Abstract
Heart failure with reduced ejection fraction (HFrEF) represents an emerging epidemic, particularly affecting frail, older, and multimorbid patients. Current therapy for the management of HFrEF includes four different classes of disease-modifying drugs, commonly referred to as ‘four pillars’, which target the neurohormonal system that is overactivated in HF and contributes to its progression. These classes of drugs include β-blockers, inhibitors of the renin-angiotensin-aldosterone system, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors. Unfortunately, these agents cannot be administered as frequently as needed to older patients because of poor tolerability and comorbidities. In addition, although these drugs have dramatically increased the survival expectations of patients with HF, their residual risk of rehospitalization and death at 5 years remains considerable. Vericiguat, a soluble guanylate cyclase (sGC) stimulator, was reported to exert beneficial effects in patients with worsening HF, including older subjects, reducing the rate of both hospitalizations and deaths, with limited adverse effects and drug interaction. In this narrative review, we present the current state of art on vericiguat, with a particular focus on elderly and frail patients.
Key Points
| Four pillar heart failure (HF) therapy is often poorly tolerated by older patients, limiting adherence to treatment. |
| Vericiguat shows promise for older and frail patients with worsening HF with reduced ejection fraction (HFrEF), targeting a new pathway in HF pathophysiology and offering a well-tolerated, more manageable option. |
| Future studies to clarify the role of vericiguat in different HFrEF subgroups, particularly in older patients, are required. |
Introduction
Despite the recent therapeutic advances, heart failure (HF) still represents a public health concern, particularly in older and frail patients, with a prevalence rate among individuals over the age of 75 years exceeding 10% [1]. HF is also linked to substantial rates of morbidity and mortality, as well as frequent hospital admissions, significantly impacting both the quality of life of patients and the financial burden on healthcare national systems and health insurance systems [1]. Notably, individuals aged 65 years or older comprise 80% of HF hospitalizations and account for 90% of HF-related deaths [2]. European Society of Cardiology (ESC) guidelines highlight that frailty, which makes one more prone to stressors, often occurs alongside HF although they are separate conditions [3]. Current therapies for HF include four disease-modifying drugs referred to as ‘pillars’: β-blockers, inhibitors of the renin-angiotensin-aldosterone system (RAAS), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose co-transporter-2 (SGLT2) inhibitors [1]. However, these drugs often show poor tolerability in older patients, and thus not allowing clinicians to properly administer the optimal medical therapy [4, 5]. Furthermore, while early initiation of guideline-directed medical therapy (GDMT) is recommended in patients with worsening HF, which occurs when symptoms of chronic HF escalate, thereby requiring urgent treatment adjustments such as intravenous diuretics or hospitalization, the benefits of this strategy in the specific group of older and complex patients are not well clarified [6–8]. Increasing lines of evidence suggest that the soluble guanylate cyclase (sGC) stimulator vericiguat is well tolerated and effective in older patients with worsening HF, reducing the rate of hospitalizations and deaths. The aim of this narrative review is to present the current state of art on vericiguat, with a particular focus on older and frail patients, and to suggest future perspectives of research.
An Overview of Vericiguat
A growing body of preclinical and clinical evidence revealed that the stimulation of the nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate (NO-sGC-cGMP) pathway by sGC stimulators represents a potential therapeutic approach for the management of HF [9]. The sGC enzyme is an intracellular nitric oxide (NO) receptor that catalyzes the synthesis of the second messenger cyclic guanosine monophosphate (cGMP), when activated [10]. Cardiac expression of sGC is decreased in HF, along with a reduced bioavailability of NO, leading to the development of myocardial and vascular dysfunction [11, 12]. Indeed, the cGMP pathway is a crucial regulator of myocardial contractility, endothelial function and smooth muscle tone [11, 12]. Restoration of the NO-sGC-cGMP pathway may improve cardiac function in HF in addition to the inhibition of the neurohormonal system [10]. Vericiguat is the first oral sGC stimulator approved to treat adults with HF with reduced ejection fraction (HFrEF) and evidence of clinical worsening [13, 14]. Vericiguat exhibits a bifunctional mechanism of action in the restoration of the cGMP pathway, and enhances both the sensitivity of sGC to suboptimal concentrations of NO and directly activates sGC in the absence of endogenous NO [12, 15]. In this regard, previous studies suggest that the efficacy of vericiguat is also evident in the presence of low concentrations of endogenous NO [12, 16]. Vericiguat acts as a potent vasodilator and also exerts anti-inflammatory and antifibrotic effects [11]. Preclinical studies also demonstrated that vericiguat improves myocardial remodeling and diastolic relaxation [17]. Oral vericiguat is approved for the treatment of worsening HFrEF in several countries, including the United States (US) and European Union (EU) [3, 11, 15, 18]. In the EU, vericiguat is indicated to treat HFrEF patients stabilized after a recent decompensation event requiring intravenous therapy [11, 15, 18]. The recommended starting dose of vericiguat is 2.5 mg taken orally once daily with food. Subsequent dose escalation is achieved by doubling the dosage every 2 weeks, until a dose of 10 mg once daily is reached [18, 19]. The half-life of vericiguat in patients with HF is estimated to be around 30 h, while the time to reach steady state is approximately 6 days. Furthermore, approximately 98% of the drug binds to plasma proteins [18, 19]. No dose modifications or specific precautions are considered necessary in older patients or in the presence of mild-to-moderate impairment of renal or hepatic function [9, 13, 20]. However, no data are available regarding the impact of vericiguat on individuals with severe renal dysfunction (stage V chronic kidney disease [CKD] or receiving dialysis) or hepatic impairment (Child–Pugh C). For these reasons, the use of vericiguat is not recommended in these patients within the EU and further investigations are needed to evaluate its safety and efficacy in these cohorts [9, 13]. While no relevant adverse effects were reported with the use of vericiguat in conjunction with other medications, its use with other sGC stimulators, such as riociguat, is contraindicated [9, 17]. Concomitant administration of vericiguat and phosphodiesterase-5 (PDE-5) inhibitors is also not recommended [9, 11]. Vericiguat should also be avoided in patients with orthostatic hypotension, since symptomatic hypotension should be prevented in patients with systolic blood pressure below 100 mmHg [9, 11]. In fact, in the VICTORIA trial, symptomatic hypotension represented a frequent adverse effect related to vericiguat administration, irrespective of the patient’s age [21]. Other adverse events associated with vericiguat include gastrointestinal disorders (e.g., nausea and dyspepsia), anemia, adverse liver events, and acute kidney injury, although these adverse events are observed with low frequencies [9, 15, 22, 23]. Preclinical studies also demonstrated that vericiguat may cause harm to the fetus, suggesting that its administration should be avoided during pregnancy [9]. No clinically significant pharmacodynamic interactions occurred when vericiguat was coadministered with an oral angiotensin receptor/neprilysin inhibitor (ARNI), aspirin, warfarin, or short-acting nitrates in preclinical and phase I drug interaction studies [9, 13, 24]. Recent data from a US registry focusing on patients hospitalized for HF with an ejection fraction of < 45% showed that the features of about 40% of these patients were consistent with the features of patients in the VICTORIA trial, and more than 90% met the criteria for vericiguat treatment based on US FDA guidelines [25]. A nationwide longitudinal cohort study in Germany showed that in a real-world context, adherence and persistence to vericiguat across various age categories was satisfactory and that the initiation of vericiguat treatment was associated with the intensification of concurrent GDMT [26]. Furthermore, participants in the VICTORIA trial received appropriate treatment for HFrEF, and the effectiveness of vericiguat remained stable regardless of the underlying treatment, with adherence to guidelines being notably high [27].
The Nitric Oxide-Soluble Guanylate Cyclase-Cyclic Guanosine Monophosphate (NO-sGC-cGMP) Pathway
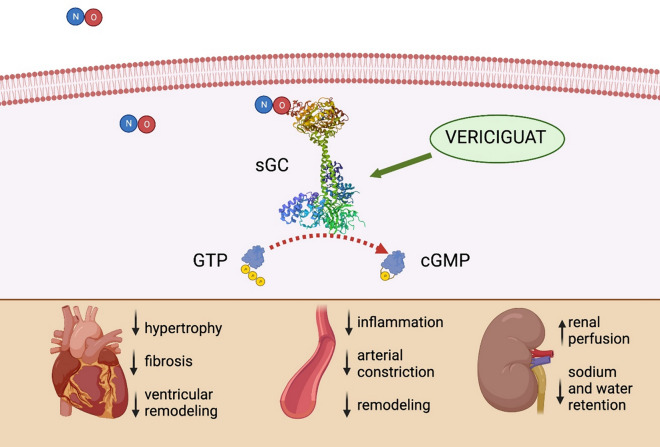
The NO-sGC-cGMP pathway plays a significant role in regulating several physiological processes, especially in endothelial and vascular muscle cells (Fig. 1) [10, 15]. NO is generated from L-arginine in endothelial cells and rapidly spreads into vascular smooth muscle cells (VSMCs), where it binds to the heme subunit of sGC, thereby catalyzing the conversion of guanosine triphosphate (GTP) into cGMP, which serves as a second intracellular messenger [15, 28]. Once produced, cGMP exerts vasodilatory effects and is also involved in the inhibition of platelet aggregation and VSMC relaxation [15, 28]. Impairment of the NO-sGC-cGMP pathway is a hallmark of several pathological conditions, such as hypertension, HF, and erectile dysfunction [15, 28]. Impairment of NO-sGC-cGMP signaling in HF is the cause of endothelial dysfunction and leads to inflammation and oxidative stress and also to reduced NO bioavailability, which in turn contributes to sGC oxidization and lack of response to NO [15, 28]. Three main causes of NO reduction can be identified in HF: (1) decreased bioavailability of l-arginine; (2) downregulation or uncoupling of endothelial nitric oxide synthase (eNOS), the enzyme responsible for the majority of the NO produced by endothelial cells; and (3) inactivation of NO by superoxide anion, a type of reactive oxygen species (ROS) [19, 28]. Therefore, inadequate stimulation of sGC in systemic, pulmonary, coronary, and renal vessels leads to impaired protection in response to ischemia/reperfusion, myocardial dysfunction and adverse left ventricular remodeling [15, 28, 29]. The reduced cGMP availability observed in advanced HF leads to vasoconstriction and vascular stiffness, and thus to decreased renal and coronary blood flow, worsening the respective organ dysfunction [28, 29]. Vericiguat may also exert a mild inotropic effect, since cGMP also induces titin phosphorylation by protein kinase G (PKG), enhancing cardiac index and attenuating left ventricular remodeling [19, 30]. Among extracardiac and pleiotropic effects, some evidence also suggests that the impairment of this pathway is involved in the development of renal fibrosis [31]. Therefore, restoration of the NO-sGC-cGMP signaling pathway activity is a promising therapeutic strategy for HF, in order to arrest the pathophysiological response observed in HF beyond inhibition of the neurohormonal system and the reduction of afterload.
Fig. 1.
Impact of vericiguat in modulating the NO-sGC-cGMP pathway. NO-sGC-cGMP nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate, GTP guanosine triphosphate
Current Recommendations from Guidelines
Gold-standard treatment of HFrEF involves the inhibition of neurohormonal activation, targeting the RAAS and sympathetic nervous system, conveying to RAAS inhibitors, ARNIs and β-blockers a paramount importance for reducing the risk of death in patients with HF [1, 32]. SGLT2 inhibitors have also acquired a major role for reducing mortality in patients with HFrEF [3, 33]. According to current guidelines for the treatment of chronic HF released by the ESC in 2021, vericiguat may be considered in worsening HFrEF (with New York Heart Association [NYHA] class II–IV) despite the concomitant administration of an angiotensin-converting enzyme inhibitor (ACEi) [or ARNI], a β-blocker, or an MRA in order to reduce cardiovascular mortality or HF hospitalization (IIB) [1, 33]. This recommendation is based on the observations derived from the recent phase III VICTORIA trial. The 2021 guidelines were updated in 2023 with a specific document that did not add any new indications for the use of vericiguat [33]. Compared with other recent clinical trials performed in patients with HFrEF, the VICTORIA trial enrolled patients with more severe disease and who were older and more prone to acute decompensation, therefore better reflecting a real-world clinical scenario [10, 15, 34, 35]. The results of the VICTORIA trial show that the addition of oral vericiguat to standard of care significantly reduces the risk of cardiovascular death or hospitalization from HF [15, 34]. However, there were no statistically significant differences in the occurrence of CV death alone and all-cause mortality among the groups. Although these results are encouraging, both the ESC guidelines and the joint guidelines of the American College of Cardiology, American Heart Association, and Heart Failure Society of America for chronic HF provided limited therapeutic significance to vericiguat (Table 1). These limitations are mainly attributable to the lack of a significant effect on CV death, a smaller number of enrolled patients (5050), and a shorter median follow-up duration (11 months) compared with other landmark studies such as the PARADIGM-HF trial (which included 8442 patients and had a median follow-up period of 27 months) aimed at testing ARNI versus enalapril in HFrEF [32]. These factors contributed to a cautious approach in current guidelines for the use of vericiguat in patients with HFrEF [34, 36]. It is plausible to hypothesize that a longer follow-up and a larger cohort may potentially unveil additional benefits on primary endpoints and mortality, improving the outcomes already achieved. However, in line with current guidelines, vericiguat represents a useful tool to reduce hospitalizations in patients already receiving gold-standard medical therapy and with a history of several previous HF decompensations, but its role, in addition to the four pillars of HFrEF, as starting therapy may need additional supporting evidence.
Table 1.
Current recommendations from guidelines
| Society | Recommendation | Class of evidence |
|---|---|---|
| ESC (2021) | Vericiguat may be considered in worsening HFrEF patients (NYHA class II–IV) despite the administration of an ACEi (or ARNI), a β-blocker, or an MRA with the aim of reducing mortality or HF hospitalization | IIB |
| AHA/ACC/HFSA (2022) | Vericiguat may be considered to reduce hospitalizations and cardiovascular death in high-risk patients with HFrEF and recent worsening HF who are already receiving OMT | IIB |
ACC American College of Cardiology, ACEi angiotensin converting enzyme inhibitor, AHA American Heart Association, ARNI angiotensin receptor/neprilysin inhibitor, ESC European Society of Cardiology, HF heart failure, HFrEF heart failure with reduced ejection fraction, HFSA Heart Failure Society of America, NYHA New York Heart Association, OMT optimal medical therapy, MRA mineralocorticoid receptor antagonist
Main Clinical Trials
In addition to the VICTORIA phase III study, the use of vericiguat in HFrEF was evaluated in the SOCRATES-REDUCED phase II study [34, 37]. Both trials suggest that vericiguat is well tolerated and effective for the treatment of HFrEF patients with recent cardiac decompensation, with an absolute event-rate reduction [34, 37]. The SOCRATES-REDUCED trial evaluated the tolerability and optimal dose of vericiguat in HFrEF patients in addition to standard therapy [37]. The inclusion criteria were a diagnosis of chronic HFrEF below 45% in NYHA functional class II–IV; already receiving optimal medical therapy for at least 30 days before the last hospitalization from HF; a recent episode of worsening chronic HF within 4 weeks of randomization (defined in terms of hospitalization for HF or outpatient administration of intravenous diuretics); signs and symptoms of congestion and elevated levels of brain natriuretic peptide (BNP); and left atrial enlargement at randomization [37]. A total of 456 patients were enrolled in the study, of whom 351 successfully completed the designated treatment. The mean left ventricular ejection fraction (LVEF) for these patients was approximately 30% [37]. All enrolled patients had already received GDMT for a minimum duration of 1 month prior to their hospitalization for HF, or administration of intravenous diuretics in an outpatient setting [37]. Patients were randomized to vericiguat at different doses (1.25, 2.5, 5 or 10 mg daily) versus placebo, for a total treatment duration of 12 weeks [37]. The primary endpoint of the study was to assess the alteration in log-transformed levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) from baseline to week 12 compared with the placebo group [37]. The primary endpoint in an initial analysis did not show any statistically significant difference between the whole pooled vericiguat group versus the placebo group; however, in a secondary exploratory analysis, performed trough linear regression analysis, higher doses of vericiguat were found to be associated with a greater reduction in NT-proBNP values, suggesting a dose-response effect [37]. Furthermore, a positive trend was observed regarding the reduction of combined cardiovascular mortality and hospitalization due to HF in subjects receiving the higher doses (5 and 10 mg) of vericiguat [37]. Finally, a slight but significant increase in LVEF was observed in patients receiving the 10 mg dose, suggesting the crucial importance of the uptitration strategy for HF therapies [37]. Vericiguat also demonstrated a favorable safety profile throughout the 12-week period, such as high tolerability and no effect on systolic and diastolic blood pressure levels and mean heart rate [15, 37]. The recent phase III VICTORIA trial was a randomized, multicenter study that evaluated, in terms of efficacy, the addition of vericiguat to optimal medical therapy versus placebo in HFrEF patients [34]. The inclusion criteria included a diagnosis of HFrEF with NYHA functional classes II–IV, with LVEF below 45% assessed within 12 months prior to randomization; BNP levels above 300 pg/mL or NT-proBNP levels above 1000 pg/mL if in sinus rhythm (BNP above 500 pg/mL or NT-proBNP above 1600 pg/mL if in atrial fibrillation) within 30 days prior to randomization; and prior hospitalization as a result of HF within 6 months, or outpatient diuretic therapy for HF within 3 months prior to randomization [34]. As previously stated, the study enrolled 5050 patients with a mean LVEF of approximately 30% who were randomized to vericiguat (1.25, 2.5, 5 or 10 mg daily) [34]. The doses were uptitrated at 2-week intervals and the target dose (10 mg) was reached in 89.2% of cases [34]. The primary endpoint included a composite of cardiovascular death or first hospitalization from HF, while the secondary outcomes were the components of the primary outcome, with a reduction in the incidence of the primary outcomes in the vericiguat group versus placebo [34]. The results showed a positive 10% relative difference reduction, with an annualized absolute risk reduction of 4.2 events per 100 patient-years; however, this positive result was basically driven by the reduction in hospitalizations rather than cardiovascular deaths [34]. A subgroup analysis also revealed that patients who were randomized after a longer duration since their last hospitalization showed the greatest benefits. This suggests that the observed effect is not attributable to the initial therapy at baseline, but rather reflects an independent effect of vericiguat [34, 38]. Indeed, concomitant use of vericiguat and sacubitril/valsartan did not show a cumulative effect [24]. The efficacy of vericiguat was also reported to be independent of renal function at baseline, for instance in patients whose glomerular filtration rate (eGFR) decreased over time [23]. In addition, a decrease in the primary composite endpoint and its components, i.e. cardiovascular death and hospitalization for HF, was noted for NT-proBNP levels up to 8000 pg/mL [34, 39]. Further insights from the VICTORIA trial revealed that the efficacy of vericiguat was unaffected by the dosage of loop diuretics or the concurrent use of sacubitril/valsartan, and was independent of age and sex [24, 40, 41]. The effects of vericiguat were also evaluated in patients with HF with preserved ejection fraction (HFpEF). The phase II SOCRATES–PRESERVED study, which analyzed the same primary outcome as the SOCRATES-REDUCED study, showed an improvement in quality of life, measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score [42]. In contrast, the phase III VITALITY trial failed to show significant differences in quality of life and exercise tolerance between patients receiving vericiguat and those receiving placebo [43]. Significant differences arise when comparing the DAPA-HF, EMPEROR-Reduced, and VICTORIA trials in terms of baseline risk profile and severity of HF at the time of randomization [34, 36, 44]. Patients from the VICTORIA trial were older and more vulnerable compared with those enrolled in the EMPEROR-Reduced and DAPA-HF studies; all patients in the VICTORIA trial had a recent episode of HF, whereas this was the case for only 62.5% and 47.5% of patients in the PARADIGM-HF and DAPA-HF studies, respectively [32, 45]. VICTORIA patients also exhibited higher values of NT-proBNP and were more symptomatic given the greater number of patients belonging to NYHA classes III–IV compared with the PARADIGM-HF and DAPA-HF trials [32, 34, 45]. A recent pooled meta-analysis combining data from the VICTORIA and VITALITY-HFpEF trials revealed that vericiguat significantly benefits HF patients with an LVEF < 45%, particularly in those with LVEF below 24% [46]. In summary, results derived from clinical trials indicate that vericiguat is a well-tolerated drug that may be useful, especially in those patients with several comorbidities and worsening HFrEF. In this cohort of patients, the beneficial effects of the current armamentarium of drugs used for the treatment of HF may be limited, and targeting an additional molecular mechanism, such as the sGC-NO-cGMP pathway, may represent a successful strategy.
All detailed features of the trials discussed are presented in Tables 2 and 3.
Table 2.
Clinical trials evaluating vericiguat in HF patients with <45% LVEF
| Trial | Inclusion criteria | Methods | Endpoints | Results |
|---|---|---|---|---|
|
SOCRATES-REDUCED Phase: IIB Year of publication: 2015 |
HF with LVEF <45% Recent episode of HF decompensation defined by three components: (1) hospitalization or outpatient administration of IV diuretics; (2) signs of congestion; (3) natriuretic peptide level |
Total patients: 351 Randomization: Placebo: 92 1.25 mg: 92 2.5 mg: 91 5 mg: 91 10 mg: 91 Follow-up: 12 weeks |
Reduction in levels of NT-proBNP at 12 weeks |
No significant difference (p = 0.15) between the vericiguat ‘pooled’ and placebo groups Secondary analyses focused on the 10 mg group evidenced a reduction in NTproBNP levels (p = 0.48); increase in LVEF (p = 0.02); and decrease of CV deaths + HF hospitalizations |
|
VICTORIA Phase: III Year of publication: 2020 |
HF with LVEF <45% (NYHA II–IV) BNP ≥300 ng/L (≥500 ng/L if AF), or NT-proBNP ≥1000 ng/L (≥1600 ng/L if AF) History of hospitalization (within 6 months) or IV diuretic use (within 3 months) |
Total patients: 5050 Randomization: Placebo: 2524 10 mg: 2526 Follow-up: 10.8 months |
Mortality from CV causes + HF hospitalization Mortality from CV causes HF hospitalization |
37.9% in the vericiguat group versus 40.9% in the placebo group (HR 0.90, 95% CI 0.83–0.98; p = 0.02) 16.4% in the vericiguat group versus 17.5% in the placebo group (HR 0.93, 95% CI 0.81–1.06) 27.4% in the vericiguat group versus 29.6% in the placebo group (HR 0.90, 95% CI 0.81–1.00) |
AF atrial fibrillation, BNP brain natriuretic peptide, CI confidence interval, CV cardiovascular, HF heart failure, HR hazard ratio, IV intravenous, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-brain natriuretic peptide, NYHA New York Heart Association
Table 3.
Clinical trials evaluating vericiguat in HF patients with >45% LVEF
| Trial | Inclusion criteria | Methods | Endpoints | Results |
|---|---|---|---|---|
|
SOCRATES-PRESERVED Phase: IIB Year of publication: 2017 |
HF with LVEF >45% (NYHA II–IV) BNP ≥100 ng/L (≥200 ng/L if AF) or NT-proBNP ≥300 ng/L (>600 ng/L if AF) at randomization LA enlargement assessed on TT echocardiogram Recent episode of HF decompensation (within <4 weeks) defined as worsening of symptoms requiring hospitalization or IV use of diuretic |
Total patients: 477 Randomization: Placebo: 93 1.25 mg: 96 2.5 mg: 95 5 mg: 95 10 mg: 96 Follow-up: 12 weeks |
Reduction in NT-proBNP levels and LA volume (mL) at 12 weeks |
No significant difference in NT-proBNP and LA volume (mL) [p = 0.15] between the vericiguat ‘pooled’ and placebo groups Secondary analyses focused on the 10 mg group evidenced: the KCCQ Clinical Summary Score improved by a mean 19.3 ± 16.3 points (median 19.8, IQR 10.4–30.7) from baseline (mean difference from placebo 9.2 points) |
|
VITALITY HFpEF Phase: IIB Year of publication: 2020 |
HF with LVEF >45% (NYHA II–IV) BNP ≥100 ng/L (≥200 ng/L if AF) or NT-proBNP ≥300 ng/L (>600 ng/L if AF) within 30 days after randomization Left atrium enlargement or left ventricular hypertrophy assessed by TT echocardiogram within 12 months after randomization Recent episode of HF decompensation (within 6 months) defined as worsening of symptoms requiring hospitalization or IV use of diuretic |
Total patients: 789 Randomization: Placebo: 262 10 mg: 263 15 mg: 264 Follow-up: 24 weeks |
Change in the KCCQ PLS Change in 6-min walking distance |
No significant difference between the three groups |
AF atrial fibrillation, BNP brain natriuretic peptide, HF heart failure, HFpEF heart failure with preserved ejection fraction, IQR interquartile range, IV intravenous, KCCQ Kansas City Cardiomyopathy Questionnaire, LA left atrium, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-brain natriuretic peptide, NYHA New York Heart Association, PLS Physical Limitation Score, TT transthoracic
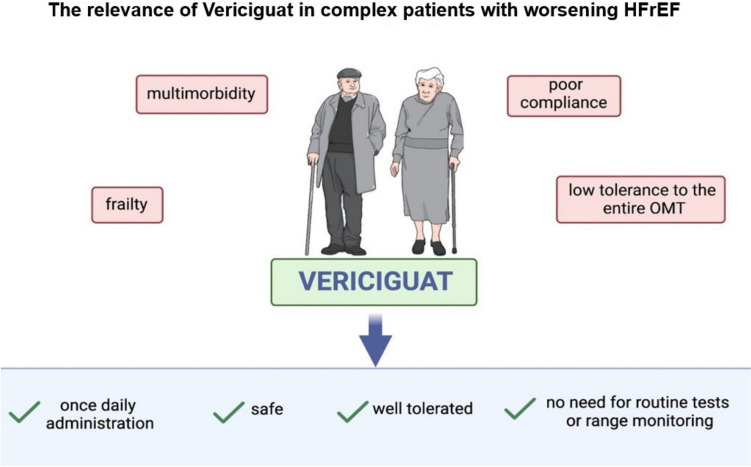
Vericiguat in Older and Complex Patients
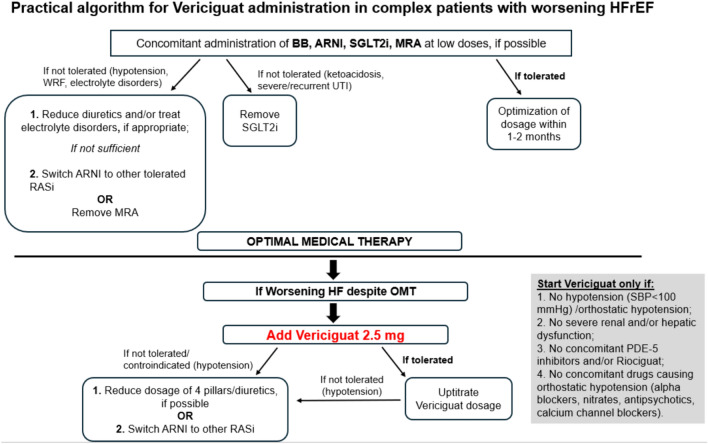
As previously stated, in the recent VICTORIA trial, patients exhibited notable differences compared with previous HFrEF trials [32, 44]. These individuals were older and showed pronounced symptoms, as well as elevated levels of NT-proBNP [34]. Compared with other therapies for HF, vericiguat only requires once daily administration, is well tolerated and does not show significant adverse events and hemodynamic impact [13]. Furthermore, vericiguat does not require routine laboratory testing or therapeutic drug monitoring [47, 48]. All these features suggest that vericiguat is a viable therapy for older and frail patients (Fig. 2). For instance, RAAS inhibitors can lead to angioedema, cough, and hypotension; MRAs can lead to hyperkalemia; and SGLT2 inhibitors can lead to urinary tract infections [3, 33]. All these drugs must be administered with caution in case of renal impairment, and their simultaneous administration is often difficult to implement [3, 33]. Vericiguat may be administered to all HFrEF patients who do not adequately respond to the classic quadruple therapy for HF due to many comorbidities (i.e. CKD or hyperkalemia) or poor compliance (i.e. patients with poor functional autonomy, hypotension) [3, 49]. In addition, it is worth noting that, according to the recent 2023 focus update on HF, while initiating and quickly uptitrating HFrEF therapy before discharge, followed by detailed follow-ups in the first 6 weeks, is recommended to reduce the risk of readmission or death, patients with worsening HFrEF continue to face a significant residual risk, even with optimal quadruple background therapy [6, 33, 50]; however, no specific recommendations for the use of vericiguat in older and frail patients have yet been provided [4, 51]. The administration of vericiguat in older and complex patients may be suggested, following the proposed algorithm shown in Fig. 3. According to our practical algorithm for managing complex patients with worsening HFrEF, the concurrent administration of low doses of β-blockers, ARNIs, SGLT2 inhibitors, and MRAs should be considered as the first step provided there are no contraindications to these drugs. Therefore, if these medications are well tolerated by the patient, it would be advisable to optimize the dosages within 1 or 2 months. However, if this initial step is not tolerated, for instance due to hypotension, worsening renal function, or electrolyte disorders, various options are available, i.e. reducing the dose of loop diuretics, switching from ARNIs to other RAS inhibitors, or removing MRAs. In the case of ketoacidosis or urinary tract infections (severe or recurrent), SGLT2 inhibitors can be removed. If the patient experiences worsening HF despite optimized medical therapy, vericiguat can be added, starting at a dose of 2.5 mg and gradually uptitrating to 10 mg, if tolerated. If the addition of vericiguat leads to hypotension, one of the initial four pillars may be reduced in dosage, or the ARNI may be switched to another RAS inhibitor. It should be kept in mind that vericiguat should be avoided in patients with severe liver and/or renal dysfunction, considering the lack of approval in these populations and the limited safety data available [18, 34]. Vericiguat is also not recommended in patients with orthostatic hypotension or those at risk of developing it, since hypotension is the main adverse effect of the drug [47, 52]. Particular attention should also be paid to the ongoing therapy, especially to nitrates, α-blockers, and calcium channel blockers [52]. Realistically, administration of the four pillars together is often not feasible, particularly in older patients [53]. In these cases, vericiguat may easily substitute the pharmacological agent, which is not well tolerated or is contraindicated. This recommendation is based on a plausible pathophysiological rationale that goes beyond guidelines; in the management of HFrEF in older patients, clinical judgment should guide the decision-making process [1, 53]. Interestingly, a recent meta-analysis suggests that vericiguat is associated with a similar risk of cardiovascular death or hospitalization for HF compared with sacubitril/valsartan, in particular in HFrEF patients [54]. Eventually, the use of vericiguat may be endorsed as an add-on therapy for complex and multimorbid HFrEF patients, particularly those with prior multiple hospitalizations or those who cannot tolerate the entire ‘four pillar’ therapy. To address this issue, data from dedicated studies are required, including the ongoing VICTOR trial [55].
Fig. 2.
Features of vericiguat in older and complex patients, HFrEF heart failure with reduced ejection fraction, OMT optimal medical therapy
Fig. 3.
Proposed algorithm for the use of vericiguat in older and complex patients. ARNI angiotensin receptor/neprilysin inhibitor, BB β-blockers, HF heart failure, HFrEF heart failure with reduced ejection fraction, MRA mineralocorticoid receptor antagonist, OMT optimal medical therapy, PDE-5 phosphodiesterase-5, RASi renin-angiotensin-aldosterone system inhibitors, SBP systolic blood pressure, SGLT2i sodium-glucose co-transporter-2 inhibitors, UTI urinary tract infection, WRF worsening renal function
Future Perspectives
In patients with HFrEF and a recent episode of acute decompensation, the VICTORIA trial has shown favorable clinical outcomes associated with the addition of vericiguat to optimal medical therapy [56]. This may represent an advancement in the management of HF in older and complex patients, since the population of the VICTORIA trial consisted of more frail and multimorbid patients compared with participants included in other recent randomized controlled trials [34]. These data may open a new scenario for a therapeutic strategy for older patients with HFrEF, in which vericiguat may assume a first-line role alongside the four pillars, particularly when one or more of these drugs are not tolerated. Although limited evidence is available regarding the potential efficacy of vericiguat in the context of HFpEF, it may become a useful strategy in patients with transthyretin cardiac amyloidosis in NYHA functional class III [19]. Given the limited therapeutic options available for these patients, who in many cases are old, frail and thus not eligible for tafamidis treatment, vericiguat may improve diastolic relaxation [15, 19, 57]. However, further clinical and preclinical investigations are necessary to validate these hypotheses. Eventually, the ongoing VICTOR trial will aim to assess the effectiveness and safety of vericiguat in individuals with chronic HFrEF who have not experienced a recent hospitalization for HF or the need for outpatient intravenous diuretics [55]. The core hypothesis of the trial is that vericiguat significantly reduces the risk of cardiovascular death or hospitalization due to HF compared with placebo [55]. Approximately 6000 patients are expected to be enrolled, with the study concluding in June 2025 [55]. Specifically, we advocate a dedicated trial focusing on older and complex patients with HFrEF. Additionally, it will be imperative to assess the efficacy and safety of vericiguat within specific subpopulations, such as patients with atrial fibrillation and cardiomyopathies, as well as those undergoing cardiotoxic chemotherapy treatments, conditions that are notably prevalent in older adults.
Conclusions
One of the foremost challenges faced by cardiologists in the 21st century is the management of frail, older, and multimorbid HFrEF patients. This population presents unique complexities that necessitate careful consideration. Vericiguat was reported to be well tolerated and efficacious, with features (minimal clinical drawbacks, limited drug interactions, once-daily administration) that align with the specific needs for these patients. Vericiguat has the potential to target an additional pathway in HF for older and frail patients with evidence of clinical worsening, going beyond the pathways currently focused on. Given its safety profile, vericiguat might be easier to handle than the standard therapies for this population. Consequently, we look forward to further results on the efficacy of vericiguat, specifically in this subgroup, which is increasingly frequent in clinical practice.
Declarations
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Conflict of interest
Luigi Spadafora, Marco Bernardi, Gianmarco Sarto, Beatrice Simeone, Maurizio Forte, Luca D’Ambrosio, Matteo Betti, Alessandra D’Amico, Vittoria Cammisotto, Roberto Carnevale, Simona Bartimoccia, Giuseppe Biondi Zoccai, Giacomo Frati, Sebastiano Sciarretta, Valentina Valenti and Erica Rocco declare they have no potential conflicts of interest that might be relevant to the contents of this manuscript. Pierre Sabouret reports consulting fees from Axis-TV, Astra-Zeneca, BMS, Les Laboratoires Servier, Novartis, Menarini and Sanofi, outside the submitted work.
Authors' contributions
LS conceived the manuscript and drafted the main text with the support of ER and SS. MB and GS reviewed the manuscript and created the figures and tables. BS, MF, LDA, MB, ADA, VC, RC, SB, PS, GBZ, GF and VV provided critical review and conducted extensive bibliographic research.
Data availability statement
Data sharing is not applicable to this manuscipt as no datasets were generated.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137(2):352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 3.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed) 2022;75(6):523. doi: 10.1016/j.rec.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Dovjak P. Polypharmacy in elderly people. Wien Med Wochenschr. 2022;172(5–6):109–113. doi: 10.1007/s10354-021-00903-0. [DOI] [PubMed] [Google Scholar]
- 5.Cacciatore S, Martone AM, Landi F, Tosato M. Acute coronary syndrome in older adults: an update from the 2022 Scientific Statement by the American Heart Association. Heart Vessels Transplant. 2023;7(1):7–10. doi: 10.24969/hvt.2023.367. [DOI] [Google Scholar]
- 6.Greene SJ, Bauersachs J, Brugts JJ, et al. Management of worsening heart failure with reduced ejection fraction: JACC focus seminar 3/3. J Am Coll Cardiol. 2023;82(6):559–571. doi: 10.1016/j.jacc.2023.04.057. [DOI] [PubMed] [Google Scholar]
- 7.D'Amato A, Prosperi S, Severino P, et al. Current approaches to worsening heart failure: pathophysiological and molecular insights. Int J Mol Sci. 2024 doi: 10.3390/ijms25031574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938–1952. doi: 10.1016/S0140-6736(22)02076-1. [DOI] [PubMed] [Google Scholar]
- 9.Chiles R, Al-Horani RA. Vericiguat: a new hope for heart failure patients. Cardiovasc Ther. 2022;2022:1554875. doi: 10.1155/2022/1554875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Marti CN, Sabbah HN, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev. 2013;18(2):123–134. doi: 10.1007/s10741-012-9323-1. [DOI] [PubMed] [Google Scholar]
- 11.Campbell N, Kalabalik-Hoganson J, Frey K. Vericiguat: a novel oral soluble guanylate cyclase stimulator for the treatment of heart failure. Ann Pharmacother. 2022;56(5):600–608. doi: 10.1177/10600280211041384. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Lin M, Maitz T, et al. Vericiguat: a novel soluble guanylate cyclase stimulator for use in patients with heart failure. Cardiol Rev. 2023;31(2):87–92. doi: 10.1097/CRD.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 13.Coats AJS, Tolppanen H. Drug treatment of heart failure with reduced ejection fraction: defining the role of vericiguat. Drugs. 2021;81(14):1599–1604. doi: 10.1007/s40265-021-01586-y. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SP, Ibrahim NE, Januzzi JL., Jr Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504. doi: 10.1001/jama.2020.10262. [DOI] [PubMed] [Google Scholar]
- 15.Vannuccini F, Campora A, Barilli M, Palazzuoli A. Vericiguat in heart failure: characteristics, scientific evidence and potential clinical applications. Biomedicines. 2022 doi: 10.3390/biomedicines10102471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31(22):2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 17.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency . VERQUVO® (vericiguat): EU summary of product characteristics. Amsterdam: European Medicines Agency; 2021. [Google Scholar]
- 19.Di Fusco SA, Alonzo A, Aimo A, et al. ANMCO Position paper: Vericiguat use in heart failure: from evidence to place in therapy [in Italian] G Ital Cardiol (Rome) 2023;24(4):323–331. doi: 10.1714/4004.39824. [DOI] [PubMed] [Google Scholar]
- 20.Rao VN, Diez J, Gustafsson F, et al. Practical patient care considerations with use of vericiguat after worsening heart failure events. J Card Fail. 2023;29(3):389–402. doi: 10.1016/j.cardfail.2022.10.431. [DOI] [PubMed] [Google Scholar]
- 21.Lam CSP, Mulder H, Lopatin Y, et al. Blood pressure and safety events with vericiguat in the VICTORIA Trial. J Am Heart Assoc. 2021;10(22):e021094. doi: 10.1161/JAHA.121.021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezekowitz JA, Zheng Y, Cohen-Solal A, et al. Hemoglobin and clinical outcomes in the vericiguat global study in patients with heart failure and reduced ejection fraction (VICTORIA) Circulation. 2021;144(18):1489–1499. doi: 10.1161/CIRCULATIONAHA.121.056797. [DOI] [PubMed] [Google Scholar]
- 23.Voors AA, Mulder H, Reyes E, et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur J Heart Fail. 2021;23(8):1313–1321. doi: 10.1002/ejhf.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senni M, Alemayehu WG, Sim D, et al. Efficacy and safety of vericiguat in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan: insights from the VICTORIA trial. Eur J Heart Fail. 2022;24(9):1614–1622. doi: 10.1002/ejhf.2608. [DOI] [PubMed] [Google Scholar]
- 25.Khan MS, Xu H, Fonarow GC, et al. Applicability of vericiguat to patients hospitalized for heart failure in the United States. JACC Heart Fail. 2023;11(2):211–223. doi: 10.1016/j.jchf.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerwagen F, Ohlmeier C, Evers T, et al. Real-world characteristics and use patterns of patients treated with vericiguat: a nationwide longitudinal cohort study in Germany. Eur J Clin Pharmacol. 2024;80(6):931–940. doi: 10.1007/s00228-024-03654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezekowitz JA, McMullan CJ, Westerhout CM, et al. Background medical therapy and clinical outcomes from the VICTORIA Trial. Circ Heart Fail. 2023;16(9):e010599. doi: 10.1161/CIRCHEARTFAILURE.123.010599. [DOI] [PubMed] [Google Scholar]
- 28.Evora PR, Evora PM, Celotto AC, Rodrigues AJ, Joviliano EE. Cardiovascular therapeutics targets on the NO-sGC-cGMP signaling pathway: a critical overview. Curr Drug Targets. 2012;13(9):1207–1214. doi: 10.2174/138945012802002348. [DOI] [PubMed] [Google Scholar]
- 29.Triposkiadis F, Xanthopoulos A, Skoularigis J, Starling RC. Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure. Heart Fail Rev. 2022;27(6):1991–2003. doi: 10.1007/s10741-022-10239-5. [DOI] [PubMed] [Google Scholar]
- 30.Premer C, Kanelidis AJ, Hare JM, Schulman IH. Rethinking endothelial dysfunction as a crucial target in fighting heart failure. Mayo Clin Proc Innov Qual Outcomes. 2019;3(1):1–13. doi: 10.1016/j.mayocpiqo.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stasch JP, Schlossmann J, Hocher B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr Opin Pharmacol. 2015;21:95–104. doi: 10.1016/j.coph.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 33.McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 35.Kaplinsky E, Perrone S, Barbagelata A. Emerging concepts in heart failure management and treatment: focus on vericiguat. Drugs Context. 2023 doi: 10.7573/dic.2022-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMurray JJV, DeMets DL, Inzucchi SE, et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF) Eur J Heart Fail. 2019;21(5):665–675. doi: 10.1002/ejhf.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;314(21):2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 38.Lam CSP, Giczewska A, Sliwa K, et al. Clinical outcomes and response to vericiguat according to index heart failure event: insights from the VICTORIA Trial. JAMA Cardiol. 2021;6(6):706–712. doi: 10.1001/jamacardio.2020.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezekowitz JA, O'Connor CM, Troughton RW, et al. N-terminal Pro-B-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8(11):931–939. doi: 10.1016/j.jchf.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Ezekowitz J, Alemayehu W, Edelmann F, et al. Diuretic use and outcomes in patients with heart failure with reduced ejection fraction: Insights from the VICTORIA trial. Eur J Heart Fail. 2024 doi: 10.1002/ejhf.3179. [DOI] [PubMed] [Google Scholar]
- 41.Lam CSP, Pina IL, Zheng Y, et al. Age, sex, and outcomes in heart failure with reduced EF: insights from the VICTORIA trial. JACC Heart Fail. 2023;11(9):1246–1257. doi: 10.1016/j.jchf.2023.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong PW, Lam CSP, Anstrom KJ, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324(15):1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial. Circulation. 2021;143(4):326–336. doi: 10.1161/CIRCULATIONAHA.120.051783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 46.Chen C, Lv J, Liu C. Vericiguat in patients with heart failure across the spectrum of left ventricular ejection fraction: a patient-level, pooled meta-analysis of VITALITY-HFpEF and VICTORIA. Front Endocrinol (Lausanne) 2024;15:1335531. doi: 10.3389/fendo.2024.1335531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agency EM. VERQUVO® (vericiguat): EU summary of product characteristics. 2021.
- 48.Kang C, Lamb YN. Vericiguat: a review in chronic heart failure with reduced ejection fraction. Am J Cardiovasc Drugs. 2022;22(4):451–459. doi: 10.1007/s40256-022-00538-5. [DOI] [PubMed] [Google Scholar]
- 49.Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128(10):1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 50.Olivella A, Almenar-Bonet L, Moliner P, et al. Role of vericiguat in management of patients with heart failure with reduced ejection fraction after worsening episode. ESC Heart Fail. 2024;11(2):628–636. doi: 10.1002/ehf2.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forman DE, Maurer MS, Boyd C, et al. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149–2161. doi: 10.1016/j.jacc.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cacciatore S, Spadafora L, Landi F. Orthostatic hypotension in elderly: do you measure orthostatic and clinostatic blood pressure? Heart Vessels Transplant. 2022;4:164–167. doi: 10.24969/hvt.2022.349. [DOI] [Google Scholar]
- 53.Unlu O, Levitan EB, Reshetnyak E, et al. Polypharmacy in older adults hospitalized for heart failure. Circ Heart Fail. 2020;13(11):e006977. doi: 10.1161/CIRCHEARTFAILURE.120.006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang DW, Kang SH, Lee K, et al. Comparative efficacy of vericiguat to sacubitril/valsartan for patients with heart failure reduced ejection fraction: systematic review and network meta-analysis. Int J Cardiol. 2024;400:131786. doi: 10.1016/j.ijcard.2024.131786. [DOI] [PubMed] [Google Scholar]
- 55.Balestrieri G, Sciatti E, D'Isa S, D'Elia E, Senni M. Heart failure therapy: the fifth card. Eur Heart J Suppl. 2023;25(Suppl B):B140–B143. doi: 10.1093/eurheartjsupp/suad099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boettcher M, Thomas D, Mueck W, et al. Safety, pharmacodynamic, and pharmacokinetic characterization of vericiguat: results from six phase I studies in healthy subjects. Eur J Clin Pharmacol. 2021;77(4):527–537. doi: 10.1007/s00228-020-03023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carla Recupero SC, Marco B, Anna MM, Francesco L. Multidisciplinary care for patients with cardiac amyloidosis: a lesson from the 2023 American College of Cardiology Expert Consensus. Heart Vessels and Transplant. 2023;11:78. doi: 10.24969/hvt.2023.388. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this manuscipt as no datasets were generated.