Abstract
Pulmonary manifestations in patients with allergic bronchopulmonary aspergillosis (ABPA) and nontuberculous mycobacterial‐pulmonary disease (NTM‐PD) include bronchiectasis and mucus plugging. A 68‐year‐old woman, treated with antibiotics and inhaled corticosteroids for NTM‐PD and asthma, presented with fever and wheezing. ABPA was diagnosed based on laboratory findings (elevated peripheral blood eosinophil counts and serum total IgE levels and positive Aspergillus‐specific IgE and IgG) and imaging observation of a high‐attenuation mucus plug. Systemic prednisolone was avoided to prevent NTM‐PD progression. Dupilumab, a monoclonal antibody that blocks IL‐4/13, was introduced to improve the clinical findings. Herein, we discuss the pathophysiological mechanisms underlying this rare comorbidity.
Keywords: allergic bronchopulmonary aspergillosis, bronchiectasis, dupilumab, mucus plug, nontuberculous mycobacterial‐pulmonary disease
The patient with allergic bronchopulmonary aspergillosis and nontuberculous mycobacterial‐pulmonary disease was successfully treated with dupilumab.
INTRODUCTION
Allergic bronchopulmonary aspergillosis (ABPA) is characterized by central bronchiectasis and recurrent pulmonary infiltrates and manifests as poorly controlled asthma, affecting an estimated 4 million patients worldwide. 1 , 2 It is a well‐recognized complication of asthma and cystic fibrosis. Therapeutic strategies include using systemic corticosteroids and antifungal agents during the initiation. Although multiple environmental factors play an important role, their pathophysiological mechanisms remain unclear.
The incidence and prevalence of nontuberculous mycobacterial‐pulmonary disease (NTM‐PD) are increasing worldwide. 3 Pulmonary manifestations include bronchiectasis and mucus plugging, similar to those observed in ABPA. Neutrophilic inflammation occurs in NTM‐PD, whereas ABPA is characterized by eosinophilic inflammation. 4 , 5 Patients with NTM‐PD often present with secondary infections by various microorganisms, including Aspergillus species. 6 Herein, we describe a patient who developed ABPA during NTM‐PD and was successfully treated with dupilumab.
CASE REPORT
A 68‐year‐old woman has been allergic to Japanese cedar and house dust mite since reaching adulthood. The patient was diagnosed with bullous pemphigoid 16 years ago, and treatment with systemic corticosteroids was initiated 13 years ago. Serum Aspergillus‐specific IgE antibody was positive 12 years ago. Asthma was diagnosed 10 years ago based on the findings of cough and rhinitis that improve with inhaled corticosteroids/long‐acting β2‐agonist. A bacteriological examination of the sputum revealed Mycobacterium avium 8 years ago, and a diagnosis of NTM‐PD was made. Imaging findings of NTM‐PD on chest radiography and computed tomography (CT) worsened 6 years ago (Figure 1A,B), and treatment with antibiotics combined with clarithromycin (800 mg/day), ethambutol (500 mg/day), and rifampicin (600 mg/day) was initiated. However, NTM‐PD was difficult to treat despite the use of multidrug therapy with the addition of amikacin and sitafloxacin to this treatment.
FIGURE 1.
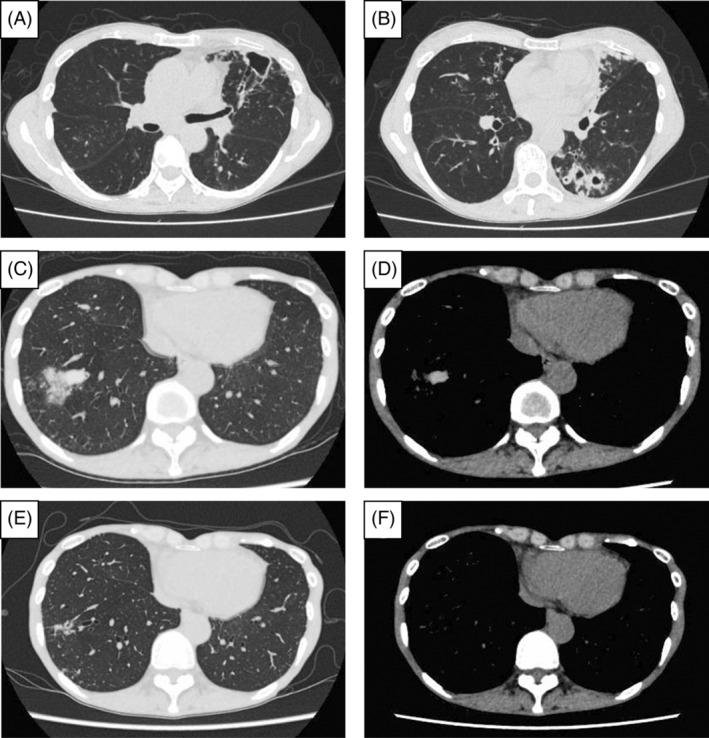
Imaging findings of chest CT at the diagnosis of NTM‐PD, at the diagnosis of ABPA, and after the initiation of dupilumab. CT showed cavities with their wall thickness and bronchiectasis in the upper and lower lobe of the left lung (A, B). At the diagnosis of ABPA, CT showed a high attenuation mucus plug in the lower lobe of the right lung (C, D). After initiating dupilumab, mucus plugging improved, and bronchiectasis was observed in the same lesion (E, F). ABPA, allergic bronchopulmonary aspergillosis; CT, computed tomography; NTM‐PD, nontuberculous mycobacterial‐pulmonary disease.
Serum Aspergillus‐specific IgG antibody became positive 3 years ago. Sputum culture revealed the presence of filamentous fungi in the previous year. This patient presented with wheezing and fever. Respiratory function test showed normal vital capacity (VC, 2.04 L; %VC, 80.3%) and forced expiratory volume in 1 s (FEV1, 1.78 L; %FEV1, 93.7%; FEV1%, 87.3%) with a high level of fractional exhaled nitric oxide (FeNO, 42 ppb). The laboratory findings are summarized in Table 1. Serum total IgE levels and peripheral blood eosinophil counts were high. Chest CT revealed a high‐attenuation mucus plug (HAM) (Figure 1C,D). Based on these findings, ABPA was diagnosed according to its diagnostic criteria. 7
TABLE 1.
Results of blood test.
| Peripheral blood | Biochemistry | ||
| White blood cells | 6900/uL | Total bilirubin | 0.7 mg/dL |
| Neutrophil | 60.0% | Aspartate transaminase | 25 U/L |
| Lymphocyte | 13.0% | Alanine transaminase | 11 U/L |
| Basophil | 2.0% | Lactate dehydrogenase | 177 U/L |
| Eosinophil | 18.0% | Alkaline phophatase | 63 U/L |
| Monocyte | 7.0% | γ‐glutamyl transpeptidase | 19 U/L |
| Eosinophil count | 1242/uL | Total protein | 7.7 g/dL |
| Haemoglobin | 13.2 g/dL | Albumin | 3.8 g/dL |
| Haematocrit | 41.5% | Urea nitrogen | 13.8 mg/dL |
| Platelets | 24.3 × 104/uL | Creatinine | 0.58 mg/dL |
| Sodium | 138.6 mEq/L | ||
| IgE‐RIST | 2933 IU/mL | Potassium | 4.3 mEq/L |
| IgE‐RAST | Chloride | 101 mEq/L | |
| Aspergillus | 25.00 UA/mL | Calcium | 9.3 mEq/L |
| Asp f1 | 0.11 UA/mL | C‐reactive protein | 0.36 mEq/L |
| Aspergillus‐specific IgG antibody | 21 AU/mL | 1,3 beta‐D glucan | 14.7 pg/mL |
| Galactomannan antigen (ELISA) | 0.2 | ||
| GPL core antibody | 7.56 U/L | ||
The use of systemic corticosteroids was avoided to prevent the exacerbation of NTM‐PD. Antifungal agents were also excluded because of their pharmacological interactions with rifampicin. Single inhaler triple therapy using inhaled corticosteroid/long‐acting β2‐agonist/long‐acting muscarinic antagonist (ICS/LABA/LAMA, fluticasone furoate (200 μg/day)/umeclidinium (62.5 μg/day)/vilanterol (25 μg/day)) and dupilumab, an anti‐IL‐4 receptor α monoclonal antibody, were initiated to treat comorbid severe eosinophilic asthma. The clinical symptoms and imaging findings improved after treatment initiation (Figure 1E,F), while serum total IgE levels and peripheral blood eosinophil counts slightly decreased (2236 IU/mL and 977/μL, respectively). Of note, there was no transient eosinophilia. No worsening of NTM‐PD has been observed following treatment with dupilumab; however, residual shadows, including bronchiectasis, remain visible on the chest CT. Figure 2 summarizes the clinical course of the disease.
FIGURE 2.
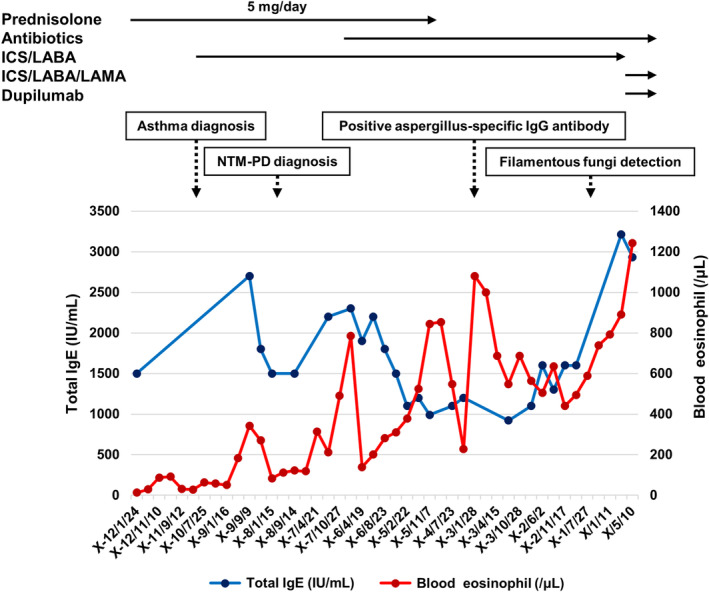
Clinical course of NTM‐PD and ABPA in the present patient. ABPA, allergic bronchopulmonary aspergillosis; ICS/LABA, inhaled corticosteroid/long‐acting β2‐agonist; ICS/LABA/LAMA, inhaled corticosteroid/long‐acting β2‐agonist/long‐acting muscarinic antagonist; IgE, immunoglobulin E; NTM‐PD, nontuberculous mycobacterial‐pulmonary disease.
DISCUSSION
This is a valuable case report of ABPA in a patient with NTM‐PD that describes the long‐term follow‐up period involving the onset of both diseases. Previous reports have shown that NTM‐PD is associated with a higher frequency of ABPA. 8 , 9 Among patients with cystic fibrosis, the incidence of ABPA is higher in those with NTM‐PD than in those without. 8 Patients with bronchiectasis also have a higher incidence of ABPA than those without NTM‐PD. 9 In contrast, the cumulative incidence of NTM‐PD increased over time in patients with ABPA and allergic bronchopulmonary mycosis (ABPM) who received oral corticosteroids as a risk factor for this complication. 10
In this case, the patient developed asthma with sensitization to Aspergillus fumigatus prior to the diagnosis of NTM‐PD. IgE‐mediated A. fumigatus sensitization aggravates respiratory conditions in patients with asthma who do not meet ABPA diagnostic criteria. Patients with A. fumigatus sensitization frequently exhibit impaired pulmonary function, mucus plugging, and bronchiectasis. 11 The positivity rate of A. fumigatus‐specific IgE increases over time in patients with asthma, and risk factors include the use of medium‐to high‐dose inhaled corticosteroids and high serum levels of total IgE. 12 Inhaled or systemic steroids are known risk factors for the development of NTM‐PD. 13 , 14 A. fumigatus may induce a Th2‐mediated immune response and reduce cytokines involved in NTM eradication. 15 These findings suggest that airway inflammation, therapeutic agents used in asthma, and sensitization to A. fumigatus may trigger the development of NTM‐PD.
Bronchiectasis often coexists with severe asthma, and A. fumigatus is frequently isolated from cultured microorganisms in such cases. 16 Bronchiectasis in NTM‐PD is associated with enhanced airway inflammation and increased cytokine levels, including IL‐1 and GM‐CSF. 17 , 18 Animal studies have demonstrated that these cytokines induce sensitization to allergens. 19 Based on these findings, NTM‐PD and bronchiectasis may promote further sensitization to Aspergillus in the airways.
A. fumigatus‐specific IgG is frequently detected in patients with NTM‐PD. A previous report showed that Aspergillus precipitating antibody‐positive patients presented with a longer duration, more severe bronchiectasis, and lower pulmonary function, and 5 of 109 patients developed ABPA. 20 Another study demonstrated that Aspergillus precipitating antibody‐positive cases were characterized by male sex, emphysema, and interstitial pneumonia, and 3 of 109 cases developed ABPA. 21 Additionally, the accumulation of neutrophils in the airways due to NTM‐PD may enhance the migratory response of eosinophils. 4 , 22 It has been reported that some patients with bronchiectasis exhibit a mixed phenotype of neutrophilic and eosinophilic inflammation. 23 These findings indicate that Aspergillus sensitization aggravates the pathogenesis of NTM‐PD and may trigger the development of ABPA.
Biologics are not currently available for ABPM cases. Case reports and series have reported that the use of biologics targeting IgE, 24 IL‐5, 25 IL‐4/IL‐13, 26 , 27 , 28 , 29 , 30 , 31 , 32 and TSLP 33 improves the disease status of ABPA complicated by severe asthma. Monoclonal antibodies against IgE have therapeutic efficacy in patients with ABPA and severe asthma, including 12 of the 25 patients with NTM‐PD. 24 Anti‐IL‐5/IL‐5 receptor antibodies are highly effective in patients with ABPA, especially for improving mucus plugging. 25 Previous case reports suggested that dupilumab is effective when switching from other biologics to this drug. 26 , 27 , 28 , 29 , 30 Similar to the present case, the therapeutic efficacy of dupilumab in patients with NTM‐PD 31 and the discontinuation of oral steroids during the use of dupilumab 32 were also observed in patients with ABPA. Of note, dupilumab treatment was associated with a reduced incidence of respiratory infections in patients with moderate‐to‐severe asthma or severe CRSwNP. 34 Since IL‐4 suppresses Th1 cells, this inhibition by dupilumab may contribute to the eradication of NTM in the lung by normalizing type 1 inflammation. 35 , 36 , 37 These findings suggestive of its usefulness in patients with infectious diseases. ABPA cases that were successfully treated with dupilumab are summarized in Table 2. It may be necessary to consider the phenotype of ABPA when selecting a specific biologic. 38
TABLE 2.
Summary of the cases with ABPA successfully treated with dupilumab.
| Age | Sex | Comorbid disease | Treatment of ABPA | Previous treatment using biologics | References |
|---|---|---|---|---|---|
| 81 | F | Asthma | OCS (prednisolone) | Mepolizumab | 16 |
| 63 | F | Asthma | Itraconazole | Benralizumab | 17 |
| 45 | M | Asthma | OCS (prednisolone) | Mepolizumab | 18 |
| 49 | F | Asthma | Itraconazole, OCS (prednisolone) | Benralizumab, omalizumab | 19 |
| 60 | F | Asthma | OCS (unknown) | Omalizumab, mepolizumab | 20 |
| 51 | F | Asthma | Itraconazole, OCS (unknown) | Mepolizumab | 20 |
| 33 | M | Asthma, Klinefelter syndrome | Voriconazole | – | 20 |
| 72 | F | Asthma, NTM‐PD | – | – | 21 |
| 54 | F | Asthma | itraconazole, OCS(prednisolone) | – | 4 |
| 68 | F | Asthma | – | ‐ | Present case |
Abbreviations: ABPA, allergic bronchopulmonary aspergillosis; NTM‐PD, non‐tuberculous mycobacterial‐pulmonary disease; OCS, oral corticosteroid.
Based on our experience with this patient, we speculated that the pathogenesis of NTM‐PD is associated with the development of ABPA in asthma. Bronchiectasis and/or inhaled/systemic corticosteroid use may be the potential causes of this comorbidity. Clinical practitioners should know about this association to decide the appropriate therapeutic management for both conditions, including biologics.
AUTHOR CONTRIBUTIONS
Ryuta Onozato and Jun Miyata conceived the idea. Koichi Fukunaga overviewed the project. Ryuta Onozato and Jun Miyata wrote the paper. Takanori Asakura, Ho Namkoong, Koichiro Asano, Naoki Hasegawa critically contributed to the accomplishment of this report.
FUNDING INFORMATION
This study did not receive any funding from any source.
CONFLICT OF INTEREST STATEMENT
Jun Miyata received lecture fees from Sanofi S.A..
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Onozato R, Miyata J, Asakura T, Namkoong H, Asano K, Hasegawa N, et al. Development of allergic bronchopulmonary aspergillosis in a patient with nontuberculous mycobacterial‐pulmonary disease successfully treated with dupilumab: A case report and literature review. Respirology Case Reports. 2024;12(7):e01432. 10.1002/rcr2.1432
Associate Editor: Young Ae Kang
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Agarwal R, Sehgal IS, Dhooria S, Aggarwal AN. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med. 2016;10(12):1317–1334. [DOI] [PubMed] [Google Scholar]
- 2. Oguma T, Taniguchi M, Shimoda T, Kamei K, Matsuse H, Hebisawa A, et al. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol Int. 2018;67(1):79–84. [DOI] [PubMed] [Google Scholar]
- 3. Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non‐tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):1900250. [DOI] [PubMed] [Google Scholar]
- 4. Alkarni M, Lipman M, Lowe DM. The roles of neutrophils in non‐tuberculous mycobacterial pulmonary disease. Ann Clin Microbiol Antimicrob. 2023;22(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asano K, Ueki S, Tamari M, Imoto Y, Fujieda S, Taniguchi M. Adult‐onset eosinophilic airway diseases. Allergy. 2020;75(12):3087–3099. [DOI] [PubMed] [Google Scholar]
- 6. Phoompoung P, Chayakulkeeree M. Chronic pulmonary aspergillosis following nontuberculous mycobacterial infections: an emerging disease. J Fungi (Basel). 2020;6(4):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asano K, Hebisawa A, Ishiguro T, Takayanagi N, Nakamura Y, Suzuki J, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147(4):1261–1268. [DOI] [PubMed] [Google Scholar]
- 8. Mussaffi H, Rivlin J, Shalit I, Ephros M, Blau H. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur Respir J. 2005;25(2):324–328. [DOI] [PubMed] [Google Scholar]
- 9. Levy I, Grisaru‐Soen G, Lerner‐Geva L, Kerem E, Blau H, Bentur L, et al. Multicenter cross‐sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients Israel. Emerg Infect Dis. 2008;14(3):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kunst H, Wickremasinghe M, Wells A, Wilson R. Nontuberculous mycobacterial disease and Aspergillus‐related lung disease in bronchiectasis. Eur Respir J. 2006;28(2):352–357. [DOI] [PubMed] [Google Scholar]
- 11. Mistry H, Ajsivinac Soberanis HM, Kyyaly MA, Azim A, Barber C, Knight D, et al. The clinical implications of Aspergillus fumigatus sensitization in difficult‐to‐treat asthma patients. J Allergy Clin Immunol Pract. 2021;9(12):4254–4267.e4210. [DOI] [PubMed] [Google Scholar]
- 12. Watai K, Fukutomi Y, Hayashi H, Nakamura Y, Hamada Y, Tomita Y, et al. De novo sensitization to Aspergillus fumigatus in adult asthma over a 10‐year observation period. Allergy. 2018;73(12):2385–2388. [DOI] [PubMed] [Google Scholar]
- 13. Brode SK, Campitelli MA, Kwong JC, Lu H, Marchand‐Austin A, Gershon AS, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50(3):1700037. [DOI] [PubMed] [Google Scholar]
- 14. Shu CC, Wei YF, Chen KH, Chuang S, Wang YH, Wang CY, et al. Inhaled corticosteroids increase risk of nontuberculous mycobacterial lung disease: a nested case‐control study and meta‐analysis. J Infect Dis. 2022;225(4):627–636. [DOI] [PubMed] [Google Scholar]
- 15. Gramegna A, Misuraca S, Lombardi A, Premuda C, Barone I, Ori M, et al. Treatable traits and challenges in the clinical management of non‐tuberculous mycobacteria lung disease in people with cystic fibrosis. Respir Res. 2023;24(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bendien SA, van Loon‐Kooij S, Kramer G, Huijgen W, Altenburg J, Ten Brinke A, et al. Bronchiectasis in severe asthma: does it make a difference? Respiration. 2020;1‐9:1136–1144. [DOI] [PubMed] [Google Scholar]
- 17. Asakura T, Okuda K, Chen G, Dang H, Kato T, Mikami Y, et al. Proximal and distal bronchioles contribute to the pathogenesis of non‐cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2024;209(4):374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu UI, Olivier KN, Kuhns DB, Fink DL, Sampaio EP, Zelazny AM, et al. Patients with idiopathic pulmonary nontuberculous mycobacterial disease have normal Th1/Th2 cytokine responses but diminished Th17 cytokine and enhanced granulocyte‐macrophage colony‐stimulating factor production. Open Forum Infect Dis. 2019;6(12):ofz484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin‐1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM‐CSF and IL‐33. J Exp Med. 2012;209(8):1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki S, Asakura T, Namkoong H, Okamori S, Yagi K, Kamata H, et al. Aspergillus precipitating antibody in patients with Mycobacterium avium complex lung disease: a cross‐sectional study. Respir Med. 2018;138:1–6. [DOI] [PubMed] [Google Scholar]
- 21. Shirai T, Furuuchi K, Fujiwara K, Nakamoto K, Tanaka Y, Ishii H, et al. Impact of Aspergillus precipitating antibody test results on clinical outcomes of patients with Mycobacterium avium complex lung disease. Respir Med. 2020;166:105955. [DOI] [PubMed] [Google Scholar]
- 22. Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, et al. Eosinophil trans‐basement membrane migration induced by interleukin‐8 and neutrophils. Am J Respir Cell Mol Biol. 2006;34(6):760–765. [DOI] [PubMed] [Google Scholar]
- 23. Choi H, Ryu S, Keir HR, Giam YH, Dicker AJ, Perea L, et al. Inflammatory molecular endotypes in bronchiectasis: a European multicenter cohort study. Am J Respir Crit Care Med. 2023;208(11):1166–1176. [DOI] [PubMed] [Google Scholar]
- 24. Tomomatsu K, Oguma T, Baba T, Toyoshima M, Komase Y, Taniguchi M, et al. Effectiveness and safety of omalizumab in patients with allergic bronchopulmonary aspergillosis complicated by chronic bacterial infection in the airways. Int Arch Allergy Immunol. 2020;181(7):499–506. [DOI] [PubMed] [Google Scholar]
- 25. Tomomatsu K, Yasuba H, Ishiguro T, Imokawa S, Hara J, Soeda S, et al. Real‐world efficacy of anti‐IL‐5 treatment in patients with allergic bronchopulmonary aspergillosis. Sci Rep. 2023;13(1):5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kai Y, Yoshikawa M, Matsuda M, Suzuki K, Takano M, Tanimura K, et al. Successful management of recurrent allergic bronchopulmonary aspergillosis after changing from mepolizumab to dupilumab: a case report. Respir Med Case Rep. 2022;39:101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotetsu Y, Ogata H, Sha K, Moriwaki A, Yoshida M. A case of allergic bronchopulmonary aspergillosis with failure of benralizumab and response to dupilumab. Cureus. 2023;15(7):e42464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikura S, Saraya T, Yoshida Y, Oda M, Ishida M, Honda K, et al. Successful treatment of mepolizumab‐ and prednisolone‐resistant allergic bronchopulmonary aspergillosis with dupilumab. Intern Med. 2021;60(17):2839–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mümmler C, Kemmerich B, Behr J, Kneidinger N, Milger K. Differential response to biologics in a patient with severe asthma and ABPA: a role for dupilumab? Allergy Asthma Clin Immunol. 2020;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramonell RP, Lee FE, Swenson C, Kuruvilla M. Dupilumab treatment for allergic bronchopulmonary aspergillosis: a case series. J Allergy Clin Immunol Pract. 2020;8(2):742–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tashiro H, Takahashi K, Kurihara Y, Sadamatsu H, Kimura S, Sueoka‐Aragane N. Efficacy of dupilumab and biomarkers for systemic corticosteroid naïve allergic bronchopulmonary mycosis. Allergol Int. 2021;70(1):145–147. [DOI] [PubMed] [Google Scholar]
- 32. Nishimura T, Okano T, Naito M, Tsuji C, Iwanaka S, Sakakura Y, et al. Complete withdrawal of glucocorticoids after dupilumab therapy in allergic bronchopulmonary aspergillosis: a case report. World J Clin Cases. 2021;9(23):6922–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogata H, Sha K, Kotetsu Y, Enokizu‐Ogawa A, Katahira K, Ishimatsu A, et al. Tezepelumab treatment for allergic bronchopulmonary aspergillosis. Respirol Case Rep. 2023;11(5):e01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geng B, Bachert C, Busse WW, Gevaert P, Lee SE, Niederman MS, et al. Respiratory infections and anti‐infective medication use from phase 3 dupilumab respiratory studies. J Allergy Clin Immunol Pract. 2022;10(3):732–741. [DOI] [PubMed] [Google Scholar]
- 35. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288. [DOI] [PubMed] [Google Scholar]
- 36. Tsai M, Thauland TJ, Huang AY, Bun C, Fitzwater S, Krogstad P, et al. Disseminated coccidioidomycosis treated with interferon‐γ and dupilumab. N Engl J Med. 2020;382(24):2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gramegna A, Lombardi A, Lorè NI, Amati F, Barone I, Azzarà C, et al. Innate and adaptive lymphocytes in non‐tuberculous mycobacteria lung disease: a review. Front Immunol. 2022;13:927049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okada N, Yamamoto Y, Oguma T, Tanaka J, Tomomatsu K, Shiraishi Y, et al. Allergic bronchopulmonary aspergillosis with atopic, nonatopic, and sans asthma‐factor analysis. Allergy. 2023;78(11):2933–2943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


