Abstract
RNA N6-methyladenosine (m6A) methylation is the most abundant and conserved RNA modification in eukaryotes. It participates in the regulation of RNA metabolism and various pathophysiological processes. Non-coding RNAs (ncRNAs) are defined as small or long transcripts which do not encode proteins and display numerous biological regulatory functions. Similar to mRNAs, m6A deposition is observed in ncRNAs. Studying RNA m6A modifications on ncRNAs is of great importance specifically to deepen our understanding of their biological roles and clinical implications. In this review, we summarized the recent research findings regarding the mutual regulation between RNA m6A modification and ncRNAs (with a specific focus on microRNAs, long non-coding RNAs, and circular RNAs) and their functions. We also discussed the challenges of m6A-containing ncRNAs and RNA m6A as therapeutic targets in human diseases and their future perspective in translational roles.
Keywords: Circular RNA, Epigenetic regulation, Long non-coding RNA, MicroRNA, Non-coding RNA, RNA m6A modification
Introduction
Epigenetic regulation is critical to many fundamental cellular processes and contributes towards the diversity of physiopathology functions.1, 2, 3 In the last few decades, histone and DNA modifications have been extensively investigated, and many excellent breakthroughs have been made from bench to clinical application.4,5 Recently, RNA modifications have gained widespread interest. An ever-increasing number of studies have identified a number of RNA modifications in the eukaryotic transcriptome, including 5-methylcytosine (m5C), pseudouridine (Ψ), N1-methyladenosine (m1A), and N6-methyladenosine (m6A) methylation.6 Among them, the RNA m6A methylation is most abundant in eukaryotes and has been observed to be consistently conserved in mammals. RNA m6A modification participates in the modulation of RNA metabolism and various pathophysiological processes, such as translation, RNA splicing, and degradation.7 Dysregulation of RNA m6A methylation is closely related to the occurrence and development of various diseases including tumorigenesis, Alzheimer's disease, and cardiovascular diseases.8, 9, 10
Non-coding RNAs (ncRNAs) are RNA molecules that are not translated into proteins. Contrary to earlier views, they have been identified to have myriad biological roles and are emerging as master regulators of cellular processes.11 Various types of ncRNAs have been reported, such as microRNAs (miRNAs), ribosomal RNAs (rRNAs), long non-coding RNAs (lncRNAs), small nuclear RNAs (snRNAs), and circular RNAs (circRNAs). With the development of more practical methods for examining RNA m6A modifications, insights into the underlying mechanisms have been uncovered in recent years.12, 13, 14 The m6A modification can be used to modify all the different types of RNAs. Although great progress has been made in the scientific approach involved in understanding the regulatory mechanism of RNA m6A modification in mRNAs, the deposition of m6A modifications in ncRNAs and its underlying regulatory roles are still in its infancy. In this review, we will discuss the research advances in the realm of RNA m6A modification and its function in ncRNAs primarily on miRNAs, lncRNAs, and circRNAs, and address the potential therapeutic strategies to target RNA m6A regulators for disease therapy, as well as outline some outstanding questions and challenges that require further exploration in this field.
Regulation of RNA m6A modification
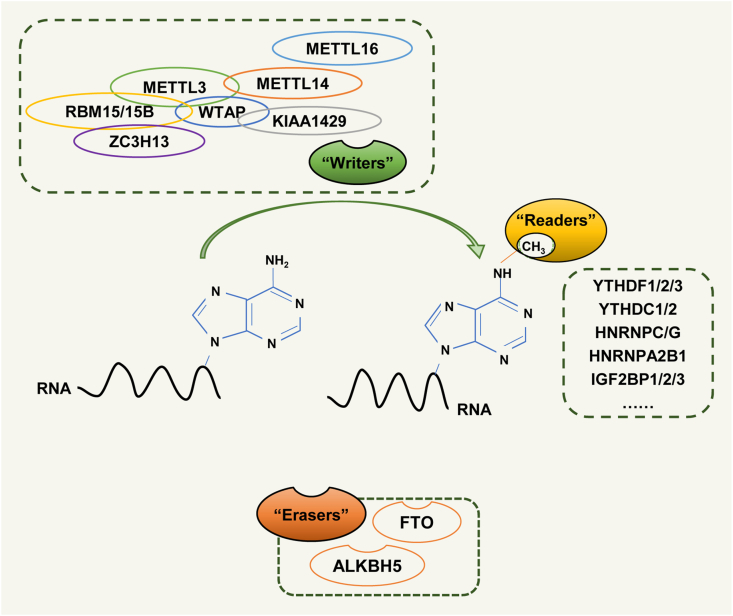
The RNA m6A modification was first discovered in mRNA fractions obtained from Novikoff-hepatoma cells.15,16 Like DNA methylation, RNA m6A methylation also has a reversible and dynamic post-transcriptional modification in mammalian.17 Both efficacy and abundance of m6A modification on RNA are regulated under the dynamic interaction among methyltransferases (“writers”), demethylases (“erasers”), and binding proteins (“readers”) (Fig. 1)7.
Figure 1.
The dynamic and reversible RNA m6A modification process. m6A modifications on RNAs are regulated by the dynamic interaction between methyltransferases (“writers”), demethylases (“erasers”), and binding proteins (“readers").
Methyltransferases are generally referred to as “writers”. They consist of nuclear methyltransferases made up of methyltransferase-like 13 (METTL3), METTL14, and WTAP, which append the m6A modification and thereby significantly influence certain physio-pathological processes.18 METTL3 and METTL14 contain a methyltransferase domain (MTD) that can catalyze the transfer of a methyl group from S-adenosylmethionine to the N6 of adenosine (A). The crystal structure of the METTL3-METTL14 heterodimer reveals that METTL3 and METTL14 are tightly bound to each other.19 WTAP has been functionally linked to its role in alternative splicing and mediates METTL3/14 complex localized to the nuclear speckles.20, 21, 22 In addition, the METTL3/METTL14/WTAP complex recruits other cofactors, such as RBM15, METTL16, and RBM15B (which directs the adenosine methylation in mRNAs and lncRNAs), KIAA1429, and ZC3H13 to mediate the methylation of methyltransferases to RNAs.23, 24, 25
Demethylases, also named “erasers”, include α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5), and fat mass and obesity-associated protein (FTO), that catalyze RNA m6A demethylation.7,26,27 Both ALKBH5 and FTO belong to the Fe (II)/α-ketoglutarate dependent dioxygenase enzyme family. FTO was the first identified RNA m6A demethylase in mammal cells,27 and ALKBH5 was the second one to be discovered. Unlike FTO, which can demethylate both DNA and RNA m6A methylations, ALKBH5 only specifically demethylated m6A-modifications of RNA.28 Besides, another alkB family protein ALKBH3 was also found to demethylate m6A modifications in tRNA.29 However, another contrasting study suggested that ALKBH3 could specifically demethylate only m1A and m3C in tRNA, but demonstrated no demethylase activity on m6A modifications.30 Therefore, if ALKBH3 has demethylase activity on tRNA m6A modification remains conflicting and requires further investigation.
The entire process of RNA m6A modifications seems to be limited to methylation and demethylation. However, these chemical modifications could have a direct effect on RNA transcription by affecting several properties such as RNA secondary structure, charge, protein-RNA interactions, and base-pairing, which in turn modulate gene expression by regulating RNA processing, localization, translation, and degradation.31 RNA m6A binding proteins, including heterogeneous nuclear ribonucleoprotein (HNRNP) proteins, YT521-B homology (YTH) domain family, and insulin-like growth factor 2 mRNA-binding protein (IGF2BP) family, are termed as “readers” that can specifically recognize m6A sites, which shows that m6A modification could affect RNA processing by recruiting specific proteins.7,32, 33, 34 The YTH protein family is composed of a series of proteins such as YTHDF1-3 and YTHDC1-2 that recognize m6A-modified transcripts. YTHDF1 selectively binds to m6A-methylated mRNA and increases mRNA translation efficiency.35 On the contrary, YTHDF2 selectively binds to m6A modification that regulates RNA degradation.36,37 Alternatively, YTHDF3 binds to m6A-modified transcripts along with either YTHDF1 or YTHDF2, indicating the close association among YTHDF1-3 proteins to regulate the turnover (translation and decay) of mRNA targets.38 Unlike YTHDFs located in the cytosol, YTHDC1 and YTHDC2 are nuclear proteins. YTHDC1 regulates m6A methylated RNA splicing and nuclear export.39,40 YTHDC2 contains a DEAH RNA helicase domain as well as a YTH m6A binding domain. Hence, the role of YTHDC2 in cells is much more complicated and remains uncertain. It has been reported that YTHDC2 could modulate the translation efficiency of the m6A-containing target mRNA and decrease its abundance.41 The IGF2BP proteins also recognize the m6A-modified mRNA, but in contrast with the YTH family proteins, enhance the mRNA stability and translation.33,42,43 With respect to HNRNP “readers”, the m6A RNA modification alters the local structure of RNA, thereby, increasing the accessibility of target RNA to bind with HNRNP proteins.44,45 Moreover, HNRNPA2B1 also participates in primary miRNA processing and alternative splicing.34
Mutual regulation between RNA m6A modification and miRNAs
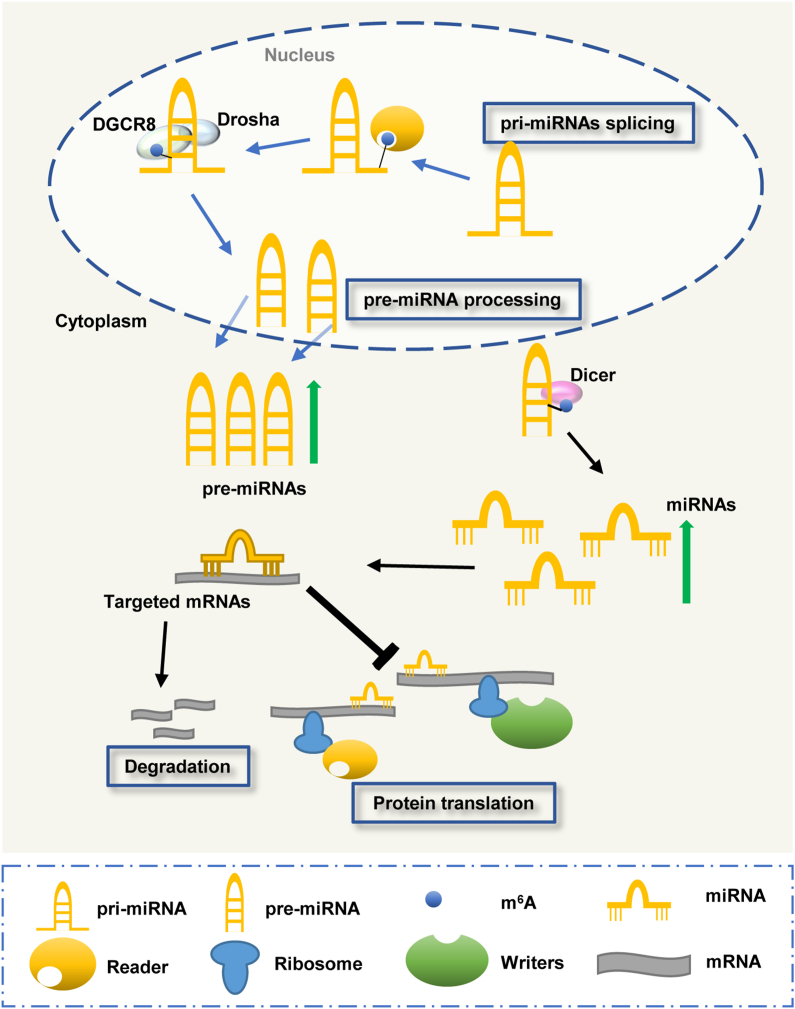
MicroRNAs (miRNAs) are a group of non-coding single-stranded RNAs with a length of about 22 nucleotides, involved in the modulation of post-transcriptional gene expression.46, 47, 48, 49 The biogenesis of miRNA is primarily produced by the transcriptional activity of RNA polymerase II or III to form the primary miRNAs (pri-miRNAs). Then the pri-miRNAs are processed by endonuclease Drosha and double-stranded RNA binding protein DGCR8 to generate precursor miRNAs (pre-miRNAs). Finally, the pre-miRNAs are transported to the cytoplasm by RanGTP and exportin-5 to form mature miRNAs, spliced by the endoribonuclease Dicer. RNA m6A modifications have been found to take part in the regulation of pri-miRNA processing and pre-miRNA splicing (Fig. 2).
Figure 2.
The regulation of RNA m6A methylation on miRNAs. RNA m6A methylation participates in the regulation of pri-miRNAs processing and pre-miRNA splicing to modulate the expression of mature miRNAs, and consequently, to regulate miRNA and target mRNA interaction.
RNA m6A methyltransferases have been reported to methylate pri-miRNAs, and consequently increase the binding and processing of pri-miRNAs by DGCR8 and increase the splicing by Dicer to pre-miRNAs.50,51 The RNA-binding protein HNRNPA2B1 has been shown to recognize the m6A-containing pri-miRNAs and affect pri-miRNAs processing.34 Altered m6A RNA modification of pri-miRNAs or pre-miRNAs not only induces the change in the expression levels of many mature miRNAs but also takes critical roles in various diseases.50,52 METTL3-mediated m6A methylation of pre-miR-320 has been found to promote osteogenic differentiation of bone marrow-derived mesenchymal stem cells.53 During the progression of pancreatic cancer, hypomethylation of the METTL3 promoter was observed to increase its expression, significantly elevating the m6A deposition of pri-miR-25 to promote the generation of mature miR-25, and consequently, aggravating the development and progression of pancreatic cancer.54 Similarly, METTL3 was seen to promote bladder cancer via accelerating pri-miR-221/222 maturation in an RNA m6A-dependent manner.52 Another study also reported that METTL3 up-regulation could contribute to the abnormal m6A modification in colorectal cancer and exacerbate tumor metastasis via the methylation of pri-miR-1246.55 On the contrary, forced expression of METTL3 was noted to alleviate colistin-induced renal injury by modulating the miR-873–5p maturation process through DGCR8 in an m6A-dependent manner.56 Unlike METTL3, METTL14-mediated m6A methylation of pri-miR-375 processing was able to up-regulate the expression and the maturation of miR-375, and thereby, inhibit colorectal cancer cell growth and metastasis.57 Moreover, METTL14/m6A was also reported to suppress tumor invasion and metastasis by regulating the processing of miR-126 by DGCR8 in hepatocellular carcinoma.58
RNA m6A demethylases also contribute to the methylation status of miRNAs. Contrary to the well-characterized role of methyltransferase in the context of miRNA-processing, the specific function of demethylases is not quite thoroughly studied. Demethylase ALKBH5 has been found to interact with DEAD-box RNA helicase DDX3 and recruited AGO2 protein to modulate the expression of methylated-miRNAs.59 Besides, ALKBH5 was also observed to negatively regulate miR-7 expression through physical interaction with HuR to suppress the process of miR-7 during epithelial ovarian cancer progression.60 Interestingly, the knockdown of another RNA m6A demethylase FTO was able to reduce miRNA expression without affecting the primary transcripts of many miRNAs.61 FTO knockdown in cardiomyocytes could increase miR-133a expression via the m6A binding protein IGF2BP2.62 Thus, further investigations to illustrate the underlying regulatory mechanisms of demethylases would have a tremendous impact on understanding their role in miRNA biogenesis.
In addition to the regulation of miRNAs by RNA m6A modification, the 3′- untranslated RNA region (UTR) of effector mRNAs can also be targeted by mature miRNAs to facilitate the degradation of targeted mRNAs and inhibit protein translation. miR-145 regulated RNA m6A modification via targeting the 3′-UTR of m6A modification reader protein YTHDF2.63 Also, miR-4429 has been found to prevent gastric cancer progression by regulating its target gene METTL3.64 Moreover, YTHDF1 was also identified to contribute to the glioma progression, through the role of miR-3436 that bound to the 3′-UTR of YTHDF1 upstream to regulate YTHDF1 expression in glioma.65 Taken together, the miRNA-m6A methylation can regulate each other in both ways. On one hand, RNA m6A methylation participates in the regulation of pri-miRNAs processing and pre-miRNA splicing to modulate mature miRNA expression. On the other hand, miRNAs could act as the upstream factor of RNA m6A methylation regulator proteins by targeting their mRNAs (Table 1).
Table 1.
Mutual regulation between RNA m6A modification and miRNAs.
| miRNAs | RNA m6A effectors | Mechanism | Biological function | References |
|---|---|---|---|---|
| The effect of RNA m6A methylation on miRNA | ||||
| miR-320 | METTL3 | METTL3 mediated m6A methylation on pre-miR-320 | Promotes osteogenic differentiation | 53 |
| miR-25 | METTL3 | METTL3 increased the m6A deposition in pri-miR-25 | Promotes progression of pancreatic cancer | 54 |
| miR-221/222 | METTL3 | METTL3 elevated the m6A deposition in pri-miR-221/222 | Promotes bladder cancer | 52 |
| miR-1246 | METTL3 | METTL3 increased the m6A deposition in pri-miR-1246 | Promotes colorectal cancer and tumor metastasis | 55 |
| miR-873–5p | METTL3 | METTL3 modulated miR-873–5p mature process through DGCR8 | Alleviates colistin-induced renal injury | 56 |
| miR-375 | METTL14 | METTL14 mediated m6A methylation on pri-miR-375 processing | Inhibits colorectal cancer cell growth and metastasis | 57 |
| miR-126 | METTL14 | METTL14/m6A regulated miR-126 processing by DGCR8 | Inhibits hepatocellular carcinoma | 58 |
| miR-7 | ALKBH5 | ALKBH5 suppressed miR-7 processing | Promotes epithelial ovarian cancer | 60 |
| miR-133a | FTO | FTO knockdown increased miR-133a expression via m6A binding protein IGF2BP2 | Prevents cardiac proliferation and promotes cardiac pathological hypertrophy | 62 |
| The effect of miRNA on RNA m6A methylation | ||||
| miR-145 | YTHDF2 | 3′-UTR of YTHDF2 targeted by miR-145 | YTHDF2 correlated with increased malignancy of hepatocellular carcinoma. | 63 |
| miR-4429 | METTL3 | 3′-UTR of METTL3 targeted by miR-4429 | Prevents gastric cancer progression | 64 |
| miR-3436 | YTHDF1 | 3′-UTR of YTHDF1 targeted by miR-3436 | High expression of YTHDF1 in glioma was associated with worse overall survival | 65 |
Mutual regulation between RNA m6A modification and lncRNAs
Accumulating evidence indicates that lncRNAs take critical roles in various biological functions and the pathophysiology of many diseases. Like messenger RNAs (mRNAs), lncRNAs are transcribed by RNA polymerase II and undergo splicing, capping, and polyadenylation.66,67 Since the discovery of lncRNAs, numerous underlying mechanisms involved in their post-transcriptional regulation have been unraveled. Based on recent research on the distribution of m6A in the transcriptome, lncRNAs with m6A modification have been identified and reported to take part in the physiology and pathology of human diseases.68, 69, 70
In recent years, an increasing number of studies have reported m6A modifications on lncRNAs and have expanded our knowledge about the regulatory mechanisms of lncRNA as well as their m6A methylation (Fig. 3). One of the proposed mechanisms is called the “m6A-switch” wherein the RNA m6A modification would alter the local structure of lncRNA and control RNA-protein interactions for biological regulation.44,45 m6A-methylated hairpin formation of the lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), one of the well-characterized lncRNAs, resulted in a much stronger binding of MALAT1 with heterogeneous nuclear ribonucleoprotein C and heterogeneous nuclear ribonucleoprotein G, thus affecting nucleus gene expression and maturation68,44,.45 Akin to MALAT1, X-inactive specific transcript (Xist) is highly methylated with many m6A modification sites.23 Several proteins which are the components of the m6A methylation complex and the m6A binding proteins, including RBM15/15B, WTAP, METTL3, and YTHDC1, have been identified to bind Xist and are in fact required for Xist-mediated transcriptional silencing of genes on the X chromosome.23,71,72
Figure 3.
The regulation of lncRNAs by RNA m6A modification. RNA m6A modification of lncRNAs has an impact on RNA-protein interaction (e.g., MALAT1) and chromatin remodeling (e.g., Xist).
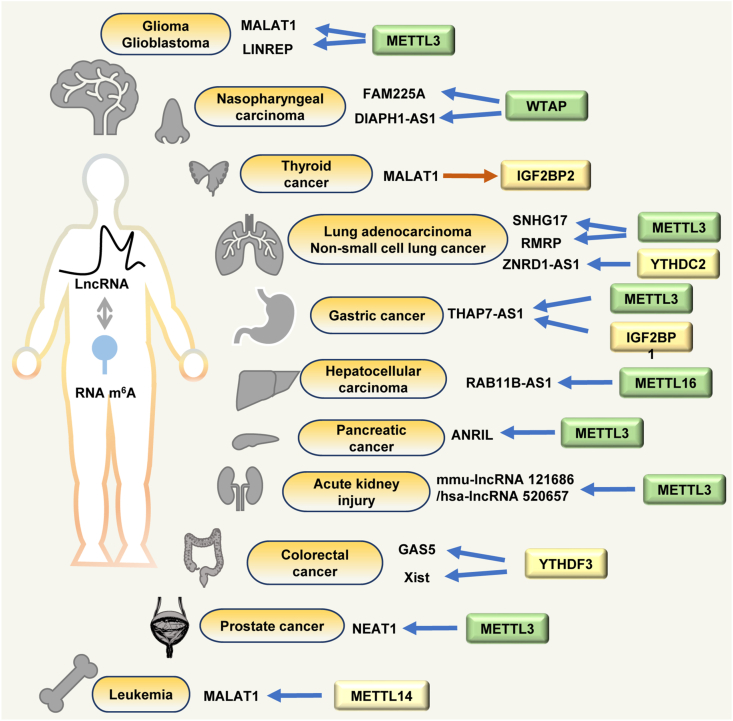
The regulation between m6A modification and lncRNAs also contributes to critical pathological roles in several diseases (Fig. 4). In glioma, METTL3 promoted the malignant progression of gliomas through up-regulation of m6A methylated-MALAT1 stability.73 Several investigations have focused on discovering the mechanism for the oncogenic role of m6A methylated-MALAT1; using mutations and super-resolution imaging, the m6A modification on MALAT1 was demonstrated to be recognized by YTHDC1 which taken an essential role in regulating the metastatic potential of cancer cells.74 One study revealed that MALAT1 bound to METTL14 mediated the interaction between fusion protein PML-RARα, and aggravated the leukemia progression by modulating chimeric mRNA export via YTHDC1 recognition.75 Similarly, METTL14-mediated RNA m6A modification regulated Xist expression in colorectal cancer and contributed towards tumor growth and invasion.76 Interestingly, another study found that Xist-mediated gene silencing is mostly attributed to synergistic actions of Spen/A-repeat and Polycomb/B-repeat, on comparison with LBR and Rbm15/m6A-methyltransferase complex that contributes minorly in mouse embryonic stem cell-based models.77 These studies appear to be inconsistent indicating that the role of m6A modification on lncRNAs might be selectively regulated or show distinct regulatory function under specific stimulants in a cell type-specific manner. Further studies focusing on the specific pathological role of m6A-methylated RNA during different diseases are essential as this would be an opportunity to better clarify the association between m6A RNA modifications and lncRNAs under specific conditions.
Figure 4.
Mutual regulation between RNA m6A and lncRNAs in human diseases. RNA m6A regulates m6A-methylated-lncRNAs and some lncRNAs are also found to regulate the expression of RNA m6A effectors to affect cellular m6A methylation and recognition in several human diseases.
In addition to MALAT1 and Xist, other m6A-methylated lncRNAs have also been identified. Colorectal cancer-associated lncRNA RP11 can be positively regulated by RNA m6A modification and enhance cancer cell metastasis.78 m6A-methylated lncRNA NEAT1 facilitated oncogenic complex, and NEAT1 with a high m6A level corelated with the bone metastasis of prostate cancer.79 Another lncRNA, FAM225A, which was an oncogenic m6A-methylated lncRNA in nasopharyngeal carcinogenesis, has been found to act as the competing endogenous RNA for miR-590–3p and miR-1275 to promote carcinoma development and progression.80 In gastric cancer, lncRNA THAP7-AS1 was up-regulated and facilitated CUL4B entry into the nucleus to promote gastric cancer cell growth, invasion, and metastasis.81 A further in-depth study revealed that METTL3-mediated m6A methylation stabilized THAP7-AS1 by m6A binding to protein IGF2BP1.81 METTL3 also increased m6A-modified lncRNA SNHG17 to promote gefitinib resistance in lung adenocarcinoma.82 RNA m6A-modified lncRNA LINREP up-regulation promoted glioblastoma multiforme progression and its up-regulation was related to poor prognosis in glioma patients.83 Mechanistically, METTL3 suppression decreased the m6A methylation level and RNA expression level of LINREP, and the stability of LINREP was enhanced by HuR in an m6A-dependent manner.83 Similarly, m6A methylation in RMRP and DIAPH1-AS1 also enhanced its stability, and RMRP or DIAPH1-AS1 elevation facilitated cancer progression.84,85 In addition to regulating expression, lncRNA splicing was also found to be regulated by RNA m6A methylation. In gemcitabine resistance pancreatic cancer cells, the m6A modification level of lncRNA ANRIL was significantly increased, and METTL3-mediated m6A methylation elevation played a pivotal role in the occurrence of SRSF3-mediated ANRIL splicing events.86 Except for oncogenic lncRNAs, lncRNAs with m6A modification have also been found to inhibit the progression of cancers. METTL16 directly bound m6A-methylated lncRNA RAB11B-AS1 and decreased its stability, RAB11B-AS1 down-regulation led to poor prognosis in patients with hepatocellular carcinoma.87 LncRNA ZNRD1-AS1 was inhibited by m6A binding protein YTHDC2, and overexpression of ZNRD1-AS1 suppressed the progression of lung cancer.88 LncRNA GAS5 was negatively regulated by the m6A reader YTHDF3 and suppressed the progression of colorectal cancer.89 WTAP-mediated m6A methylation on lncRNA NORAD can promote nucleus pulposus cells' senescence and intervertebral disc degeneration development.90 Mechanically, the stability of lncRNA NORAD was regulated by YTHDF2 in an RNA m6A-dependent manner, and histone modification H3K4me3-induced WTAP would increase NORAD's m6A methylation level and promote its degradation.90 In addition, knockdown of m6A-modified mmu-lncRNA 121686/hsa-lncRNA 520657 can attenuate acute kidney injury.91 RNA m6A reader YTHDC1 can decrease m6A-modified lncRNA FENDRR's stability to enhance pyroptosis in hypoxic pulmonary artery endothelial cells.92 Moreover, similar to miRNAs, lncRNAs could also be found to regulate the expression of RNA m6A effectors to affect cellular m6A methylation and recognition. LncRNA DNA methylation-deregulated and RNA m6A reader-cooperating lncRNA (DMDRMR) enhances the activity of IGF2BP3 thereby regulating its target genes in an m6A-dependent manner.93 MALAT1 increased IGF2BP2 expression in thyroid cancer and promoted its target gene MYC expression through m6A recognition.94 Considering all the recent research in m6A regulation of lncRNAs, like the variety of lncRNA functions and mechanisms, the m6A RNA modifications on lncRNAs have a very complex and changeable regulatory mechanism. Towards understanding this, further studies are certainly necessary to explore the role of m6A-methylated lncRNAs that could provide new insights with respect to diseases diagnosis, treatment, and prognosis, as well as expand our fundamental understanding of the “RNA metabolism” world (Table 2).
Table 2.
Mutual regulation between RNA m6A modification and lncRNAs.
| LncRNAs | RNA m6A effectors | Mechanism | Biological function | References |
|---|---|---|---|---|
| The effect of RNA m6A methylation on lncRNA | ||||
| MALAT1 | METTL3 | METTL3 up-regulates m6A-methylated MALAT1 stability | Promotes the progression of gliomas | 73 |
| MALAT1 | YTHDC1 | M6A-methylated MALAT1 recruits YTHDC1 to nuclear speckles | Promotes cancer metastasis | 74 |
| MALAT1 | YTHDC1 | MALAT1 modulates chimeric mRNAs export | Promotes leukemia progression | 75 |
| Xist | METTL14 | METTL14-mediated m6A modification down-regulates Xist | Suppresses colorectal cancer metastasis | 76 |
| RP11 | METTL3/ALKBH5 | m6A modification up-regulates RP11 expression | Promotes cancer cell metastasis | 78 |
| NEAT1 | METTL3 | RNA m6A-methylated NEAT1 facilitates oncogenic complex | Promotes bone metastasis of prostate cancer | 79 |
| FAM225A | / | FAM225A modulate ITGB3 expression by binding to miR-590-3p and miR-1275 | Promotes nasopharyngeal carcinoma tumorigenesis and metastasis | 80 |
| THAP7-AS1 | METTL3, IGF2BP1 | METTL3-mediated m6A methylation stabilizes THAP7-AS1 by IGF2BP1 | Promotes gastric cancer | 81 |
| SNHG17 | METTL3 | METTL3 increases the m6A methylation and stability of SNHG17 | Promotes gefitinib resistance in lung adenocarcinoma | 82 |
| LINREP | METTL3 | METTL3 knockdown decreases the m6A methylation level and RNA expression level of LINREP | Promotes glioblastoma multiforme progression | 83 |
| RMRP | METTL3 | RNA m6A methylation in RMRP enhances its stability | Facilitates non-small cell lung cancer progression | 84 |
| DIAPH1-AS1 | WTAP | RNA m6A methylation in DIAPH1-AS1 enhances its stability | Facilitates nasopharyngeal carcinoma growth and metastasis | 85 |
| ANRIL | METTL3 | METTL3-mediated m6A methylation elevation takes a crucial role in the occurrence of SRSF3-mediated ANRIL splicing events | Promotes gemcitabine resistance in pancreatic cancer | 86 |
| RAB11B-AS1 | METTL16 | METTL16-mediated m6A methylation on lncRNA RAB11B-AS1 and decreases its stability | Suppresses the progression of hepatocellular carcinoma | 87 |
| ZNRD1-AS1 | YTHDC2 | YTHDC2 down-regulates ZNRD1-AS1 | Suppresses the progression of lung cancer | 88 |
| GAS5 | YTHDF3 | YTHDF3 facilitates m6A-modified GAS5 degradation | Suppresses the progression of colorectal cancer | 89 |
| NORAD | WTAP, YTHDF2 | WTAP-mediated m6A methylation on lncRNA NORAD promotes the degradation of lncRNA NORAD in a YTHDF2-dependent manner | Inhibits nucleus pulposus cells senescence | 90 |
| mmu-lncRNA 121686/hsa-lncRNA 520657 | METTL3 | METTL3 knockdown decreases the m6A methylation level of mmu-lncRNA 121686/hsa-lncRNA 520657 | Aggravates acute kidney injury | 91 |
| FENDRR | YTHDC1 | YTHDC1 can decrease m6A-modified FENDRR's stability | Inhibits hypoxic pulmonary artery endothelial cell pyroptosis | 92 |
| The effect of lncRNA on RNA m6A methylation | ||||
| DMDRMR | IGF2BP3 | DMDRMR enhances the activity of IGF2BP3 to regulate target genes | Facilitates tumor growth and metastasis | 93 |
| MALAT1 | IGF2BP2 | MALAT1 up-regulates IGF2BP2 and enhances MYC expression via m6A modification recognition | Promotes thyroid cancer progression | 94 |
Mutual regulation between RNA m6A modification and circular RNAs
Apart from traditional ncRNAs, circular RNAs (circRNAs) are emerging as a novel class of endogenous ncRNAs that form covalently closed circle structures. CircRNAs are widely distributed throughout the body, across different types of tissues and organs, and participate in diverse biological and pathophysiological processes.95, 96, 97 CircRNAs have been found to function as miRNA decoys and bind to RNA-binding proteins.95 Some circRNAs can be translated into proteins or peptides to exert their regulatory roles.98 Like mRNAs and other ncRNAs, mutual regulation between RNA m6A and circRNAs also contributes to circRNAs' biology and function.99,100
A recent study on genome-wide profiling revealed that RNA m6A modifications were widespread in circRNAs, and exhibited distinct m6A patterns compared with mRNAs.101 CircRNAs can also be translated into proteins in a cap-independent translation mechanism through an internal ribosomal entry site or RNA m6A modification.98,102 Therefore, the translation efficiency of m6A-methylated circRNAs, which followed a non-canonical translation pathway for protein synthesis, was positively regulated by the m6A levels. Specifically, this m6A-driven translation was initiated by the binding of YTHDF3 and translation initiation factors eIF4G2 and eIF3A.98 Circ-ZNF609 was the most well-studied translated circRNA, which was translated into a protein in response to heat shock.103 RNA m6A regulates circ-ZNF609 translation via YTHDF3 and eIF4G2.99 Recently, our group reported that down-regulation of circ-ZNF609 could promote heart repair.104 Mechanistically, m6A modified-circ-ZNF609 was reported to exert its regulatory role via regulating another m6A modified mRNA, Yap expression, demonstrating the critical role of m6A modification in circRNA regulatory function.104 M6A-modified circRNA circE7 was also identified to be bound to polysomes and translated to E7 oncoprotein, which was essential for the transforming potential of human papillomaviruses.105 In eukaryotic cells, apart from the canonical cap-dependent translation initiation machinery, non-canonical translation mechanisms have been detected and proposed to be associated with stress responses.106 The fundamental role of m6A-driven translation of circRNAs remains largely unknown, and any further investigations on m6A modifications and circRNA translation, as well as their regulatory roles correlated with human health, would be of great significance.
CircRNAs are more stable and have a longer half-life compared with their parent linear mRNAs due to their cyclized structure, along with the fact that circRNA degradation mechanisms are more complex and specific (Fig. 5). CircCDR1 can be cleaved and degraded in a miRNA-dependent manner.107 During innate immune responses, circRNAs have been observed to be degraded by endoribonuclease RNase L.108 Additionally, RNA m6A methylation participates in the regulation of m6A-methylated circRNA decay.109 Usually, m6A containing circRNAs are recognized by YTHDF2 in an HRSP12-dependent way, and therefore, degraded by RNase P/MRP. In addition, highly structured circRNA degradation can also be mediated by UPF1 and its binding partner G3BP1.110 In fact, RNA m6A-binding proteins YTHDFs are important for stress granule formation.111 Because G3BP1 is the marker gene of stress granule formation, RNA m6A modification might also contribute to structure-mediated circRNA decay. Therefore, other potential regulatory mechanisms in which RNA m6A modification is involved in circRNA decay are certainly worth exploring.
Figure 5.
The regulation of circRNAs by RNA m6A modification. RNA m6A methylation regulates the translation and degradation of circRNAs.
Aberrant expression of circRNA is closely related to many diseases.96,112,113 Also, dysregulation of m6A RNA modifications has been observed in various pathological processes. Despite a limited number of investigations on the function of m6A-methylated circRNAs in diseases, a certain level of understanding regarding this process has been reached (Fig. 6). Endogenous m6A-containing circRNA can be recognized by YTHDF2 and inhibit innate immunity.100 Also, circRNAs with m6A modification exerted crucial roles during cancer progression. circNSUN2 has been found to be up-regulated in colorectal carcinoma patients with liver metastasis, wherein the RNA m6A modification of circNSUN2 was seen to promote its cytoplasmic export, and consequently, stabilized Hmga2 mRNA to facilitate the aggressiveness of cancer.114 Similarly, another circRNA circ3823 promoted colorectal cancer progression via sponging miR-30c-5p. Mechanically, the RNA m6A modification that occurred in circ3823, regulated its decay via YTHDF3 and ALKBH5.115 Besides, the expression of circCUX1 was observed to be increased in head and neck squamous cell carcinoma patients resistant to radiotherapy. Here, METTL3-mediated m6A modification on circCUX1 stabilized its expression and predicted a poor survival outcome.116 A similar phenomenon was observed with circRNA-SORE, circMDK, and circCCDC134.117, 118, 119 Elevated RNA m6A level in circRNA-SORE increased its stability and sustained sorafenib resistance in hepatocellular carcinoma.117 Also, IGF2BP1 stabilized m6A-methylated circMDK (hsa_circ_0095,868) and promoted tumorigenesis in hepatocellular carcinoma.118 ALKBH5-mediated m6A modification on circCCDC134 controlled its stability and can enhance cervical cancer metastasis in a YTHDF2-dependent manner.119 In addition to direct methylation on circRNAs, RNA m6A methylation also occurred in the flanking sequences of circ1662 affecting its biogenesis in colorectal cancer.120 Moreover, circRNAs took part in the dynamic regulation of m6A by altering the activity or expression of RNA m6A effectors. CircPTPRA was identified to disrupt the m6A-modified RNA recognition of IGF2BP1 by interacting with IGF2BP1 KH domains to inhibit bladder cancer progression.121 CircZBTB20 enhanced the interaction between RNA m6A demethylases ALKBH5 and Nr4a1 mRNA to maintain group 3 innate lymphoid cell homeostasis.122 CircEZH2 increased the stability of m6A reader IGF2BP2 and aggravated colorectal cancer.123 CircPDE5A bound to WTAP and inhibited WTAP-mediated m6A modification of Eif3c mRNA to suppress prostate cancer metastasis.124 Notably, circSTAG1 ameliorated depressive behavior in mice by decreasing the translocation of ALKBH5 to the nucleus and further promoted the fatty acid amide hydrolase mRNA m6A methylation.125 Another m6A demethylase, FTO, directly bound to circRAB11FIP1 facilitating its mRNA expression in ovarian cancer.126 Along with reader and demethylases, m6A methyltransferase METTL3 was found to be suppressed by circMEG3. Increased expression of circMEG3 was observed to act as an anti-tumor factor in human liver cancer.127 Moreover, circRNA-m6A modification can form a feedback loop to mutually regulate each other and exert function in cells.128,129 M6A-modified circGPR137B inhibited hepatocellular carcinoma via forming a feedback loop with FTO.129 Similarly, RNA m6A-regulated circ-ZNF609 alleviated doxorubicin-induced cardiotoxicity by promoting FTO expression.128 Collectively, these studies implicate that the regulatory interaction between RNA m6A modification and circRNAs could be highly associated with human health, and future research in this direction could lead to better therapeutic intervention for many diseases (Table 3).
Figure 6.
Mutual regulation between RNA m6A and circRNAs in human diseases. Aberrant expression of m6A-methylated circRNAs in human diseases. circRNAs are also involved in the dynamic regulation of RNA m6A.
Table 3.
Mutual regulation between RNA m6A modification and circRNAs.
| circRNAs | RNA m6A effectors | Mechanism | Biological function | References |
|---|---|---|---|---|
| The effect of RNA m6A methylation on circRNA | ||||
| circ-ZNF609 | YTHDF3, eIF4G2 | m6A regulates circZNF609 translation via YTHDF3 and eIF4G2 | Regulates myoblast proliferation | 98, 99, 103 |
| circ-ZNF609 | YTHDF3 | m6A-modified circ-ZNF609 regulates the expression of m6A-modified Yap via binding to YTHDF3 | Regulates cardiomyocyte survival | 104 |
| circE7 | / | m6A-modified circE7 translated to produce E7 oncoprotein | Highly associated with human papillomaviruses | 105 |
| circNSUN2 | YTHDC1, IGF2BP2 | m6A modification of circNSUN2 increases export to the cytoplasm and enhances the stability of HMGA2 mRNA via IGF2BP2 | Promotes colorectal cancer metastasis progression | 114 |
| circ3823 | YTHDF3, ALKBH5 | m6A modification presents in circ3823 and regulates its decay | Promotes colorectal cancer progression | 115 |
| circCUX1 | METTL3 | METTL3 mediated m6A methylation of circCUX1 and stabilizes its expression | Predicts a poor survival outcome of hypopharyngeal squamous cell carcinoma | 116 |
| circRNA-SORE | / | m6A modification of circRNA-SORE increases its stability | Maintenance of sorafenib resistance in hepatocellular carcinoma | 117 |
| circMDK | IGF2BP1 | IGF2BP1 stabilizes m6A-methylated circMDK | Promotes tumorigenesis in hepatocellular carcinoma | 118 |
| circCCDC134 | ALKBH5 | ALKBH5-mediated m6A modification on circCCDC134 controlled its stability | Enhances cervical cancer metastasis | 119 |
| circ1662 | METTL3 | METTL3 induces circ1662 expression by binding its flanking sequences and installing m6A modifications | Promotes colorectal cancer metastasis | 120 |
| The effect of circRNA on RNA m6A methylation | ||||
| circPTPRA | IGF2BP1 | CircPTPRA interacts with IGF2BP1 KH domains to disturb IGF2BP1's m6A-modified RNA recognition | Suppresses bladder cancer progression | 121 |
| circZbtb20 | ALKBH5 | circZbtb20 enhances the interaction of Alkbh5 with Nr4a1 mRNA | Maintenance of group 3 innate lymphoid cells homeostasis | 122 |
| circEZH2 | IGF2BP2 | circEZH2 increases the stability of m6A reader IGF2BP2 | Aggravates colorectal cancer | 123 |
| circPDE5A | WTAP | circPDE5A binds to WTAP and inhibits WTAP-mediated m6A modification of Eif3c mRNA | Suppresses prostate cancer metastasis | 124 |
| circSTAG1 | ALKBH5 | circSTAG1 captures ALKBH5 and decreases the translocation of ALKBH5 into nucleus, leading to ALKBH5′S targeted mRNA degradation | Attenuates depressive-like behaviors in mice | 125 |
| CircRAB11FIP1 | FTO | circRAB11FIP1 directly binds to Fto mRNA and promotes its expression | CircRAB11FIP1 induces autophagy accelerating proliferation and invasion in epithelial ovarian cancer | 126 |
| CircMEG3 | METTL3 | CircMEG3 inhibits METTL3 expression | Inhibits human liver cancer growth | 127 |
| CircGPR137B | FTO | m6A-demethylation promotes circGPR137B and circGPR137B up-regulates FTO | Suppresses hepatocellular carcinoma | 129 |
| Circ-ZNF609 | METTL14, FTO | m6A-methylation promotes circ-ZNF609 and circ-ZNF609 inhibits FTO | Aggravates doxorubicin-induced cardiotoxicity | 128 |
Therapeutic strategies to modulate the RNA m6A regulators
RNA modification plays important roles in normal physiological conditions and various diseases. Targeting epigenetic regulators has provided new therapeutic strategies for human diseases.130,131 Like histone modification and DNA methylation, small molecules targeting RNA m6A regulators have been developed. Rhein was the first identified FTO small molecule inhibitor, and rhein caused the increase of mRNA m6A levels in cells.132 However, rhein bound to the nucleic acid-binding site and demonstrated little selectivity for the AlkB subfamily. Using a high-throughput fluorescence polarization assay, meclofenamic acid has been discovered to inhibit RNA m6A demethylase FTO.133 Different from the 2-oxoglutarate-tethering screen strategy, meclofenamic acid specifically inhibits FTO over ALKBH5, which would provide opportunities for specific targeting FTO for biological and therapeutic studies. Other FTO inhibitors, FB23 and FB23-2, displayed therapeutic effects in acute myeloid leukemia.134 In addition to targeting RNA m6A demethylase, small molecule targeting RNA m6A methyltransferase inhibitors were also developed. S-adenosylhomocysteine (SAH) demonstrated inhibitory effects on METTL3-METTL14 complex.135 UZH1a was a METTL3 inhibitor with high–nanomolar activity, UZH1a treatment reduced cellular m6A level and induced acute myeloid leukemia MOLM-13 cell apoptosis.136 Besides, STM2457, a bioavailable inhibitor of METTL3, has been characterized and developed as a therapeutic strategy against acute myeloid leukemia.137 Notably, STM2457 was also the first known small molecule inhibitor of an RNA methyltransferase which demonstrated in vivo activity and therapeutic efficacy. In addition, inhibitors that were targeted to RNA m6A binding proteins can also provide new therapeutic opportunities for diseases. Tegaserod has been identified as a specific YTHDF1 inhibitor and Tegaserod treatment inhibited acute myeloid leukemia progression via YTHDF1.138 Despite some scientific advances, understanding epigenetic therapies for disease treatment is only just beginning. Further studies to develop new inhibitors targeting RNA m6A regulators with improved specificity and much more favorable pharmacokinetic profiles would provide better opportunities for RNA m6A regulators-based therapeutic interventions.
Conclusions and perspectives
In the past few years, thanks to the tremendous progress of new technologies, precise detection of m6A-depositions and efficient m6A methylation installation systems have been developed.139, 140, 141 An increasing number of investigations have demonstrated the important roles of m6A RNA modification in physiological and pathological processes. Recently, more attention has been bestowed on studying the regulation of m6A deposition on RNA, especially in the context of ncRNAs. Studying m6A RNA modifications on ncRNAs is undoubtedly of great importance specifically to deepen our understanding of their biological roles and clinical implications.
Though tremendous progress has been made in recent times, to better uncover this RNA m6A-ncRNAs epigenetic regulation network, several questions need to be further explored. First, the understanding of the underlying mechanisms regarding the m6A modification and ncRNAs remains limited. This is more relevant, especially in a specific context, wherein the RNA m6A modification and ncRNAs regulation always exhibit cell- or tissue-specific interaction. Future studies to address the cell-type specific regulation under a particular context (disease or normal) represent an important area of investigation. Second, RNA m6A modifications and ncRNA dysregulation are closely associated with many human diseases. However, since most of the elaborate studies are still focused on the field of cancer, there is a growing need to explore the biological functions and clinical implications of RNA m6A modifications in other diseases. Future efforts to develop new sequencing and detection technologies for not only RNA m6A modification but also ncRNAs, especially lncRNAs and circRNA, would surely move this field forward and provide greater insight into understanding exact mechanisms as well as a potential strategy for the treatment of human diseases.
While there have been reviews summarizing the research progress of RNA m6A over the past few years, it is important to note that our understanding of RNA m6A and its potential applications in medical research is constantly evolving with the rapid advancements in literature. In this present review, we summarized the latest information about the mutual regulation between RNA m6A modification and ncRNAs, with a specific focus on miRNAs, lncRNAs, and circRNAs. Also, we have discussed the challenges of m6A-containing ncRNAs and RNA m6A as therapeutic targets in human diseases and their future perspective in translational roles. The growing understanding of RNA m6A modification has led to the development of RNA m6A -based therapeutic interventions. RNA m6A-modified genes and their regulatory factors are expected to be potential therapeutic targets. One of the attractive areas is the development of new inhibitors targeting m6A-regulatory factors, including RNA m6A methyltransferase, demethylase, and specific binding protein, and exploration of the relationship between enzyme/reader inhibition and disease phenotypes. Considering the complexity and global effects of RNA m6A methylation regulation and recognition, comprehensive experiments and population-based data are required before the therapeutic potential of those inhibitors can be clinically evaluated. With the rapid progresses in gene editing technologies, gene editing-based technologies on RNA modifications have been designed and remarkable progress has been achieved.142,143 Using gene editing technology to regulate specific target transcript's m6A methylation level is another promising therapeutic strategy for RNA m6A -based therapeutic interventions. Further investigation to apply gene-editing based- RNA m6A control in living organisms might offer a precise tool to regulate RNA m6A modification with high efficiency and specificity.
Author contributions
All authors contributed to the design and interpretation of this work. Gui-e Xu: conceptualization, data curation, methodology, writing-original draft. Xuan Zhao: conceptualization, data curation, methodology. Guoping Li: conceptualization, writing-reviewing, and editing. Priyanka Gokulnath: conceptualization, writing-reviewing, and editing. Lijun Wang: conceptualization, supervision, funding acquisition, writing-original draft, writing-reviewing, and editing. Junjie Xiao: conceptualization, supervision, project administration, funding acquisition, writing-reviewing, and editing. All authors read and approved the final version of the manuscript.
Conflict of interests
The authors declare no competing interests.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82020108002 & 82225005 to JJ Xiao, 82270291 to LJ Wang), the Science and Technology Commission of Shanghai, China (No. 23410750100, 20DZ2255400 & 21XD1421300 to JJ Xiao), and the Natural Science Foundation of Shanghai, China (No. 23ZR1423000 to LJ Wang).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Lijun Wang, Email: lijunwang@shu.edu.cn.
Junjie Xiao, Email: junjiexiao@shu.edu.cn.
References
- 1.Wu G., Zhang X., Gao F. The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci. 2021;10(6):648–659. doi: 10.1016/j.jshs.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiyama A., Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37(11):1012–1027. doi: 10.1016/j.tig.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Wu Q., Schapira M., Arrowsmith C.H., Barsyte-Lovejoy D. Protein arginine methylation: from enigmatic functions to therapeutic targeting. Nat Rev Drug Discov. 2021;20(7):509–530. doi: 10.1038/s41573-021-00159-8. [DOI] [PubMed] [Google Scholar]
- 4.Bhat K.P., Ümit Kaniskan H., Jin J., Gozani O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat Rev Drug Discov. 2021;20(4):265–286. doi: 10.1038/s41573-020-00108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L., Zhang H., Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13(12):877–919. doi: 10.1007/s13238-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Vásquez E., Alata Jimenez N., Vázquez N.A., Strobl-Mazzulla P.H. Emerging role of dynamic RNA modifications during animal development. Mech Dev. 2018;154:24–32. doi: 10.1016/j.mod.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y., Hsu P.J., Chen Y.S., Yang Y.G. Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28(6):616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S., Zhang S., Wu X., Zhou X. m6A RNA methylation in cardiovascular diseases. Mol Ther. 2020;28(10):2111–2119. doi: 10.1016/j.ymthe.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livneh I., Moshitch-Moshkovitz S., Amariglio N., Rechavi G., Dominissini D. The m6A epitranscriptome: transcriptome plasticity in brain development and function. Nat Rev Neurosci. 2020;21(1):36–51. doi: 10.1038/s41583-019-0244-z. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.S., Ouyang X.P., Yu X.H., et al. N6-adenosine methylation (m6A) RNA modification: an emerging role in cardiovascular diseases. J Cardiovasc Transl Res. 2021;14(5):857–872. doi: 10.1007/s12265-021-10108-w. [DOI] [PubMed] [Google Scholar]
- 11.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X., Liu B., Nie Z., et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Targeted Ther. 2021;6(1):74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., Lee G. Metabolic control of m6A RNA modification. Metabolites. 2021;11(2):80. doi: 10.3390/metabo11020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H., Weng H., Chen J. The biogenesis and precise control of RNA m6A methylation. Trends Genet. 2020;36(1):44–52. doi: 10.1016/j.tig.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desrosiers R.C., Friderici K.H., Rottman F.M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- 17.Yue Y., Liu J., He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29(13):1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun T., Wu R., Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108613. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Bei Y., Huang P., et al. Inhibition of miR-155 protects against LPS-induced cardiac dysfunction and apoptosis in mice. Mol Ther Nucleic Acids. 2016;5:e374. doi: 10.1038/mtna.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiuchi K., Kawamura T., Iwanari H., et al. Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288(46):33292–33302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Yue Y., Han D., et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping X.L., Sun B.F., Wang L., et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil D.P., Chen C.K., Pickering B.F., et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz S., Mumbach M.R., Jovanovic M., et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8(1):284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen J., Lv R., Ma H., et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(6):1028–1038. doi: 10.1016/j.molcel.2018.02.015. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng G., Dahl J.A., Niu Y., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia G., Fu Y., Zhao X., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng G., Dahl J.A., Niu Y., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda Y., Ooshio I., Fusamae Y., et al. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7 doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z., Qi M., Shen B., et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47(5):2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theler D., Dominguez C., Blatter M., Boudet J., Allain F.H.T. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 2014;42(22):13911–13919. doi: 10.1093/nar/gku1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H., Weng H., Sun W., et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20(3):285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Zhao B.S., Roundtree I.A., et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Lu Z., Gomez A., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du H., Zhao Y., He J., et al. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7 doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H., Wang X., Lu Z., et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao W., Adhikari S., Dahal U., et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Roundtree I.A., Luo G.Z., Zhang Z., et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6 doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu P.J., Zhu Y., Ma H., et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller S., Glaß M., Singh A.K., et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Chim B., Su Y., et al. Enhancement of LIN28B-induced hematopoietic reprogramming by IGF2BP3. Genes Dev. 2019;33(15–16):1048–1068. doi: 10.1101/gad.325100.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu N., Zhou K.I., Parisien M., Dai Q., Diatchenko L., Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45(10):6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartel D.P. microRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Lv Y., Li G., Xiao J. microRNAs in heart and circulation during physical exercise. J Sport Health Sci. 2018;7(4):433–441. doi: 10.1016/j.jshs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Q., Chen L., Liang X., et al. Exercise attenuates angiotensinⅡ-induced muscle atrophy by targeting PPARγ/miR-29b. J Sport Health Sci. 2022;11(6):696–707. doi: 10.1016/j.jshs.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lou J., Wu J., Feng M., et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. J Sport Health Sci. 2022;11(4):495–508. doi: 10.1016/j.jshs.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H., Deng Q., Lv Z., et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18(1):181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Alarcón C.R., Lee H., Goodarzi H., Halberg N., Tavazoie S.F. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han J., Wang J.Z., Yang X., et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(1):110. doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan G., Yuan Y., He M., et al. m6A methylation of precursor-miR-320/RUNX2 controls osteogenic potential of bone marrow-derived mesenchymal stem cells. Mol Ther Nucleic Acids. 2020;19:421–436. doi: 10.1016/j.omtn.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Bai R., Li M., et al. Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10(1):1858. doi: 10.1038/s41467-019-09712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng W., Li J., Chen R., et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res. 2019;38(1):393. doi: 10.1186/s13046-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J., Ishfaq M., Xu L., Xia C., Chen C., Li J. METTL3/m6A/miRNA-873-5p attenuated oxidative stress and apoptosis in colistin-induced kidney injury by modulating Keap1/Nrf2 pathway. Front Pharmacol. 2019;10:517. doi: 10.3389/fphar.2019.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X., Xu M., Xu X., et al. METTL14 suppresses CRC progression via regulating N6-methyladenosine-dependent primary miR-375 processing. Mol Ther. 2019;28:599–612. doi: 10.1016/j.ymthe.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Ma J.Z., Yang F., Zhou C.C., et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary microRNA processing. Hepatology. 2016;65(2):529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 59.Shah A., Rashid F., Awan H.M., et al. The DEAD-box RNA helicase DDX3 interacts with m6A RNA demethylase ALKBH5. Stem Cell Int. 2017;2017 doi: 10.1155/2017/8596135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu H., Gan X., Jiang X., Diao S., Wu H., Hu J. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res. 2019;38(1):163. doi: 10.1186/s13046-019-1159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berulava T., Rahmann S., Rademacher K., Klein-Hitpass L., Horsthemke B. N6-adenosine methylation in MiRNAs. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian B., Wang P., Zhang D., Wu L. m6A modification promotes miR-133a repression during cardiac development and hypertrophy via IGF2BP2. Cell Death Dis. 2021;7(1):157. doi: 10.1038/s41420-021-00552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z., Li J., Feng G., et al. microRNA-145 modulates N6-methyladenosine levels by targeting the 3'-untranslated mRNA region of the N6-methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292(9):3614–3623. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He H., Wu W., Sun Z., Chai L. miR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m6A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517(4):581–587. doi: 10.1016/j.bbrc.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 65.Xu C., Yuan B., He T., Ding B., Li S. Prognostic values of YTHDF1 regulated negatively by mir-3436 in Glioma. J Cell Mol Med. 2020;24(13):7538–7549. doi: 10.1111/jcmm.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lara-Pezzi E. Understanding cardiac physiological hypertrophy in a LncRNA way. J Cardiovasc Transl Res. 2022;15(1):3–4. doi: 10.1007/s12265-022-10212-5. [DOI] [PubMed] [Google Scholar]
- 67.Sun J., Wang R., Chao T., Wang C. Long noncoding RNAs involved in cardiomyocyte apoptosis triggered by different stressors. J Cardiovasc Transl Res. 2022;15(3):588–603. doi: 10.1007/s12265-021-10186-w. [DOI] [PubMed] [Google Scholar]
- 68.Liu N., Parisien M., Dai Q., Zheng G., He C., Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19(12):1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 70.Huang H., Weng H., Chen J. m6A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37(3):270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu C., Zhang Q.C., da Rocha S.T., et al. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161(2):404–416. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moindrot B., Cerase A., Coker H., et al. A pooled shRNA screen identifies Rbm15, spen, and wtap as factors required for xist RNA-mediated silencing. Cell Rep. 2015;12(4):562–572. doi: 10.1016/j.celrep.2015.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang Y.Z., Chai R.C., Pang B., et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-κB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021;511:36–46. doi: 10.1016/j.canlet.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Wang X., Liu C., Zhang S., et al. N6-methyladenosine modification of MALAT1 promotes metastasis via reshaping nuclear speckles. Dev Cell. 2021;56(5):702–715. doi: 10.1016/j.devcel.2021.01.015. e8. [DOI] [PubMed] [Google Scholar]
- 75.Chen Z.H., Chen T.Q., Zeng Z.C., et al. Nuclear export of chimeric mRNAs depends on an lncRNA-triggered autoregulatory loop in blood malignancies. Cell Death Dis. 2020;11:566. doi: 10.1038/s41419-020-02795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang X., Zhang S., He C., et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19(1):46. doi: 10.1186/s12943-020-1146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nesterova T.B., Wei G., Coker H., et al. Systematic allelic analysis defines the interplay of key pathways in X chromosome inactivation. Nat Commun. 2019;10(1):3129. doi: 10.1038/s41467-019-11171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Y., Yang X., Chen Z., et al. m6A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18(1):87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen S., Wei Y., Zen C., Xiong W., Niu Y., Zhao Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol Cancer. 2020;19(1):171. doi: 10.1186/s12943-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Z.Q., Li Z.X., Zhou G.Q., et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and upregulate ITGB3. Cancer Res. 2019;79(18):4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 81.Liu H.T., Zou Y.X., Zhu W.J., et al. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 2022;29(3):627–641. doi: 10.1038/s41418-021-00879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H., Wang S.Q., Wang L., et al. m6A methyltransferase METTL3-induced lncRNA SNHG17 promotes lung adenocarcinoma gefitinib resistance by epigenetically repressing LATS2 expression. Cell Death Dis. 2022;13(7):657. doi: 10.1038/s41419-022-05050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ji X., Liu Z., Gao J., et al. N6-Methyladenosine-modified lncRNA LINREP promotes glioblastoma progression by recruiting the PTBP1/HuR complex. Cell Death Differ. 2023;30(1):54–68. doi: 10.1038/s41418-022-01045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin H., Chen L., Piao S., et al. M6A RNA methylation-mediated RMRP stability renders proliferation and progression of non-small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2023;30(3):605–617. doi: 10.1038/s41418-021-00888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z.X., Zheng Z.Q., Yang P.Y., et al. WTAP-mediated m6A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. 2022;29(6):1137–1151. doi: 10.1038/s41418-021-00905-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z.W., Pan J.J., Hu J.F., et al. SRSF3-mediated regulation of N6-methyladenosine modification-related lncRNA ANRIL splicing promotes resistance of pancreatic cancer to gemcitabine. Cell Rep. 2022;39(6) doi: 10.1016/j.celrep.2022.110813. [DOI] [PubMed] [Google Scholar]
- 87.Dai Y.Z., Liu Y.D., Li J., et al. METTL16 promotes hepatocellular carcinoma progression through downregulating RAB11B-AS1 in an m6A-dependent manner. Cell Mol Biol Lett. 2022;27(1):41. doi: 10.1186/s11658-022-00342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J., Tan L., Yu X., et al. lncRNA ZNRD1-AS1 promotes malignant lung cell proliferation, migration, and angiogenesis via the miR-942/TNS1 axis and is positively regulated by the m6A reader YTHDC2. Mol Cancer. 2022;21(1):229. doi: 10.1186/s12943-022-01705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ni W., Yao S., Zhou Y., et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18(1):143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li G., Ma L., He S., et al. WTAP-mediated m6A modification of lncRNA NORAD promotes intervertebral disc degeneration. Nat Commun. 2022;13(1):1469. doi: 10.1038/s41467-022-28990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pan J., Xie Y., Li H., et al. mmu-lncRNA 121686/hsa-lncRNA 520657 induced by METTL3 drive the progression of AKI by targeting miR-328-5p/HtrA3 signaling axis. Mol Ther. 2022;30(12):3694–3713. doi: 10.1016/j.ymthe.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X., Li Q., He S., et al. LncRNA FENDRR with m6A RNA methylation regulates hypoxia-induced pulmonary artery endothelial cell pyroptosis by mediating DRP1 DNA methylation. Mol Med. 2022;28(1):126. doi: 10.1186/s10020-022-00551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gu Y., Niu S., Wang Y., et al. DMDRMR-mediated regulation of m6A-modified CDK4 by m6A reader IGF2BP3 drives ccRCC progression. Cancer Res. 2021;81(4):923–934. doi: 10.1158/0008-5472.CAN-20-1619. [DOI] [PubMed] [Google Scholar]
- 94.Ye M., Dong S., Hou H., Zhang T., Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2021;23:1–12. doi: 10.1016/j.omtn.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21(8):475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 96.Lu D., Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol. 2019;16(11):661–674. doi: 10.1038/s41569-019-0218-x. [DOI] [PubMed] [Google Scholar]
- 97.Li B., Li Y., Hu L., et al. Role of circular RNAs in the pathogenesis of cardiovascular disease. J Cardiovasc Transl Res. 2020;13(4):572–583. doi: 10.1007/s12265-019-09912-2. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y., Fan X., Mao M., et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Timoteo G., Dattilo D., Centrón-Broco A., et al. Modulation of circRNA metabolism by m6A modification. Cell Rep. 2020;31(6) doi: 10.1016/j.celrep.2020.107641. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y.G., Chen R., Ahmad S., et al. N6-methyladenosine modification controls circular RNA immunity. Mol Cell. 2019;76(1):96–109.e9. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou C., Molinie B., Daneshvar K., et al. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20(9):2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y., Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21(2):172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Legnini I., Di Timoteo G., Rossi F., et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L., Yu P., Wang J., et al. Downregulation of circ-ZNF609 promotes heart repair by modulating RNA N6-methyladenosine-modified Yap expression. Research. 2022;2022 doi: 10.34133/2022/9825916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao J., Lee E.E., Kim J., et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10(1):2300. doi: 10.1038/s41467-019-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leprivier G., Rotblat B., Khan D., Jan E., Sorensen P.H. Stress-mediated translational control in cancer cells. Biochim Biophys Acta. 2015;1849(7):845–860. doi: 10.1016/j.bbagrm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 107.Hansen T.B., Wiklund E.D., Bramsen J.B., et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30(21):4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C.X., Li X., Nan F., et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177(4):865–880.e21. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 109.Park O.H., Ha H., Lee Y., et al. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol Cell. 2019;74(3):494–507.e8. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 110.Fischer J.W., Busa V.F., Shao Y., Leung A.K.L. Structure-mediated RNA decay by UPF1 and G3BP1. Mol Cell. 2020;78(1):70–84.e6. doi: 10.1016/j.molcel.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fu Y., Zhuang X. m6A-binding YTHDF proteins promote stress granule formation. Nat Chem Biol. 2020;16(9):955–963. doi: 10.1038/s41589-020-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haque S., Harries L.W. Circular RNAs (circRNAs) in health and disease. Genes (Basel) 2017;8(12):353. doi: 10.3390/genes8120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L., Xu G.E., Spanos M., et al. Circular RNAs in cardiovascular diseases: regulation and therapeutic applications. Research. 2023;6:38. doi: 10.34133/research.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen R.X., Chen X., Xia L.P., et al. N6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10(1):4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo Y., Guo Y., Chen C., et al. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer. 2021;20(1):93. doi: 10.1186/s12943-021-01372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu P., Fang X., Liu Y., et al. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12(4):298. doi: 10.1038/s41419-021-03558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu J., Wan Z., Tang M., et al. N6-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol Cancer. 2020;19(1):163. doi: 10.1186/s12943-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Du A., Li S., Zhou Y., et al. M6A-mediated upregulation of circMDK promotes tumorigenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. Mol Cancer. 2022;21(1):109. doi: 10.1186/s12943-022-01575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang L., Zhu Y., Li J., Zeng J., Wu L. ALKBH5-mediated m6A modification of circCCDC134 facilitates cervical cancer metastasis by enhancing HIF1A transcription. J Exp Clin Cancer Res. 2022;41(1):261. doi: 10.1186/s13046-022-02462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen C., Yuan W., Zhou Q., et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11(9):4298–4315. doi: 10.7150/thno.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xie F., Huang C., Liu F., et al. CircPTPRA blocks the recognition of RNA N6-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20(1):1–17. doi: 10.1186/s12943-021-01359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu B., Liu N., Zhu X., et al. Circular RNA circZbtb20 maintains ILC3 homeostasis and function via Alkbh5-dependent m6A demethylation of Nr4a1 mRNA. Cell Mol Immunol. 2021;18(6):1412–1424. doi: 10.1038/s41423-021-00680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yao B., Zhang Q., Yang Z., et al. CircEZH2/miR-133b/IGF2BP2 aggravates colorectal cancer progression via enhancing the stability of m6A-modified CREB1 mRNA. Mol Cancer. 2022;21(1):140. doi: 10.1186/s12943-022-01608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ding L., Wang R., Zheng Q., et al. circPDE5A regulates prostate cancer metastasis via controlling WTAP-dependent N6-methyladenisine methylation of EIF3C mRNA. J Exp Clin Cancer Res. 2022;41(1):187. doi: 10.1186/s13046-022-02391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang R., Zhang Y., Bai Y., et al. N6-methyladenosine modification of fatty acid amide hydrolase messenger RNA in circular RNA STAG1-regulated astrocyte dysfunction and depressive-like behaviors. Biol Psychiatr. 2020;88(5):392–404. doi: 10.1016/j.biopsych.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Z., Zhu H., Hu J. CircRAB11FIP1 promoted autophagy flux of ovarian cancer through DSC1 and miR-129. Cell Death Dis. 2021;12(2):219. doi: 10.1038/s41419-021-03486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang X., Xing L., Chen Y., et al. CircMEG3 inhibits telomerase activity by reducing Cbf5 in human liver cancer stem cells. Mol Ther Nucleic Acids. 2021;23:310–323. doi: 10.1016/j.omtn.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu P., Wang J., Xu G.E., et al. RNA m6A-regulated circ-ZNF609 suppression ameliorates doxorubicin-induced cardiotoxicity by upregulating FTO. JACC (J Am Coll Cardiol) 2023;8(6):677–698. doi: 10.1016/j.jacbts.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L., Gu M., Ma J., et al. CircGPR137B/miR-4739/FTO feedback loop suppresses tumorigenesis and metastasis of hepatocellular carcinoma. Mol Cancer. 2022;21(1):149. doi: 10.1186/s12943-022-01619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hogg S.J., Beavis P.A., Dawson M.A., Johnstone R.W. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19(11):776–800. doi: 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Rong D., Li B., Wang Y. Targeting epigenetic regulators with covalent small-molecule inhibitors. J Med Chem. 2021;64(12):7900–7925. doi: 10.1021/acs.jmedchem.0c02055. [DOI] [PubMed] [Google Scholar]
- 132.Chen B., Ye F., Yu L., et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134(43):17963–17971. doi: 10.1021/ja3064149. [DOI] [PubMed] [Google Scholar]
- 133.Huang Y., Yan J., Li Q., et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43(1):373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang Y., Su R., Sheng Y., et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35(4):677–691. doi: 10.1016/j.ccell.2019.03.006. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li F., Kennedy S., Hajian T., et al. A radioactivity-based assay for screening human m6A-RNA methyltransferase, METTL3-METTL14 complex, and demethylase ALKBH5. J Biomol Screen. 2016;21(3):290–297. doi: 10.1177/1087057115623264. [DOI] [PubMed] [Google Scholar]
- 136.Moroz-Omori E.V., Huang D., Kumar Bedi R., et al. METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem. 2021;16(19):3035–3043. doi: 10.1002/cmdc.202100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yankova E., Blackaby W., Albertella M., et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593(7860):597–601. doi: 10.1038/s41586-021-03536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hong Y.G., Yang Z., Chen Y., et al. The RNA m6A reader YTHDF1 is required for acute myeloid leukemia progression. Cancer Res. 2023;83(6):845–860. doi: 10.1158/0008-5472.CAN-21-4249. [DOI] [PubMed] [Google Scholar]
- 139.Wilson C., Chen P.J., Miao Z., Liu D.R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat Biotechnol. 2020;38(12):1431–1440. doi: 10.1038/s41587-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X., Xiong X., Yi C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat Methods. 2016;14(1):23–31. doi: 10.1038/nmeth.4110. [DOI] [PubMed] [Google Scholar]
- 141.Garcia-Campos M.A., Edelheit S., Toth U., et al. Deciphering the “m6A code” via antibody-independent quantitative profiling. Cell. 2019;178(3):731–747.e16. doi: 10.1016/j.cell.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 142.Wilson C., Chen P.J., Miao Z., Liu D.R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat Biotechnol. 2020;38(12):1431–1440. doi: 10.1038/s41587-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun X., Wang D.O., Wang J. Targeted manipulation of m6A RNA modification through CRISPR-Cas-based strategies. Methods. 2022;203:56–61. doi: 10.1016/j.ymeth.2022.03.006. [DOI] [PubMed] [Google Scholar]