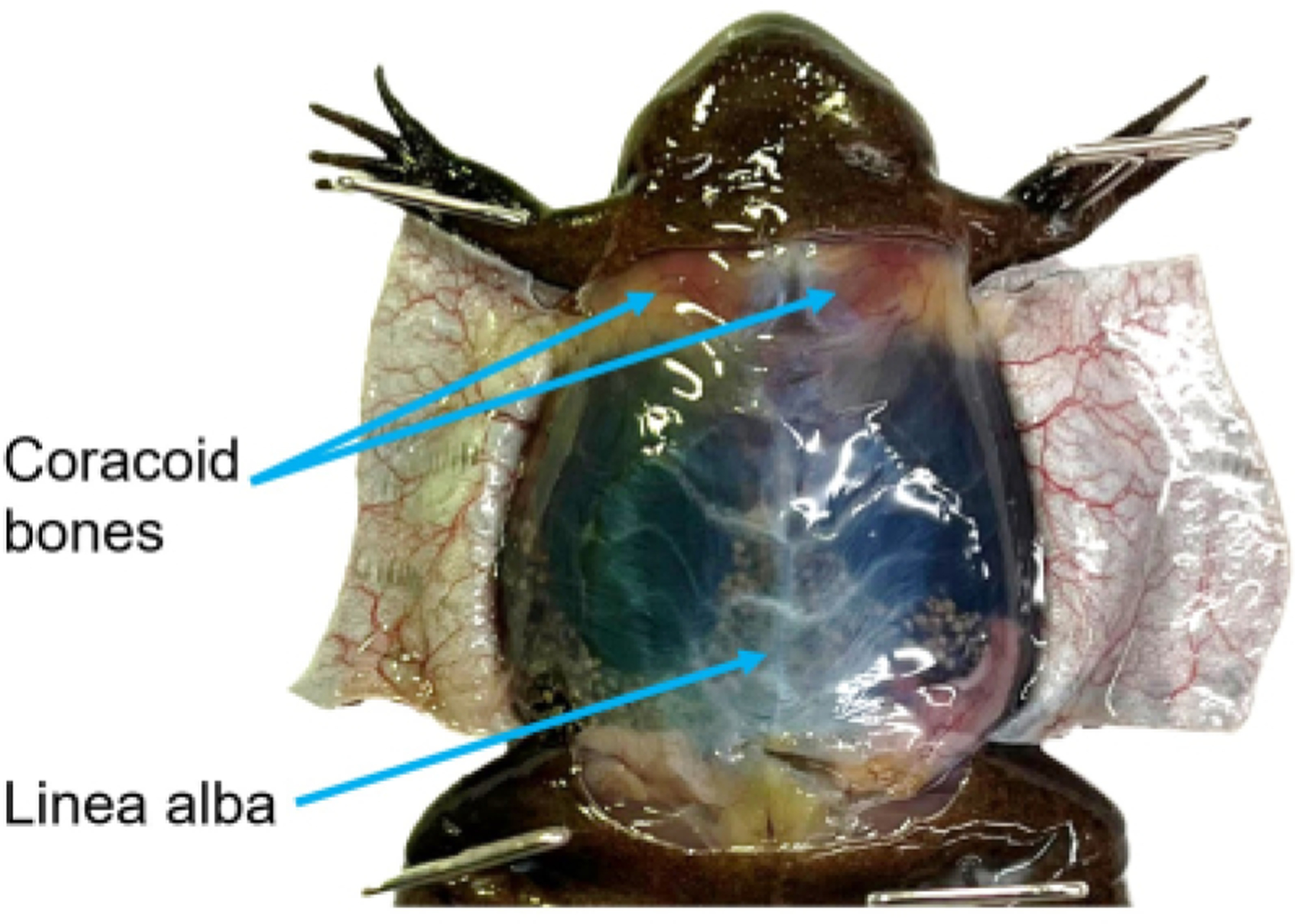
With the ventral skin open but the muscular wall intact, the linea alba is visible. To reduce the likelihood of damaging the underlying tissues, grasp the linea alba and pull it taught prior to cutting. The coracoid bones are visible through the peritoneum. Once the coelomic cavity has been opened, these bones should be reduced to give better access to the heart.
2. Perfusion
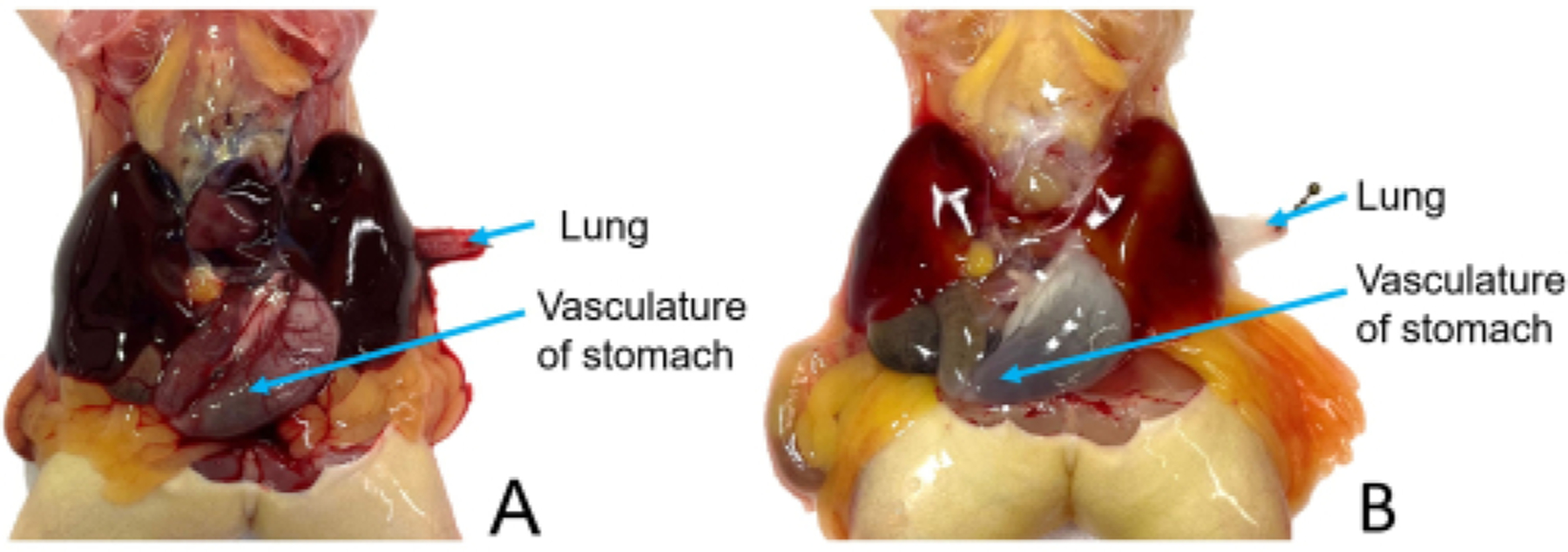
Identify the stomach and gently shift it so that it is on top of the left lobe of the liver (on the viewer’s right), with its vasculature visible for the duration of the procedure. Identify a lung and grasp it by its tip using tissue forceps. Pull the lung outside of the coelomic cavity and pin it through the tip (Figure 4). Do this gently, as broken blood vessels do not perfuse well. Note if blood is visible within the lobe, as this will affect the ability to determine the completion of the procedure.
Take an image of the coelomic cavity to better assess perfusion efficiency and potentially identify abnormal tissues at a later date.
Identify the thin pericardium and pull it taught with tissue forceps (Figure 5). Gently perforate the pericardium using the tip of the iridectomy scissors, being careful not to cut the underlying tissues. Peel the pericardium up away from the three chambers of the heart.
Use forceps to gently grasp the ventricle by its apex. Apply limited pressure so that there is enough room between the traction surfaces of the forceps for the perfusion needle to pass (Figure 6).
-
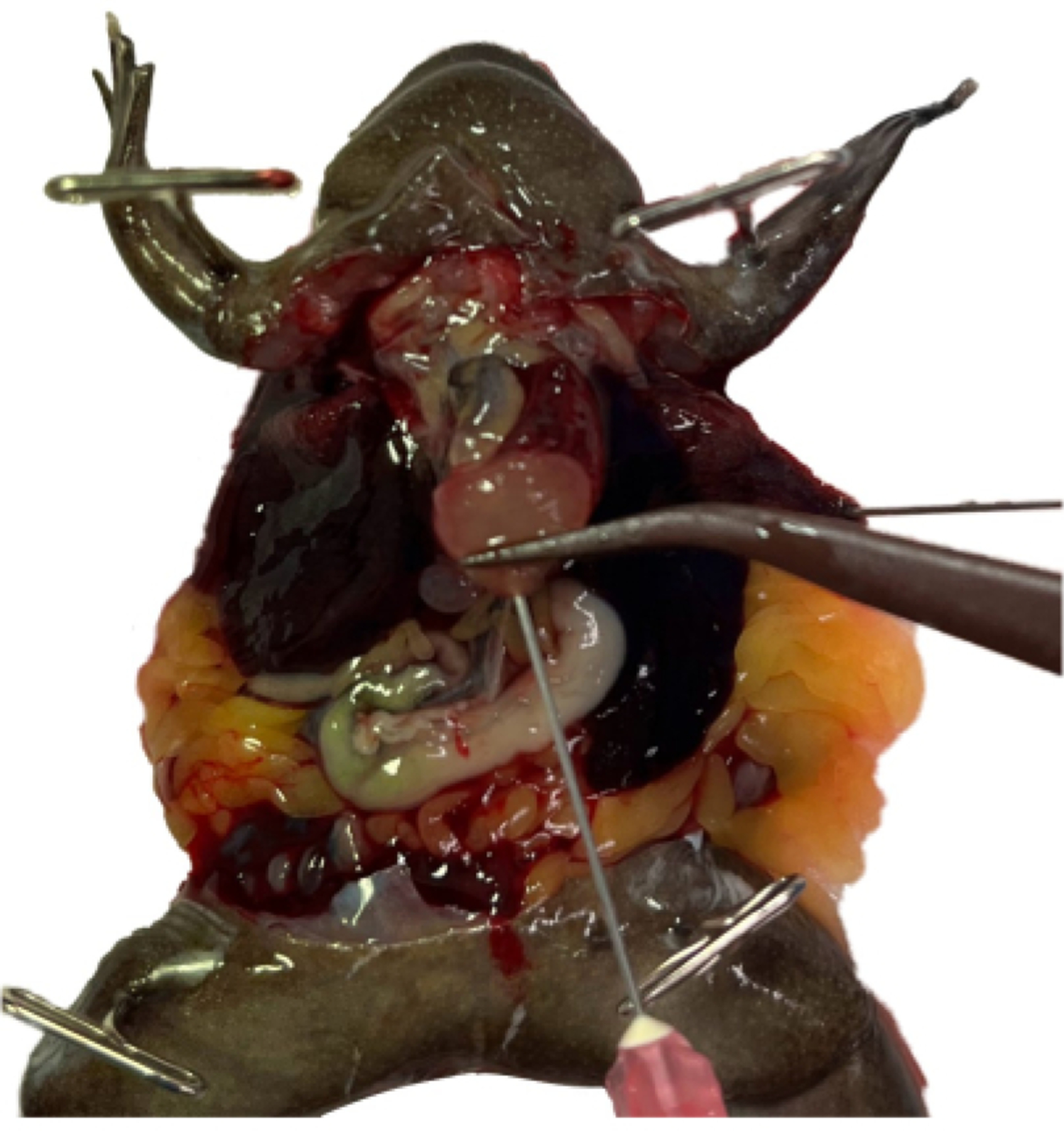
Insert the needle through the closure of the forceps into the chamber of the ventricle, being careful not to perforate through the ventricle (Figure 7). Clamp the tissue forceps in place using a needle holder by using a hemostat.
NOTE: This technique stabilizes the position of the needle, which is still sharp. Clamping the needle directly to the ventricle will also cause unnecessary damage, making reclamping more difficult should it be required (see Table 1).
Start the flow of the pump at approximately 5 mL/min. The three chambers of the heart and arterial trunk will engorge (Figure 8; see Table 1).
With scissors, carefully lance the right auricle (on the viewer’s left); blood will pour out. Set the flow rate at 5 mL/min or increase it to 10 mL/min.
Continue until the vasculature of the stomach blanches (see Table 1), then lance the left auricle of the heart (on the viewer’s right). If the flow rate is still 5 mL/min, increase it to approximately 10 mL/min.
Use a transfer pipette to rinse the coelomic cavity in perfusion media, to help maintain visibility and to better assess the color of the perfusate flowing from the auricles.
Keep the needle in place until the perfusate flowing from the auricles is clear (see Table 1) and the lung has lost its red hue (see Table 1; Figure 9).
Figure 4: The coelomic cavity of a mature X. tropicalis male.
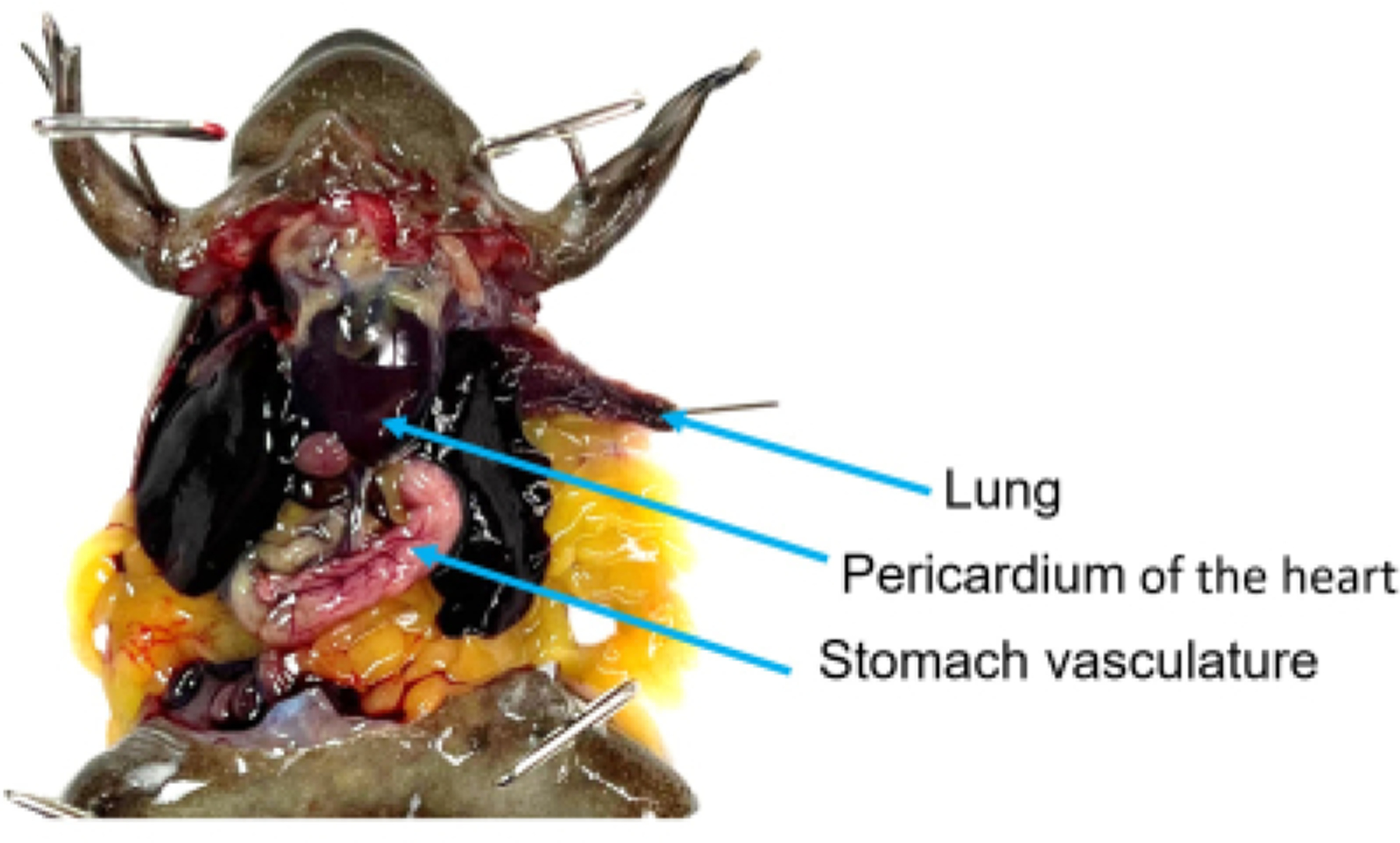
The coracoid bones have been reduced, providing access to the pericardium-enclosed heart. The stomach has been shifted in front of the left lobe of the liver, and its vasculature is clearly visible. The left lung has been pulled out of the coelomic cavity by its tip and pinned to ensure that it does not retract during the rinsing process.
Figure 5: Pericardium.
The pericardium is a thin, tough membrane enclosing the heart. Using tissue forceps, gently grasp the pericardium and then use the tip of iridectomy scissors to perforate it. Once perforated, peel it up, away from the heart.
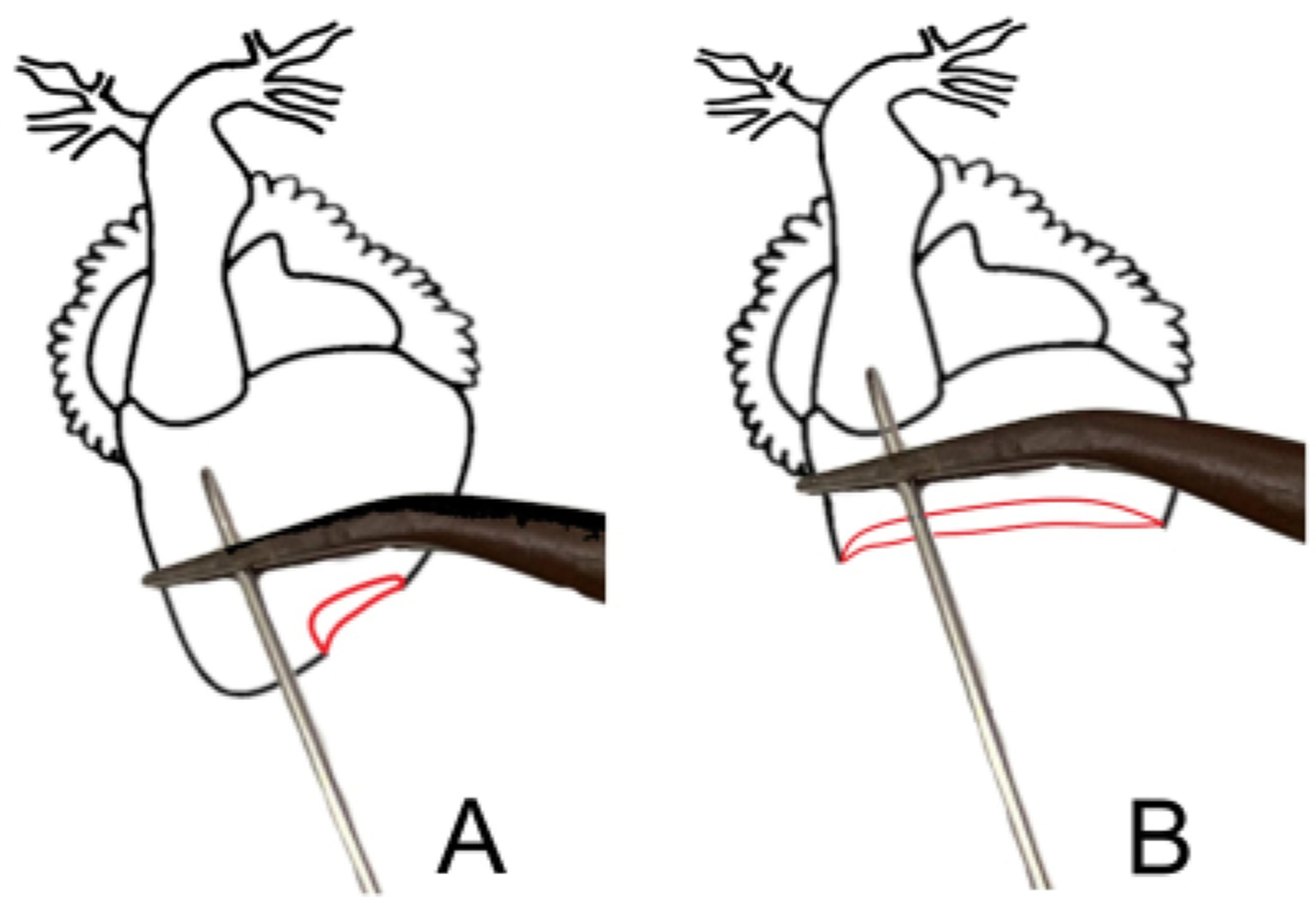
Figure 6: Heart anatomy needle placement diagrams.
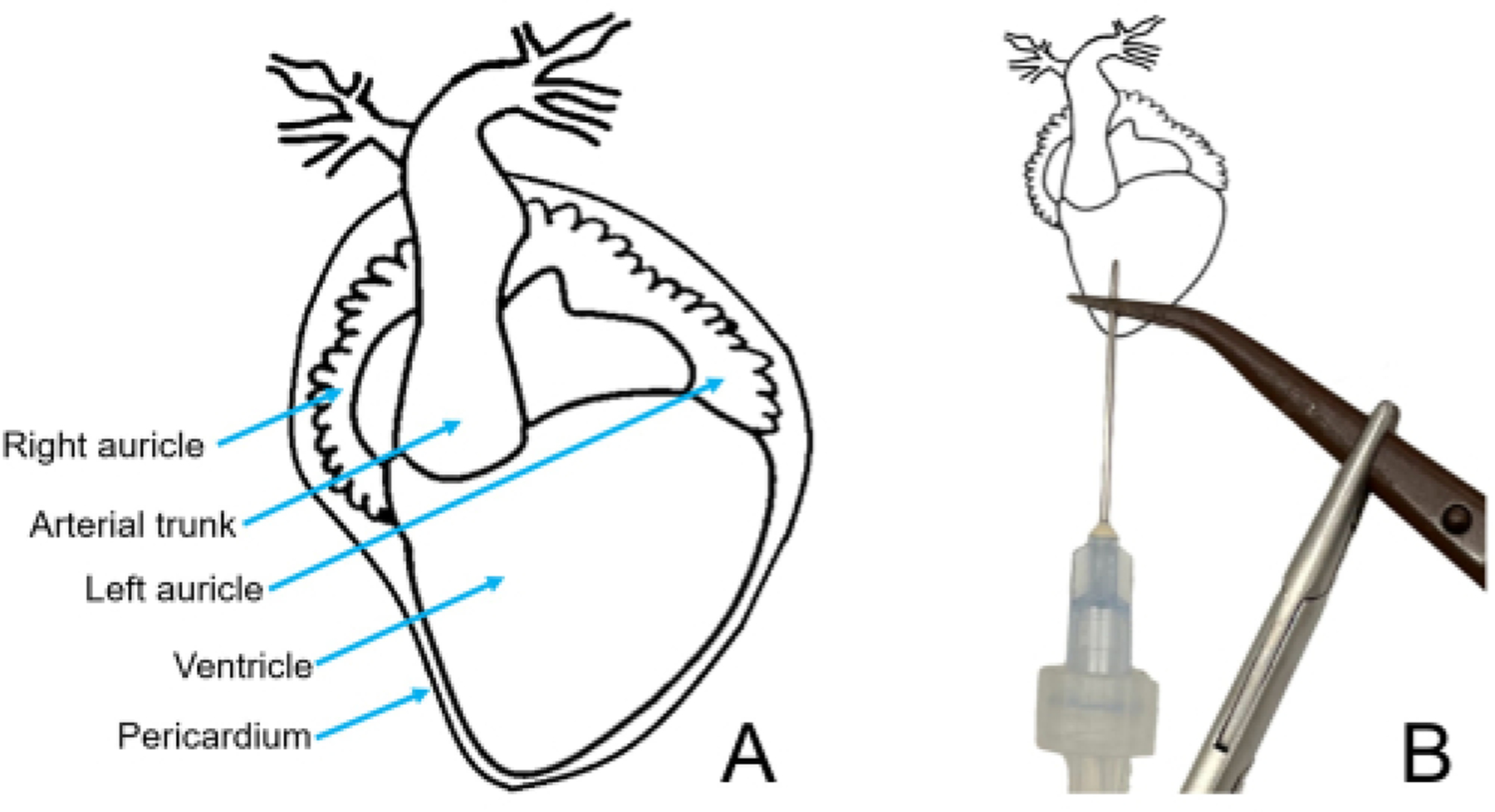
(A) Ventral diagram of an X. laevis heart. (B) Heart diagram, with the pericardium removed, showing the correct needle and clamp placement.
Figure 7: Heart anatomy and needle placement photograph.
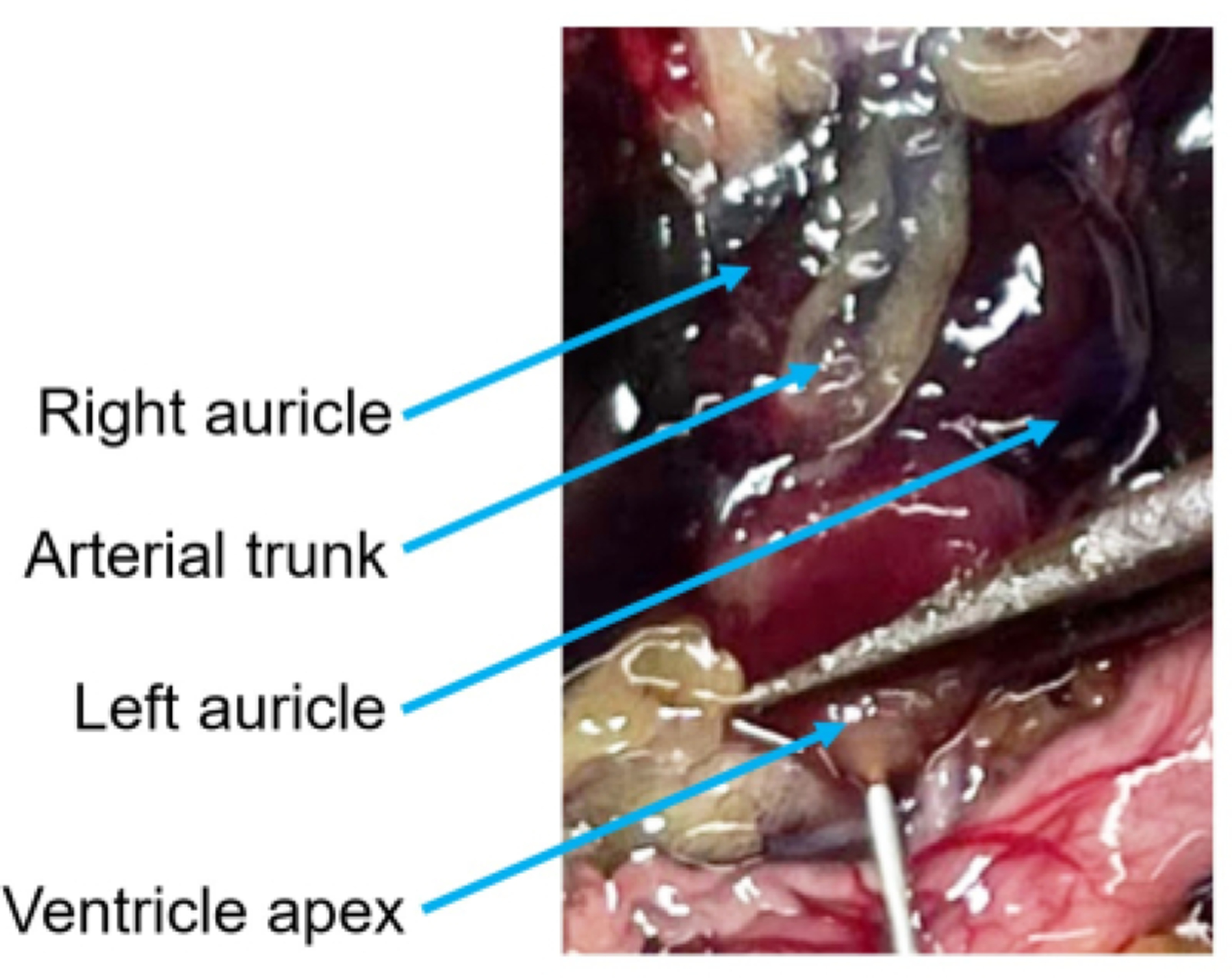
With the pericardium removed, the three chambers of the heart and arterial trunk are easily visible. Use forceps to gently grasp the ventricle by its apex and then insert the needle through the forceps. Be careful not to cause unnecessary damage to the ventricle or other chambers as this will compromise the perfusion efficiency.
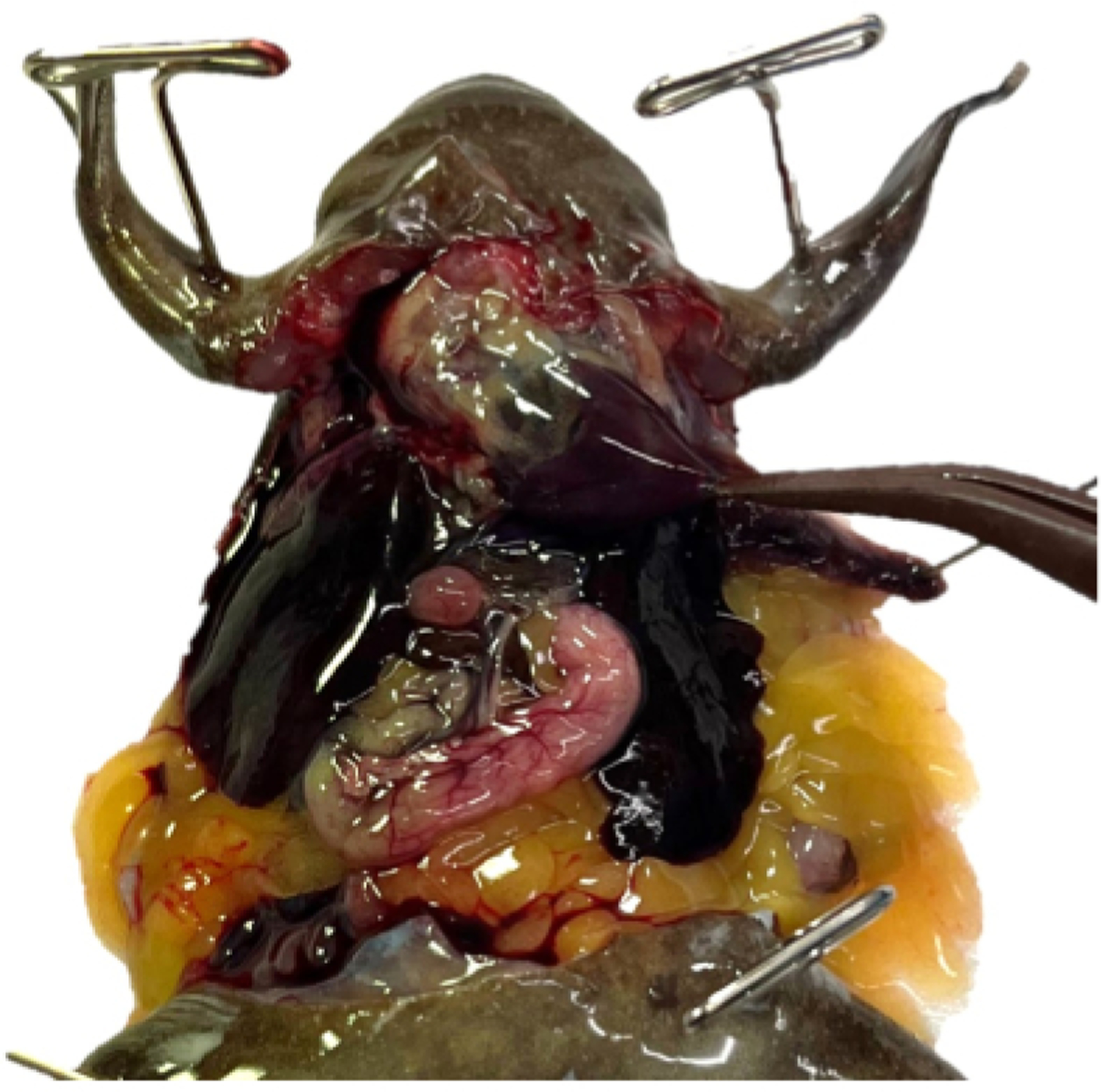
Figure 8: Perfusion is underway.
The right auricle has been lanced, and the ventricle, arterial trunk, and left auricle are visibly engorged. The stomach is blanching and both the media running from the animal and the lung tissue are heavily saturated with blood.
Figure 9: The coelomic cavity following successful rapid perfusion and rinsing.
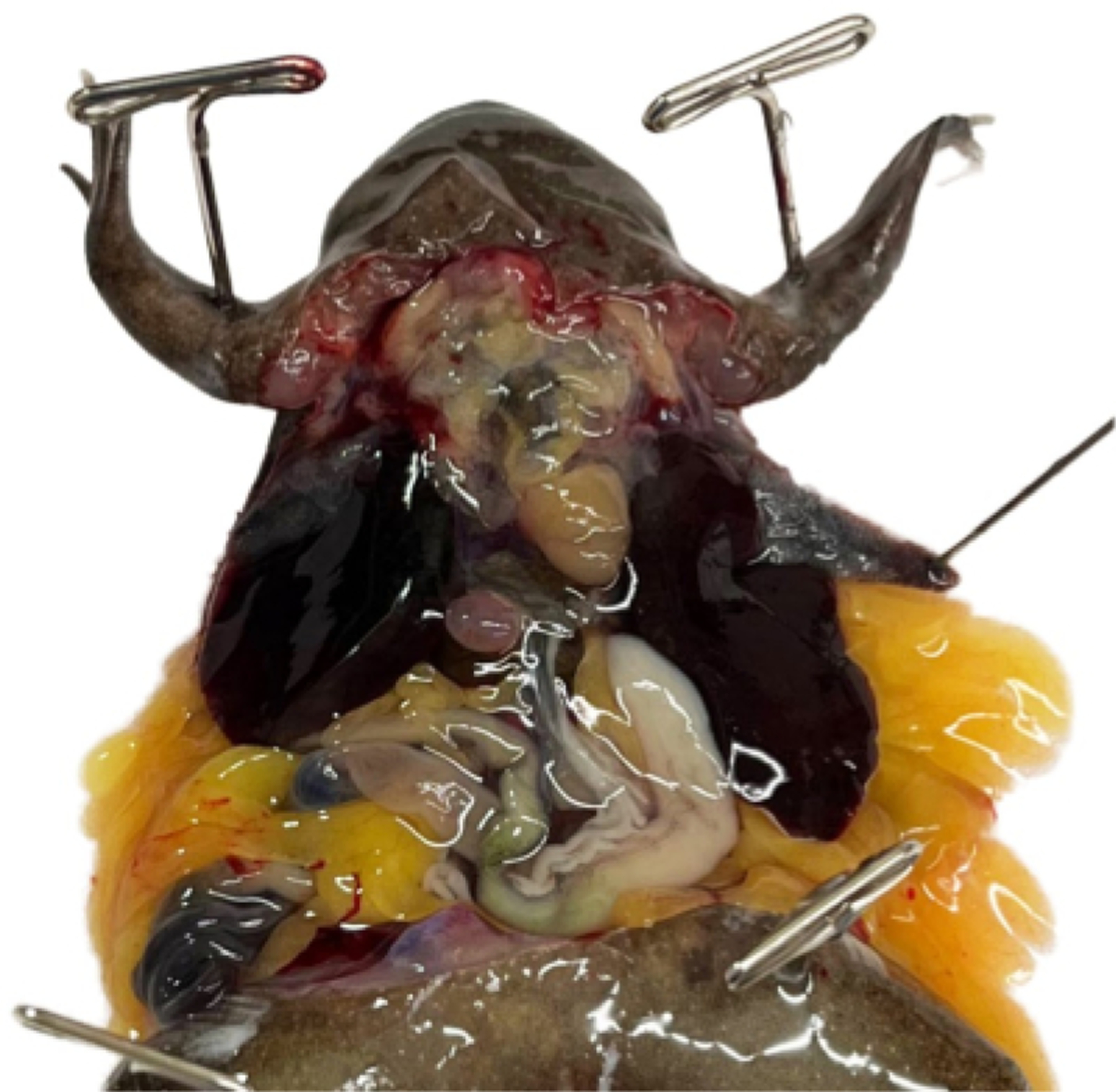
The vasculature of the stomach and other organs is no longer easily visible. Unless the Xenopus is albino, the liver will remain heavily pigmented.
Representative Results
Following successful perfusion, all tissues (excluding the liver in pigmented Xenopus) will be distinctly lighter and less saturated with blood. Major blood vessels will become less noticeable (Figure 10), and tissues (excluding the liver) will rinse cleanly in the buffer after being sampled. While the successful execution of the protocol can ultimately only be confirmed by the quality of the data from exsanguinated tissue samples, several typical problems, their possible causes, and suggested remedial actions are provided in the troubleshooting table (Table 1 and Figure 11).
Figure 10: Tissues samples from an unperfused and perfused albino male X. laevis.
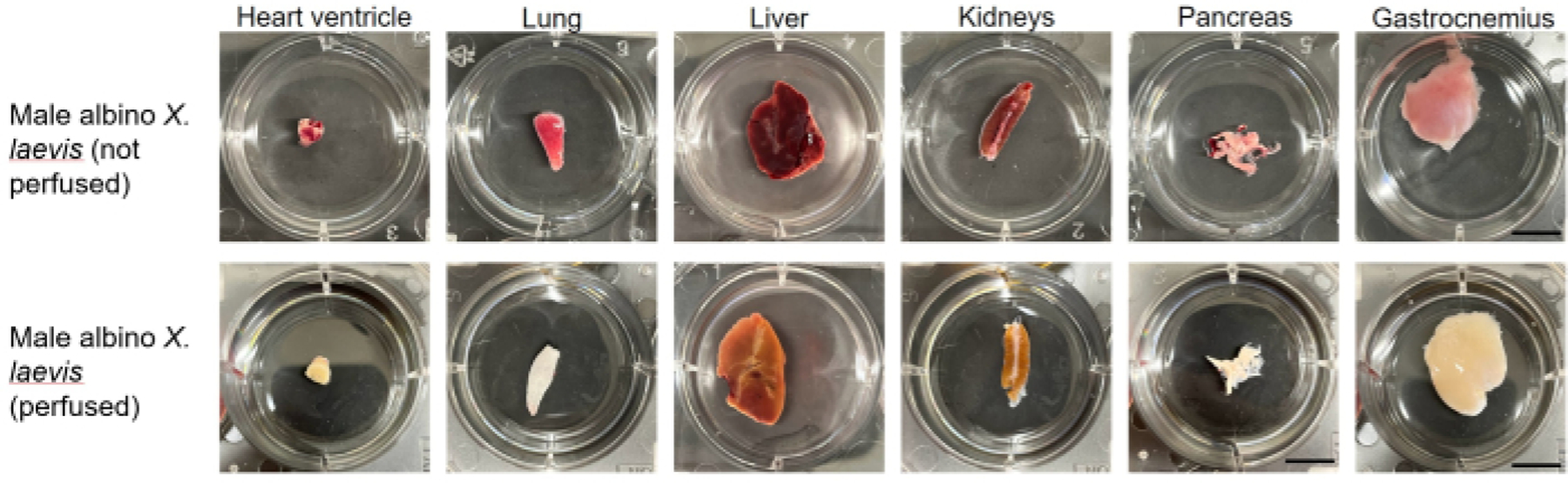
The differences in pigmentation and visibility of the vasculature are pronounced. All samples are within 3.5 cm diameter wells.
Figure 11: Troubleshooting diagrams of a Xenopus heart.
(A) The ventricle has perforation (in red); this perforation is isolated by the forceps and will not affect the perfusion efficiency. (B) A heart with a severely damaged ventricle. The needle can be guided into the arterial trunk and clamped in place. It is especially important to ensure the needle is well-blunted when using this technique14.
Discussion
This protocol describes traditional dissection techniques for accessing the coelomic cavity. Other techniques are also acceptable, provided they cause minimal damage to the tissues, the heart is accessible, and the lung and stomach are visible. Similarly, most dissection tools listed can be easily substituted with comparable items.
While attempts have been made to optimize the efficacy of this procedure, results may vary depending on one’s experience and variability between individual frogs. One interesting aspect of blood perfusion that remained outside of the scope of this paper is how this procedure compares to alternative ways of perfusion for animals that undergo surgery. Another unexplored variable is how blood perfusion would work in very young animals or animals of advanced age where vasculature might be excessively fragile. Additional remarks are provided to facilitate the application of this protocol. Several typical problems, their possible causes, and suggested remedial actions are provided in Table 1.
A limitation of this procedure is that the perfusion efficiency can be negatively impacted by its speed. If perfusion efficiency takes precedence over rapid perfusion, adapting an axolotl technique is recommended1 (Saltman et al. use the term aorta to refer to the arterial trunk).
The duration of the procedure and volume of media used are dependent on a number of variables. Generally, X. tropicalis males take between 2–3 min to successfully perfuse with 15–25 mL of media, while X. tropicalis females take between 3–4 min with 25–40 mL of media. Significantly more animal-to-animal variation was found when perfusing X. laevis. Though a higher flow rate would lessen the length of time required to perfuse larger animals, the increased line pressure can easily lead to the tube fittings becoming dislodged and pump failure.
Naturally, it is much easier to assess perfusion efficiency in albino animals. The difference is especially apparent in the lung and liver tissues (Figure 12). Thus, the use of albinos is recommended, especially when first attempting perfusion or undergoing training.
Figure 12: Assessing perfusion efficiency in albinos.
An albino X. laevis both before (A) and after (B) rapid perfusion. The albinism makes it easier to determine the proficiency of the perfusion than it would be in a pigmented animal. This is especially apparent in the lung and liver tissues.
By adjusting the flow rate and needle size, the protocol is adaptable for all species of Xenopus. Owing to the homology in heart anatomy and blood circulation between Xenopus and most other amphibians15, as well as non-crocodilian reptiles, this technique may be modified for full-body rapid perfusion of other models with three-chambered hearts16. If a non-crocodilian reptile model is used that exclusively requires the perfusion of one of the aortic arches, other protocols are recommended17.
Acknowledgments
This work was supported by NIH’s OD R24 grant OD031956 and NICHD R01 grant HD073104. We thank Darcy Kelly for helpful discussions and initial input on this protocol. We would also like to thank Samantha Jalbert, Jill Ralston, and Wil Ratzan for their assistance and support as well as our three anonymous peer reviewers for their feedback.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/65287.
Citation
Jonas-Closs, R.A., Peshkin, L. Effective Rapid Blood Perfusion in Xenopus. J. Vis. Exp. (), e65287, doi:10.3791/65287 (2023).
Disclosures
The authors declare no competing interests.
References
- 1.Saltman AJ, Barakat M, Bryant DM, Brodovskaya A, Whited JL DiI perfusion as a method for vascular visualization in Ambystoma mexicanum. Journal of Visualized Experiments. (124), 55740 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lametschwandtner A, Minnich B Microvascular anatomy of the brain of the adult pipid frog, Xenopus laevis (Daudin): A scanning electron microscopic study of vascular corrosion casts. Journal of Morphology. 279 (7), 950–969 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lametschwandtner A, Minnich B Microvascular anatomy of the urinary bladder in the adult African clawed toad, Xenopus laevis: A scanning electron microscope study of vascular casts. Journal of Morphology. 282 (3), 368–377 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lametschwandtner A et al. Microvascular anatomy of the gallbladder of the adult South African clawed toad, Xenopus laevis Daudin: A scanning electron microscope study of vascular corrosion casts. Microscopy and Microanalysis. 13 (S02), 492–493 (2007). [Google Scholar]
- 5.Lametschwandtner A, Spornitz U, Minnich B Microvascular anatomy of the non-lobulated liver of adult Xenopus laevis: A scanning electron microscopic study of vascular casts. Anatomical Record. 305 (2), 243–253 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miodoński AJ, Bär T Arterial supply of the choriocapillaris of anuran amphibians (Rana temporaria, Rana esculenta). Scanning electron-microscopic (SEM) study of microcorrosion casts. Cell and Tissue Research. 249 (1), 101–109 (1987). [DOI] [PubMed] [Google Scholar]
- 7.Nenni MJ et al. Xenbase: Facilitating the use of Xenopus to model human disease. Frontiers in Physiology. 10, 154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandon P, Conlon F, Furlow JD, Horb ME Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Developmental Biology. 426 (2), 325–335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peshkin L et al. The protein repertoire in early vertebrate embryogenesis. bioRxiv. 10.1101/571174 (2019). [DOI] [Google Scholar]
- 10.Briggs JA et al. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science. 360 (6392), eaar5780 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao Y et al. Cell landscape of larval and adult Xenopus laevis at single-cell resolution. Nature Communications. 13 (1), 4306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.AVMA (American Veterinary Medical Association). AVMA guidelines for the euthanasia of animals, 2020 edition. AMVA, Schaumburg, Illinois. 37 (2020). [Google Scholar]
- 13.Navarro K, Jampachaisri K, Chu D, Pacharinsak C Bupivacaine as a euthanasia agent for African Clawed Frogs (Xenopus laevis). PLoS One. 17 (12), e0279331 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J et al. Transcardiac perfusion of the mouse for brain tissue dissection and fixation. Bio-Protocol. 11 (5), e3988 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinz-Taheny KM Cardiovascular physiology and diseases of amphibians. Veterinary clinics of North America. The Veterinary Clinics of North America. Exotic Animal Practice. 12 (1), 39–50 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Stephenson A, Adams JW, Vaccarezza M The vertebrate heart: an evolutionary perspective. Journal of Anatomy. 231 (6), 787–797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoops D A perfusion protocol for lizards, including a method for brain removal. MethodsX. 2, 165–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


