Summary
Many addictive drugs increase stress hormone levels. They also alter the propensity of organisms to prospectively select actions based on long-term consequences. We hypothesized that cocaine causes inflexible action by increasing circulating stress hormone levels, activating the glucocorticoid receptor (GR). We trained mice to generate two nose pokes for food and then required them to update action-consequence associations when one response was no longer reinforced. Cocaine delivered in adolescence or adulthood impaired the capacity of mice to update action strategies, and inhibiting CORT synthesis rescued action flexibility. Next, we reduced Nr3c1, encoding GR, in the orbitofrontal cortex (OFC), a region of the brain responsible for interlacing new information into established routines. Nr3c1 silencing preserved action flexibility and dendritic spine abundance on excitatory neurons, despite cocaine. Spines are often considered substrates for learning and memory, leading to the discovery that cocaine degrades the representation of new action memories, obstructing action flexibility.
Subject areas: Natural sciences, Biological sciences, Neuroscience, Behavioral neuroscience, Molecular neuroscience, Cellular neuroscience
Graphical abstract
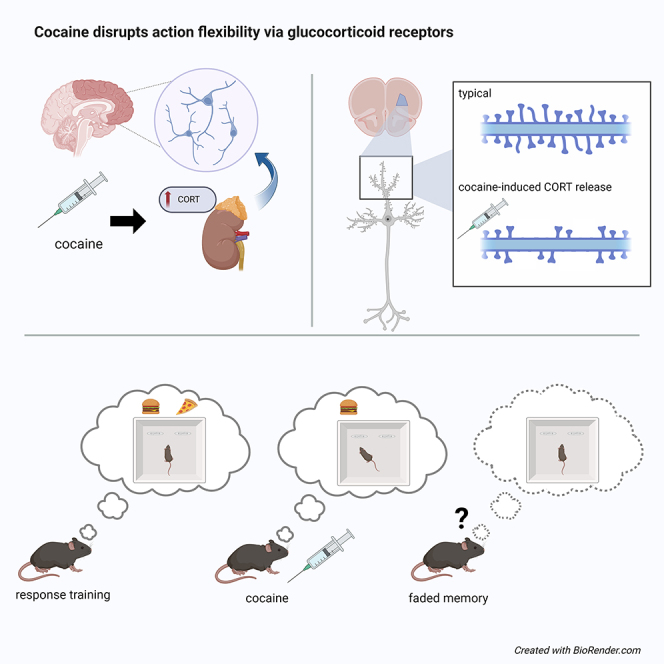
Highlights
-
•
Cocaine induces corticosteroid hormone release and decision-making biases
-
•
We find that hormone binding in the orbital cortex induces dendritic spine loss
-
•
It also contributes to habit-like decision-making biases
-
•
One mechanism is by impairing the ability of mice to access reward memories
Natural sciences; Biological sciences; Neuroscience; Behavioral neuroscience; Molecular neuroscience; Cellular neuroscience
Introduction
Stress is considered both a causal factor in, and consequence of, many neuropsychiatric illnesses including substance use disorders. In experimental animals, social defeat stress increases cocaine self-administration,1 while cocaine elicits a stress response.2,3,4,5 Stress may play a role in inducing and exacerbating many characteristics of cocaine use disorder, including modifications in decision-making strategies that perpetuate drug seeking. For instance, repeated cocaine,6,7,8,9,10 chronic stress,11,12 and exogenous corticosterone (CORT)13,14 degrade the capacity of organisms to select actions based on their consequences, causing a deferral to habit-like behavior. For example, Dias-Ferreira et al. subjected rats to repeated restraint stress, which caused them to be unable to make choices based on whether a response was likely to be reinforced or based on the value of the likely outcome.11 Later investigation revealed that response biases are attributable, at least in part, to CORT release.13 A history of stressor exposure even appears to contribute to cocaine-induced decision-making biases in humans,9 but the consequences of drug-elicited stress hormone release are not well understood.
CORT is a primary stress hormone (cortisol in humans). It binds to high-affinity mineralocorticoid receptors (MR) at baseline and then additionally, low-affinity glucocorticoid receptors (GR) upon stress-induced adrenal CORT release. CORT readily crosses the blood brain barrier and binds neuronal GRs,15,16 including in the orbitofrontal cortex (OFC). This brain region is important for integrating new learning into existing knowledge, enabling adaptive modification of behavioral action strategies when familiar expectancies change. Stressors, CORT, and psychostimulants all commonly cause dendritic spine attrition on excitatory OFC neurons and also obstruct action flexibility.4,7,17,18,19,20,21,22 Meanwhile, mice that are resilient to cocaine-induced behavioral inflexibilities have enlarged spine heads on excitatory OFC neurons,8 and drugs that improve flexible behavior cause spine head enlargement23 or spinogenesis.24 Spines are often considered substrates for learning and memory, and these patterns recently led to the discovery that the OFC forms memory traces for new action memories, which are necessary for later action flexibility.25
Here, we tested the hypothesis that repeated cocaine, as would occur in individuals suffering from substance misuse, causes action inflexibilities by increasing circulating stress hormones and activating GRs. We find that cocaine-elicited CORT release and binding to GRs in the OFC robustly contributes to response inflexibilities. Further, these inflexibilities can be attributed to the inability of the OFC to use action memory traces to update action strategies, and not obviously impulsive- or anhedonic-like behavior.
Results
Cocaine induces CORT excess, which occludes action updating in mice
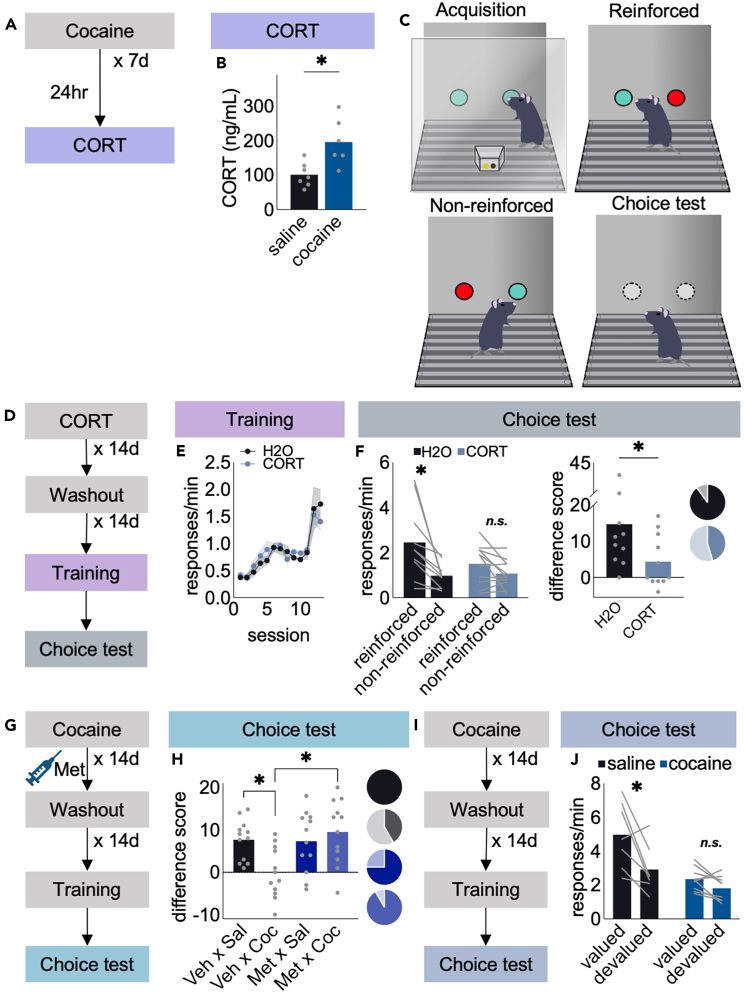
The over-arching hypothesis of this project was that cocaine-induced CORT contributes to cocaine-induced modifications in reward-seeking behavior. We thus first confirmed that repeated cocaine increases circulating CORT relative to saline injection [t11 = 3.25, p = 0.008] (Figures 1A and 1B), as previously reported.2,3,4
Figure 1.
Cocaine-induced action inflexibilities appear attributable to CORT release
(A) Timeline.
(B) Cocaine increased circulating CORT. n = 6–7 mice/group.
(C) Mice were trained to respond on 2 nose poke apertures for food. Then, pellets associated with 1 nose poke were delivered independently of nose poking (“non-reinforced” condition). Responding at the other port remained reinforced. Behavioral flexibility was assessed the following day in a brief choice test.
(D) Timeline.
(E) Mice were trained to nose poke, with no differences between groups here or in other experiments.
(F) Excess CORT blocked action flexibility, indicated by non-preferential responding at the choice test. The same data can be represented as difference scores (number of responses at the “reinforced”-“non-reinforced” aperture). Zero indicates no preference. Pie charts represent the number of mice/group that preferentially responded at the “reinforced” aperture. n = 10–11 mice/group.
(G) Timeline.
(H) Cocaine caused the same response biases as CORT exposure, and blocking CORT synthesis prior to cocaine prevented those inflexibilities. n = 12 mice/group.
(I) Timeline.
(J) Effects of cocaine on sensitivity to reinforcer devaluation were also assessed. Control mice favored a valued over devalued pellet, but cocaine-exposed mice did not. n = 7–9 mice/group. ∗p ≤ 0.05 following t-test when comparing 2 groups at a single time point and ANOVA when comparing >2 groups and/or multiple time points. Bars and connected dots represent means (±SEMs if indicated), and gray dots and lines represent individual mice.
We next used a task in which mice must update actions when familiar expectancies are violated. We trained mice to nose poke for food reinforcers and then reduced the likelihood that responding at 1 of 2 apertures would be reinforced, thus breaking the association between nose poking at that aperture and food delivery (Figure 1C). Successfully updating this association is evidenced by preferential responding during a subsequent choice test on the aperture at which the action-outcome association remained intact. In this experiment, mice were first delivered exogenous CORT, increasing blood serum CORT to a degree similar to that following cocaine,26 followed by a washout period to assess long-term consequences (Figure 1D). Groups did not differ during response training [no main effect of group F < 1, no interaction p = 0.53] (Figure 1E); however, mice with prior CORT exposure were unable to update choice behavior, responding equivalently at both apertures even when one response was unlikely to be reinforced [aperture × group interaction F(1,19) = 4.28, p = 0.05] (Figure 1F). Response rates appeared lower overall in the CORT-exposed mice [though the effect of group was non-significant: F(1,19) = 1.42, p = 0.25], consistent with evidence that prolonged CORT induces chronic amotivation.13,26,27
The same data can be converted to difference scores, referring to responses on the “reinforced”-“non-reinforced” ports. Scores >0 indicate preferential responding, as in the control group, whereas CORT-exposed mice had lower scores approximating 0 – no preference [t(19) = 2.06, p = 0.05] (Figure 1F). Difference scores are reported in the rest of the main text. Acquisition curves (i.e., response training), which never differed between groups, and response rates in the choice tests are hereafter reported in the supplementary materials (Figure S1).
Next, we assessed the effects of cocaine in the same task, anticipating that any behavioral inflexibilities would be attributable to cocaine-elicited CORT. To test this possibility, we turned to the CORT synthesis inhibitor, Metyrapone (Met), administered prior to cocaine (Figure 1G). Later testing revealed that cocaine obstructed action flexibility, as expected. Meanwhile, inhibiting CORT synthesis rescued flexible, preferential responding in cocaine-exposed mice [cocaine × Met interaction F(1,44) = 7.28, p = 0.01] (Figure 1H). Notably, we also observed the same effect in mice given cocaine in adolescence and then tested in adulthood (Figure S2), suggesting that cocaine-induced CORT release impacts action flexibility across multiple ages and drug administration procedures.
This pattern of cocaine-induced response inflexibility has been framed as habit-like behavior. To substantiate this perspective, we assessed whether the same cocaine exposure procedure impacted the ability of mice to update action strategies when outcome values changed (Figure 1I). In this task, mice must integrate the sensory properties of rewards into goal representations to engage in flexible goal seeking. A failure to do so is classically considered habitual behavior.28 Mice were trained to nose poke for food reinforcers, then given ad libitum access to pellets, thus devaluing pellets by virtue of satiety (“devalued” condition). As a control, mice were separately given free access to vivarium chow, thus leaving pellet value intact (“valued” condition). Control mice respond more in the valued condition, while cocaine-exposed mice responded equivalently in both conditions [cocaine x value condition interaction F(1,14) = 4.85, p = 0.045] (Figure 1J), despite intact motivation for food reinforcers, as assessed using a progressive ratio test (Figure S3).
Activation of GRs in the VLO drives cocaine-induced inflexibilities
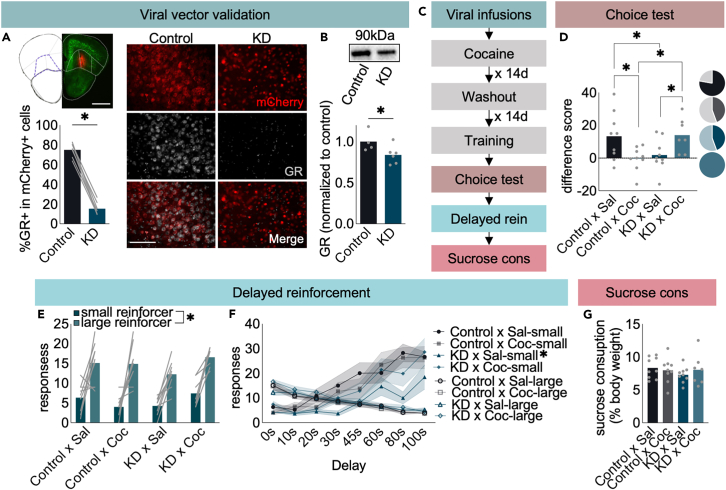
The OFC is an expansive and functionally heterogeneous structure.29 We focused here on the ventrolateral OFC (VLO), which appears to be sensitive to changes in reward availability and updating action strategies, compared to far lateral or posterior subregions.25,30 We hypothesized that CORT binding to low-affinity GRs in the VLO may be responsible for the disruption of action flexibility. We site-selectively silenced Nr3c1, which encodes GR, resulting in near complete ablation of GR in transduced CaMKII+ neurons [t(6) = 19.14, p < 0.001] (Figure 2A). This nevertheless accounted for only ∼20% loss of gross GR protein in VLO-containing tissue punches [t(8) = 2.02, p = 0.04] (Figure 2B), presumably due to the ubiquity of GR on multiple cell types that were spared transduction (like glial cells).31 Next, we behaviorally tested mice with VLO-selective GR reduction (Figure 2C). Mice were trained to respond for food, then had to update the association between nose poking and pellet delivery when one response was no longer reinforced, as above. Cocaine-exposed mice exhibited non-preferential responding, as expected. Meanwhile, GR reduction rescued preferential responding in cocaine-exposed mice. Interestingly, GR reduction in naive mice had the opposite effect, ablating response flexibility [cocaine x Nr3c1 condition interaction F(1,30) = 11.78, p = 0.002] (Figure 2D).
Figure 2.
GR presence in the VLO controls action flexibility
(A) Representative mCherry-expressing viral vector in the VLO of a YFP-expressing mouse. Cre infusion into “floxed” Nr3c1 mice reduced GR immunofluorescence in CaMKII+ neurons relative to infusion of a control viral vector in the opposite hemisphere. n = 7 mice, with comparison across hemispheres. Scale bars = 100μm.
(B) Cre infusion into “floxed” Nr3c1 mice also reduced GR protein levels in gross tissue punches. n = 4–6 mice/group.
(C) Timeline.
(D) Cocaine induced inflexible choice, as before, but GR silencing in the VLO prevented inflexible behavior. Interestingly, GR reduction induced inflexible behavior in cocaine-naïve mice. Pie charts represent the number of mice/group that preferentially responded at the “reinforced” aperture.
(E) Impulsive-like behavior was next assessed in the same mice using a delayed reinforcement task. Mice could preferentially respond for a large over small reinforcer.
(F) When a delay was introduced between responding for the large reinforcer and reinforcer delivery, mice switched preference to the small reinforcer. Drug-naïve mice with GR silencing generated lower response rates overall, though cocaine was without effect.
(G) Groups did not differ in sucrose consumption. n = 7–9 mice/group. ∗p < 0.05 following t-test when comparing 2 groups at a single time point and ANOVA when comparing >2 groups and/or multiple time points. Bars and connected dots represent means (±SEMs if indicated), and gray dots and lines represent individual mice.
Cocaine does not induce impulsive- or anhedonic-like behavior
Response inflexibilities following cocaine could conceivably be attributable to multiple stress-related sequelae, including impulsive- or anhedonic-like behavior. To disentangle these possibilities, mice were next trained to nose poke at 1 aperture, resulting in the delivery of 5 pellets (large reinforcer) or another aperture, resulting in 1 pellet (small reinforcer). All mice, regardless of group, preferentially responded at the aperture associated with the large reinforcer [main effect of reinforcer magnitude F(1,30) = 67.97, p < 0.001, no interaction, all other Fs<1] (Figure 2E). Following this training, a delay between nose poking for the large reinforcer and pellet delivery was introduced, increasing across sessions. We identified no effects of cocaine [aperture × cocaine interaction F < 1; delay × cocaine interaction F < 1], suggesting that cocaine did not induce impulsive-like responding. Interestingly, GR reduction alone reduced responding for the small reinforcer across the delay phase [aperture x delay x Nr3c1 condition interaction F(7,24) = 5.03, p = 0.001] (Figure 2F). This outcome was unexpected, given that reducing brain GR levels in mice by ∼ half hinders their ability to inhibit responding during a waiting period32 so further investigation may be warranted.
We next tested mice for anhedonic-like behavior using a sucrose consumption test, revealing no group differences [no main effect of cocaine F < 1; no main effect of Nr3c1 condition F < 1; no cocaine x Nr3c1 condition interaction F(1,30) = 0.85, p = 0.36] (Figure 2G).
Cocaine destabilizes action memory
Another explanation for cocaine-induced response inflexibilities, aside from impulsive- or anhedonic-like behavior, is deficiencies in the learning and memory processes required for mice to update action strategies. A neuronal ensemble in the VLO that encodes action memories was recently identified. These cells are active when mice experience unexpected non-reinforcement, and then must be re-activated later in order for mice to flexibly deviate from familiar response strategies.25 We hypothesized that cocaine degrades new action strategy memories. To test this possibility, we induced Gq DREADDs in cocaine-naïve mice in cells that were active following the non-reinforced session, when new expectancies were violated and mice inhibited a non-reinforced behavior (Figure S1G), which allowed us to then stimulate these memory trace neurons following cocaine exposure. We rationalized that if cocaine deteriorates new memory or weakens memory retrieval, stimulating this memory trace population may restore action flexibility. If cocaine instead ablates action memory, which is another possibility, then stimulation would have no effects.
We first confirmed that administration of the DREADDs ligand increased c-Fos in mice bearing Cre-dependent DREADDs vs. Cre-dependent fluorophores, as expected [t(12) = −2.31, p = 0.04] (Figures 3A–3C). With viral vectors thus validated, we proceeded to behavioral testing. Cocaine alone caused non-preferential responding even when one familiar behavior was no longer reinforced, as expected. This was despite reactivation of memory trace neurons at the choice test, as measured by c-Fos in viral transduced cells (Figure S4). Nevertheless, chemogenetically stimulating memory trace cells rescued flexible behavior in cocaine-exposed mice [cocaine × DREADD interaction F(1,25) = 4.10, p = 0.05] (Figure 3D). And interestingly, Gq DREADDs in naive mice had the opposite effects, ablating response flexibility (Figures 3D and S1H).
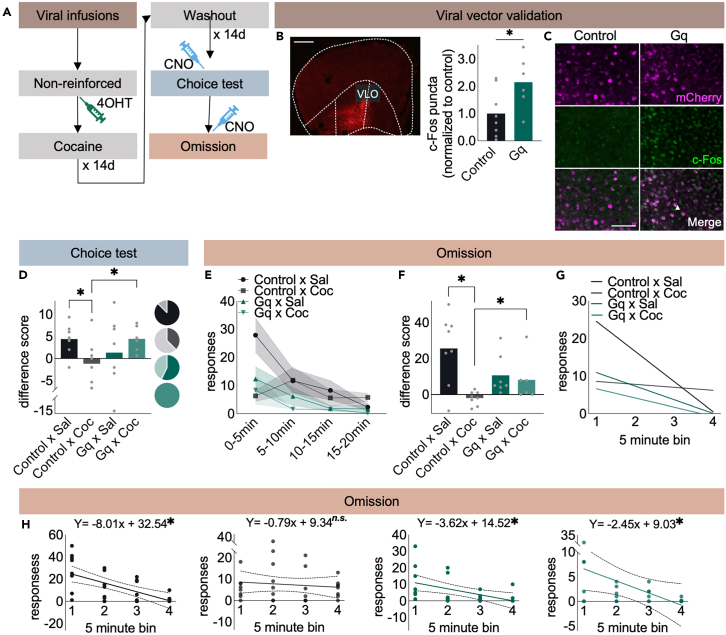
Figure 3.
Cocaine degrades action-outcome memory
(A) Timeline. Cre-dependent Gq DREADDs were delivered to the VLO, and 4OHT induced Cre in cells active following unexpected non-reinforcement. Mice were then administered cocaine, followed by a washout period, and finally, Gq DREADDs+ cells were stimulated during a choice test. The rationale was that if cocaine weakened action memory representations, then stimulating these neurons may reinstate action flexibility. If cocaine instead ablated action memory, then Gq DREADDs should have no effects.
(B) Representative viral vector infusion. Gq DREADDs increased c-Fos, as expected. n = 6–8 mice/group. Scale bar = 100μm.
(C) Representative c-Fos+ puncta. Arrows indicating co-localization of c-Fos puncta and DREADD. Scale bar = 100 μm.
(D) Control mice favored a reinforced behavior, and cocaine obstructed response preference, as expected. Gq DREADDs stimulation, though, rescued flexible action in cocaine-exposed mice. Pie charts represent the number of mice/group that preferentially responded at the “reinforced” aperture.
(E) Response patterns when mice were next tested in omission.
(F) Response rates dropped considerably from the beginning to end of the session in control mice, reflected by large difference scores. Meanwhile, cocaine-alone mice responded similarly in the first and last time bins (scores ∼0), and Gq DREADDs boosted difference scores in cocaine-exposed mice.
(G) Line fits corresponding to e.
(H) Line fits with individual data points. The slope of the cocaine-alone group did not differ from 0, indicating no change across time. n = 6–8 mice/group. ∗p < 0.05 following t-test when comparing 2 groups at a single time point and ANOVA when comparing >2 groups and/or multiple time points. Simple linear regression analyses were also applied (bottom row). Bars and connected dots represent means (±SEMs if indicated), and unconnected dots represent individual mice.
To summarize, cocaine destabilizes action memories – likely memory to inhibit a familiar response.25 To substantiate this perspective, we next assessed the ability of the mice to update responding in an instrumental omission task. In this case, reinforcers are delivered only when mice inhibit responding, so the adaptive response is to depress responding. Across the session, mice decreased responding [main effect of time bin F(3,75) = 11.48, p < 0001]. Cocaine-alone mice did not change responding as much, however, and activating the action memory trace cells facilitated adaptive responding, decreasing responding in cocaine-exposed mice [bin x drug × DREADD interaction F(3,75) = 3.30, p = 0.03] (Figure 3E). We next calculated the difference between response rates during the last 5 min relative to the first 5 min. Again, cocaine-alone mice did not change their responding across time, but stimulating action memory trace cells caused cocaine-exposed mice to suppress responding [cocaine × DREADD interaction F(1,25) = 6.19, p = 0.02] (Figure 3F).
To assess response rates in another way, we used simple linear regression analyses to fit lines to each group. The line for each group was significantly different [F(3,108) = 4.75, p = 0.004] (Figure 3G), indicating that the groups responded differently across the session. We then assessed whether each line was significantly different than zero, indicating a change in responding. All groups except the cocaine-alone mice had a slope significantly different than zero, indicating that all but the cocaine-alone mice decreased responding across time [mCherry x Sal F(1,30) = 18.69, p = 0.0002; Gq x Sal F(1,26) = 8.44, p = 0.007; Gq x Coc F(1,22) = 4.79, p = 0.04; mCherry x Sal F(1,30) = 0.34, p = 0.56] (Figure 3H). Taken together, our findings indicate that cocaine impedes the ability of OFC neurons to stabilize novel action strategies, as opposed to ablating action memory entirely.
GRs in the VLO drive cocaine-induced dendritic spine loss
In addition to their overlapping effects on behavioral responses, both cocaine and CORT cause attrition of dendritic spines on excitatory neurons in the VLO. Given our findings that cocaine imperils memory, and dendritic spines are often considered substrates of learning and memory, we lastly hypothesized that cocaine-induced spine loss was due to activation of GRs. To test this hypothesis, we imaged YFP+mCherry+ dendrites from the mice used in Figure 2. Here, YFP identifies excitatory layer V neurons and enables high-resolution single cell imaging (Figure 4A), and mCherry signifies viral vector transduction. Dendritic spine densities were lower in cocaine-exposed mice, which was rescued by viral-mediated GR reduction. Interestingly, GR reduction alone (in the absence of cocaine) also reduced dendritic spine density, bringing to mind poor learning in these mice (cf. Figure 2) [cocaine x Nr3c1 condition interaction F(1,24) = 11.47, p = 0.002] (Figure 4B). In other words, both groups that struggled to update action strategies also suffered spine attrition on excitatory OFC neurons.
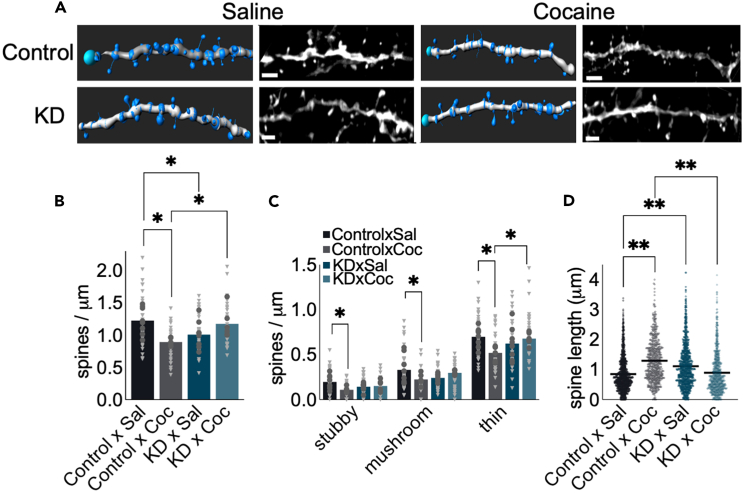
Figure 4.
Cocaine-induced dendritic spine loss in the VLO is GR-dependent
(A) Representative images and 3D reconstructions adjacent. Scale bar = 2μm.
(B) Cocaine caused a loss of dendritic spines in the VLO that was prevented by GR reduction. GR reduction alone also reduced overall dendritic spine densities in the VLO.
(C) Cocaine caused attrition of all spine subtypes, with prevention of thin-type spine loss by GR reduction.
(D) Cocaine and GR reduction alone also increased dendritic spine lengths. Meanwhile, cocaine-induced lengthening was prevented by GR reduction. n = 6–8 mice/group. Bars represent means. Dark gray dots represent individual mice, and light gray triangles represent individual dendrites in B and C. Dots represent individual dendrites in D. ∗p < 0.05, ∗∗p < 0.0001 following interaction effects detected by ANOVA or K-S comparisons (in D).
Next, we compared dendritic spine morphological subtypes across groups – stubby-type, mushroom-type, versus thin-type. Cocaine alone caused a loss of stubby-type and mushroom-type spines [stubby-type spines cocaine x Nr3c1 condition interaction F(1,24) = 4.97, p = 0.035; mushroom-type spines cocaine x Nr3c1 condition F(1,24) = 4.92, p = 0.036]. Cocaine also caused a loss of thin-type spines, which was rescued by GR reduction [cocaine x Nr3c1 condition F(1,24) = 5.92, p = 0.023] (Figure 4C). We then assessed the distribution of spine lengths, agnostic to the spine types. Dendritic spine lengths were longer in cocaine-exposed mice and mice that had GR reduction alone [K-S p’s < 0.0001] (Figure 4D). Other morphometric measures did not differ between groups (not shown).
Discussion
Here, we examined mechanisms by which cocaine biases organisms toward inflexible, familiar action strategies. Specifically, we evaluated the contribution of cocaine-elicited CORT release, finding that inhibiting CORT synthesis prevents inflexible behavior in cocaine-exposed mice, and this effect appears to be mediated by GR presence on CaMKII+ neurons in the VLO. Cocaine-induced response biases were not obviously attributable to impulsive- or anhedonic-like behavior, sequelae linked to stress systems, but rather, the destabilization of memory traces in the VLO that store action memories for later retrieval and action flexibility.
Cocaine-induced CORT blunts action updating
Here, we investigated the learning and memory processes by which behaviors are flexibly updated in dynamic environments. Mice were first trained to respond in two nose-poke apertures for food pellets delivered into a separate magazine. Then, responding was no longer reinforced at one aperture, and instead, pellets were delivered non-contingently. Thus, for one nose-poking behavior, familiar reinforcement conditions were maintained, while for the other, those expectations were violated. Nose-poking is instrumental in nature, as opposed to a Pavlovian response based on the stimulus properties of the nose-poke aperture.25 As such, during a brief choice test, typical mice preferentially engage the aperture where nose-poking had remained reinforced, demonstrating sustained, flexible deviation from the equivalent responding at both apertures established during training.
Cocaine causes failures in action updating in this and similar tasks in rodents6,7,33 and humans9,10 alike. Prior stressor exposure appears to be a major contributor in humans,9 and is itself sufficient to induce the same behavioral inflexibilities.11,12 Given that cocaine elicits a stress response,2,3,4 and cocaine and stress have strikingly similar effects in tests of action flexibility, we hypothesized that cocaine acts through a CORT-mediated mechanism. Indeed, blocking CORT synthesis prior to cocaine administration fully rescued flexible behavior in cocaine-exposed mice. Notably, CORT synthesis inhibition can also blunt cocaine-induced locomotor sensitization, as well as self-administration and reinstatement of drug seeking.34,35,36,37 And in individuals with cocaine dependency, a combination of the CORT synthesis inhibitor Met and a benzodiazepine reduced cocaine craving and use.38 Thus, cocaine-elicited CORT may contribute to multiple behaviors associated with cocaine misuse, including drug seeking and drug-induced sensitization and action inflexibilities.
One important aspect of this report is that we replicated evidence that cocaine increases circulating CORT, as previously reported with repeated2,3,4 and acute34 exposure. We measured CORT levels halfway through our repeated cocaine exposure procedure to emphasize that GRs are likely being repeatedly activated with repeated cocaine exposure. Cocaine-induced CORT release may be due to cocaine increasing monoamines in the synapse. For instance, norepinephrine binding to receptors on neurons that release corticotropin-releasing hormone in the paraventricular nucleus of the hypothalamus leads to adrenocorticotropin hormone binding in the adrenal glands, ultimately causing CORT release.39,40 Another possibility, relevant here, is that detected CORT may have been released in an anticipatory or conditioned response, given that blood was collected at the time of day when mice would have typically received cocaine.
What might account for cocaine-induced action inflexibility? A number of possibilities are plausible, particularly given this discovery of stress hormone involvement. It is conceivable that cocaine caused impulsive-like behavior,41,42 which caused mice to be unable to preferentially respond at the previously reinforced aperture. We tested this possibility with a delayed reinforcement task and found that cocaine was without effect at the time of testing, which was notably well after cocaine exposure. Another consideration is that mice here could have been primed to detect changes in task parameters, given their experiences with other behavioral tests.
It is also conceivable that cocaine caused response biases by inducing anhedonic-like behavior.43 In this case, mice may not have favored the reinforced behavior because the food reinforcers had lost hedonic value. Yet, we found no effects in a sucrose consumption test well-suited to detect anhedonic-like behavior,26 if it exists.
Action inflexibility in the task used here has often been interpreted as being habit-like – meaning, driven by stimulus-response associations that are insensitive to outcomes. To substantiate this perspective, we turned to a classical reinforcer devaluation task, in which case reinforcer value is reduced and habitual behavior is inferred if mice do not modify their behaviors accordingly.44 As anticipated, cocaine obstructed action flexibility here, as well as in a classical omission task, suggesting that cocaine-induced response biases are habitual in nature. We included female mice throughout this report because prior reports overwhelmingly utilized males. To our knowledge, ours is amongst the first time this phenomenon has been comprehensively demonstrated in adult, female mice, as opposed to adult males or adolescent mice of both sexes.6,33
Mammals may perform habitual behaviors by virtue of extensive familiarity with a task, or because they are unable to learn, maintain, or recall the action-outcome links that support competing goal-directed actions. Medial prefrontal cortical regions like the prelimbic cortex form action-outcome links, and the OFC, particularly ventrolateral compartment, is necessary for updating and maintaining associations when they change and retrieving new memories, thus enabling action flexibility.25,30,45,46 We found that reducing GR presence selectively in the VLO rescued the ability of cocaine-exposed mice to modify their action strategies when familiar behaviors were no longer reinforced. Thus, excess stimulation of GRs in the VLO impedes action flexibility.
VLO neurons form stable representations of new action strategies when familiar expectations are violated. These memory traces are then retrieved when mice seek rewards in the future.25 We envisioned that cocaine could ablate or degrade action memory or obstruct its retrieval. To disentangle these possibilities, we selectively induced chemogenetic constructs in cells active when familiar expectations were violated, which allowed us to stimulate those memory trace cells later, after cocaine exposure. Stimulating memory trace cells in cocaine-exposed mice improved action flexibility. Thus, cocaine did not ablate action memory, since it could be made accessible by virtue of chemogenetic cell stimulation. Cocaine also did not grossly obstruct the reactivation of memory trace neurons, since these neurons expressed c-Fos upon memory retrieval. Thus, we imagine that the representation of action memories is corrupted by cocaine, such that greater stimulation than typical is required for memory retrieval. One consideration, though, is that c-Fos offers limited resolution by which to discern differences in degrees of activity in neurons,47,48 so it is possible that cocaine impacts memory trace neuron reactivation in a subtle fashion.
Cocaine and CORT both cause a loss of dendritic spines on excitatory neurons in the OFC,49,50 including projection-defined neurons necessary for memory retrieval.25 Meanwhile, recovery of spine densities has been linked to successful action updating in cocaine-exposed mice.7 One mechanism by which excess GR binding upon cocaine exposure may cause dendritic spine attrition is through its interactions with Brain-derived Neurotrophic Factor (BDNF). CORT exposure reduces Bdnf mRNA in the OFC51 and BDNF binding to its high-affinity receptor tropomyosin receptor kinase B in the OFC is necessary for successful action updating.24,33,52,53 Arango-Lievano et al.54 find that the function of BDNF and GRs must be coordinated to promote dendritic spine plasticity. Perturbations in either or both systems could result in dendritic spine loss. Importantly, BDNF in the VLO is necessary for the encoding and retrieval of action memories25 and is therefore, in conjunction with its role in dendritic spine plasticity, likely to be involved in action memory stability and its recall. Another potential mechanism by which cocaine-induced CORT release destabilizes memory is by modulating memory traces through changes in excitatory/inhibitory signaling.55 For example, CORT exposure in another report increased recruitment of neurons into a memory trace in the dentate gyrus and increased the excitability of these neurons, which increased fear memory expression.56 Chronic stressor exposure increases inhibitory signaling in the prefrontal cortex,57,58 which could also conceivably affect memory stability.
Limitations of the study
GRs play an important role in learning and memory processes, including long-term memory storage and retrieval.59,60,61,62 Thus, it is perhaps unsurprising that GR loss in the VLO of cocaine-naïve mice obstructed action flexibility here, as with systemic administration of GR antagonists in prior reports.13,63 Optimal performance in this task appears to require dendritic spine plasticity on excitatory deep-layer neurons,25,45 a process likely facilitated by homeostatic GR occupancy and blunted in its absence.64,65,66,67 This observation, plus evidence that stimulation of excitatory VLO neurons in drug-naïve organisms obstructs action flexibility in the same task (Figure 3 here and ref; 25 and 68), altogether reinforce the notion that expectancy updating within the VLO appears to follow a nonlinear, inverted-U-shaped relationship with “too little” or “too much” activity of a given biological factor hindering optimal performance. An alternative consideration is that cocaine may cause NR3C1 downregulation due to repeated CORT release.69,70 This would account for similarities between cocaine-exposed mice and drug-naïve mice with GR silencing here. Relatively little is known about GR (or mineralocorticoid receptor) levels following cocaine exposure. Future investigations could help disentangle these possibilities.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-GR | Abcam | Cat. #ab18312; RRID:AB_2833234 |
| Rabbit anti-cFos | Abcam | Cat. #ab190289; RRID:AB_2737414 |
| Rabbit anti-cFos | Cell Signaling Technology | Cat. #2250S; RRID:AB_2247211 |
| Bacterial and virus strains | ||
| AAV2.CAMKII-mCherry | UNC Viral Vector Core | N/A |
| AAV8-CAMKII-mCherry-Cre | UNC Viral Vector Core | N/A |
| AAV5-hSyn-DIO-mCherry ± HM3D(Gq) | Addgene; Bryan Roth | Cat. #44361 |
| Chemicals, peptides, and recombinant proteins | ||
| Cocaine hydrochloride | Sigma-Aldrich | Cat. #C5776 |
| Clozapine-N-oxide | Sigma-Aldrich | Cat. #C0832 |
| 4-hydroxytamoxifen | Sigma-Aldrich | Cat. #H6278 |
| Metyrapone | Santa Cruz Biotechnology | Cat. #sc-200597 |
| 4-pregen-11β 21-DIOL-3 20-DIONE 12-hemisuccinate (CORT) | Steraloids | Cat. #Q1562 |
| Critical commercial assays | ||
| Corticosterone ELISA | Enzo Life Sciences | Cat. #ADI-900-097; RRID:AB_2307314 |
| Deposited data | ||
| Analyzed Data | This paper | Emory University DataVerse: https://doi.org/10.15139/S3/09JFIC |
| Experimental models: Organisms/strains | ||
| Mouse: B6.129S6-Nr3c1tm2.1Ljm/J | The Jackson Laboratory | Strain #:012914; RRID:IMSR_JAX:012914 |
| Mouse: B6.Cg-Tg(Thy1-YFP)HJrs/J | The Jackson Laboratory | Strain #:003782; RRID:IMSR_JAX:003782 |
| Mouse: Fostm2.1(icre/ERT2)Luo/J | The Jackson Laboratory | Strain #:030323; RRID:IMSR_JAX:030323 |
| Software and algorithms | ||
| Imaris v.8 | Oxford Instruments | http://imaris.oxinst.com |
| ImageJ | Wayne Rasband | http://imagej.nih.gov/ij/ |
| SPSS v.28 | IBM | http://www.ibm.com/products/spss-statistics |
| GraphPad Prism v.10 | GraphPad Software | https://www.graphpad.com |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Shannon Gourley (shannon.l.gourley@emory.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Data will be deposited to the Emory University Dataverse: https://doi.org/10.15139/S3/09JFIC and thus publicly available upon publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper may be made available from the lead contact upon request.
Experimental model and study participant details
Mice were: wildtype C57BL/6 mice, double-transgenic mice expressing Thy1-YFP-H71 and Nr3c1flox,72 or Fos2A-iCreER (“TRAP2”)73 transgenic mice. Mutant mice were bred on a C57BL/6 background. Mice were ≥6 weeks old except in experiments with adolescent mice, in which case manipulations began at postnatal day (P) 31. Initial pharmacological studies (Figure 1) utilized female mice, replicating prior, foundational experiments conducted in males (see discussion for references). Subsequent experiments used both sexes. Sex differences were not detected. Mice were maintained on a 14-h light cycle (07:00 on) and provided food ad libitum except during instrumental conditioning when body weights were reduced to ∼90–93% baseline. Experiments took place between 09:00 and 13:00. Procedures were approved by the Emory University Institutional Animal Care and Use Committee, protocol 201700227.
Method details
Intracranial surgery
Experiments that required intracranial surgery used adeno-associated viruses (AAV) expressing Cre recombinase (Cre) (AAV2/8-CAMKII-mCherry ± Cre; University of North Carolina Viral Vector Core) or Cre-dependent Gq-coupled Designer Receptor Exclusively Activated by Designer Drugs (DREADDs) (AAV5-hSyn-DIO-mCherry ± HM3D(Gq); AddGene).
Mice were anesthetized with a 100 mg/kg ketamine/1 mg/kg xylazine mixture, i.p., and then placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL). The head was cleaned, skin cut, and skull leveled. For VLO infusions, infusion needles were centered at bregma and a hole was drilled in the skull corresponding to +2.6 AP, +/−1.2 ML, −2.8 DV.18,74 Viral vectors were bilaterally infused (0.5 μL per hemisphere) over 10 min with the needle left in place for an additional 5 min. Mice were sutured and allowed to recover for 3 weeks, allowing for viral vector expression.
Drug administration
Drugs were administered in a volume of 1 mL/100 g unless otherwise noted.
Cocaine HCl
Cocaine (30 mg/kg, i.p., in saline; Sigma-Aldrich) or vehicle was administered to adult mice daily for 7 or 14 days, as indicated in figure timelines. In adolescent mice, cocaine (10 mg/kg) was administered from P31-P35 followed by a 21-day washout period to allow the mice to age to adulthood. These doses and timing were derived from prior investigations revealing that cocaine impacts response strategies, biasing rodents towards habit-based behaviors.6,7
Metyrapone (Met)
Met (30 mg/kg in adults, 10 mg/kg in adolescents, i.p., in saline; Sigma-Aldrich) or vehicle was administered 30 min before cocaine or vehicle. The 30 mg/kg dose used here would be expected to reduce CORT in cocaine-exposed mice by roughly 35%.34 The 10 mg/kg dose was chosen by scaling down the 30 mg/kg dose to align with the lower cocaine dose used in adolescents.
4-hydroxytamoxifen (4OHT)
4OHT (40 mg/kg, i.p., in 2% Tween80, 5% DMSO, and saline; Sigma-Aldrich) was administered immediately after the non-reinforced session. 4OHT was administered in a volume of 2 mL/100 g.
Clozapine N-oxide (CNO)
CNO (1 mg/kg, i.p., in 2% DMSO and saline, Sigma-Aldrich) was delivered 30 min before test. All mice received CNO, regardless of condition, to equally expose animals to any unintended consequences of CNO. Importantly, this dose does not by itself impact responding in this task.25 When mice were euthanized with CNO on-board, euthanasia occurred 1 h following injection.
CORT
4-pregen-11β 21-DIOL-3 20-DIONE 12-hemisuccinate (Steraloids, Newport, RI) was dissolved in tap water (35 μg/mL free base;75), and CORT-infused water replaced regular water. Control mice consumed tap water. Water bottles were weighed daily, and mice weighed every other day. Using these values, we were able to calculate the amount of liquid displaced/total weight of all mice in the cage. We were then able to calculate the approximate dose of CORT ingested (6–9 mg/kg/day). Water bottles were refilled with fresh water or newly prepared CORT solution every 3 days. Mice were exposed to CORT for 14 days, followed by a 14-day washout period.
Blood serum CORT
Blood serum CORT was collected and measured 1 day following the final cocaine injection, as described.14 Briefly, mice were rapidly anesthetized with isoflurane between the 10:00-11:00 h, decapitated and trunk blood was collected in chilled Eppendorph tubes. Samples were centrifuged at 4°C for 30 min and serum was extracted. CORT levels were measured in duplicate via enzyme-linked immunosorbent assay (ELISA; Assay Designs) in accordance with the manufacturer’s instructions except for the extraction step, which was omitted.
Instrumental response training
Mice were trained to nose poke for food pellets (20mg, Bio-Serv, Flemington, NJ) in Med-Associates operant conditioning chambers (St. Albans, VT) equipped with 2 nose poke apertures and a food magazine. Mice were trained using a fixed ratio (FR) 1 schedule of reinforcement wherein 30 pellets were available for responding on each aperture for a maximum of 60 pellets/session. Mice were considered to have “acquired” when they received 25 pellets for responding at both apertures. The program ended when mice had received 30 pellets/side or at 70 min. Mice required 10–14 sessions to acquire, and the last 10 sessions are shown. For experiments in Figures 1 and S2, mice received 2 additional days of training according to a random interval (RI) 30-s schedule of reinforcement to increase response rates. Mice then proceeded to satiety-specific devaluation or the test of response flexibility.
Satiety-specific devaluation
Satiety-specific devaluation was used to assess whether mice could modify response strategies due to changes in the value of expected outcomes. After instrumental response training and immediately before a choice test, mice were allowed unlimited access in a novel environment to the pellets used in training, thus reducing the pellet value by virtue of satiety (referred to as “devalued”). As a control, mice were allowed unlimited access to standard laboratory chow on another day, leaving the value of the pellets unaffected (“valued” condition). Following ad libitum feeding (90 min), mice were placed in the operant conditioning chambers for a 10 min choice test conducted in extinction. Mice that successfully update their response strategies will decrease responding for the devalued pellet. Pellet vs. chow prefeeding sessions were counter-balanced, and groups did not differ in the amount of pellets or chow consumed during the prefeeding period.
Test of response flexibility
This test was conducted after instrumental response training, first using a single reinforcer (purified grain-based pellet; for Figures 1C–1G), then with 2 separate, equally preferred reinforcers (purified grain and chocolate) for the rest of the manuscript, given that goal-seeking behavior is often thought to involve the integration of specific reward features into goal representations. In a 25 min “reinforced” session, 1 aperture was occluded, and responding on the other aperture was reinforced using an FR1 schedule of reinforcement. In a 25 min “non-reinforced” session, the following day, the opposite aperture was occluded, and pellets were delivered at a rate matched to each animal’s reinforcement rate during the “reinforced” session. Responses at the available aperture resulted in no programmed consequence. The following day, both apertures were available in a 10 min choice test conducted in extinction. Preferential responding at the nose poke that was previously reinforced indicates a flexible response strategy, deviating from equivalent responding during training. Meanwhile, comparable responding at both apertures indicates a failure to update action strategies.
Timeline for chemogenetic experiment
Mice in the final behavioral experiment reported here underwent the 2 25 min sessions described above, and 4OHT was administered following the non-reinforced session to induce chemogenetic constructs in neurons active during this period. These neurons form memory traces for new action strategies, and chemogenetic receptors allow for their later manipulation.25 Mice then underwent cocaine administration. Mice were then re-trained daily for 5 days using an FR1 schedule of reinforcement to reignite responding. The test of response flexibility protocol was repeated as described above, with the reinforced and non-reinforced apertures held constant. A choice test followed the next day, with CNO delivered prior to the test. Finally, mice were again re-trained using an FR1 schedule of reinforcement for 2 days to reignite responding before an omission test, described below.
Delayed reinforcement (delayed discounting)
A delayed reinforcement task was used to assess impulsive-like behavior. Following the “Test for Response Flexibility,” mice were placed in the same operant conditioning chambers. First, mice had access to 2 active nose poke apertures for 30 min. Nose poking at 1 aperture resulted in the delivery of 5 pellets (large reinforcer) while nose poking at the other aperture resulted in delivery of a single pellet (small reinforcer). Following nose poke responses, there was a 25s time-out. Mice were considered to have acquired once they preferentially responded for the large reinforcer. Mice then began the delay phase of the task. Across 7 days, they experienced an increasing delay (10s, 20s, 30s, 45s, 60s, 80s, 100s) between nose poking for the large reinforcer and delivery of the pellets, and responding was monitored. Conditions were unchanged for the small reinforcer.
Instrumental omission
Instrumental omission was used to further assess the ability of mice to update responding after a change in the relationship between nose poking and pellet delivery. Following the “Test for Response Flexibility,” responding on one aperture was reinstated using 2 sessions and an FR1 schedule of reinforcement. The other aperture was occluded and not available for responding. Then, the omission procedure commenced on the available aperture as described,76 in which case pellets were scheduled to be delivered every 20s but the counter was reset after a nose poke response, delaying delivery of the reinforcer. Thus, mice had to inhibit responding in order to receive pellets. The session was 20 min long.
Progressive ratio
Behaviorally naive mice were trained to nose poke at one aperture using an FR1 schedule of reinforcement wherein 30 pellets were available/session for responding. Mice were considered to have “acquired” when they received 25 pellets/session. Then, they were trained for 5 sessions according to an RI 30-s schedule of reinforcement. The program ended when mice had received 30 pellets or at 70 min. Mice were then tested using a progressive ratio schedule of reinforcement in which the response requirement increased by 4 with each pellet delivery (i.e. 1,5,9, x+4). Sessions ended at 180 min or when mice did not respond for 5 min. Break point ratios, the highest number of responses the mice were willing to complete to receive a pellet, are reported, averaged across 3 sessions conducted on 3 consecutive days.
Sucrose consumption
Sucrose consumption was used to assess anhedonic-like behavior.26 Mice were placed individually into clean cages with access to a water bottle filled with 1% w/v sucrose. Mice were acclimated to the cage and sucrose for 4 h and then water-restricted for 19 h. The water bottles containing the sucrose solution were returned to the cage for 1 h. The bottles were weighed immediately before and after this 1-h period. We were thereby able to calculate % body weight consumed.
Western blotting
Mice were briefly anesthetized by isoflurane and euthanized by rapid decapitation 3 weeks after viral vector infusion. Brains were extracted and frozen at −80°C, then sectioned at 1mm. A single experimenter centered a 1mm corer within the ventrolateral OFC under a fluorescence dissection microscope for dissection. Tissue was homogenized by sonication, and protein content was measured by Bradford colorimetric assay. 10 μg of protein/sample was separated by SDS-PAGE on a 4–15% gradient tris-glycine stain-free gel (Bio-rad). Following transfer to PVDF membrane, membranes were blocked in 5% nonfat milk.
Membranes were incubated in primary antibody, Anti-GR (1:2000, Abcam, ab183127, lot# GR3259213-11) overnight and then in horseradish peroxidase-conjugated goat anti-rabbit (Vector; 1:5000) secondary antibody. Immunoreactivity was assessed using a chemiluminescence substrate (Pierce) and measured using a ChemiDoc Imager (Bio-rad). All gels were normalized to their corresponding total protein. The signals were normalized to the control sample mean from the same membrane to control for variance between gels. Samples were run twice to ensure replication.
Immunofluorescence
Viral vector localization
Mice were deeply anesthetized and euthanized by intracardiac perfusion 4 days after the conclusion of behavioral experiments. Brains were extracted and submerged in 4% paraformaldehyde for 48 h and then transferred to 30% w/v sucrose. 50 μm-thick sections were prepared on a microtome held at −21°C ± 1. Viral vector infusion sites were verified and characterized by imaging mCherry.
c-Fos immunostaining and quantification
To validate Gq DREADDs, mice were euthanized 1 h following CNO injection by deep anaesthesia and intracardiac perfusion. Brains were prepared as above.
Sections were blocked in a solution containing PBS, 2% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.03% Triton X-100 (Sigma Aldrich) for 90 min at room temperature. Then, sections were incubated with the primary antibody solution containing anti-c-Fos (1:1000, Abcam, ab190289, lot# GR3443853-1), 2% NGS, and 0.003% Triton X-100 at 4°C overnight. Then, sections were incubated in a secondary solution containing Alexafluor 488 (1:1000, Life Technologies, A11070, lot# 234906), 2% NGS, and 0.03% Triton X-100 at room temperature for 90 min. Sections were mounted and coverslipped with Fluromount-G with DAPI (ThermoFisher Scientific).
In another experiment, mice were euthanized 1 h after the choice test, and c-Fos was visualized in memory trace neurons, identified by viral-mediated mCherry expression. Brains were prepared as above.
Sections were blocked in a solution containing PBS, 2% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.03% Triton X-100 (Sigma Aldrich) for 90 min at room temperature. Then, sections were incubated with the primary antibody solution containing anti-c-Fos (1:1000, Cell Signaling Technology, 2250S, lot# 12), 2% NGS, and 0.003% Triton X-100 at 4°C overnight. Then, sections were incubated in a secondary solution containing Alexafluor 680 (1:1000, Jackson ImmunoResearch Laboratories, 111-625-144, lot# 163593), 2% NGS, and 0.03% Triton X-100 at room temperature for 1 h. Sections were mounted and coverslipped with Fluromount-G with DAPI (ThermoFisher Scientific).
Immunostained sections were imaged using a Keyence BZ-X710 microscope at 40× magnification. Uniform exposure parameters were used throughout, and anatomical landmarks were used to ensure images were similarly localized. 3–4 images were collected per mouse. When normalized, values were normalized to the control sample mean. For c-Fos quantification, analyses were performed using CellProfiler. The analysis pipeline included thresholding (Otsu method) and identifying primary objects,77 defined as viral transduced neurons.
GR immunostaining and quantification
Mice were deeply anesthetized and euthanized by intracardiac perfusion 3 weeks after viral vector infusion. Brains were prepared as above. Sections were blocked in a solution containing PBS, 2% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.03% Triton X-100 (Sigma Aldrich) for 90 min at room temperature. Then, sections were incubated with the primary antibody solution containing anti-GR (1:1000, Cell Signaling Technology, 3660S, lot# 5), 2% NGS, and 0.003% Triton X-100 at 4°C overnight. Then, sections were incubated in a secondary solution containing Alexafluor 680 (1:1000, Jackson ImmunoResearch Laboratories, 111-625-144, lot# 163593), 2% NGS, and 0.03% Triton X-100 at room temperature for 1 h. Sections were mounted and coverslipped with Fluromount-G with DAPI (ThermoFisher Scientific).
Immunostained sections were imaged using a Keyence BZ-X710 microscope at 40× magnification. Uniform exposure parameters were used throughout, and anatomical landmarks were used to ensure images were similarly localized. 3–4 images were collected per hemisphere. Analyses were performed using CellProfiler. The analysis pipeline included thresholding (Otsu method) and identifying primary objects,77 defined as viral transduced neurons.
Dendritic spine imaging
YFP-expressing mice were deeply anaesthetized and euthanized by intracardiac perfusion 4 days after the conclusion of behavioral experiments. YFP in these mice labels layer V neurons. Brains were extracted and submerged in 4% paraformaldehyde for 48 h and then transferred to 30% w/v sucrose. 50 μm-thick sections were prepared on a microtome held at −21°C ± 1. Images of transduced neurons were acquired on a Leica DM5500B microscope equipped with a spinning disk confocal (VisiTech International) and a Hamamatsu Orca R2 camera using a 100x 1.4 NA objective. Z-stacks of dendritic segments were acquired using a 0.1 μm step size. 4–8 dendrites per mouse were acquired bilaterally from independent neurons. The location of the imaged segments within target regions was confirmed by zooming out to a low magnification. Care was taken to image second-order or higher apical OFC dendrites 50–150 μm from soma. A single blinded user generated all images.
Dendrite reconstruction
The FilamentTracer module of Imaris (Bitplane AG) was used: A dendritic segment 19–30 μm in length was drawn with the autodepth function. Dendritic spine head location was manually indicated, and FilamentTracer processing algorithms were used to calculate morphological parameters. Morphological classification of dendritic spines was determined using parameters modified from ref.78 Spines with a head:neck diameter ratio ≥1.1 and head diameter ≥0.7 μm were classified as mushroom-type or otherwise classified as thin-type. Spines with a head:neck diameter ratio <1.1 and a length:neck diameter ratio ≥2.5 were classified as thin-type or otherwise classified as stubby-type. A single blinded individual quantified all dendritic spines within a given experiment.
Quantification and statistical analysis
Nose poke rates, difference scores, and dendritic spine densities were compared by ANOVA, with nose poke aperture, viral vector, and/or drug(s) as factors, and with repeated measures when appropriate. Difference scores were calculated as responses on the “reinforced” port minus responses on the “non-reinforced” port, or responses in the last 5-min epoch relative to the first in the case of omission. In the case of significant interactions, post-hoc comparisons were made with Tukey’s tests, and results are indicated graphically. In the behavioral choice tests, an interaction between nose poke aperture, drug, ± viral vector is required to conclude that a given manipulation affected flexible behavior. For analyses of responding across the instrumental omission session, simple linear regression was also used to generate line of best fit.
For western blot, blood serum CORT, and c-Fos analyses in the main text, each mouse contributed a single value (each animal’s mean value from multiple gels/samples/sections). c-Fos analyses in the supplementary figure were compared on a per-section basis to maximally explore between- and within-group variability. Comparisons were made by unpaired t-tests.
For GR immunofluorescence, the control viral vector was infused into the VLO of one hemisphere and the Cre-expressing viral vector into the other hemisphere. Each hemisphere contributed a single value (each hemisohere’s mean percentage value from multiple images). Comparisons were made by paired t-tests across hemispheres.
Dendritic spine morphometric analyses were conducte by Kolmogorov-Smirnov (K-S) comparisons.
Throughout, SPSS and GraphPad Prism were used, and p ≤ 0.05 was considered significant, except in the case of K-S comparisons, in which p < 0.0001 was considered significant. Comparisons were 2-tailed except for the western blot validating Nr3c1 knockdown, which was 1-tailed based on the a priori hypothesis that gene silencing would reduce protein levels. Group sizes were determined based on power analyses and preexisting datasets. n values for each individual group are reported in the figure captions. All experiments were conducted at least twice.
Acknowledgments
This research project was supported in part by the Emory University Integrated Cellular Imaging Core, Children’s Healthcare of Atlanta, and the Emory University Research Council. This work was also supported in part by NIH MH117103, DA044297, and DA051184. The Emory National Primate Research Center is supported by NIH OD011132. Finally, this work was assisted in part by a grant from the NIH-funded Emory Specialized Center of Research Excellence in Sex Differences (NIH U54AG062334). We thank Dr. Brian Dias for generously sharing resources. We thank Aylet Allen for assistance with the devaluation test and graphical abstract.
Author contributions
Conceptualization and methodology, M.K.S. and S.L.G.; Investigation, M.K.S., K.M.S., E.H.S., and S.Y.T.; Writing, M.K.S. and S.L.G.; Funding acquisition, M.K.S. and S.L.G.; Supervision, S.L.G.
Declaration of interests
The authors declare no competing interests.
Published: May 28, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110148.
Supplemental information
References
- 1.Miczek K.A., Nikulina E.M., Shimamoto A., Covington H.E., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J. Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowsky B., Kuhn C.M. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J. Pharmacol. Exp. Therapeut. 1991;256:204–210. [PubMed] [Google Scholar]
- 3.Moldow R.L., Fischman A.J. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- 4.Radley J.J., Anderson R.M., Cosme C.V., Glanz R.M., Miller M.C., Romig-Martin S.A., LaLumiere R.T. The Contingency of Cocaine Administration Accounts for Structural and Functional Medial Prefrontal Deficits and Increased Adrenocortical Activation. J. Neurosci. 2015;35:11897–11910. doi: 10.1523/JNEUROSCI.4961-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantsch J.R., Yuferov V., Mathieu-Kia A.-M., Ho A., Kreek M.J. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 6.Schoenbaum G., Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cerebr. Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- 7.DePoy L.M., Zimmermann K.S., Marvar P.J., Gourley S.L. Induction and Blockade of Adolescent Cocaine-Induced Habits. Biol. Psychiatr. 2017;81:595–605. doi: 10.1016/j.biopsych.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePoy L.M., Allen A.G., Gourley S.L. Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ersche K.D., Gillan C.M., Jones P.S., Williams G.B., Ward L.H.E., Luijten M., de Wit S., Sahakian B.J., Bullmore E.T., Robbins T.W. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–1471. doi: 10.1126/science.aaf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ersche K.D., Lim T.V., Murley A.G., Rua C., Vaghi M.M., White T.L., Williams G.B., Robbins T.W. Reduced Glutamate Turnover in the Putamen Is Linked With Automatic Habits in Human Cocaine Addiction. Biol. Psychiatr. 2021;89:970–979. doi: 10.1016/j.biopsych.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias-Ferreira E., Sousa J.C., Melo I., Morgado P., Mesquita A.R., Cerqueira J.J., Costa R.M., Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 12.Patterson T.K., Craske M.G., Knowlton B.J. The effect of early-life stress on memory systems supporting instrumental behavior. Hippocampus. 2013;23:1025–1034. doi: 10.1002/hipo.22174. [DOI] [PubMed] [Google Scholar]
- 13.Gourley S.L., Swanson A.M., Jacobs A.M., Howell J.L., Mo M., Dileone R.J., Koleske A.J., Taylor J.R. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci. USA. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barfield E.T., Gerber K.J., Zimmermann K.S., Ressler K.J., Parsons R.G., Gourley S.L. Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 16.Joels M. Corticosteroids and the brain. J. Endocrinol. 2018;238:R121–R130. doi: 10.1530/JOE-18-0226. [DOI] [PubMed] [Google Scholar]
- 17.Gourley S.L., Swanson A.M., Koleske A.J. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whyte A.J., Trinoskey-Rice G., Davies R.A., Woon E.P., Foster S.L., Shapiro L.P., Li D.C., Srikanth K.D., Gil-Henn H., Gourley S.L. Cell adhesion factors in the orbitofrontal cortex control cue-induced reinstatement of cocaine seeking and amygdala-dependent goal seeking. J. Neurosci. 2021;41:5923–5936. doi: 10.1523/JNEUROSCI.0781-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barfield E.T., Sequeira M.K., Parsons R.G., Gourley S.L. Morphological Responses of Excitatory Prelimbic and Orbitofrontal Cortical Neurons to Excess Corticosterone in Adolescence and Acute Stress in Adulthood. Front. Neuroanat. 2020;14:45. doi: 10.3389/fnana.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crombag H.S., Gorny G., Li Y., Kolb B., Robinson T.E. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebr. Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 21.Helmeke C., Seidel K., Poeggel G., Bredy T.W., Abraham A., Braun K. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163:790–798. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Gourley S.L., Olevska A., Warren M.S., Taylor J.R., Koleske A.J. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J. Neurosci. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp W.G., Allen A.G., Stubbs K.H., Criado K.K., Sanders R., McCracken C.E., Parsons R.G., Scahill L., Gourley S.L. Successful pharmacotherapy for the treatment of severe feeding aversion with mechanistic insights from cross-species neuronal remodeling. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann K.S., Yamin J.A., Rainnie D.G., Ressler K.J., Gourley S.L. Connections of the Mouse Orbitofrontal Cortex and Regulation of Goal-Directed Action Selection by Brain-Derived Neurotrophic Factor. Biol. Psychiatr. 2017;81:366–377. doi: 10.1016/j.biopsych.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D.C., Dighe N.M., Barbee B.R., Pitts E.G., Kochoian B., Blumenthal S.A., Figueroa J., Leong T., Gourley S.L. A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory. Nat. Neurosci. 2022;25:1213–1224. doi: 10.1038/s41593-022-01148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourley S.L., Kiraly D.D., Howell J.L., Olausson P., Taylor J.R. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol. Psychiatr. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourley S.L., Wu F.J., Kiraly D.D., Ploski J.E., Kedves A.T., Duman R.S., Taylor J.R. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatr. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balleine B.W., O’Doherty J.P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izquierdo A. Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. J. Neurosci. 2017;37:10529–10540. doi: 10.1523/JNEUROSCI.1678-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkes S.L., Ravassard P.M., Cerpa J.-C., Wolff M., Ferreira G., Coutureau E. Insular and Ventrolateral Orbitofrontal Cortices Differentially Contribute to Goal-Directed Behavior in Rodents. Cerebr. Cortex. 2018;28:2313–2325. doi: 10.1093/cercor/bhx132. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steckler T., Sauvage M., Holsboer F. Glucocorticoid receptor impairment enhances impulsive responding in transgenic mice performing on a simultaneous visual discrimination task. Eur. J. Neurosci. 2000;12:2559–2569. doi: 10.1046/j.1460-9568.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- 33.Pitts E.G., Barfield E.T., Woon E.P., Gourley S.L. Action-Outcome Expectancies Require Orbitofrontal Neurotrophin Systems in Naïve and Cocaine-Exposed Mice. Neurotherapeutics. 2020;17:165–177. doi: 10.1007/s13311-019-00752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinelli M., Rougé-Pont F., De Jesus-Oliveira C., Le Moal M., Piazza P.V. Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology. 1997;16:156–161. doi: 10.1016/S0893-133X(96)00169-8. [DOI] [PubMed] [Google Scholar]
- 35.Piazza P.V., Marinelli M., Jodogne C., Deroche V., Rougé-Pont F., Maccari S., Le Moal M., Simon H. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine-induced locomotion and relapse of cocaine self-administration. Brain Res. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- 36.Mantsch J.R., Saphier D., Goeders N.E. Corticosterone facilitates the acquisition of cocaine self-administration in rats: opposite effects of the type II glucocorticoid receptor agonist dexamethasone. J. Pharmacol. Exp. Therapeut. 1998;287:72–80. [PubMed] [Google Scholar]
- 37.Mantsch J.R., Baker D.A., Serge J.P., Hoks M.A., Francis D.M., Katz E.S. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008;33:814–826. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- 38.Kablinger A.S., Lindner M.A., Casso S., Hefti F., DeMuth G., Fox B.S., McNair L.A., McCarthy B.G., Goeders N.E. Effects of the combination of metyrapone and oxazepam on cocaine craving and cocaine taking: a double-blind, randomized, placebo-controlled pilot study. J. Psychopharmacol. 2012;26:973–981. doi: 10.1177/0269881111430745. [DOI] [PubMed] [Google Scholar]
- 39.Goeders N.E. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- 40.Dunn A.J., Swiergiel A.H. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur. J. Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon N.W., Mendez I.A., Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav. Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez G., Oleson E.B., Gentry R.N., Abbas Z., Bernstein D.L., Arvanitogiannis A., Cheer J.F. Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates. Biol. Psychiatr. 2014;75:487–498. doi: 10.1016/j.biopsych.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garfield J.B.B., Lubman D.I., Yücel M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust. N. Z. J. Psychiatr. 2014;48:36–51. doi: 10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- 44.Woon E.P., Sequeira M.K., Barbee B.R., Gourley S.L. Involvement of the rodent prelimbic and medial orbitofrontal cortices in goal-directed action: A brief review. J. Neurosci. Res. 2020;98:1020–1030. doi: 10.1002/jnr.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whyte A.J., Kietzman H.W., Swanson A.M., Butkovich L.M., Barbee B.R., Bassell G.J., Gross C., Gourley S.L. Reward-related expectations trigger dendritic spine plasticity in the mouse ventrolateral orbitofrontal cortex. J. Neurosci. 2019;39:4595–4605. doi: 10.1523/JNEUROSCI.2031-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gourley S.L., Taylor J.R. Going and stopping: Dichotomies in behavioral control by the prefrontal cortex. Nat. Neurosci. 2016;19:656–664. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovács K.J. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 48.Appleyard S.M. Lighting up neuronal pathways: the development of a novel transgenic rat that identifies Fos-activated neurons using a red fluorescent protein. Endocrinology. 2009;150:5199–5201. doi: 10.1210/en.2009-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DePoy L.M., Gourley S.L. Synaptic Cytoskeletal Plasticity in the Prefrontal Cortex Following Psychostimulant Exposure. Traffic. 2015;16:919–940. doi: 10.1111/tra.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sequeira M.K., Gourley S.L. The stressed orbitofrontal cortex. Behav. Neurosci. 2021;135:202–209. doi: 10.1037/bne0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gourley S.L., Kedves A.T., Olausson P., Taylor J.R. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pitts E.G., Li D.C., Gourley S.L. Bidirectional coordination of actions and habits by TrkB in mice. Sci. Rep. 2018;8:4495. doi: 10.1038/s41598-018-22560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gourley S.L., Olevska A., Zimmermann K.S., Ressler K.J., Dileone R.J., Taylor J.R. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur. J. Neurosci. 2013;38:2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arango-Lievano M., Lambert W.M., Bath K.G., Garabedian M.J., Chao M.V., Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc. Natl. Acad. Sci. USA. 2015;112:15737–15742. doi: 10.1073/pnas.1509045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeanneteau F., Coutellier L. The glucocorticoid footprint on the memory engram. Curr. Opin. Endocr. Metab. Res. 2022;25 doi: 10.1016/j.coemr.2022.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lesuis S.L., Brosens N., Immerzeel N., van der Loo R.J., Mitrić M., Bielefeld P., Fitzsimons C.P., Lucassen P.J., Kushner S.A., van den Oever M.C., Krugers H.J. Glucocorticoids Promote Fear Generalization by Increasing the Size of a Dentate Gyrus Engram Cell Population. Biol. Psychiatr. 2021;90:494–504. doi: 10.1016/j.biopsych.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 57.McKlveen J.M., Morano R.L., Fitzgerald M., Zoubovsky S., Cassella S.N., Scheimann J.R., Ghosal S., Mahbod P., Packard B.A., Myers B., et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol. Psychiatr. 2016;80:754–764. doi: 10.1016/j.biopsych.2016.03.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newton D.F., Oh H., Shukla R., Misquitta K., Fee C., Banasr M., Sibille E. Chronic Stress Induces Coordinated Cortical Microcircuit Cell-Type Transcriptomic Changes Consistent With Altered Information Processing. Biol. Psychiatr. 2022;91:798–809. doi: 10.1016/j.biopsych.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plast. 1998;6:41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atsak P., Guenzel F.M., Kantar-Gok D., Zalachoras I., Yargicoglu P., Meijer O.C., Quirarte G.L., Wolf O.T., Schwabe L., Roozendaal B. Glucocorticoids mediate stress-induced impairment of retrieval of stimulus-response memory. Psychoneuroendocrinology. 2016;67:207–215. doi: 10.1016/j.psyneuen.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Schwabe L., Joëls M., Roozendaal B., Wolf O.T., Oitzl M.S. Stress effects on memory: an update and integration. Neurosci. Biobehav. Rev. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 63.Swanson A.M., Shapiro L.P., Whyte A.J., Gourley S.L. Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Commun. Integr. Biol. 2013;6:e26068. doi: 10.4161/cib.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arango-Lievano M., Borie A.M., Dromard Y., Murat M., Desarmenien M.G., Garabedian M.J., Jeanneteau F. Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc. Natl. Acad. Sci. USA. 2019;116:13097–13106. doi: 10.1073/pnas.1903203116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liston C., Cichon J.M., Jeanneteau F., Jia Z., Chao M.V., Gan W.B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liston C., Gan W.B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barfield E.T., Gourley S.L. Prefrontal cortical trkB, glucocorticoids, and their interactions in stress and developmental contexts. Neurosci. Biobehav. Rev. 2018;95:535–558. doi: 10.1016/j.neubiorev.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinton E.A., Li D.C., Allen A.G., Gourley S.L. Social Isolation in Adolescence Disrupts Cortical Development and Goal-Dependent Decision-Making in Adulthood, Despite Social Reintegration. eNeuro. 2019;6 doi: 10.1523/ENEURO.0318-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kluwe-Schiavon B., Schote A.B., Vonmoos M., Hulka L.M., Preller K.H., Meyer J., Baumgartner M.R., Grünblatt E., Quednow B.B. Psychiatric symptoms and expression of glucocorticoid receptor gene in cocaine users: A longitudinal study. J. Psychiatr. Res. 2020;121:126–134. doi: 10.1016/j.jpsychires.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 70.Schote A.B., Jäger K., Kroll S.L., Vonmoos M., Hulka L.M., Preller K.H., Meyer J., Grünblatt E., Quednow B.B. Glucocorticoid receptor gene variants and lower expression of NR3C1 are associated with cocaine use. Addiction Biol. 2019;24:730–742. doi: 10.1111/adb.12632. [DOI] [PubMed] [Google Scholar]
- 71.Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 72.Brewer J.A., Khor B., Vogt S.K., Muglia L.M., Fujiwara H., Haegele K.E., Sleckman B.P., Muglia L.J. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat. Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- 73.DeNardo L.A., Liu C.D., Allen W.E., Adams E.L., Friedmann D., Fu L., Guenthner C.J., Tessier-Lavigne M., Luo L. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 2019;22:460–469. doi: 10.1038/s41593-018-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DePoy L.M., Shapiro L.P., Kietzman H.W., Roman K.M., Gourley S.L. β1-Integrins in the Developing Orbitofrontal Cortex Are Necessary for Expectancy Updating in Mice. J. Neurosci. 2019;39:6644–6655. doi: 10.1523/JNEUROSCI.3072-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mekiri M., Gardier A.M., David D.J., Guilloux J.P. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp. Clin. Psychopharmacol. 2017;25:94–104. doi: 10.1037/pha0000112. [DOI] [PubMed] [Google Scholar]
- 76.Rossi M.A., Yin H.H. Methods for studying habitual behavior in mice. Curr. Protoc. Neurosci. 2012;Chapter 8 doi: 10.1002/0471142301.ns0829s60. Unit 8.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stirling D.R., Swain-Bowden M.J., Lucas A.M., Carpenter A.E., Cimini B.A., Goodman A. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinf. 2021;22:433. doi: 10.1186/s12859-021-04344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radley J.J., Anderson R.M., Hamilton B.A., Alcock J.A., Romig-Martin S.A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33:14379–14391. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data will be deposited to the Emory University Dataverse: https://doi.org/10.15139/S3/09JFIC and thus publicly available upon publication.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper may be made available from the lead contact upon request.