Abstract
Background and Aims
As practice patterns and hepatitis C virus (HCV) genotypes (GT) vary geographically, a global real-world study from both East and West covering all GTs can help inform practice policy toward the 2030 HCV elimination goal. This study aimed to assess the effectiveness and tolerability of DAA treatment in routine clinical practice in a multinational cohort for patients infected with all HCV GTs, focusing on GT3 and GT6.
Methods
We analyzed the sustained virological response (SVR12) of 15,849 chronic hepatitis C patients from 39 Real-World Evidence from the Asia Liver Consortium for HCV clinical sites in Asia Pacific, North America, and Europe between 07/01/2014–07/01/2021.
Results
The mean age was 62±13 years, with 49.6% male. The demographic breakdown was 91.1% Asian (52.9% Japanese, 25.7% Chinese/Taiwanese, 5.4% Korean, 3.3% Malaysian, and 2.9% Vietnamese), 6.4% White, 1.3% Hispanic/Latino, and 1% Black/African-American. Additionally, 34.8% had cirrhosis, 8.6% had hepatocellular carcinoma (HCC), and 24.9% were treatment-experienced (20.7% with interferon, 4.3% with direct-acting antivirals). The largest group was GT1 (10,246 [64.6%]), followed by GT2 (3,686 [23.2%]), GT3 (1,151 [7.2%]), GT6 (457 [2.8%]), GT4 (47 [0.3%]), GT5 (1 [0.006%]), and untyped GTs (261 [1.6%]). The overall SVR12 was 96.9%, with rates over 95% for GT1/2/3/6 but 91.5% for GT4. SVR12 for GT3 was 95.1% overall, 98.2% for GT3a, and 94.0% for GT3b. SVR12 was 98.3% overall for GT6, lower for patients with cirrhosis and treatment-experienced (TE) (93.8%) but ≥97.5% for treatment-naive patients regardless of cirrhosis status. On multivariable analysis, advanced age, prior treatment failure, cirrhosis, active HCC, and GT3/4 were independent predictors of lower SVR12, while being Asian was a significant predictor of achieving SVR12.
Conclusions
In this diverse multinational real-world cohort of patients with various GTs, the overall cure rate was 96.9%, despite large numbers of patients with cirrhosis, HCC, TE, and GT3/6. SVR12 for GT3/6 with cirrhosis and TE was lower but still excellent (>91%).
Keywords: Hepatitis C virus, Liver cirrhosis, Hepatocellular carcinoma, Genotype, DAA, REAL-C
Graphical abstract
Introduction
Hepatitis C virus (HCV) infection is a significant global health problem. Chronic hepatitis C (CHC) can result in the progression of liver disease, including liver cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality.1 As of 2020, an estimated 56.8 million individuals worldwide are affected by chronic HCV infection, indicating a decrease from the 71 million estimated in 2015.2 However, despite this decline, the goal of globally eliminating HCV by 2030 remains distant. The advent of direct-acting antiviral (DAA) medications has revolutionized the treatment of HCV infection by allowing shorter treatment durations and enhancing the efficiency of sustained virological response (SVR). Achieving SVR improves patient outcomes by reducing the risk of liver disease progression and its associated complications.3–6
Globally, HCV genotypes (GTs) 1, 2, 3, 4, and 6 are the most prevalent. HCV GTs can play a crucial role in predicting the risk of disease progression and the response to treatment. For example, GT3 has been associated with an increased risk of treatment failure and the emergence of resistance-associated substitutions.7,8 HCV GT distribution also varies geographically, with GT1 accounting for the highest proportion of HCV cases globally (46.2%) and being the predominant GT in most Western countries. GT3 is the second most common genotype (30.1%) and is more prevalent in South Asia and Western China. Other GTs, including GT2 (9.1%), GT4 (8.3%), and GT6 (5.4%), are more common in East and Southeast Asia, North Africa, and the Middle East.9–11 Geographical variations in HCV GT distribution, access to healthcare, and the availability of effective treatment options all contribute to the complexity of treatment outcomes. Consequently, conducting a global real-world HCV study encompassing data from both East and West is important and can guide medical practice and public health policy to achieve the 2030 HCV elimination goal.
In this study, using a large cohort of patients with CHC derived from the Real-World Evidence from the Asia Liver Consortium for HCV (REAL-C) database, we aimed to assess the effectiveness and tolerability of DAA treatment in routine clinical practice for patients infected with all HCV GTs. Since GT3 is still more difficult to treat, especially for patients with GT3b and cirrhosis, and there are still conflicting data on low SVR rates for GT6,7,8,12–14 this study focused on SVR outcomes and factors associated with lower treatment response for patients with GT3 and GT6 infections.
Methods
Study design and study patients
This multinational, non-interventional study extracted patient data from the REAL-C database using a standardized case report form and unified variable definitions at each study center. REAL-C is an international consortium comprising 39 study centers from North America, Europe, and the Asia-Pacific region. Details of this registry have been described previously and include CHC patients monitored and treated in routine practice.15 The study was conducted by the Declaration of Helsinki and approved by the Institutional Review Board at Stanford University, Stanford, CA, USA, and at each participating study center.
Patients were included if they were adults (18 years or older), received any interferon (IFN)-free DAA regimen for the treatment of CHC of any GT between July 1, 2014, and July 1, 2021, regardless of a history of cirrhosis or HCC, prior treatment, and had adequate medical records for the primary endpoint assessment. Patients with a prognosis of < 12 months were excluded.
Study assessment
Patient demographic information, clinical characteristics, and laboratory data were collected at baseline. The demographic information included age, gender, and race/ethnicity. The clinical characteristics included the presence of cirrhosis, active/inactive HCC, co-infection with HBV/HIV, comorbidities, treatment history, and the specific DAA treatment regimens administered. Patients with inactive HCC were defined as those who had undergone local ablation (such as alcohol injection, radiofrequency ablation, or microwave ablation), surgical resection, or liver transplantation and had no evidence of HCC recurrence on imaging within the three months preceding the initiation of DAA treatment. The choice of antiviral therapy was solely determined by the treating physician, taking into consideration factors such as HCV GTs, local preferences, insurance coverage, prior treatment history, and the presence of cirrhosis.
The baseline laboratory data included HCV GT, platelet count, total bilirubin, alanine aminotransferase, aspartate aminotransferase, albumin, creatinine, estimated glomerular filtration rate, fibrosis-4 index, and aspartate aminotransferase to platelet ratio index. The HCV RNA viral load was determined both at the time of DAA initiation and 12 weeks after the end of treatment using a real-time reverse transcriptase polymerase chain reaction assay.
The primary endpoint was the achievement of SVR12, defined as an undetectable HCV RNA level (with a detection threshold of 25 IU/mL or lower) at 12 weeks after the completion of treatment. The secondary endpoint was the assessment of tolerability, including treatment-related adverse events (AEs), treatment discontinuation, and death.
Statistical analysis
Categorical variables were expressed as numbers (percentages) and evaluated by χ2 or Fisher’s exact test as appropriate. Continuous variables were expressed as mean±standard deviation and evaluated by Student’s t-test for normally distributed data or Mann-Whitney U-tests if not. Logistic regression analysis was used to identify any independent baseline factors influencing treatment response. We estimated the odds ratio (OR) associated with achieving SVR12 in each subgroup and its 95% confidence intervals (95% CI). Factors included in the logistic regression models were chosen based on prior knowledge, and those with an univariable OR value of p < 0.1 were considered for inclusion in the multivariable model. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 17.0 (StataCorp LLC, USA).
Results
Patient characteristics and treatment regimens
Our study analyzed a total of 15,849 patients treated with IFN-free DAA in routine practice from 39 centers across North America, Europe, and the Asia-Pacific region. As shown in Table 1, the mean age of the cohort was 62±13 years, 49.6% were male, and most were Asian (91.1%: 52.9% Japanese, 25.7% Chinese and Taiwanese, 5.4% Korean, 3.3% Malaysian, and 2.9% Vietnamese), followed by White (6.4%), Hispanic/Latino (1.3%), Black/African-American (1.0%), American Indian/Alaska Native (0.1%), and others (0.3%). In this study, 24.9% were treatment-experienced (TE: 20.7% with IFN regimens and 4.3% with DAA), 34.8% had cirrhosis (10.7% of these with decompensated cirrhosis, classified as CTP class B in 9.1% and CTP class C in 1.6%), 8.6% had preexisting HCC (78.6% inactive, 21.4% active), and 3.0% and 1.7% were co-infected with HBV or HIV, respectively. In addition, common comorbidities included hypertension (37.6%), diabetes (18.9%), dyslipidemia (8.8%), and cardiovascular disease (6.7%) (Table 1).
Table 1. Baseline characteristics of patients who initiated antiviral therapy.
| Characteristics | All Genotypes (n=15,849) | Genotype 1 (n=10,246) | Genotype 2 (n=3,686) | Genotype 3 (n=1,151) | Genotype 4 (n=47) | Genotype 5 (n=1) | Genotype 6 (n=457) | Untyped GTs (n=261) |
|---|---|---|---|---|---|---|---|---|
| Age, year | 62±13 | 64±13 | 61±13 | 50±10 | 58±11 | 48 | 60±13 | 54±13 |
| Male, n (%) | 7,866 (49.6) | 4,803 (46.9) | 1,696 (46.0) | 921 (80.0) | 28 (59.6) | 1 (100) | 244 (53.4) | 173 (66.3) |
| Ethnicity, n (%) (n=15,663) | ||||||||
| Asian | 14,263 (91.1) | 9,110 (89.5) | 3,602 (97.9) | 951 (83.3) | 16 (35.6) | – | 454 (99.3) | 130 (80.3) |
| White | 995 (6.4) | 763 (7.5) | 54 (1.5) | 150 (13.1) | 9 (20.0) | 0 | 19 (11.7) | |
| Black/African-American | 149 (1.0) | 125 (1.2) | 5 (0.1) | 3 (0.3) | 13 (28.9) | 0 | 3 (1.9) | |
| Hispanic/Latino | 197 (1.3) | 135 (1.3) | 16 (0.4) | 31 (2.7) | 6 (13.3) | 0 | 9 (5.6) | |
| American Indian/Alaska Native | 9 (0.1) | 8 (0.1) | 0 | 1 (0.1) | 0 | 0 | 0 | |
| Others | 50 (0.3) | 37 (0.4) | 2 (0.1) | 6 (0.5) | 1 (2.2) | 3 (0.7) | 1 (0.6) | |
| Treatment experienced, n (%) (n=15,725) | 3,922 (24.9) | 2,995 (29.5) | 691 (18.9) | 99 (8.6) | 13 (28.3) | 0 | 76 (16.7) | 48 (19.2) |
| Interferon regimens | 3,249 (20.7) | 2,455 (24.2) | 614 (16.8) | 65 (5.7) | 11 (23.9) | 0 | 67 (14.8) | 37 (14.8) |
| DAA regimens | 699 (4.3) | 537 (5.3) | 77 (2.1) | 34 (3.0) | 2 (4.4) | 0 | 8 (1.8) | 11 (4.4) |
| HBV co-infection, n (%) (n=11,835) | 353 (3.0) | 218 (2.9) | 73 (2.7) | 29 (2.7) | 1 (3.2) | – | 22 (5.7) | 10 (7.4) |
| HIV co-infection, n (%) (n=7,363) | 124 (1.7) | 32 (0.8) | 11 (0.7) | 49 (4.7) | 1 (3.9) | – | 6 (1.7) | 25 (12.6) |
| HCC, n (%) (n=15,811) | 1,352 (8.6) | 992 (9.7) | 242 (6.6) | 72 (6.3) | 2 (4.3) | – | 18 (3.9) | 26 (10.0) |
| HCC status, n (%) (n=1,312) | ||||||||
| Inactive | 1,031 (78.6) | 778 (80.2) | 176 (76.2) | 42 (60.0) | 2 (100) | – | 13 (72.2) | 20 (95.2) |
| Active | 281 (21.4) | 192 (19.8) | 55 (23.8) | 28 (40.0) | 0 | – | 5 (27.8) | 1 (4.8) |
| Cirrhosis, n (%) | 5,502 (34.8) | 3,748 (34.6) | 1,007 (27.3) | 468 (40.8) | 21 (44.7) | – | 156 (34.2) | 102 (39.2) |
| Cirrhosis stages, n (%) (n=3297) | ||||||||
| CTP A | 2,947 (89.4) | 2,091 (89.7) | 583 (92.7) | 202 (80.2) | 10 (90.9) | 38 (90.5) | 23 (69.7) | |
| CTP B | 299 (9.1) | 207 (8.9) | 41 (6.5) | 40 (15.9) | 1 (9.1) | 3 (7.1) | 7 (21.2) | |
| CTP C | 51 (1.6) | 32 (1.4) | 5 (0.8) | 10 (4.0) | 0 | 1 (2.4) | 3 (9.1) | |
| AST, IU/L | 45 (30–70) | 45 (31–69) | 41 (27–72) | 52 (35–80) | 34 (24–72) | 30 | 44 (29–72) | 45 (29–74) |
| ALT, IU/L | 43 (27–77) | 43 (27–72) | 40 (23–79) | 58 (34–96) | 40 (25–90) | 35 | 53 (29–90) | 66 (40–99) |
| Platelet count, ×103 U/L | 164±70 | 159±68 | 172±69 | 175±78 | 181±74 | – | 179±70 | 181±80 |
| Albumin, g/dL | 4.1±0.5 | 4.0±0.5 | 4.1±0.5 | 4.1±0.7 | 4.0±0.5 | – | 4.1±0.5 | 4.1±0.7 |
| Total bilirubin, mg/dL | 0.7 (0.6–1) | 0.7 (0.6–1) | 0.7 (0.5–1) | 0.8 (0.5–1.1) | 0.6 (0.4–0.8) | – | 0.7 (0.5–1) | 0.7 (0.5–1) |
| FIB–4 | 2.8 (1.7–4.9) | 3.1 (1.9–5.2) | 2.5 (1.5–4.4) | 2.0 (1.2–4.0) | 2.4 (1.2–3.3) | – | 2.0 (1.4–3.9) | 2.0 (1.1–3.7) |
| APRI | 0.7 (0.4–1.5) | 0.8 (0.4–1.5) | 0.6 (0.3–1.4) | 0.8 (0.5–1.6) | 0.6 (0.3–1.3) | – | 0.6 (0.4–1.3) | 0.6 (0.4–1.5) |
| Creatinine, mg/dL | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.7 (0.6–0.9) | 0.9 (0.7–1.0) | 0.8 (0.6–1.0) | – | 0.8 (0.7–1.0) | 0.8 (0.6–1.0) |
| eGFR, mL/m/1.73 m2 | 88±22 | 86±22 | 89±23 | 93±20 | 95±23 | – | 85±23 | 92±26 |
| HCV RNA, log10 IU/mL | 6.0±0.8 | 6.0±0.8 | 5.9±1.0 | 6.0±0.9 | 5.8±0.8 | 6.9 | 6.3±0.9 | 6.1±0.9 |
| >800,000 IU/mL | 9,758 (63.2) | 6,438 (64.2) | 2,076 (57.5) | 745 (68.7) | 24 (53.3) | 1 (100) | 309 (72.0) | 165 (66.5) |
| Comorbidity, n (%) | – | |||||||
| Diabetes (n=14,674) | 2,772 (18.9) | 1,898 (20.1) | 660 (19.1) | 89 (8.4) | 10 (23.3) | 73 (17.0) | 42 (17.0) | |
| Hypertension (n=11,674) | 4,387 (37.6) | 2,991 (39.2) | 1,043 (37.1) | 125 (23.0) | 20 (51.3) | 146 (34.3) | 62 (27.9) | |
| Dyslipidemia (n=11,630) | 1,026 (8.8) | 656 (8.7) | 254 (8.9) | 45 (8.5) | 5 (13.2) | 49 (11.5) | 17 (7.8) | |
| Cardiovascular disease (n=9,491) | 636 (6.7) | 443 (7.3) | 146 (6.6) | 27 (5.1) | 1 (2.8) | 11 (2.6) | 8 (3.7) | |
| DAA treatment regimens | – | |||||||
| DCV/ASV | 2,357 (14.9) | 2,351 (23.0) | 5 (0.1) | 1 (0.1) | 0 | 0 | 0 | |
| PrOD±RBV | 1,603 (10.1) | 1,527 (14.9) | 75 (2.0) | 0 | 0 | 0 | 1 (0.4) | |
| EBR/GZR | 847 (5.3) | 825 (8.1) | 3 (0.1) | 0 | 6 (12.8) | 2 (0.4) | 11 (4.2) | |
| SOF/LDV±RBV | 4,856 (30.6) | 4,375 (42.7) | 132 (3.6) | 24 (2.1) | 25 (53.2) | 1 (100) | 219 (47.9) | 80 (30.7) |
| SOF/RBV | 2,785 (17.6) | 74 (0.7) | 2,593 (70.4) | 82 (7.1) | 2 (4.3) | 22 (4.8) | 12 (4.6) | |
| SOF/DCV±RBV | 382 (2.4) | 96 (0.9) | 149 (4.0) | 102 (8.9) | 0 | 16 (3.5) | 19 (7.3) | |
| GLE/PIB | 1,219 (7.7) | 530 (5.2) | 494 (13.4) | 68 (5.9) | 5 (10.6) | 70 (15.3) | 52 (19.9) | |
| SOF/VEL±RBV | 1,640 (10.4) | 381 (3.7) | 205 (5.6) | 851 (73.9) | 8 (17.0) | 122 (26.7) | 73 (28.0) | |
| Others | 160 (1.0) | 87 (0.9) | 30 (0.8) | 23 (2.0) | 1 (2.1) | 6 (1.3) | 13 (5.0) |
HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus; HBV, Hepatitis B virus; HIV, Human immunodeficiency virus; DAA, Direct-acting antiviral; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; FIB-4, Fibrosis-4 index; APRI, AST to platelet ratio index; CTP, Child-Turcotte-Pugh; eGFR, Estimated glomerular filtration rate; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; ASV, Asunaprevir; LDV, Ledipasvir; PrOD, Paritaprevir/ritonavir, ombitasvir + dasabuvir; EBR, Elbasvir; GZR, Grazoprevir; GLE, Glecaprevir; PIB, Pibrentasvir.
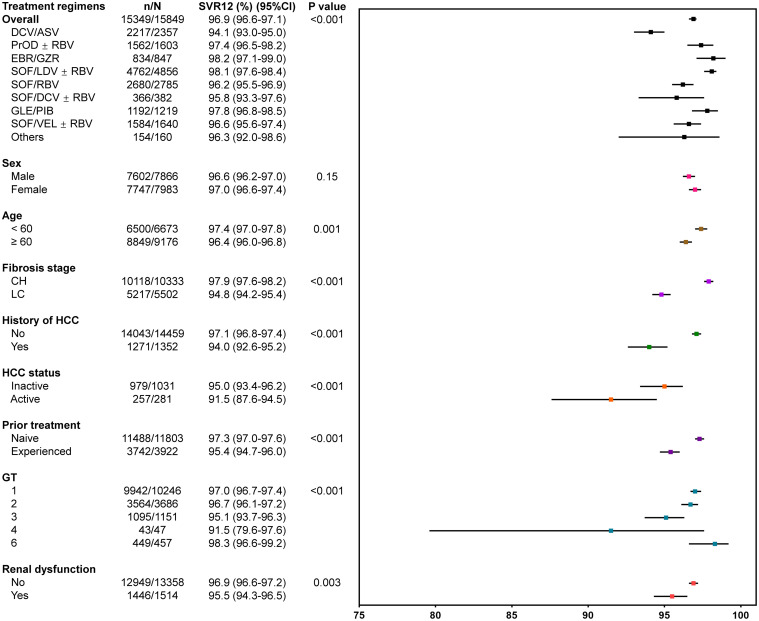
Patients were primarily infected with HCV GT1 (10,246 [64.6%]), followed by GT2 (3,686 [23.2%]), GT3 (1,151 [7.2%]), GT6 (457 [2.8%]), GT4 (47 [0.3%]), untyped GTs (261 [1.6%]), and there was only 1 case of GT5 (1 [0.006%]). Prescribed DAA regimens were also diverse, with the most commonly used being sofosbuvir/ledipasvir (SOF/LDV)±ribavirin (RBV) (30.6%), followed by SOF + RBV (17.6%), daclatasvir + asunaprevir (DCV/ASV) (14.9%), SOF/velpatasvir (VEL)±RBV (10.4%), paritaprevir/ritonavir, ombitasvir + dasabuvir (PrOD)±RBV (10.1%), glecaprevir/pibrentasvir (GLE/PIB) (7.7%), elbasvir/grazoprevir (EBR/GZR) (5.3%), SOF + DCV±RBV (2.4%), and others (1.0%).
SVR12 outcomes
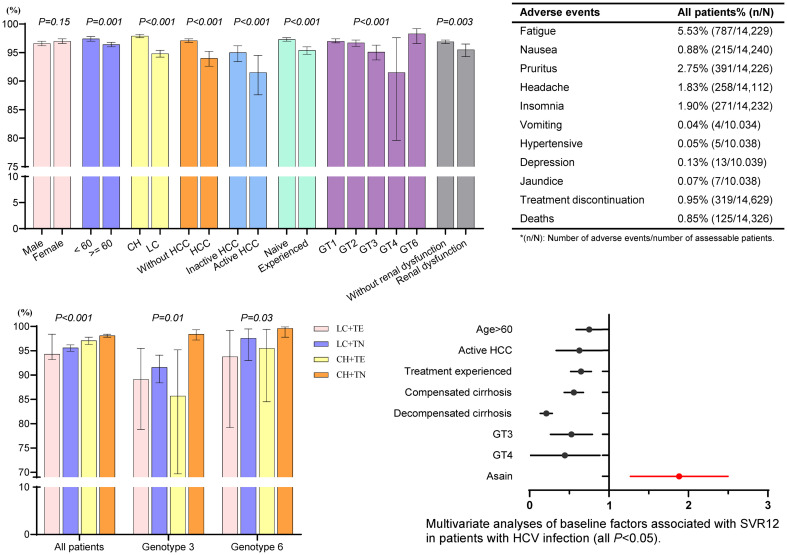
Overall, SVR12 was achieved in 96.9% (95% CI: 96.6–97.1%, all DAA-based regimens and all GTs combined). Figure 1 displayed the SVR12 outcomes by demographic characteristics and DAA regimens. The SVR12 rates were generally comparable for both males (96.6%) and females (97.0%). By age group, patients older than 60 years had a slightly lower SVR12 rate (96.4%) compared to those younger than 60 years (97.4%) (p=0.001). By GTs, SVR12 was >95% for GT1/2/3/6, while it was 91.5% for GT4 (p < 0.001). Moreover, cirrhosis (94.8% vs. 97.9% without cirrhosis; p < 0.001), history of HCC (94.0% vs. 97.1% without HCC; p < 0.001), especially for active HCC (91.5% vs. 95.0% inactive HCC; p < 0.001), prior treatment failure (95.4% vs. 97.3% with treatment-naive; p < 0.001), as well as renal dysfunction (95.5% vs. 96.9% without renal dysfunction; p=0.003), all had negative impacts on SVR12 rates, though the incremental differences were not large.
Fig. 1. Sustained virologic response at week 12 after end-of-treatment (SVR12) rates for patients with chronic hepatitis C.
Renal dysfunction was defined as an estimated glomerular filtration rate (eGFR) less than 60 (mL/m/1.73 m2). HCC, Hepatocellular carcinoma; CH, Chronic hepatitis; LC, Liver cirrhosis; GT, Genotype; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; ASV, Asunaprevir; LDV, Ledipasvir; PrOD, Paritaprevir/ritonavir, ombitasvir + dasabuvir; EBR, Elbasvir; GZR, Grazoprevir; GLE, Glecaprevir; PIB, Pibrentasvir; CI, Confidence interval.
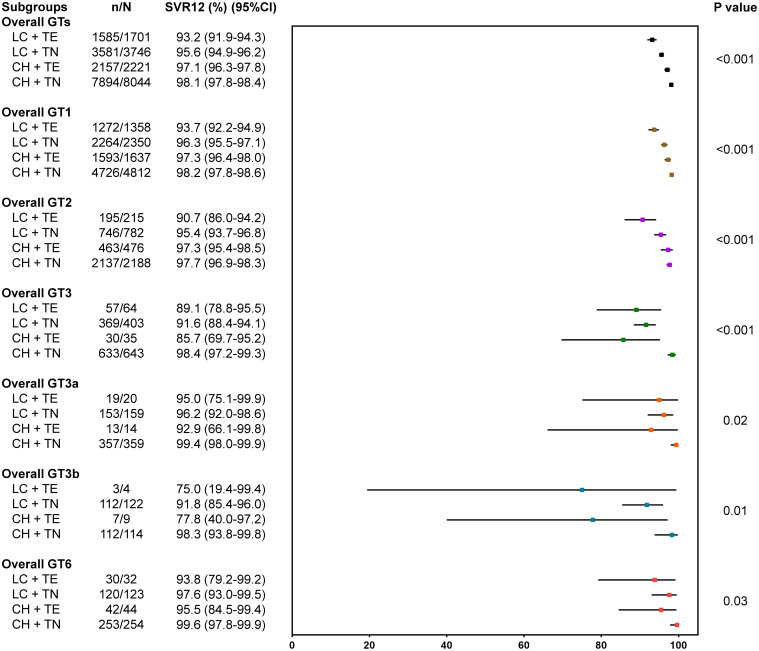
Figure 2 displayed SVR12 outcomes for patients stratified by GTs, liver cirrhosis, and prior treatment history. Overall, patients with TE cirrhosis had the lowest SVR12 (93.2%, 95% CI: 91.9–94.3%), compared with treatment-naive cirrhotic (95.6%, 95% CI: 94.9–96.2%), TE non-cirrhotic (97.1%, 95% CI: 96.3–97.8%), and treatment-naive non-cirrhotic (98.1%, 95% CI: 97.8–98.4%) (p < 0.001). Among GT3-infected patients, although the overall SVR12 was 95.1% (1,095/1,151, 95% CI: 93.7–96.3%), significantly lower SVR12 was observed in TE patients, regardless of cirrhosis status (85.7–89.1%). Notably, GT3b patients had lower SVR12 than those with GT3a (94.0% vs. 98.2%; p < 0.001). For GT3b-infected, TE patients had significantly lower SVR12 (10/13, 76.9%, 95% CI: 46.2–95.0%) compared to treatment-naive patients (224/236, 94.9%, 95% CI: 91.3–97.4%). However, for GT3a-infected, the lowest SVR12 was observed in patients with TE CHC while it was still over 92%. Among GT6-infected patients, the overall SVR12 rate was 98.3% (449/457, 95% CI: 96.6–99.2%) (Fig. 1), with slightly lower response at 93.8% (95% CI: 79.2–99.2%) among those with TE cirrhosis, while SVR12 for all treatment-naive patients was 97.5% regardless of cirrhosis status. Additionally, among patients with GT1 or GT2 infection, significantly lower treatment responses were observed in patients with TE cirrhosis, though still over 90%.
Fig. 2. Sustained virologic response at week 12 after end-of-treatment (SVR12) rates for patients with chronic hepatitis C virus stratified by genotypes (GTs), liver fibrosis stage, and prior treatment.
CH, Chronic hepatitis; LC, Liver cirrhosis; TE, Treatment-experienced; TN, Treatment-naive; CI, Confidence interval.
The treatment responses in patients stratified by GT3 or GT6 and different regimens were shown in Supplementary Table 1. Among patients with GT3 infection, the best SVR12 was 96.2% with SOF/VEL±RBV (95% CI: 94.7–97.4%), followed by 95.8% with SOF/LDV±RBV (95% CI: 78.9–99.9%), 94.1% with GLE/PIB (95% CI: 85.6–98.4%), 91.5% with SOF + RBV (95% CI: 83.2–96.5%), and 88.2% with SOF + DCV±RBV (95% CI: 80.4–93.8%). Among patients with GT6 infection, the SVR12 rates were ≥ 97.5% with SOF-based regimens and 100% with GLE/PIB.
Factors associated with treatment response
Using a multivariable logistic regression analysis, significant independent factors associated with lower SVR12 included age over 60 years (adjusted odds ratio [aOR], 0.74; p=0.01), prior treatment failure (aOR, 0.64; p < 0.001), cirrhosis (compensated: aOR, 0.55; p < 0.001; decompensated: aOR, 0.20; p<0.001), active HCC (aOR, 0.58; p=0.03, compared with non-HCC), and HCV GT3 and GT4 (aOR, 0.48; p=0.01 and aOR, 0.30; p=0.04; compared with GT1). However, being Asian (aOR, 1.81; p < 0.001) compared to non-Asian was a significant independent predictor for achieving SVR12, and there was no significant difference between inactive HCC and non-HCC (aOR, 0.86 [0.62–1.18]; p=0.34) (Table 2).
Table 2. Univariate and multivariate analyses of baseline factors associated with SVR12 in patients with HCV infection (all GTs)§.
| Factors | Category | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | ||
| Age, years | ≤60 | 1 (Referent) | 1 (Referent) | ||
| >60 | 0.72 (0.60–0.87) | 0.001 | 0.74 (0.59–0.92) | 0.01 | |
| Sex | Female | 1 (Referent) | 1 (Referent) | ||
| Male | 0.88 (0.73–1.05) | 0.15 | 0.91 (0.74–1.11) | 0.33 | |
| Ethnicity | Non-Asian | 1 (Referent) | 1 (Referent) | ||
| Asian | 1.94 (1.51–2.49) | < 0.001 | 1.81 (1.30–2.53) | < 0.001 | |
| Prior treatment | No | 1 (Referent) | 1 (Referent) | ||
| Yes | 0.57 (0.47–0.69) | < 0.001 | 0.64 (0.52–0.78) | < 0.001 | |
| Liver fibrosis | Non-cirrhosis | 1 (Referent) | 1 (Referent) | ||
| Compensated cirrhosis | 0.45 (0.37–0.55) | < 0.001 | 0.55 (0.44–0.68) | < 0.001 | |
| Decompensated cirrhosis | 0.18 (0.13–0.24) | < 0.001 | 0.20 (0.14–0.29) | < 0.001 | |
| HCC | Non-HCC | 1 (Referent) | 1 (Referent) | ||
| Ablated HCC | 0.56 (0.42–0.75) | < 0.001 | 0.86 (0.62–1.18) | 0.35 | |
| Active HCC | 0.32 (0.21–0.49) | < 0.001 | 0.58 (0.36–0.94) | 0.03 | |
| CKD, eGFR < 60 | No | 1 (Referent) | 1 (Referent) | ||
| Yes | 0.67 (0.52–0.87) | 0.003 | 0.85 (0.63–1.13) | 0.26 | |
| HBV co-infection | No | 1 (Referent) | |||
| Yes | 1.54 (0.72–3.27) | 0.27 | – | – | |
| HIV co-infection | No | 1 (Referent) | |||
| Yes | 4.25 (0.59–30.6) | 0.15 | – | – | |
| HCV RNA, IU/mL | ≤800,000 | 1 (Referent) | 1 (Referent) | ||
| >800,000 | 1.17 (0.97–1.41) | 0.10 | 1.01 (0.83–1.24) | 0.91 | |
| Genotypes | GT1 | 1 (Referent) | 1 (Referent) | ||
| GT2 | 0.89 (0.72–1.11) | 0.30 | 0.68 (0.42–1.09) | 0.11 | |
| GT3 | 0.60 (0.47–0.80) | 0.001 | 0.48 (0.29–0.81) | 0.01 | |
| GT4 | 0.33 (0.12–0.92) | 0.03 | 0.30 (0.09–0.94) | 0.04 | |
| GT6 | 1.72 (0.85–3.48) | 0.14 | 0.96 (0.43–2.18) | 0.93 | |
| DAA regimens | DCV/ASV | 1 (Referent) | 1 (Referent) | ||
| PrOD±RBV | 2.41 (1.69–3.43) | < 0.001 | 2.96 (2.00–4.36) | < 0.001 | |
| EBR/GZR | 3.50 (2.04–6.00) | < 0.001 | 3.73 (1.95–7.11) | < 0.001 | |
| SOF/LDV±RBV | 3.20 (2.45–4.17) | < 0.001 | 3.54 (2.63–4.76) | < 0.001 | |
| SOF/RBV | 1.61 (1.24–2.09) | < 0.001 | 1.96 (1.15–3.35) | 0.01 | |
| SOF/DCV±RBV | 1.44 (0.85–2.45) | 0.17 | 2.55 (1.27–5.12) | 0.01 | |
| GLE/PIB | 2.79 (1.84–4.23) | < 0.001 | 3.29 (1.95–5.57) | < 0.001 | |
| SOF/VEL±RBV | 1.79 (1.30–2.45) | < 0.001 | 3.07 (1.79–5.27) | < 0.001 | |
| Others | 1.62 (0.70–3.73) | 0.26 | 3.46 (1.34–8.93) | 0.01 | |
§5,995 patients with data for all variables, and 13,971 of 15,849 (88.2%) patients were included in the multivariable model, which does not include HBV and HIV. HBV and HIV were not used in the multivariable models since they were not significant predictors in the univariable analysis. HCV, Hepatitis C virus; HBV, Hepatitis B virus; HIV, Human immunodeficiency virus; CKD, Chronic kidney disease; eGFR, Estimated glomerular filtration rate; HCC, Hepatocellular carcinoma; GT, Genotype; SVR, Sustained virological response; DAA, Direct-acting antiviral; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; ASV, Asunaprevir; LDV, Ledipasvir; PrOD, Paritaprevir/ritonavir, ombitasvir + dasabuvir; EBR, Elbasvir; GZR, Grazoprevir; GLE, Glecaprevir; PIB, Pibrentasvir; CI, Confidence interval.
Among GT3-infected patients, significant factors independently associated with lower SVR12 included decompensated cirrhosis, with patients being 81% less likely to achieve SVR12 compared to those without cirrhosis (aOR, 0.19; p < 0.001), while Asians were significant independent predictors for achieving SVR12 (aOR, 4.05; p=0.01) (Table 3). For patients with GT3b, TE (OR 0.12, 95% CI 0.03–0.57; p=0.01) and HCV RNA >800,000 IU/mL (OR 3.94, 95% CI 1.14–13.61; p=0.03) were associated with treatment failure (Table 4). Among GT6-infected patients, active HCC was the strongest independent predictor for treatment failure (OR 0.01, 95% CI 0.0002–0.36; p=0.01), but this was not true for inactive HCC (p=0.62). Moreover, TE patients were 83% less likely to achieve SVR than treatment-naive patients (OR, 0.17; p=0.03) (Table 5).
Table 3. Univariate and multivariate analysis of baseline factors associated with SVR12 in patients with HCV GT3§.
| Factors | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | ||
| Age, years | ≤60 | 1 (Referent) | 1 (Referent) | ||
| >60 | 0.48 (0.26–0.90) | 0.02 | 1.16 (0.52–2.62) | 0.71 | |
| Sex | Female | 1 (Referent) | 1 (Referent) | ||
| Male | 0.86 (0.43–1.74) | 0.68 | 0.72 (0.31–1.64) | 0.43 | |
| Ethnicity | Non-Asian | 1 (Referent) | 1 (Referent) | ||
| Asian | 5.19 (2.98–9.03) | < 0.001 | 4.05 (1.43–11.5) | 0.01 | |
| Prior treatment | No | 1 (Referent) | 1 (Referent) | ||
| Yes | 0.32 (0.16–0.62) | 0.001 | 0.49 (0.20–1.19) | 0.12 | |
| Liver fibrosis | Non cirrhosis | 1 (Referent) | 1 (Referent) | ||
| Compensated cirrhosis | 0.40 (0.20–0.81) | 0.01 | 0.49 (0.22–1.10) | 0.08 | |
| Decompensated cirrhosis | 0.10 (0.05–0.21) | < 0.001 | 0.19 (0.08–0.46) | < 0.001 | |
| HCC | Non-HCC | 1 (Referent) | 1 (Referent) | ||
| Ablated HCC | 0.29 (0.11–0.75) | 0.01 | 0.81 (0.25–2.58) | 0.72 | |
| Active HCC | 0.18 (0.07–0.49) | 0.001 | 0.37 (0.20–1.37) | 0.14 | |
| CKD, eGFR < 60 | No | 1 (Referent) | |||
| Yes | 0.54 (0.21–1.35) | 0.18 | – | – | |
| HBV co-infection | No | 1 (Referent) | |||
| Yes | 2.98 (0.18–49.5) | 0.45 | – | – | |
| HIV co-infection | No | 1 (Referent) | |||
| Yes | 1.57 (0.30–8.20) | 0.59 | – | – | |
| HCV RNA, IU/mL | ≤800,000 | 1 (Referent) | 1 (Referent) | ||
| >800,000 | 2.04 (1.17–3.55) | 0.01 | 1.02 (0.52–2.03) | 0.95 | |
| Genotypes | GT3a | 1 (Referent) | 1 (Referent) | ||
| GT3b | 0.29 (0.13–0.65) | 0.003 | 0.55 (0.19–1.58) | 0.27 | |
| GT3 unspecified | 0.19 (0.10–0.39) | < 0.001 | 0.59 (0.21–1.62) | 0.30 | |
| DAA regimens | SOF/DCV±RBV | 1 (Referent) | 1 (Referent) | ||
| SOF/RBV | 1.39 (0.53–3.61) | 0.50 | 0.93 (0.29–2.91) | 0.90 | |
| GLE/PIB | 1.98 (0.64–6.09) | 0.23 | 1.68 (0.43–6.56) | 0.45 | |
| SOF/VEL±RBV | 3.48 (1.75–6.92) | < 0.001 | 1.32 (0.49–3.60) | 0.59 | |
| SOF/LDV | 2.16 (0.37–12.5) | 0.39 | 2.54 (0.36–17.7) | 0.35 | |
§861 patients with data for all variables, and 1,001 of 1,151 (87.0%) patients were included in the multivariable model, which does not include HBV and HIV. HBV and HIV were not used in the multivariable models since they were not significant predictors in the univariable analysis. HCV, Hepatitis C virus; HBV, Hepatitis B virus; HIV, Human immunodeficiency virus; CKD, Chronic kidney disease; eGFR, Estimated glomerular filtration rate; HCC, Hepatocellular carcinoma; GT, Genotype; SVR, Sustained virological response; DAA, Direct-acting antiviral; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; LDV, Ledipasvir; GLE, Glecaprevir; PIB, Pibrentasvir; CI, Confidence interval.
Table 4. Univariate and multivariate analysis of baseline factors associated with SVR12 in patients with HCV GT3b§.
| Factors | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | ||
| Age, years | ≤60 | 1 (Referent) | 1 (Referent) | ||
| >60 | 0.64 (0.13–3.02) | 0.57 | 0.49 (0.10–2.48) | 0.39 | |
| Sex | Female | 1 (Referent) | 1 (Referent) | ||
| Male | 1.34 (0.44–4.06) | 0.61 | 1.09 (0.30–3.96) | 0.90 | |
| Prior treatment | No | 1 (Referent) | 1 (Referent) | ||
| Yes | 0.18 (0.04–0.73) | 0.02 | 0.12 (0.03–0.57) | 0.01 | |
| Liver fibrosis | Non cirrhosis | 1 (Referent) | – | – | |
| Compensated cirrhosis | 0.82 (0.15–4.64) | 0.83 | – | – | |
| Decompensated cirrhosis | 0.40 (0.07–2.33) | 0.31 | – | – | |
| HCC | No | 1 (Referent) | – | – | |
| Yes | 0.60 (0.13–2.87) | 0.53 | – | – | |
| CKD, eGFR < 60 | No | 1 (Referent) | |||
| Yes | 1.24 (0.15–9.99) | 0.84 | – | – | |
| HIV co-infection | No | 1 (Referent) | |||
| Yes | 1.84 (0.23–14.6) | 0.56 | – | – | |
| HCV RNA, IU/mL | ≤800,000 | 1 (Referent) | 1 (Referent) | ||
| >800,000 | 3.87 (1.16–13.0) | 0.03 | 3.94 (1.14–13.61) | 0.03 | |
| DAA regimens | SOF/VEL±RBV | 1 (Referent) | – | – | |
| Others | 1.37 (0.42–4.43) | 0.60 | – | – | |
§167 patients with data for all variables, and 236 of 249 (94.8%) patients were included in the multivariable model, which does not include HIV. There are not enough patients for variables of HBV and active/ablated HCC. HCV, Hepatitis C virus; HBV, Hepatitis B virus; HIV, Human immunodeficiency virus; CKD, Chronic kidney disease; eGFR, Estimated glomerular filtration rate; HCC, Hepatocellular carcinoma; GT, Genotype; SVR, Sustained virological response; DAA, Direct-acting antiviral; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; LDV, Ledipasvir; GLE, Glecaprevir; PIB, Pibrentasvir; CI, Confidence interval.
Table 5. Univariate and multivariate analysis of baseline factors associated with SVR12 in patients with HCV GT6§.
| Factors | Category | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | ||
| Age, years | ≤60 | 1 (Referent) | 1 (Referent) | ||
| >60 | 1.02 (0.27–3.83) | 0.97 | 1.35 (0.26–7.14) | 0.73 | |
| Sex | Female | 1 (Referent) | 1 (Referent) | ||
| Male | 0.43 (0.10–1.89) | 0.27 | 0.56 (0.11–2.91) | 0.50 | |
| Ethnicity | Non-Asian | 1 (Referent) | |||
| Asian | 7.54 (0.36–156.86) | 0.19 | – | – | |
| Prior treatment | No | 1 (Referent) | 1 (Referent) | ||
| Yes | 0.19 (0.05–0.73) | 0.02 | 0.17 (0.03–0.87) | 0.03 | |
| Liver fibrosis | Non cirrhosis | 1 (Referent) | 1 (Referent) | ||
| Compensated cirrhosis | 0.30 (0.07–1.23) | 0.09 | 0.64 (0.12–3.36) | 0.60 | |
| Decompensated cirrhosis | 0.48 (0.02–9.66) | 0.63 | 1.86 (0.03–117.17) | 0.77 | |
| HCC | Non-HCC | 1 (Referent) | 1 (Referent) | ||
| Ablated HCC | 0.12 (0.02–0.80) | 0.03 | 0.45 (0.02–10.6) | 0.62 | |
| Active HCC | 0.04 (0.01–0.33) | 0.002 | 0.01 (0.0002–0.36) | 0.01 | |
| CKD, eGFR < 60 | No | 1 (Referent) | |||
| Yes | 0.77 (0.13–4.52) | 0.77 | – | – | |
| HBV co-infection | No | 1 (Referent) | |||
| Yes | 0.26 (0.04–1.60) | 0.15 | – | – | |
| HIV co-infection | No | 1 (Referent) | |||
| Yes | 0.29 (0.02–5.68) | 0.42 | – | – | |
| HCV RNA, IU/mL | ≤800,000 | 1 (Referent) | |||
| >800,000 | 1.17 (0.26–5.29) | 0.84 | – | – | |
| DAA regimens | SOF/DCV±RBV | 1 (Referent) | 1 (Referent) | ||
| SOF/LDV±RBV | 1.45 (0.07–28.1) | 0.81 | 2.41 (0.09–62.5) | 0.60 | |
| SOF/VEL±RBV | 1.03 (0.05–20.9) | 0.98 | 2.44 (0.07–83.7) | 0.62 | |
§260 patients with data for all variables, and 338 of 457 (74.0%) patients were included in the multivariable model, which does not include HBV and HIV. HBV and HIV were not used in the multivariable models since they were not significant predictors in the univariable analysis. HCV, Hepatitis C virus; HBV, Hepatitis B virus; HIV, Human immunodeficiency virus; CKD, Chronic kidney disease; eGFR, Estimated glomerular filtration rate; HCC, Hepatocellular carcinoma; GT, Genotype; SVR, Sustained virological response; DAA, Direct-acting antiviral; SOF, Sofosbuvir; VEL, Velpatasvir; RBV, Ribavirin; DCV, Daclatasvir; LDV, Ledipasvir; CI, Confidence interval.
Among GT1-infected patients, significant factors independently associated with lower SVR12 were age over 60 years (aOR, 0.69; p=0.01), prior treatment failure (aOR, 0.64; p < 0.001), decompensated and compensated cirrhosis (aOR, 0.19; p < 0.001 and aOR, 0.56; p < 0.001; respectively) (Supplementary Table 2). Among GT2-infected patients, decompensated cirrhosis (OR, 0.27; p=0.003) and compensated cirrhosis (OR, 0.47; p < 0.001) were significant factors independently associated with lower SVR12 (Supplementary Table 3).
Treatment tolerability
Supplementary Table 4 displayed the safety profile for patients stratified by different regimens. The overall incidence of AEs was low, with the most common AEs being fatigue (5.5%), pruritus (2.7%), insomnia (1.9%), headache (1.8%), and nausea (1.5%). AEs by DAA regimens are shown in Supplementary Table 4. Additionally, 319 patients (2.18%) discontinued treatment prematurely. The most common causes for early discontinuation were liver injury (0.3%), non-compliance (0.2%), and viral breakthrough (0.2%), with other reasons including patient choice, loss to follow-up, HCC diagnosis/recurrence, and other AEs such as fever, nausea, vomiting, and pruritus. Higher rates of treatment interruption were seen in patients receiving DCV/ASV (6.4%, 144/2,249), PrOD±RBV (3.2%, 43/1,346), and GLE/PIB (2.2%, 24/1,115), while it was less than 1.3% for other regimens. Serious adverse events (SAEs) occurred in 90 patients (0.6%), with the most common SAEs being decompensation (0.06%), HCC progression (0.03%), renal events (0.02%), cerebral hemorrhage (0.02%), and anemia (0.02%).
Discussion
The emergence of DAAs has dramatically transformed the HCV treatment landscape, but clinical practice patterns and HCV GT distribution vary by geographic region. We presented a large, multinational study including patients infected with HCV GTs 1–6 who received DAAs in a "real-world" setting. Overall, the SVR12 rate was 96.9%, with rates exceeding 90% in almost all subgroups, including patients with advanced age, renal dysfunction, prior treatment failure, cirrhosis, and HCC. Among the GTs, the cure rates were 97.0% for GT1, 96.7% for GT2, 95.1% for GT3, 91.5% for GT4, and 98.3% for GT6 (Fig. 1). The single patient with GT5 was treatment-naive, noncirrhotic, and was cured with SOF/LDV. Independent predictors of lower SVR12 included age over 60 years, prior treatment failure, cirrhosis, active HCC, and GT3 or GT4. Conversely, being Asian was a significant predictor of achieving SVR12, and there was no significant difference in SVR12 between patients with non-HCC and inactive HCC. The study also found that the overall incidence of AEs was low, with common AEs including fatigue, pruritus, insomnia, headache, and nausea. SAEs were rare (0.6%), and none led to death. Early treatment discontinuation was also uncommon in this large cohort (2.2%).
GT3 is the second most common HCV GT worldwide, and patients infected with GT3, especially the 3b subtype, remain a difficult-to-cure population.12,13 Our results showed an overall high SVR12 rate of 95.1% for GT3 patients, but a lower SVR12 in TE patients with or without cirrhosis (85.7–89.1%), largely due to TE patients infected with GT3b (75–77%). Moreover, decompensated cirrhosis, rather than compensated cirrhosis, was an independent predictor of treatment failure in GT3 patients, and a high HCV viral load increased the risk of treatment failure by 3.9 times compared to patients with a low HCV RNA. A large cohort study from Thailand showed an overall SVR12 of 96.7% in HCV GT3 patients who received SOF-based±RBV regimens, but it was 88.4% in patients with decompensated cirrhosis, and 57.1% for TE decompensated cirrhotic patients with GT3b.12 A multicenter phase 3 clinical trial in Asia showed an overall SVR12 of 86% in GT3 patients treated with SOF/VEL, while it was only 50% in GT3b patients with cirrhosis.13
On the other hand, our study found that SOF-based±RBV regimens were highly efficacious in HCV GT3-infected patients, with the highest SVR12 of 96.2% achieved by SOF/VEL±RBV (98.1% for GT3a and 93.4% for GT3b) (Supplementary Table 1). This supports the current recommendation for GT3 treatment with an IIB evidence grade by the 2023 HCV guidelines of the American Association for the Study of Liver Diseases and the Infectious Disease Society of America.16 SOF/VEL, as a protease inhibitor-free regimen, was recommended for treatment-naive or non-cirrhotic TE patients with GT3 infection for 12 weeks, or in combination with RBV for decompensated cirrhosis for 12/24 weeks.16,17 A multicenter phase 2 clinical study across Australia, New Zealand, and the United States found that SOF/VEL combination therapy for TE GT3 patients was highly effective, with an SVR12 of 88% without RBV and 96% with RBV.18 A phase 2 clinical study from Spain showed an SVR12 of 91% with SOF/VEL and 96% with SOF/VEL+RBV in GT3 patients with compensated cirrhosis.19 Furthermore, a phase 3 clinical study in the United States showed that 12-week SOF/VEL, 24-week SOF/VEL, or 12-week SOF/VEL + RBV in GT3 patients with decompensated cirrhosis achieved SVR12 rates of 50%, 50%, and 85%, respectively.20 Notably, SOF/VEL + RBV treatment of refractory GT3 patients can still achieve a high SVR12 rate, including patients with GT3b (93.4% in our study). High SVR12 rates among GT3 patients have been shown in real-world settings using SOF/VEL; however, few patients with GT3b were included.21 In settings where the cost and availability of HCV genotyping may be limited (e.g., HCV microelimination through outreach programs) and the prevalence of GT3b is low, pangenotypic SOF-based DAA regimens could be a reasonable and cost-effective approach. However, the efficacy of this regimen in GT3b patients with treatment experience or cirrhosis needs further evaluation. Therefore, despite the relatively high SVR12 based on DAA achieved in patients with GT3, the treatment of HCV 3b subtype infection remains a clinical challenge, especially when combined with cirrhosis and/or prior treatment failure, as well as high HCV viral load.
Our study of over 15,000 diverse patients, including 1,352 HCC patients (281 with active HCC) from both the East and the West, confirmed an important finding: inactive HCC was not associated with a lower SVR12 compared to non-HCC patients. Thus, the presence of HCC should not discourage providers from treating viremic HCV patients with DAAs though treatment should generally be delayed until HCC has been adequately treated since active HCC was associated with lower SVR, consistent with prior reports.22,23 The current study expanded on prior knowledge of the interaction between HCC and DAA treatment, including findings from an earlier study from REAL-C that included only patients from Asia which compared SVR12 rates of 436 pairs of matched patients with HCV/HCC and HCV/non-HCC patients and found that SVR12 for active HCC was significantly lower than that with inactive HCC (85.5% vs. 93.7%; p=0.03). However, there was no statistically significant difference between HCC (92.7%) and non-HCC (95.0%) groups.24 It should also be noted that the SVR12 for active HCC in our study was still fairly high at 91.5% (Fig.1), supporting prior suggestions that HCV-related HCC should be treated with DAAs based on a risk-benefit evaluation.25,26 However, DAA-based treatment recommendations for patients with HCV-related HCC have not been well-established across guidelines,16,17 despite increasing evidence that HCV-cured HCC patients have significantly improved overall and liver-related survival compared to those without SVR12.27,28 Therefore, additional patient and provider education and clearer guideline recommendations are needed. This is especially important given the dismally poor median 20-month overall survival for HCV-HCC patients. Those who received DAA treatment had a higher five-year survival rate compared to those untreated (47.2% vs. 35.2%). Additionally, the DAA utilization rate was low at 23.5% in a nationwide cohort of 3,922 U.S. patients with private insurance.29,30
Globally, data regarding the clinical features and treatment of HCV GT6 are limited. In this multicenter cohort, our study included 457 patients with HCV GT6, 99% of whom were from Asia (59.5% Vietnamese and 37.4% Chinese). Our study reported an excellent SVR12 rate of 98.3% among patients with GT6 infection, including those with TE cirrhosis (93.8%). HCV GT6 is mostly found in Southern China and Southeast Asia and has a higher prevalence in certain populations, such as intravenous drug users.31 A study from Hong Kong showed that the proportion of HCV GT6 infection among intravenous drug users was approximately 58%, significantly higher than among non-intravenous drug users (23%).32 Given the high cure rate of HCV GT6 patients receiving DAA treatment, including those with TE cirrhosis, it is recommended that people at high risk of HCV GT6 infection, especially intravenous drug users, should be screened and treated early to achieve the 2030 hepatitis C elimination target.
There were some limitations in our study. Firstly, this study is a real-world investigation, and patients were not randomly assigned to a specific DAA regimen. Instead, DAA treatment options were chosen based on the availability of DAAs in different regions at the time and the judgment of clinicians. Despite this, our overall SVR12 remained high. Secondly, although we performed multivariable regression analyses to adjust for potential confounders, residual confounding effects may remain, and not all patients had detailed records of important factors such as co-infection with HBV or HIV.
Conclusion
This large international multicenter observational study of patients with diverse GTs showed an overall SVR12 rate of 96.9%, despite including many patients with cirrhosis, HCC, prior treatment failure, and the use of older DAAs. Our study demonstrates that for refractory GT3 patients, including those with TE cirrhosis, SOF/VEL plus RBV can achieve high cure rates, lending further support to recent practice guideline recommendations. However, the efficacy of this regimen for the GT3b subtype with prior treatment failure or cirrhosis and high HCV viral load remains to be evaluated. Meanwhile, the study observed a high overall SVR12 for GT6, a prevalent GT among injection drug users who should be screened and promptly treated. Our study also confirmed high SVR12 rates for patients with HCC, though slightly lower for those with active HCC, underscoring the need for additional guideline recommendations to increase DAA utilization in this population.
Supporting information
Ethical statement
The study was conducted according to the Helsinki Declaration of 1975, as revised in 2008, and was approved by the Institutional Review Board at Stanford University, Stanford, California, USA (No. 40878, No. 13927), and participating study centers. The written informed consent was obtained from the patients.
Data sharing statement
Due to privacy policy, data are not publicly available.
References
- 1.Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14(2):122–132. doi: 10.1038/nrgastro.2016.176. [DOI] [PubMed] [Google Scholar]
- 2.Polaris Observatory HCV Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa E, Chien N, Kam L, Yeo YH, Ji F, Huang DQ, et al. Association of Direct-Acting Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in Patients With Chronic Hepatitis C. JAMA Intern Med. 2023;183(2):97–105. doi: 10.1001/jamainternmed.2022.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvaruso V, Craxì A. Hepatic benefits of HCV cure. J Hepatol. 2020;73(6):1548–1556. doi: 10.1016/j.jhep.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 5.McCombs J, Matsuda T, Tonnu-Mihara I, Saab S, Hines P, L’italien G, et al. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med. 2014;174(2):204–212. doi: 10.1001/jamainternmed.2013.12505. [DOI] [PubMed] [Google Scholar]
- 6.Yeo YH, He X, Lv F, Zhao Y, Liu Y, Yang JD, et al. Trends of Cirrhosis-related Mortality in the USA during the COVID-19 Pandemic. J Clin Transl Hepatol. 2023;11(3):751–756. doi: 10.14218/JCTH.2022.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CY, Huang CF, Cheng PN, Tseng KC, Lo CC, Kuo HT, et al. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021;41(6):1265–1277. doi: 10.1111/liv.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Wei L. Direct-acting Antiviral Regimens for Patients with Chronic Infection of Hepatitis C Virus Genotype 3 in China. J Clin Transl Hepatol. 2021;9(3):419–427. doi: 10.14218/JCTH.2020.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Yu C, Yin X, Guo X, Wu S, Hou J. Hepatitis C virus genotypes and subtypes circulating in Mainland China. Emerg Microbes Infect. 2017;6(11):e95. doi: 10.1038/emi.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji F, Li J, Liu L, Liang J, Wang X, Liu J, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b. J Gastroenterol Hepatol. 2021;36(3):767–774. doi: 10.1111/jgh.15192. [DOI] [PubMed] [Google Scholar]
- 12.Charatcharoenwitthaya P, Wongpaitoon V, Komolmit P, Sukeepaisarnjaroen W, Tangkijvanich P, Piratvisuth T, et al. Real-world effectiveness and safety of sofosbuvir and nonstructural protein 5A inhibitors for chronic hepatitis C genotype 1, 2, 3, 4, or 6: a multicentre cohort study. BMC Gastroenterol. 2020;20(1):47. doi: 10.1186/s12876-020-01196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4(2):127–134. doi: 10.1016/S2468-1253(18)30343-1. [DOI] [PubMed] [Google Scholar]
- 14.Hlaing NKT, Nangia G, Tun KT, Lin S, Maung MZ, Myint KT, et al. High sustained virologic response in genotypes 3 and 6 with generic NS5A inhibitor and sofosbuvir regimens in chronic HCV in myanmar. J Viral Hepat. 2019;26(10):1186–1199. doi: 10.1111/jvh.13133. [DOI] [PubMed] [Google Scholar]
- 15.Wong YJ, Tran S, Huang CF, Hsu YC, Preda C, Toyoda H, et al. Real-world treatment outcome with protease inhibitor direct-acting antiviral in advanced hepatitis C cirrhosis: a REAL-C study. Hepatol Int. 2023;17(5):1150–1161. doi: 10.1007/s12072-023-10547-4. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya D, Aronsohn A, Price J, Lo Re V, AASLD-IDSA HCV Guidance Panel Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2023:ciad319. doi: 10.1093/cid/ciad319. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver, EASL Governing Board representative, Panel members EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73(5):1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Pianko S, Flamm SL, Shiffman ML, Kumar S, Strasser SI, Dore GJ, et al. Sofosbuvir Plus Velpatasvir Combination Therapy for Treatment-Experienced Patients With Genotype 1 or 3 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163(11):809–817. doi: 10.7326/M15-1014. [DOI] [PubMed] [Google Scholar]
- 19.Esteban R, Pineda JA, Calleja JL, Casado M, Rodríguez M, Turnes J, et al. Efficacy of Sofosbuvir and Velpatasvir, With and Without Ribavirin, in Patients With Hepatitis C Virus Genotype 3 Infection and Cirrhosis. Gastroenterology. 2018;155(4):1120–1127.e4. doi: 10.1053/j.gastro.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 21.Wong YJ, Thurairajah PH, Kumar R, Tan J, Fock KM, Law NM, et al. Efficacy and safety of sofosbuvir/velpatasvir in a real-world chronic hepatitis C genotype 3 cohort. J Gastroenterol Hepatol. 2021;36(5):1300–1308. doi: 10.1111/jgh.15324. [DOI] [PubMed] [Google Scholar]
- 22.Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis. J Hepatol. 2019;71(3):473–485. doi: 10.1016/j.jhep.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Saberi B, Dadabhai AS, Durand CM, Philosophe B, Cameron AM, Sulkowski MS, et al. Challenges in treatment of hepatitis C among patients with hepatocellular carcinoma. Hepatology. 2017;66(2):661–663. doi: 10.1002/hep.29126. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa E, Toyoda H, Iio E, Jun DW, Huang CF, Enomoto M, et al. Hepatitis C Virus Cure Rates Are Reduced in Patients With Active but Not Inactive Hepatocellular Carcinoma: A Practice Implication. Clin Infect Dis. 2020;71(11):2840–2848. doi: 10.1093/cid/ciz1160. [DOI] [PubMed] [Google Scholar]
- 25.Reig M, Cabibbo G. Antiviral therapy in the palliative setting of HCC (BCLC-B and -C) J Hepatol. 2021;74(5):1225–1233. doi: 10.1016/j.jhep.2021.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Ji F, Li T, Nguyen MH. Improved survival and high sustained virologic response with DAA therapy in patients with HCV-related HCC: A call for expanded use. J Gastroenterol Hepatol. 2021;36(6):1721–1722. doi: 10.1111/jgh.15420. [DOI] [PubMed] [Google Scholar]
- 27.Dang H, Yeo YH, Yasuda S, Huang CF, Iio E, Landis C, et al. Cure With Interferon-Free Direct-Acting Antiviral Is Associated With Increased Survival in Patients With Hepatitis C Virus-Related Hepatocellular Carcinoma From Both East and West. Hepatology. 2020;71(6):1910–1922. doi: 10.1002/hep.30988. [DOI] [PubMed] [Google Scholar]
- 28.Singal AG, Rich NE, Mehta N, Branch AD, Pillai A, Hoteit M, et al. Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection Is Associated With Increased Survival in Patients With a History of Hepatocellular Carcinoma. Gastroenterology. 2019;157(5):1253–1263.e2. doi: 10.1053/j.gastro.2019.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karim MA, Singal AG, Kum HC, Lee YT, Park S, Rich NE, et al. Clinical Characteristics and Outcomes of Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma in the United States. Clin Gastroenterol Hepatol. 2023;21(3):670–680.e18. doi: 10.1016/j.cgh.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kam LY, Yeo YH, Ji F, Henry L, Cheung R, Nguyen MH. Treatment rates and factors associated with direct-acting antiviral therapy for insured patients with hepatitis C-related hepatocellular carcinoma - A real-world nationwide study. Aliment Pharmacol Ther. 2024;59(3):350–360. doi: 10.1111/apt.17794. [DOI] [PubMed] [Google Scholar]
- 31.Bunchorntavakul C, Chavalitdhamrong D, Tanwandee T. Hepatitis C genotype 6: A concise review and response-guided therapy proposal. World J Hepatol. 2013;5(9):496–504. doi: 10.4254/wjh.v5.i9.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou DX, Tang JW, Chu IM, Cheung JL, Tang NL, Tam JS, et al. Hepatitis C virus genotype distribution among intravenous drug user and the general population in Hong Kong. J Med Virol. 2006;78(5):574–581. doi: 10.1002/jmv.20578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.