Abstract
Antibody to the capsid (PORF2) protein of hepatitis E virus (HEV) is sufficient to confer immunity, but knowledge of B-cell epitopes in the intact capsid is limited. A panel of murine monoclonal antibodies (MAbs) was generated following immunization with recombinant ORF2.1 protein, representing the C-terminal 267 amino acids (aa) of the 660-aa capsid protein. Two MAbs reacted exclusively with the conformational ORF2.1 epitope (F. Li, J. Torresi, S. A. Locarnini, H. Zhuang, W. Zhu, X. Guo, and D. A. Anderson, J. Med. Virol. 52:289–300, 1997), while the remaining five demonstrated reactivity with epitopes in the regions aa 394 to 414, 414 to 434, and 434 to 457. The antigenic structures of both the ORF2.1 protein expressed in Escherichia coli and the virus-like particles (VLPs) expressed using the baculovirus system were examined by competitive enzyme-linked immunosorbent assays (ELISAs) using five of these MAbs and HEV patient sera. Despite the wide separation of epitopes within the primary sequence, all the MAbs demonstrated some degree of cross-inhibition with each other in ORF2.1 and/or VLP ELISAs, suggesting a complex antigenic structure. MAbs specific for the conformational ORF2.1 epitope and a linear epitope within aa 434 to 457 blocked convalescent patient antibody reactivity against VLPs by approximately 60 and 35%, respectively, while MAbs against epitopes within aa 394 to 414 and 414 to 434 were unable to block patient serum reactivity. These results suggest that sequences spanning aa 394 to 457 of the capsid protein participate in the formation of strongly immunodominant epitopes on the surface of HEV particles which may be important in immunity to HEV infection.
Hepatitis E virus (HEV) is responsible for epidemic and sporadic cases of enterically transmitted viral hepatitis, particularly in the developing world (17, 31). HEV is a single-stranded, positive-sense RNA virus, with the genome encoding three open reading frames (ORFs), of which ORF2 encodes the major structural or capsid protein, PORF2. Antibody is sufficient to confer immunity to HEV infection (38), but little is known of the structure of the viral particle or of the antibody specificities which contribute to humoral immunity, which could pose a major hurdle in the development and clinical evaluation of effective vaccines.
The use of peptide scanning has led to the identification of a number of linear epitopes within the capsid protein of HEV, with many of these being type specific (4, 12, 13, 15). More recently, the use of larger overlapping peptides has revealed some conformational epitopes which are reactive with acute-phase sera (16). Linkage of a number of such peptide epitopes from different strains of HEV into an artificial “mosaic” protein improves the detection of acute-phase HEV antibody (5, 14), but the antibodies induced with this protein do not appear to be neutralizing in a cell culture system which measures virus-cell binding (26). In addition, it is not known whether antibodies to any of these linear and conformational peptide epitopes can bind to intact viral particles, or indeed whether this antibody repertoire is maintained during the convalescent phase after HEV infection and thus contributes to humoral immunity.
Expression of PORF2 with an N-terminal truncation of 111 amino acids (aa) in the baculovirus system results in the production of virus-like particles (VLPs), which, in contrast to synthetic peptides or full-length PORF2, appear to mimic the antigenicity and immunogenicity of the native virus (24, 25, 32). Most significantly, immunization of macaques with VLPs confers immunity to both homologous and heterologous virus challenge (37, 38, 43). The improved antibody reactivity and immunogenicity of VLPs have been attributed to conformational epitopes which are not presented by synthetic peptides, full-length PORF2, and most HEV proteins expressed in Escherichia coli (24, 25), but the relevant epitopes have not been identified.
Expression of the ORF2.1 fragment of PORF2 (aa 394 to 660) in E. coli also results in the presentation of conformational epitopes (2, 22, 23). Since antisera from animals immunized with ORF2.1 are able to inhibit the reactivity of HEV patient sera against VLPs by as much as 97% (21), it appears likely that the major epitopes within VLPs and the ORF2.1 antigen expressed in E. coli may be the same or closely overlapping.
In this study we have used monoclonal antibodies (MAbs) to study the antigenic structure of HEV in more detail. We show that the conformational ORF2.1 epitope involving aa residues 394 to 457 and a linear epitope in the region of aa 434 to 457 are not only present on the surface of VLPs, but immunodominant in the humoral immune response of convalescent HEV patients.
MATERIALS AND METHODS
Preparation of antigen.
The AC2.1 fragment from pGEX-AC2.1 (23) was subcloned into the pET-30a(+) vector (Novagen Inc.), to encode a fusion protein (ET-2.1) comprising the ORF2.1 fragment (aa 394 to 660) of PORF2 with a hexahistidine tag. ET-2.1 protein was expressed in E. coli, solubilized in 5 M urea, and purified in the presence of 5 M urea using TALON resin (Clontech Laboratories, Palo Alto, Calif.) as described previously for the protein GST-ORF2.1 (GST-ORF2.1-6xHis) (2). The purified ET-2.1 protein was then refolded by dialysis in 20 mM Tris-HCl (pH 8.0) (21).
Production of MAbs.
Eight-week-old female BALB/c mice were immunized at 0 and 4 weeks by intraperitoneal inoculation with equal volumes of antigen (100 μg in 100 μl) and Hunter's Titermax (Sigma Chemicals, St. Louis, Mo.). Four weeks later, a final boost of equal volumes of antigen and 0.85% saline was administered into the tail vein. Three days after the final intravenous inoculation, mouse spleen cells were fused with sp2/0-Ag 14 mouse myeloma cells using polyethylene glycol 1500 (50% [wt/vol]) (Boehringer, Mannheim, Germany) essentially as described by Adler and Faine (1).
Samples of supernatant from wells positive for hybridoma growth were screened by enzyme-linked immunosorbent assay (ELISA) using the GST-ORF2.1 antigen as described below. Hybridomas secreting specific antibodies to HEV were subcloned three times by limiting dilution, after which they were considered to be monoclonal. Antibodies in culture supernatants were isotyped using the Mouse Monoclonal Antibody Isotyping kit (Amersham, Little Chalfont, Buckinghamshire, U.K.) in accordance with the manufacturer's protocol.
Hybridomas were grown in bulk in stationary flasks (Nunc, Roskilde, Denmark) using Opti-Mem reduced-serum medium (Gibco-BRL, Grand Island, N.Y.) without serum. Supernatant was harvested and filtered once cells appeared dead (between 7 and 14 days), and antibodies were purified using HiTrap protein G affinity columns (Pharmacia Biotech AB, Uppsala, Sweden) and stored at −70°C. MAb 1H1, an immunoglobulin G2a (IgG2a) specific for the L protein of duck hepatitis B virus (a gift from J. Pugh), was prepared in the same way and included as a negative control in all subsequent experiments.
Biotinylation of antibodies.
Purified MAbs were concentrated to 1 mg/ml in phosphate-buffered saline (PBS) using 30K Nanosep microconcentrators (Pall Filtron, Northborough, Mass.). MAbs were then biotinylated using EZ-Link Sulfo-NHS(N-hydroxysuccinimide)-LC-Biotin (Pierce, Rockford, Ill.) in accordance with the manufacturer's protocol, separated from free biotin by gel filtration over PD-10 columns (Pharmacia), and stored at −70°C until use.
Immunoassays. (i) ELISAs for human and murine IgG anti-HEV.
ELISA plates coated with GST-ORF2.1 (2) or baculovirus-expressed VLPs (24) were prepared as previously described. Samples (0.1 ml) containing undiluted hybridoma supernatants or purified MAbs or human convalescent patient sera diluted in C-PBST (1% casein, PBS [pH 7.4], 0.5% Tween 20) were added to duplicate ELISA wells for 1 h at 37°C (VLP) or 21°C (GST-ORF2.1). Plates were washed with PBST (PBS [pH 7.4], 0.05% Tween 20), and bound IgG was detected with horseradish peroxidase (HRPO)-conjugated sheep anti-mouse IgG (Amersham International) diluted 1:5,000 in C-PBST or with HRPO-conjugated sheep anti-human IgG (Amersham) diluted 1:6,000 in C-PBST for 1 h at 21°C. Antibody complexes were detected using tetramethylbenzidine substrate (AMRAD Biotech, Melbourne, Australia), reactions were stopped with H2SO4, and absorbance was read at 450 and 620 nm.
(ii) Blocking ELISAs.
The spatial relationships of epitopes recognized by each of the MAbs were investigated by a blocking ELISA in which antigen was incubated with saturating levels of unlabeled MAbs (as determined in the experiment shown in Fig. 2) prior to the addition of biotinylated MAbs, and residual binding of biotinylated MAbs was detected using HRPO-conjugated streptavidin. Samples of unlabeled MAbs (100 μl, 20 μg/ml in C-PBST) were added to VLP- or GST-ORF2.1-coated plates for 1 h at 37 or 21°C, respectively, followed by the addition of 100 μl of biotinylated MAbs diluted in C-PBST for a further 1 h at the same temperature. Biotinylated MAbs were used at a dilution which gave an ELISA result of approximately 2.0 optical density (OD) units against the corresponding antigen type (results not shown). Plates were then washed with PBST, and residual binding of biotinylated antibodies was detected using HRPO-conjugated streptavidin (Amersham) diluted 1:1,000 in C-PBST. To quantitate the residual binding of each biotinylated MAb, dilutions of biotinylated MAbs representing 10% activity (90% inhibition), 30% activity (70% inhibition), 70% activity (30% inhibition), and 100% activity were prepared in C-PBST containing 10 μg of irrelevant MAb 1H1 per ml and assayed in the same plate.
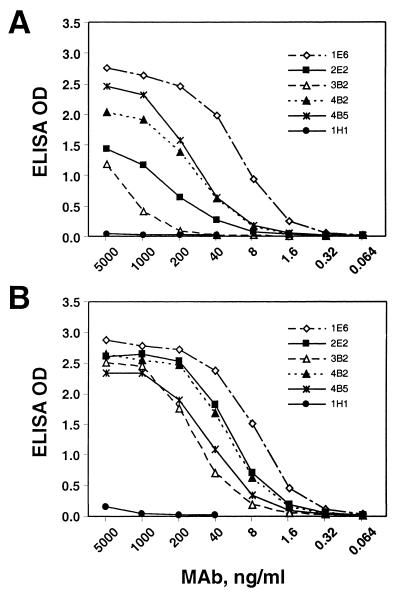
FIG. 2.
Endpoint titration of MAb reactivities against GST-ORF2.1 antigen (A) and VLP antigen (B). ELISA plates were incubated with the indicated concentrations of each MAb for 1 h, and bound antibody detected with HRPO-conjugated anti-mouse IgG. 1H1, irrelevant MAb.
The proportion of convalescent patient antibody directed against each of the MAb epitopes present on VLPs was determined by a second blocking ELISA, in which antigen was incubated with saturating levels of MAbs prior to the addition of patient sera, and residual binding of human IgG was detected using HRPO-conjugated anti-human IgG. Samples of MAbs (75 μl, 10 μg/ml in C-PBST) were added to VLP-coated plates for 1 h at 37°C, followed by the addition of 75 μl of patient serum diluted 1:150 (patients G31 and G37 from Nepal) or 1:500 (patient NIH116 from Mexico) in C-PBST for a further 1 h. Dilutions of patient sera were chosen in order to give an ELISA result of approximately 1.2 OD units (results not shown). Plates were then washed with PBST, and residual binding of patient IgG was detected as before using HRPO-conjugated sheep anti-human IgG, which was shown to be unreactive with murine IgG (results not shown). To quantitate the residual binding of patient IgG, each plate included in triplicate a dilution series of the International Reference HEV Serum 95/584 (100 IU/ml), from which a standard curve was constructed to relate ELISA OD to HEV-specific IgG reactivity in milli-international units per milliliter (2). Any difference in the level of human IgG binding between the control (irrelevant MAb 1H1) and specific MAbs is therefore a measure of the proportion of total HEV-specific IgG directed against epitopes which are altered or blocked by binding of the specific MAb.
SDS-PAGE and Western immunoblotting.
The epitope specificities of MAbs were determined by Western immunoblotting using GST-ORF2 fusion proteins representing different fragments of full-length ORF2 as described previously (23). Briefly, equimolar amounts of each fusion protein were subjected to sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE), transferred to nitrocellulose membranes, and probed with MAb culture supernatant diluted 1:10 in C-PBST. Immune complexes were detected with HRPO-conjugated sheep anti-mouse IgG and enhanced chemiluminescence (Amersham). For clarity, PORF2 fragments with the exception of ORF2.1 will be referred to by their N- and C-terminal amino acids: for example, P394-473 was previously described as 2.1Δ2.1-4 (23) and represents aa 394 to 473 of PORF2.
Indirect immunofluorescence.
Because HEV cannot be grown in cell culture, the full-length PORF2 protein was expressed in HepG2 cells using plasmid pCI-ORF2. The coding sequence of full-length PORF2 was released from plasmid pSFV1/ORF2K (37) by partial digestion with EcoRI and cloned into the EcoRI site of plasmid pCI-neo (Promega, Madison, Wis.), generating plasmid pCI-ORF2, and the insert was confirmed by restriction enzyme digestions and sequencing. HepG2 cells were grown on glass coverslips until 60% confluent, transfected with 1 μg of DNA per cm2 using the calcium phosphate method (6), and incubated for 40 h at 37°C. Cultures were fixed in acetone for 2 min at 4°C, dried, and then stained with HEV-specific MAbs or MAb 1H1, specific for duck hepatitis B virus L protein (30), at a final concentration of 5 μg/ml in PBS containing 2% fetal calf serum for 1 h at 21°C. The slides were washed for 30 min in PBS and counterstained with a 1:50 dilution of fluorescein-conjugated anti-mouse IgG (Dako, Copenhagen, Denmark) and 5 μg of propidium iodide (Sigma) per ml for 1 h. The slides were washed for a further 30 min, mounted with PBS-buffered glycerol, and examined with an Axiovert 100 microscope (Zeiss, Jena, Germany).
RESULTS
Isolation and biophysical characterization of MAbs.
The ORF2.1 fragment of PORF2 in the form of protein QE2.1 can induce antibodies against immunodominant epitopes in HEV (21), and an analogous protein, ET-2.1, containing the same HEV-specific amino acids but with a shorter N-terminal fusion protein, was used here as the immunogen for production of MAbs to facilitate studies of HEV antigenic structure. Splenocytes from BALB/c mice immunized with purified ET-2.1 protein were fused with murine myeloma cells to generate hybridomas secreting anti-ORF2.1. Seven hybridomas giving initial ELISA reactivities against GST-ORF2.1 protein of >0.25 OD were identified and subcloned; five MAbs were IgG1 (1C7, 3B2, 4B5, 2E2, and 4B2), and two were IgG2b (1E6 and 1E7).
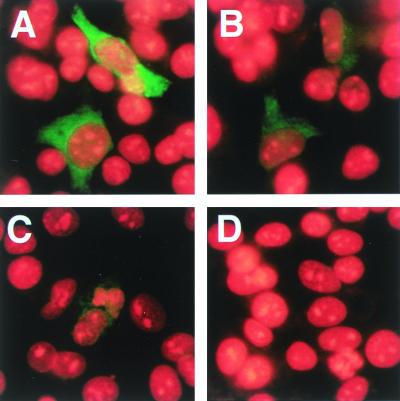
As a first step in examining the specificity of the MAbs, they were used in indirect immunofluorescence against full-length PORF2 expressed in HepG2 cells using plasmid pCI-ORF2 (Fig. 1). MAb 4B2 was only weakly reactive (Fig. 1B), and the reactivity of MAb 2E2 was barely detectable (Fig. 1C), whereas the other MAbs showed very strong reactivity, as shown for MAb 1E6 (Fig. 1A and results not shown). This difference in reactivities for 4B2 and 2E2 was found using a variety of fixation conditions and was also seen using PORF2 expressed using the Semliki Forest virus expression system (36) (results not shown) and is therefore due to inefficient presentation of the 4B2 and 2E2 epitopes in full-length PORF2. It has been shown previously that full-length PORF2 is less reactive with patient sera than is the truncated PORF2 assembled into VLPs (24, 25, 32), suggesting that the epitopes for MAbs 4B2 and 2E2 are of particular interest.
FIG. 1.
Indirect immunofluorescence staining of full-length PORF2 protein with MAbs against PORF2. HepG2 cells were transfected with pCI-ORF2 for 40 h, fixed, and stained with MAbs followed by fluorescein-conjugated anti-mouse IgG (green) together with propidium iodide to counterstain nuclei (red). Transfected cells were stained with HEV-specific MAb 1E6 (A), 4B2 (B), or 2E2 (C) or with irrelevant MAb 1H1 (D). Magnification, ×200.
Five of the MAbs were purified and used in endpoint titration against both the GST-ORF2.1 and VLP antigens (Fig. 2). The MAbs displayed a wide variation in apparent affinity for the GST-ORF2.1 antigen (Fig. 2A), which is unlikely to be due to the GST fusion because the GST-ORF2.1 and PinPoint-ORF2.1 (22), QE-2.1 (21), and ET-2.1 fusions (results not shown) have almost identical reactivities. Interestingly, the MAbs displayed much less variation with the VLPs (Fig. 2B), largely as a result of an approximately 25-fold increase in reactivity for both 3B2 and 2E2 against VLPs compared to GST-ORF2.1. It is clear that each of the MAbs reacts with epitopes which are well exposed on the surface of purified HEV VLPs (Fig. 2B), even though the immune response was initiated with a bacterial fusion protein, and are thus well suited to the study of HEV structure. Notably, both 4B2 and 2E2 are highly reactive against VLPs, in contrast to their weak reactivity against full-length PORF2 (Fig. 1).
Epitope mapping.
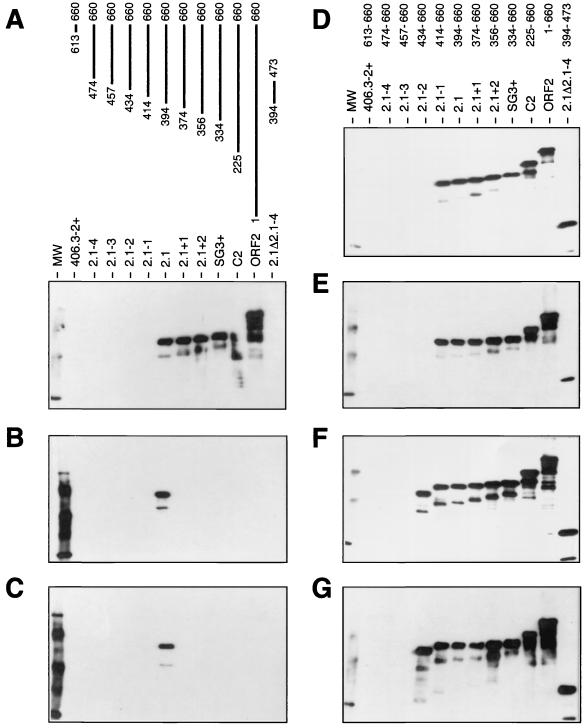
To determine the epitope specificities of the MAbs, a series of GST fusion proteins containing either full-length PORF2 or partially overlapping fragments of PORF2 (2, 21, 22) were probed with each MAb in Western blots (Fig. 3). Two MAbs each were reactive with proteins having N-terminal truncations extending to aa 414 to 660 or aa 434 to 660, consistent with epitopes in the regions of aa 414 to 434 (MAbs 1C7 and 3B2; Fig. 3D and E) and aa 434 to 457 (MAbs 1E6 and 1E7; Fig. 3F and G), respectively. All of these MAbs were reactive with all larger fragments of PORF2 and with P394-473 and are thus directed against linear peptide epitopes. MAbs 2E2 and 4B2 were exclusively reactive with the ORF2.1 fragment, in a manner identical to convalescent-phase sera analyzed by this method (2, 21), and are thus directed against the conformational ORF2.1 epitope, which is ablated when smaller or larger fragments of PORF2 are expressed in E. coli (2, 21, 22). MAb 4B5 was reactive with all fragments beginning upstream of aa 394, and we conclude that it is directed against an epitope in the region of aa 394 to 414, but its lack of reactivity with P394-473 suggests that this epitope is not correctly modeled in the absence of C-terminal sequences. We therefore consider that this epitope is conformational but within aa 394 to 414.
FIG. 3.
Mapping of MAb epitopes within PORF2. Equimolar amounts of fusion proteins (GST with the indicated fragments of PORF2) were separated on SDS-PAGE gels and transferred to nitrocellulose membranes, and each membrane was reacted with a single MAb. Immune complexes were detected by enhanced chemiluminescence. (A) MAb 4B5 and schematic of truncated PORF2 fragments in each lane. (B) MAb 2E2; (C) MAb 4B2; (D) MAb 1C7; (E) MAb 3B2; (F) MAb 1E6; (G) MAb 1E7. Note the exclusive reactivity of 2E2 and 4B2 with the ORF2.1 fragment. Lane MW, size markers.
Antigenic structure of HEV.
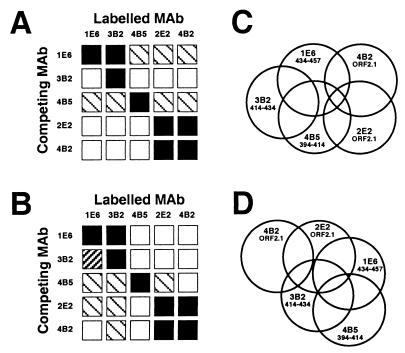
The availability of MAbs with known specificities allowed us to examine the antigenic structure of HEV. Five of the MAbs were purified, biotinylated, and used in blocking ELISAs to determine the spatial relationship of the antigenic sites in both the purified GST-ORF2.1 fusion protein and, more importantly, VLPs which mimic the native particle structure. For this purpose, saturating levels of unlabeled MAbs were bound to GST-ORF2.1 (Fig. 4A) or VLPs (Fig. 4B) prior to the addition of labeled MAbs, and the level of residual binding was estimated by comparison with the binding achieved with 100, 70, 30, and 10% of the corresponding labeled MAb.
FIG. 4.
Competition matrix between HEV-specific MAbs tested on GST-ORF2.1 (A) and VLP (B) ELISAs. ELISA wells were reacted with saturating amounts of the competing MAb before addition of biotin-labeled MAbs, the residual binding of which was then detected with HRPO-conjugated streptavidin and quantitated by comparison with dilutions of each biotin-labeled MAb alone. Symbols: □, <30%;  , 30 to 70%;
, 30 to 70%;  , 70 to 90%; ■, >90% inhibition of binding. (C and D) Two-dimensional surface-like maps of epitopes on GST-ORF2.1 (C) and VLPs (D). Overlap indicates more than 30% inhibition of ELISA reactivity between pairs of MAbs for each antigen.
, 70 to 90%; ■, >90% inhibition of binding. (C and D) Two-dimensional surface-like maps of epitopes on GST-ORF2.1 (C) and VLPs (D). Overlap indicates more than 30% inhibition of ELISA reactivity between pairs of MAbs for each antigen.
As expected, blocking of homologous MAbs was complete. The MAbs specific for the conformational ORF2.1 epitope (2E2 and 4B2) also demonstrated complete, reciprocal blocking on both GST-ORF2.1 and VLP antigens despite the differences observed between their levels of reactivity against both full-length PORF2 (Fig. 1) and GST-ORF2.1 (Fig. 2A). Patterns of blocking were more complex for other combinations of MAbs; for example, 1E6 and 3B2 caused more than 70% blocking of each other on VLPs, but 3B2 failed to block 1E6 on GST-ORF2.1, demonstrating subtle antigenic differences between the fusion protein and VLPs.
The corresponding results are summarized in two-dimensional surface-like maps (18), showing overlap of the epitopes for GST-ORF2.1 (Fig. 4C) and VLPs (Fig. 4D). In this representation of the data, overlap in the Venn diagram indicates significant spatial overlap of antigenic sites, defined as more than 30% inhibition between MAbs in one or both directions. These results allow us to draw two preliminary conclusions. First, sequences in the region of aa 394 to 457 are exposed on the surface of HEV VLPs and are most likely highly folded, such that binding of antibody to widely separated linear sequences is mutually exclusive. Second, these sequences most likely contribute to the conformational ORF2.1 epitope (Fig. 4D).
Comparison of MAb epitope specificities with those of HEV-infected patients.
To compare the epitope specificities of these MAbs with those of convalescent-phase patient IgG, we performed similar blocking ELISAs in which saturating amounts of MAbs were added to plates coated with VLPs prior to the addition of patient sera. Two patients (G31 and G37) were infected in Nepal, while patient NIH116 was infected with the divergent Mexico strain of HEV. The residual binding of patient IgG was detected with human IgG-specific secondary antibodies and quantitated by comparison with a standard curve (Fig. 5).
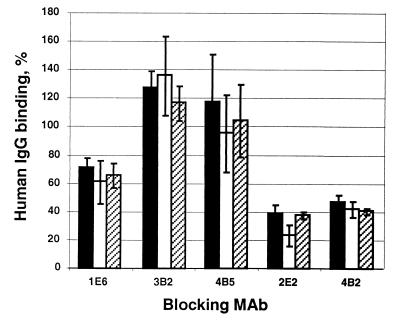
FIG. 5.
Competition between HEV-specific MAbs and convalescent-phase patient sera for binding to VLPs. VLP ELISA wells were reacted with saturating amounts of competing MAb before addition of patient sera, the binding of which was then detected with HRPO-conjugated anti-human IgG. Residual human anti-HEV binding was quantitated by comparison with a standard curve and calculated as a percentage of binding in the presence of irrelevant MAb 1H1. The mean and error of two separate experiments are shown. Solid bars, patient G31 (Nepal); open bars, patient G37 (Nepal); hatched bars, patient NIH116 (Mexico).
MAbs 3B2 and 4B5 failed to block patient antibody reactivity with VLPs and in some cases appeared to stimulate binding, although this varied between experiments. Conversely, MAb 1E6 (aa 434 to 457) reduced the level of patient IgG reactivity by 30 to 40%, while MAbs 2E2 and 4B2 (ORF2.1 conformational epitope) reduced patient reactivity by about 60% (Fig. 5), demonstrating that these epitopes are strongly immunodominant in the convalescent humoral response to HEV infection. The very similar results for the three patient sera, including NIH116, further suggest that this immunodominant antigenic region of the virus is highly conserved.
The characteristics of each of the HEV-specific MAbs are summarized in Table 1. Strikingly, the most efficient blocking of patient IgG binding to VLPs was achieved with 2E2 and 4B2, which demonstrated very weak reactivity with full-length PORF2 expressed in HepG2 cells, whereas 1E6 (moderate blocking activity) and other MAbs with no blocking activity showed very high reactivity with full-length PORF2.
TABLE 1.
Summary of MAb characteristics
| MAb | Isotype | Epitopea | PORF2 IFb | Blocking (%)c |
|---|---|---|---|---|
| 4B5 | IgG1 | 394–414, conformational | ++++ | 0–4 |
| 3B2 | IgG1 | 414–434 | ++++ | 0 |
| 1C7 | IgG1 | 414–434 | ++++ | NT |
| 1E6 | IgG2b | 434–457 | ++++ | 28–38 |
| 1E7 | IgG2b | 434–457 | ++++ | NT |
| 4B2 | IgG1 | ORF2.1, conformational | + | 52–59 |
| 2E2 | IgG1 | ORF2.1, conformational | +/− | 60–76 |
Epitope range in aa. Data are from Fig. 3.
Indirect immunofluorescence reactivity against HepG2 cells expressing full-length PORF2. ++++, very strong reactivity; +, weak reactivity; +/−, very weak reactivity. Data are from Fig. 1 and results not shown.
Blocking of convalescent-phase patient IgG binding to VLPs: range between respective means for three patient sera. Data are from Fig. 5. NT, not tested.
DISCUSSION
This study provides detailed information on the antigenic structure of HEV particles, implicating aa residues between 394 and 457 of PORF2 in the formation of immunodominant epitopes, including the conformational ORF2.1 epitope, on the surface of VLPs. It must be noted that such VLPs differ in size and some other physical properties from authentic HEV virions, including arrangement of subunits in a T=1 capsid containing 60 molecules of PORF2 rather than the T=3 180 molecules of PORF2 suspected in virions (41), and the PORF2 in VLPs is truncated at both the N and C termini (24), whereas nothing is known of the processing of PORF2 in virions; however, in the absence of reliable cell culture models for HEV and more detailed physical information on the infectious viral particle, they represent a useful model with which to study HEV antigenicity (24). We have previously shown that polyclonal antisera induced with the ORF2.1 protein can inhibit both acute-phase and convalescent-phase reactivity to VLPs by between 74 and 97% (21), but it is clear from the present study that the majority of the convalescent IgG repertoire is in fact directed to just a few epitopes in the viral capsid, with a minimum 60% directed against the conformational ORF2.1 epitope defined by MAbs 2E2 and 4B2 and at least 28% against the partially overlapping linear epitope within aa 434 to 457 defined by MAb 1E6 (Fig. 5).
It has been noted previously that VLPs have unique antigenic properties compared to full-length PORF2 expressed in eukaryotic cells, presumably due to the formation of conformational epitopes during assembly of the truncated PORF2 into particles (24, 25, 32). Interestingly, VLPs had approximately equivalent reactivity with saturating levels of all the MAbs studied (Fig. 2), whereas full-length PORF2 expressed in HepG2 cells had very low levels of reactivity with MAbs 2E2 and 4B2 compared with the other MAbs (Fig. 1). Taken together with the strong inhibition of VLP ELISA reactivity by MAbs 2E2 and 4B2 (Fig. 5), these results suggest that the conformational ORF2.1 epitope contributes most of the increased antigenic reactivity of VLPs compared to full-length PORF2.
Extensive competition was observed between the MAbs for binding to VLPs or GST-ORF2.1 (Fig. 4) despite the wide separation of epitopes, with the sequences recognized by 4B5 and 1E6 separated by a minimum of 19 nonoverlapping aa. These results suggest that the sequences between aa 394 and 457 are highly folded in order to bring supposedly distal epitopes close together, as well as contributing to the formation of the conformational ORF2.1 epitope. Computer-assisted secondary structure and hydrophilicity predictions of PORF2 (MacVector 3.5; Stratagene) do not reveal any striking features in this region, with beta sheets from aa 410 to 415 and 437 to 442 and random coils elsewhere, although the epitopes recognized by 3B2 (aa 414 to 434) and 1E6 (aa 434 to 457) fall within regions containing prominent peaks of hydrophilicity and flexibility (results not shown). However, the requirement for more C-terminal sequences in the correct formation of the ORF2.1 epitope (indicated by the lack of reactivity for P394-473; Fig. 3) suggests that more distal sequences may thus contribute to the overall structure of the epitope, perhaps by forming a scaffold for correct folding of sequences within individual protein chains or by promoting the juxtaposition of multiple protein chains in subunit-subunit interactions. Interestingly, the structure of HEV VLPs has recently been solved at 22 Å resolution by cryoelectron microscopy, revealing a T=1 particle with protruding dimers at the icosahedral twofold axes (16), and it is therefore possible that the ORF2.1 epitope is formed in the process of dimerization.
The linear epitopes recognized by MAbs in this study lie within domain 4 of PORF2, as defined by Khudyakov and colleagues (16), but domain 4 represents only a small fraction of the acute-phase response detected in that study, with much greater reactivity found in the N-terminal (domain 1) and extreme C-terminal (domain 6) regions of the protein. These results suggest that the humoral response to PORF2 is initially broad but then matures to a very limited spectrum of epitopes, of which the conformational ORF2.1 epitope on the surface of VLPs is immunodominant. This has obvious implications for the serological detection of past HEV infections, and the presence of the conformational ORF2.1 epitope undoubtedly contributes to the high sensitivity of both VLP (7, 24, 42) and ORF2.1 (2) ELISA for this purpose.
An understanding of antigenic structure will be important in the clinical evaluation of HEV vaccines. The development and implementation of effective vaccines for hepatitis A virus and hepatitis B virus over the past 20 years have been aided by serological assays which are highly predictive of immunity. For hepatitis A virus, it was shown that the majority of total antibody was directed against a small number of epitopes, with good correlation between reactivity in ELISA (and other serological assays) and protective, neutralizing antibody (19, 20, 27, 28, 34), while for hepatitis B virus, it is known that antibody to the highly immunodominant a determinant of small surface antigen is protective (10, 11, 39, 40). No such information is available for HEV, but if the ORF2.1 conformational and/or aa 434 to 457 epitopes are protective, then it is obviously important to measure their specific responses. Conversely, if these epitopes are not protective, then reactivity to these immunodominant epitopes might obscure the measurement of more important but minor responses. Experimental vaccines based on VLPs are clearly effective in animal models of HEV infection (37, 38, 43), and further study of the antibody responses induced by these and other vaccines with respect to the epitopes identified here will be useful for determining protective epitopes. The use of MAbs to block patient reactivity to one or more epitopes might prove useful in monitoring the responses of human vaccinees.
The abundance in convalescent-phase patient sera of antibody to the conformational ORF2.1 epitope leads us to speculate that vaccines which elicit a strong response to this epitope might represent the most promising candidates for broadly protective HEV vaccines, and we have shown that purified QE2.1 protein elicits such responses (21). Although the corresponding studies have not yet been performed for animals receiving the candidate VLP (37, 38, 43) or TrpE-C2 vaccines (30), we note that VLPs are more reactive with MAbs 2E2 and 4B2 than is the GST-ORF2.1 protein (Fig. 2) and might thus be expected to induce strong responses to the conformational ORF2.1 epitope, whereas C2 (aa 225 to 660) is completely unreactive with these MAbs, at least in Western immunoblotting (Fig. 3). DNA vaccines have been the focus of intense research, and this approach has also been used for HEV (9). Subsequent work by the same group has demonstrated that antibodies induced with a plasmid encoding full-length PORF2 are able to bind authentic HEV particles (8), but our results suggest that the majority of such antibodies are unlikely to be directed to the conformational ORF2.1 epitope, as this is very poorly modeled when full-length PORF2 is expressed in mammalian cells (Fig. 1). In addition, the majority of full-length PORF2 is rapidly degraded following heterologous expression in mammalian cells (35), and such rapid degradation tends to favor the induction of cellular rather than humoral immune responses (33). In order to induce antibodies to the conformational ORF2.1 epitope, we believe that a DNA or protein vaccine against HEV may need to be based on truncated forms of PORF2, such as aa 112 to 660 or ORF2.1. Preliminary studies have shown that the ORF2.1 fragment expressed in mammalian cells is indeed highly reactive with all the MAbs used in this study (F. Li and D. A. Anderson, unpublished data).
In this and previous studies (2, 21, 22), we have speculated that antibodies of the most abundant specificities during convalescence are likely to be protective, but it must be noted that while passive immunization with high-titer convalescent-phase macaque plasma was protective against HEV disease, it did not prevent infection (38), and passive immunization with IgG from a single convalescent-phase patient serum also failed to protect macaques from HEV infection (3). These results may suggest the importance of less immunodominant epitopes (and thus less abundant antibodies) and/or cellular immune responses in preventing HEV infection. Whether or not antibody to the conformational ORF2.1 epitope proves to be protective against disease, its strong immunodominance must be taken into account in studies towards the further development of HEV vaccines, and the MAbs described here should also prove useful in further studies of HEV biology, replication, and assembly.
ACKNOWLEDGMENTS
We thank Tian-Cheng Li, Naokazu Takeda, and Tatsuo Miyamura for the gift of purified VLPs; Morag Ferguson for the international reference HEV serum; Robert Purcell for patient sera; Scott Bowden for advice on MAb production; Heng-Fong Seow and Bo Lin for helpful discussions; Elizabeth Grgacic for critical reading of the manuscript; and Jenalle Chandler, Michelle Snooks, and Raquel Cowan for technical assistance.
These studies were supported in part by Project Grant number 950876 (D.A.A.) and the Dora Lush Postgraduate Research Scholarship (F.L.) from the National Health and Medical Research Council of Australia and by the Research Fund of the Macfarlane Burnet Centre for Medical Research.
REFERENCES
- 1.Adler B, Faine S. A pomona serogroup-specific, agglutinating antigen in Leptospira, identified by monoclonal antibodies. Pathology. 1983;15:247–250. doi: 10.3109/00313028309083501. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D A, Li F, Riddell M A, Howard T, Seow H-F, Torresi J, Perry G, Sumarsidi D, Shrestha S M, Shrestha I L. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J Virol Methods. 1999;81:131–142. doi: 10.1016/s0166-0934(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan A, Dilawari J B, Sharma R, Mukesh M, Saroa S R. Role of long-persisting human hepatitis E virus antibodies in protection. Vaccine. 1998;16:755–756. doi: 10.1016/s0264-410x(97)00252-1. [DOI] [PubMed] [Google Scholar]
- 4.Coursaget P, Buisson Y, Depril N, le Cann P, Chabaud M, Molinie C, Roue R. Mapping of linear B cell epitopes on open reading frames 2- and 3-encoded proteins of hepatitis E virus using synthetic peptides. FEMS Microbiol Lett. 1993;109:251–255. doi: 10.1111/j.1574-6968.1993.tb06176.x. [DOI] [PubMed] [Google Scholar]
- 5.Favorov M O, Khudyakov Y E, Mast E E, Yashina T L, Shapiro C N, Khudyakova N S, Jue D L, Onischenko G G, Margolis H S, Fields H A. IgM and IgG antibodies to hepatitis E virus (HEV) detected by an enzyme immunoassay based on an HEV-specific artificial recombinant mosaic protein. J Med Virol. 1996;50:50–58. doi: 10.1002/(SICI)1096-9071(199609)50:1<50::AID-JMV10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Gazina E V, Fielding J E, Lin B, Anderson D A. Core protein phosphorylation modulates pregenomic RNA encapsidation to different extents in human and duck hepatitis B viruses. J Virol. 2000;74:4721–4728. doi: 10.1128/jvi.74.10.4721-4728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghabrah T M, Tsarev S, Yarbough P O, Emerson S U, Strickland G T, Purcell R H. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134–137. [PubMed] [Google Scholar]
- 8.He J, Binn L N, Caudill J D, Asher L V, Longer C F, Innis B L. Antiserum generated by DNA vaccine binds to hepatitis E virus (HEV) as determined by PCR and immune electron microscopy (IEM): application for HEV detection by affinity-capture RT-PCR. Virus Res. 1999;62:59–65. doi: 10.1016/s0168-1702(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 9.He J, Hoffman S L, Hayes C G. DNA inoculation with a plasmid vector carrying the hepatitis E virus structural protein gene induces immune response in mice. Vaccine. 1997;15:357–362. doi: 10.1016/s0264-410x(97)00201-6. [DOI] [PubMed] [Google Scholar]
- 10.Howard C R, Allison L M. Hepatitis B surface antigen variation and protective immunity. Intervirology. 1995;38:35–40. doi: 10.1159/000150412. [DOI] [PubMed] [Google Scholar]
- 11.Iwarson S, Tabor E, Thomas H C, Goodall A, Waters J, Snoy P, Shih J W, Gerety R J. Neutralization of hepatitis B virus infectivity by a murine monoclonal antibody: an experimental study in the chimpanzee. J Med Virol. 1985;16:89–96. doi: 10.1002/jmv.1890160112. [DOI] [PubMed] [Google Scholar]
- 12.Kaur M, Hyams K C, Purdy M A, Krawczynski K, Ching W M, Fry K E, Reyes G R, Bradley D W, Carl M. Human linear B-cell epitopes encoded by the hepatitis E virus include determinants in the RNA-dependent RNA polymerase. Proc Natl Acad Sci USA. 1992;89:3855–3858. doi: 10.1073/pnas.89.9.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khudyakov Y E, Favorov M O, Jue D L, Hine T K, Fields H A. Immunodominant antigenic regions in a structural protein of the hepatitis E virus. Virology. 1994;198:390–393. doi: 10.1006/viro.1994.1048. [DOI] [PubMed] [Google Scholar]
- 14.Khudyakov Y E, Favorov M O, Khudyakova N S, Cong M E, Holloway B P, Padhye N, Lambert S B, Jue D L, Fields H A. Artificial mosaic protein containing antigenic epitopes of hepatitis E virus. J Virol. 1994;68:7067–7074. doi: 10.1128/jvi.68.11.7067-7074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khudyakov Y E, Khudyakova N S, Fields H A, Jue D, Starling C, Favorov M O, Krawczynski K, Polish L, Mast E, Margolis H. Epitope mapping in proteins of hepatitis E virus. Virology. 1993;194:89–96. doi: 10.1006/viro.1993.1238. [DOI] [PubMed] [Google Scholar]
- 16.Khudyakov Y E, Lopareva E N, Jue D L, Crews T K, Thyagarajan S P, Fields H A. Antigenic domains of the open reading frame 2-encoded protein of hepatitis E virus. J Clin Microbiol. 1999;37:2863–2871. doi: 10.1128/jcm.37.9.2863-2871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krawczynski K. Hepatitis E. Hepatology. 1993;17:932–941. [PubMed] [Google Scholar]
- 18.Lee J W, Kim K, Jung S H, Lee K J, Choi E C, Sung Y C, Kang C Y. Identification of a domain containing B-cell epitopes in hepatitis C virus E2 glycoprotein by using mouse monoclonal antibodies. J Virol. 1999;73:11–18. doi: 10.1128/jvi.73.1.11-18.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemon S M. Immunologic approaches to assessing the response to inactivated hepatitis A vaccine. J Hepatol. 1993;18(Suppl. 2):S15–S19. doi: 10.1016/s0168-8278(05)80372-1. [DOI] [PubMed] [Google Scholar]
- 20.Lemon S M, Murphy P C, Provost P J, Chalikonda I, Davide J P, Schofield T L, Nalin D R, Lewis J A. Immunoprecipitation and virus neutralization assays demonstrate qualitative differences between protective antibody responses to inactivated hepatitis A vaccine and passive immunization with immune globulin. J Infect Dis. 1997;176:9–19. doi: 10.1086/514044. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Riddell M A, Seow H F, Takeda N, Miyamura T, Anderson D A. Recombinant subunit ORF2.1 antigen and induction of antibody against immunodominant epitopes in the hepatitis E virus capsid protein. J Med Virol. 2000;60:379–386. doi: 10.1002/(sici)1096-9071(200004)60:4<379::aid-jmv3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Torresi J, Locarnini S A, Zhuang H, Zhu W, Guo X, Anderson D A. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J Med Virol. 1997;52:289–300. doi: 10.1002/(sici)1096-9071(199707)52:3<289::aid-jmv10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Zhuang H, Kolivas S, Locarnini S A, Anderson D A. Persistent and transient antibody responses to hepatitis E virus detected by Western immunoblot using open reading frame 2 and 3 and glutathione S-transferase fusion proteins. J Clin Microbiol. 1994;32:2060–2066. doi: 10.1128/jcm.32.9.2060-2066.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T C, Yamakawa Y, Suzuki K, Tatsumi M, Razak M A, Uchida T, Takeda N, Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–7213. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAtee C P, Zhang Y, Yarbough P O, Bird T, Fuerst T R. Purification of a soluble hepatitis E open reading frame 2-derived protein with unique antigenic properties. Protein Expr Purif. 1996;8:262–270. doi: 10.1006/prep.1996.0099. [DOI] [PubMed] [Google Scholar]
- 26.Meng J, Pillot J, Dai X, Fields H A, Khudyakov Y E. Neutralization of different geographic strains of the hepatitis E virus with anti-hepatitis E virus-positive serum samples obtained from different sources. Virology. 1998;249:316–324. doi: 10.1006/viro.1998.9346. [DOI] [PubMed] [Google Scholar]
- 27.Ping L H, Jansen R W, Stapleton J T, Cohen J I, Lemon S M. Identification of an immunodominant antigenic site involving the capsid protein VP3 of hepatitis A virus. Proc Natl Acad Sci USA. 1988;85:8281–8285. doi: 10.1073/pnas.85.21.8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ping L H, Lemon S M. Antigenic structure of human hepatitis A virus defined by analysis of escape mutants selected against murine monoclonal antibodies. J Virol. 1992;66:2208–2216. doi: 10.1128/jvi.66.4.2208-2216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugh J C, Di Q, Mason W S, Simmons H. Susceptibility to duck hepatitis B virus infection is associated with the presence of cell surface receptor sites that efficiently bind viral particles. J Virol. 1995;69:4814–4822. doi: 10.1128/jvi.69.8.4814-4822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purdy M A, McCaustland K A, Krawczynski K, Spelbring J, Reyes G R, Bradley D W. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV) J Med Virol. 1993;41:90–94. doi: 10.1002/jmv.1890410118. [DOI] [PubMed] [Google Scholar]
- 31.Reyes G R, Purdy M A, Kim J P, Luk K C, Young L M, Fry K E, Bradley D W. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 32.Robinson R A, Burgess W H, Emerson S U, Leibowitz R S, Sosnovtseva S A, Tsarev S, Purcell R H. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75–84. doi: 10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez F, Zhang J, Whitton J L. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton J T, Raina V, Winokur P L, Walters K, Klinzman D, Rosen E, McLinden J H. Antigenic and immunogenic properties of recombinant hepatitis A virus 14S and 70S subviral particles. J Virol. 1993;67:1080–1085. doi: 10.1128/jvi.67.2.1080-1085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torresi J, Li F, Locarnini S A, Anderson D A. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J Gen Virol. 1999;80:1185–1188. doi: 10.1099/0022-1317-80-5-1185. [DOI] [PubMed] [Google Scholar]
- 36.Torresi J, Meanger J, Lambert P, Li F, Locarnini S A, Anderson D A. High level expression of the capsid protein of hepatitis E virus in diverse eukaryotic cells using the Semliki Forest virus replicon. J Virol Methods. 1997;69:81–91. doi: 10.1016/s0166-0934(97)00142-0. [DOI] [PubMed] [Google Scholar]
- 37.Tsarev S A, Tsareva T S, Emerson S U, Govindarajan S, Shapiro M, Gerin J L, Purcell R H. Recombinant vaccine against hepatitis E: dose response and protection against heterologous challenge. Vaccine. 1997;15:1834–1838. doi: 10.1016/s0264-410x(97)00145-x. [DOI] [PubMed] [Google Scholar]
- 38.Tsarev S A, Tsareva T S, Emerson S U, Govindarajan S, Shapiro M, Gerin J L, Purcell R H. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci USA. 1994;91:10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters J A, Brown S E, Steward M W, Howard C R, Thomas H C. Analysis of the antigenic epitopes of hepatitis B surface antigen involved in the induction of a protective antibody response. Virus Res. 1992;22:1–12. doi: 10.1016/0168-1702(92)90085-n. [DOI] [PubMed] [Google Scholar]
- 40.Waters J A, O'Rourke S M, Richardson S C, Papaevangelou G, Thomas H C. Qualitative analysis of the humoral immune response to the “a” determinant of HBs antigen after inoculation with plasma-derived or recombinant vaccine. J Med Virol. 1987;21:155–160. doi: 10.1002/jmv.1890210207. [DOI] [PubMed] [Google Scholar]
- 41.Xing L, Kato K, Li T, Takeda N, Miyamura T, Hammar L, Cheng R H. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology. 1999;265:35–45. doi: 10.1006/viro.1999.0005. [DOI] [PubMed] [Google Scholar]
- 42.Yarbough P O, Garza E, Tam A W, Zhang Y, McAtee P, Fuerst T R. Assay development of diagnostic tests for IgM and IgG antibody to hepatitis E virus. In: Buisson Y, Coursaget P, Kane M, editors. Enterically-transmitted hepatitis viruses. Joue-les-Tours, France: La Simarre; 1996. pp. 294–296. [Google Scholar]
- 43.Yarbough P O, Krawczynski K, Tam A W, McAtee C P, McCaustland K A, Zhang Y, Garcon N, Spellbring J, Carson D, Myriam F, Lifson J D, Slaoui M, Prieels J P, Margolis H, Fuerst T R. Prevention of hepatitis E using r62K ORF 2 subunit vaccine: full protection against heterologous HEV challenge in cynomolgus macaques. In: Rizzetto M, Purcell R H, Gerin J L, Verme G, editors. Viral hepatitis and liver disease. Turin, Italy: Edizioni Minervi Medica; 1997. pp. 650–655. [Google Scholar]