Abstract
Background:
Various scoring systems have been developed to assess the risk of bleeding in medical settings. HAS-BLED and HEMORR2HAGES risk scores are commonly used to estimate bleeding risk in patients receiving anticoagulation for atrial fibrillation, but data on their predictive value in patients undergoing percutaneous coronary intervention (PCI) are limited.
Methods:
This study evaluated and compared the predictive abilities of the HAS-BLED and HEMORR2HAGES bleeding risk scores in all-comer patients undergoing PCI. The PARIS score, specifically designed for patients undergoing PCI, was used as a comparator. The scores were calculated at baseline and compared with the occurrence of events during a 2-year clinical follow-up period. Between 2015 and 2017, all consecutive patients undergoing PCI we re prospectively enrolled and divided into risk tertiles based on bleeding risk scores. The primary end points were hierarchical major bleeding events, defined by Bleeding Academic Research Consortium types 3 through 5, and patient-oriented composite end points according to Bleeding Academic Research Consortium classification, which were assessed during the 2-year follow-up period.
Results:
A total of 1,080 patients completed the follow-up period. Two years after index, 189 patients (17.5%) had experienced any bleeding, with 48 events (4.4%) classified as Bleeding Academic Research Consortium types 3 to 5. All bleeding risk scores showed statistically significant predictive ability for bleeding events. The HEMORR2HAGES score (C statistic, 0.73) was more effective than the HAS-BLED score (C statistic, 0.66; P = .07) and the PARIS score (C statistic, 0.66; P = .06) in predicting risk of major bleeding. Patients in high-risk bleeding groups also experienced a higher incidence of patient-oriented composite end points.
Conclusions:
The HEMORR2HAGES, HAS-BLED, and PARIS risk scores exhibited good predictive abilities for bleeding events following PCI. Patients at high risk of bleeding also demonstrated increased ischemic risk and higher mortality during the 2-year follow-up period.
Keywords: Hemorrhage, percutaneous coronary intervention, ischemia, risk factors, risk assessment
Key Points
HAS-BLED, HEMORR2HAGES, and PARIS risk scores demonstrated statistically significant predictive ability for risk of bleeding events after PCI, particularly major bleeding events, during a 2-year clinical follow-up period.
Despite being initially designed for patients with AF, the HEMORR2HAGES risk score exhibited the highest accuracy in predicting the risk of bleeding events for patients who had undergone PCI.
All 3 risk scores modestly predicted POCEs.
High bleeding risk was also associated with high ischemic risk and an increased mortality rate.
Introduction
Dual antiplatelet therapy (DAPT) is mandatory after percutaneous coronary intervention (PCI) but carries an increased risk of bleeding.1,2 The selection of DAPT type and duration is based on a balance between ischemic and bleeding risks.3 Patients at high ischemic risk and low bleeding risk benefit from extended DAPT, whereas patients at high bleeding risk but low ischemic risk do better with shorter DAPT durations.4,5
Over the years, patient and procedural factors that increase ischemic risk have been identified, including diabetes, kidney failure, acute coronary syndromes, heavily calcified lesions, bifurcated lesions, extensive stent length, small stent diameter, and the presence of incomplete stent expansion or apposition.6-8
Many factors that increase the risk of bleeding have also been identified. Various scoring systems have been proposed to assist clinicians in estimating bleeding risk in their patients. Some scoring systems are specific to patients who have undergone PCI, such as the Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients (PARIS) score, but the Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol (HAS-BLED) score and the Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke (HEMORR2HAGES) score remain widely used in clinical practice. Both the HAS-BLED9 and HEMORR2HAGES10 scores were developed to gauge the risk of major bleeding in people treated with oral anticoagulants (OACs) for atrial fibrillation (AF). The prognostic value of these AF-specific bleeding scores after PCI in all-comer patients is not yet known.
This study aimed to assess and compare the predictive abilities of 2 AF-specific bleeding risk scores (HAS-BLED and HEMORR2HAGES) with 1 PCI-specific bleeding risk score (PARIS) in unselected patients undergoing PCI in a single institution.
Patients and Methods
Study Population and Data Collection
Cardio-FR is a single-center, all-comers registry. All patients who underwent PCI at the study institution between June 2015 and July 2017 and who provided informed consent were included in the registry. The only exclusion criteria were an inability to sign the informed consent paperwork and an unwillingness to participate in clinical follow-up. The indication for PCI was based on established European guidelines.11 There were no limitations on the type, number, or length of lesions treated. Treatment modalities and antithrombotic management were administered at the physician’s discretion and according to the local standard of care at the time of intervention. Clinical follow-up was scheduled at 1, 2, 5, and 10 years. The Cardio-FR registry complied with the Helsinki Declaration and was approved by the local ethics committee (No. 003-REP-CER-FR). All patients provided written informed consent.
Risk Scores Categorization
Each patient in the study cohort was assigned 3 bleeding risk scores: HAS-BLED, HEMORR2HAGES, and PARIS. For each score, the patients were allocated to 1 of 3 risk groups to allow for comparison among the scores.8-10 The strata were low risk (HAS-BLED, 0 points; HEMORR2HAGES, 0-1 point; PARIS, 0-3 points); intermediate risk (HAS-BLED, 1-2 points; HEMORR2HAGES, 2-3 points; PARIS, 4-7 points), and high risk (HAS-BLED, 3-9 points; HEMORR2HAGES, 4-12 points; PARIS, 8-15 points). Bleeding risk was assessed at the time of PCI.
Clinical End Points
Clinical end points were reported at 2-year follow-up. Bleeding was defined by the Bleeding Academic Research Consortium (BARC) classification system, with types 3 through 5 considered “major bleeding.”12 Ischemic outcomes were defined as a BARC-related patient-oriented composite end point (POCE): all-cause mortality, any myocardial infarction, and any coronary revascularization. All in-hospital and postdischarge events were prospectively collected and adjudicated by a clinical event committee for study analysis.
Statistical Analysis
Categorical variables were reported using numbers and percentages; continuous variables were reported using mean (SD) values. Continuous variables were analyzed using a t test or the Wilcoxon rank sum test per distribution. Categorical variables were compared using χ2 tests or Fisher exact tests as appropriate. Survival free from the occurrence of clinical end points was assessed by computation of Kaplan-Meier curves. Survival rates were compared using the log-rank test, and clinical outcomes were reported as Kaplan-Meier failure estimations.
To better illustrate the incremental risk of bleeding conditioned by an increasing number of criteria, subgroup analysis that allocated patients to groups according to the number of criteria was performed for each score. Every subgroup was then univariately compared with the reference group, which comprised patients without any criteria, using the Cox proportional hazards regression model.
All statistical analyses were performed using Stata 17 software (StataCorp LLC). P < .05 was considered statistically significant.
Results
Baseline Patient Characteristics
During the inclusion period, 1,080 patients were enrolled in the registry. Baseline patient characteristics are summarized in Table I. During the 2-year follow-up period, 891 patients stayed free of bleeding events, 189 had at least 1 bleeding event, and 48 of these 189 patients had a major bleeding event. Patients with major bleeding events during follow-up were more likely to be older (median [IQR] age, 76 [70-83] years vs 67 [58-74] years; P < .01), have a lower estimated glomerular filtration rate (median [IQR] rate, 61.9 [47.2-79.6] mL/ min/1.73 m2 vs 83.1 [63.5-107.7] mL/min/1.73 m2; P < .01), and have anemia (median [IQR] hemoglobin level, 12.1 [10.8-13.9] g/dL vs 14.3 [13.1-15.3] g/dL; P < .01) than patients without major bleeding events during clinical follow-up.
TABLE I.
Baseline Patient Characteristics
| All (N = 1,080) | No major bleeding (n = 1,032) | BARC types 3-5a (n = 48) | P value | |
|---|---|---|---|---|
| Age, median (IQR), y | 67 (58-75) | 67 (58-74) | 76 (70-83) | <.01 |
| Male sex, No. (%) | 824 (76.3) | 791 (76.6) | 33 (68.8) | .21 |
| Hypertension, No. (%) | 664 (61.5) | 626 (60.7) | 38 (79.2) | .01 |
| Diabetes, No. (%) | 263 (24.4) | 245 (23.7) | 18 (37.5) | .03 |
| Insulin dependent, No. (%) | 78 (7.2) | 73 (7.1) | 5 (10.4) | .38 |
| Smoking, No. (%) | 305 (28.2) | 297 (28.8) | 8 (16.7) | .07 |
| Dyslipidemia, No. (%) | 501 (46.4) | 483 (46.8) | 18 (37.5) | .21 |
| Body mass index, median (IQR) | 27.0 (24.2-29.8) | 27 (24.2-29.8) | 27.4 (24.7-29.4) | .92 |
| Estimated glomerular filtration rate, median (IQR), mL/min/1.73 m2 | 82.2 (62.3-107.2) | 83.1 (63.5-107.7) | 61.9 (47.2-79.6) | <.01 |
| Hemoglobin, median (IQR), g/dL | 14.2 (12.9-15.3) | 14.3 (13.1-15.3) | 12.1 (10.8-13.9) | <.01 |
| Thrombocytes, median (IQR), ×109/L | 232.0 (193.0-275.5) | 232 (194-275) | 228 (177-300) | .62 |
| Family history of myocardial infarction,b No. (%) | 227 (21.0) | 224 (21.7) | 3 (6.3) | .01 |
| Previous PCI, No. (%) | 319 (29.5) | 304 (29.5) | 15 (31.3) | .79 |
| Previous coronary artery bypass graft, No. (%) | 115 (10.6) | 109 (10.6) | 6 (12.5) | .67 |
| Previous myocardial infarction, No. (%) | 137 (12.7) | 132 (12.8) | 5 (10.4) | .63 |
BARC, Bleeding Academic Research Consortium; PCI, percutaneous coronary intervention.
P < .05 was considered statistically significant.
SI conversion factor: To convert g/dL to g/L, multiply by 10.
The following are the BARC type 3-5 definitions. Type 3a: overt bleeding plus a hemoglobin drop of 3-5 g/dL (provided the hemoglobin drop is related to the bleeding event); any transfusion with overt bleeding. Type 3b: overt bleeding plus a hemoglobin drop of 5 g/dL (provided the hemoglobin drop is related to bleed); cardiac tamponade; bleeding requiring surgical intervention for control (excluding dental, nasal, skin, and hemorrhoid); bleeding requiring intravenous vasoactive agents. Type 3c: intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation, does include intraspinal); subcategories confirmed by autopsy or imaging or lumbar puncture; intraocular bleed compromising vision. Type 4: bleeding related to coronary artery bypass grafting; perioperative intracranial bleeding within 48 hours; reoperation after closure of sternotomy for the purpose of controlling bleeding; transfusion of 5 units of whole blood or packed red blood cells within a 48-hour period; chest tube output 2 L within a 24-hour period. Type 5a: probable fatal bleeding; no autopsy or imaging confirmation but clinically suspicious. Type 5b: definite fatal bleeding; overt bleeding or autopsy, or imaging confirmation.
“Family history of myocardial infarction” indicates a first-degree relative diagnosed with myocardial infarction before age 55 years (men) or 65 years (women).
Bleeding Scores
HAS-BLED Score
The incidence of each criterion constituting the HASBLED score in the whole cohort and its subsets is summarized in Supplemental Table I. The criteria statistically significantly associated with the occurrence of bleeding were being older than 65 years of age; prior bleeding or predisposition to bleeding; and, to a lesser extent, alcohol consumption and kidney failure (creatinine clearance >221 µmol/L or chronic dialysis or kidney transplant). Because all patients received an antiplatelet agent at discharge, no patients were considered to be at low risk using the HAS-BLED score. The patients were stratified into intermediate-risk (675 patients [62.5%]) and high-risk (405 patients [37.5%]) categories.
HEMORR2HAGES Score
The incidence of each criterion constituting the HEMORR2HAGES score in the whole cohort and its subsets is summarized in Supplemental Table II. The criteria statistically significantly associated with bleeding were hepatic or kidney disease, a history of malignancies, being older than 75 years of age, a history of bleeding events, having anemia, and having a high fall risk. The patients were stratified into low-risk (475 patients [44.0%]), intermediate-risk (521 [48.2%]), and high-risk (84 patients [7.8%]) categories.
PARIS Score
The incidence of each criterion constituting the PARIS score in the whole cohort and its subsets is summarized in Supplemental Table III. The criteria significantly associated with bleeding risk were age (5.8% of patients in the ≤50 years of age category experienced any bleeding vs 18.0% of patients in the >50 years of age category), anemia, and kidney failure (creatinine clearance <60 mL/min). The patients were stratified into low-risk (454 patients [42.0%]), intermediate-risk (483 [44.7%]), and high-risk (143 patients [13.2%]) categories.
Clinical Bleeding End Points
Clinical bleeding end points are summarized in Table II, Figure 1, and Supplemental Figure 1. At 2-year followup, 189 (17.5%) patients had had a bleeding event, of which 48 (4.4%) were major bleeding events. Among patients classified as having a high bleeding risk by the HAS-BLED score, 97 (24.0%) experienced any bleeding and 33 (8.1%) experienced major bleeding, while 92 patients (13.6%) at intermediate risk had any bleeding and 15 (2.2%) had major bleeding (P < .01). The higher the HEMORR2HAGES score, the higher the incidence of any bleeding (high risk, 32 [38.1%] vs intermediate risk, 103 [19.8%] vs low risk, 54 [11.4%]; P < .01) and of major bleeding (17 [20.2%] vs 25 [4.8%] vs 6 [1.3%]; P < .01). The PARIS score also showed a statistically significant association between risk category and the occurrence of both any bleeding event and a major bleeding event. In the high-risk category, 45 patients (31.5%) experienced any bleeding compared with 81 (16.8%) in the intermediate-risk category and 63 (13.9%) in the low-risk category (P < .01), and 19 high-risk patients (13.3%) experienced major bleeding events compared with 17 (3.5%) in the intermediate-risk category and 12 (2.6%) in the low-risk category (P < .01). As Table II shows, each additional point in the score seems to confer an increased risk of bleeding.
TABLE II.
Bleeding Outcome at 2-Year Follow-Up According to Bleeding Risk Scorea
| Risk group | Patients, No. (%) (N = 1080) | No bleeding, No. (%) (n = 891) | Any bleeding, No. (%) (n = 189) | No major bleeding, No. (%) (n = 1,032) | Major bleeding BARC types 3-5, No. (%) (n = 48) |
|---|---|---|---|---|---|
| HAS-BLED score (mean [SD], 2.23 [0.97]) | |||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 270 (25) | 241 (89.3) | 29 (10.7) | 267 (98.9) | 3 (1.1) |
| 2 | 405 (37.5) | 342 (84.4) | 63 (15.6) | 393 (97) | 12 (3) |
| 3 | 311 (28.8) | 249 (80.1) | 62 (19.9) | 297 (95.5) | 14 (4.5) |
| 4 | 74 (6.9) | 44 (59.5) | 30 (40.5) | 56 (75.7) | 18 (24.3) |
| 5 | 19 (1.8) | 14 (73.7) | 5 (26.3) | 18 (94.7) | 1 (5.3) |
| 6 | 1 (0.1) | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| P value | <.01 | <.01 | |||
| Low (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intermediate (1-2) | 675 (62.5) | 583 (86.4) | 92 (13.6) | 660 (97.8) | 15 (2.2) |
| High (≥3) | 405 (37.5) | 308 (76.0) | 97 (24.0) | 372 (91.9) | 33 (8.1) |
| P value | <.01 | <.01 | |||
| HEMORR2HAGES score (mean [SD], 2.05 [1.37]) | |||||
| 0 | 4 (0.4) | 4 (100) | 0 (0) | 4 (100) | 0 (0) |
| 1 | 471 (43.6) | 417 (88.5) | 54 (11.5) | 465 (98.7) | 6 (1.3) |
| 2 | 373 (34.5) | 310 (83.1) | 63 (16.9) | 361 (96.8) | 12 (3.2) |
| 3 | 148 (13.7) | 108 (73.0) | 40 (27.0) | 135 (91.2) | 13 (8.8) |
| 4 | 59 (5.5) | 40 (67.8) | 19 (32.2) | 51 (86.4) | 8 (13.6) |
| 5 | 18 (1.7) | 7 (38.9) | 11 (61.1) | 10 (55.6) | 8 (44.4) |
| 6 | 5 (0.5) | 3 (60.0) | 2 (40.0) | 4 (80.0) | 1 (20.0) |
| 7 | 2 (0.2) | 2 (100) | 0 (0) | 2 (100) | 0 (0) |
| P value | <.01 | <.01 | |||
| Low (0-1) | 475 (44) | 421 (88.6) | 54 (11.4) | 469 (98.7) | 6 (1.3) |
| Intermediate (2-3) | 521 (48.2) | 418 (80.2) | 103 (19.8) | 496 (95.2) | 25 (4.8) |
| High (≥4) | 84 (7.8) | 52 (61.9) | 32 (38.1) | 67 (79.8) | 17 (20.2) |
| P value | <.01 | <.01 | |||
| PARIS score (mean [SD], 4.44 [2.55]) | |||||
| 0 | 21 (1.9) | 20 (95.2) | 1 (4.8) | 21 (100) | 0 (0) |
| 1 | 66 (6.1) | 58 (87.9) | 8 (12.1) | 65 (98.5) | 1 (1.5) |
| 2 | 164 (15.2) | 138 (84.1) | 26 (15.9) | 163 (99.4) | 1 (0.6) |
| 3 | 203 (18.8) | 175 (86.2) | 28 (13.8) | 193 (95.1) | 10 (4.9) |
| 4 | 179 (16.6) | 155 (86.6) | 24 (13.4) | 176 (98.3) | 3 (1.7) |
| 5 | 145 (13.4) | 121 (83.4) | 24 (16.6) | 144 (99.3) | 1 (0.7) |
| 6 | 88 (8.1) | 68 (77.3) | 20 (22.7) | 77 (87.5) | 11 (12.5) |
| 7 | 71 (6.6) | 58 (81.7) | 13 (18.3) | 69 (97.2) | 2 (2.8) |
| 8 | 61 (5.6) | 49 (80.3) | 12 (19.7) | 59 (96.7) | 2 (3.3) |
| 9 | 28 (2.6) | 17 (60.7) | 11 (39.3) | 23 (82.1) | 5 (17.9) |
| 10 | 24 (2.2) | 16 (66.7) | 8 (33.3) | 21 (87.5) | 3 (12.5) |
| 11 | 19 (1.8) | 9 (47.4) | 10 (52.6) | 12 (63.2) | 7 (36.8) |
| 12 | 3 (0.3) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 1 (33.3) |
| 13 | 7 (0.6) | 5 (71.4) | 2 (28.6) | 6 (85.7) | 1 (14.3) |
| 14 | 1 (0.1) | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| P value | <.01 | <.01 | |||
| Low (0-3) | 454 (42.0) | 391 (86.1) | 63 (13.9) | 442 (97.4) | 12 (2.6) |
| Intermediate (4-7) | 483 (44.7) | 402 (83.2) | 81 (16.8) | 466 (96.5) | 17 (3.5) |
| High (≥8) | 143 (13.2) | 98 (68.5) | 45 (31.5) | 124 (86.7) | 19 (13.3) |
| P value | <.01 | <.01 | |||
BARC, Bleeding Academic Research Consortium; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol; HEMORR2HAGES, Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke; PARIS, Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients.
P < .05 was considered statistically significant.
Percentages in table may not total 100.0% due to rounding.
Fig. 1.
Kaplan-Meier estimates show survival without major bleeding events at 2-year follow-up for (A) HAS-BLED, (B) HEMORR2HAGES, and (C) PARIS risk scores. Major bleeding events are defined as BARC types 3 through 5.
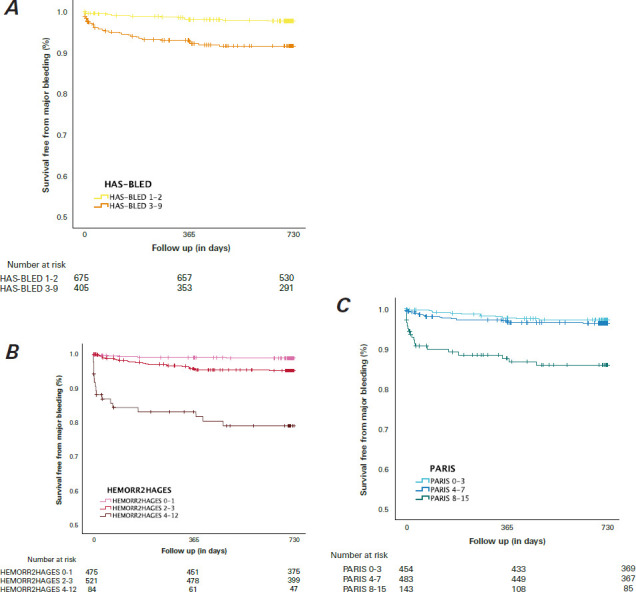
BARC, Bleeding Academic Research Consortium; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol; HEMORR2HAGES, Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke; PARIS, Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients.
Clinical Ischemic End Points
The POCEs (all-cause death, myocardial infarction, and repeated revascularization) are summarized in Table III and Figure 2. During the 2-year follow-up period, 230 (21%) patients experienced any POCE, and 72 (7%) patients died. Though a high score on 1 of the scoring systems was statistically significantly associated with an increase in the incidence of POCEs (P < .01) and death (P < .01), only a high HAS-BLED score was associated with a higher rate of myocardial infarction (P = .01).
TABLE III.
POCEs at 2-Year Follow-Up According to Bleeding Risk Score
| Risk group | All patients, No. (%)(N = 1080) | Any POCE, No. (%) (n = 230) | All-cause death, No. (%) (n = 72) | Myocardial infarction, No. (%) (n = 54) | Repeat revascularization, No. (%) (n = 150) |
|---|---|---|---|---|---|
| HAS-BLED score | |||||
| Low (0) | 0 (0) | 0 | 0 | 0 | 0 |
| Intermediate (1-2) | 675 (62.5) | 120 (17.8) | 23 (3.4) | 25 (3.7) | 92 (13.6) |
| High (≥3) | 405 (37.5) | 110 (27.2) | 49 (12.1) | 29 (7.2) | 58 (14.3) |
| P value | <.01 | <.01 | .01 | .79 | |
| HEMORR2HAGES score | |||||
| Low (0-1) | 475 (44) | 84 (17.7) | 14 (3) | 16 (3.4) | 67 (14.1) |
| Intermediate (2-3) | 521 (48.2) | 118 (22.6) | 40 (7.7) | 33 (6.3) | 71 (13.6) |
| High (≥4) | 84 (7.8) | 28 (33.3) | 18 (21.4) | 5 (6.0) | 12 (14.3) |
| P value | <.01 | <.01 | .09 | .97 | |
| PARIS scorea | |||||
| Low (0-3) | 454 (42.0) | 81 (17.8) | 11 (2.4) | 17 (3.7) | 66 (14.5) |
| Intermediate (4-7) | 483 (44.7) | 105 (21.7) | 28 (5.8) | 26 (5.4) | 72 (14.9) |
| High (≥8) | 143 (13.2) | 44 (30.8) | 33 (23.1) | 11 (7.7) | 12 (8.4) |
| P value | <.01 | <.01 | .15 | .12 | |
HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol; HEMORR2HAGES, Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke; PARIS, Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients; POCE, patient-oriented composite end point.
P < .05 was considered statistically significant.
Percentages of all patients’ PARIS scores may not total 100.0% due to rounding.
Fig. 2.
Kaplan-Meier estimates show survival without POCEs at 2-year follow-up for (A) HAS-BLED, (B) HEMORR2HAGES, and (C) PARIS risk scores.
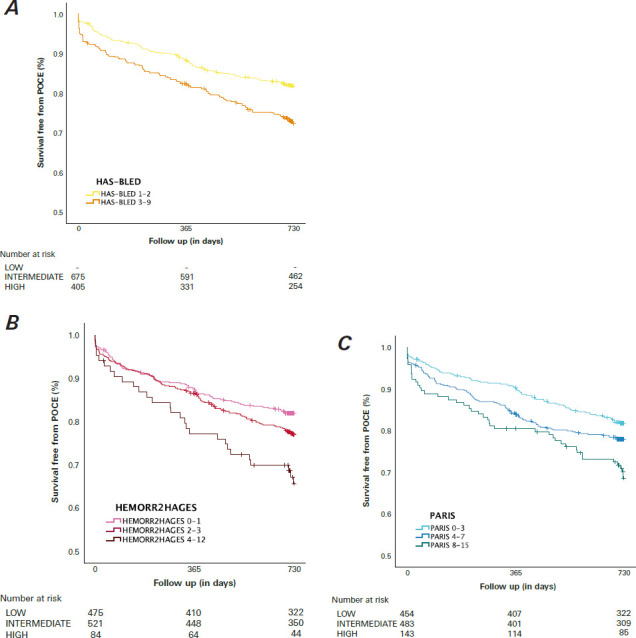
HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol; HEMORR2HAGES, Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke; PARIS, Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients; POCE, patient-oriented composite end point.
Predictive Performance of Each Score
Receiver operating characteristic curves analyzing the performance of each score to predict any bleeding and BARC types 3 through 5 bleeding can be found in Supplemental Figure 2 and Figure 3, respectively. Regarding the occurrence of any bleeding event (Supplemental Figure 2), the scores were statistically significantly, albeit modestly, discriminative (C statistics: HAS-BLED, 0.614; HEMORR2HAGES, 0.635; PARIS, 0.599). The scores were slightly better at predicting the risk of major bleeding events (Figure 3) (C statistics: HAS-BLED, 0.727; HEMORR2HAGES, 0.767; PARIS, 0.716). No score demonstrated statistically significant superiority compared with the others, but the HEMORR2HAGES score was observed to have a trend toward superiority at predicting major bleeding over the HAS-BLED score (P = .07) and the PARIS score (P = .06). Finally, and to a lesser extent, all 3 scores predicted the occurrence of POCEs (C statistics: HAS-BLED, 0.566; HEMORR2HAGES, 0.559; PARIS, 0.559).
Fig. 3.
The line graph shows the area under the receiver operating characteristic curve for major bleeding events. P < .05 was considered statistically significant.
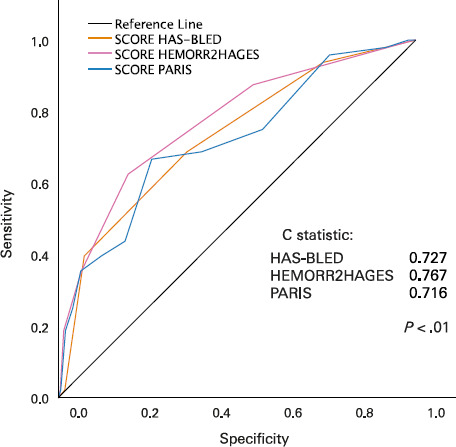
HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol; HEMORR2HAGES, Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke; PARIS, Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients.
Performance of Scores in Patients on OACs
In this study, 104 patients (9.6%) were on oral anticoagulation. The 2 risk scores developed to estimate the risk of major bleeding in patients treated with OACs for AF (HAS-BLED and HEMORR2HAGES) did not show statistically significantly better performance in patients on OACs vs patients not on OACs (C statistics: HAS-BLED, 0.673 vs 0.657, P = .86; HEMORR2HAGES, 0.724 vs 0.732, P = .95). The PCI-specific bleeding risk score (PARIS) was not better at predicting the risk of major bleeding among patients on OACs vs patients not on OACs (0.613 vs 0.661; P = .74).
Discussion
During a 2-year follow-up after PCI, 189 patients (17.5%) experienced at least 1 bleeding event, of which 48 (4.4%) were classified as major according to BARC criteria. The HAS-BLED, HEMORR2HAGES, and PARIS scores demonstrated a statistically significant ability to predict bleeding events, particularly major bleeding events. Although the HEMORR2HAGES score was intended for use in patients with AF, it showed the highest accuracy in predicting risk of bleeding events for PCI. Finally, all 3 scoring systems modestly predicted POCEs.
HAS-BLED Score for Estimating Patients at Risk of Bleeding After PCI
Although the HAS-BLED score was originally developed for patients with AF, several studies have evaluated its performance in patients undergoing PCI.
Puurunen et al13 first demonstrated in a group of 929 patients with AF receiving OACs that the modified HAS-BLED score was not associated with an increased risk of BARC-defined bleeding events at 1-year follow-up. In fact, a modified score greater than 2 even had a protective effect on the bleeding rate in this study. Yoshida et al14 found that unmodified HAS-BLED scores were associated with an increased 3-year bleeding rate in a population of 302 patients on OACs (area under the curve [AUC] = 0.62]. In patients not on OACs, Chen et al15 and Yildirim et al16 demonstrated that the HAS-BLED score can be used to estimate the risk of short-term bleeding (in-hospital or 30-day), with study AUCs of 0.67 and 0.68, respectively. Costa et al17 and Konishi et al,18 using larger cohorts (Costa, N = 1,946; Konishi, N = 1,207) and longer clinical follow-up periods (Costa, 2 years; Konishi, 3.6 years), confirmed the usefulness of the HAS-BLED score in predicting bleeding events and mortality in patients with and without AF. These findings are consistent with the results of the current study (AUC = 0.66), which demonstrated statistically significant predictive ability for bleeding risk, particularly for major bleeding events.
HEMORR2HAGES Score for Estimating Patients at Risk of Bleeding After PCI
The HEMORR2HAGES score, similar to the HAS-BLED score, was initially designed for patients with AF,9,10 but no studies had specifically examined the performance of the HEMORR2HAGES score in a broad population undergoing PCI until the present study.
PARIS Score for Estimating Patients at Risk of Bleeding After PCI
The PARIS score is a PCI-specific bleeding risk score. Its performance in predicting bleeding risk after PCI has been assessed in various studies, including those by Raposeiras-RoubÍn et al,19 Montalto et al,20 Yoshida et al,14 and Bianco et al.21 These studies reported AUCs of 0.56, 0.64, 0.64, and 0.59, respectively. These values are somewhat comparable to the results of the present study (C statistic, 0.66). The present study did not find a statistically significant difference between the scores in predicting bleeding events, but Yoshida et al14 concluded from their data that the HAS-BLED score was significantly better at predicting bleeding events than the PARIS score. It should be noted that in their study, all patients were treated with an OAC and at least 1 antiplatelet agent.
The ARC for High Bleeding Risk (ARC-HBR) recently identified major and minor risk factors associated with bleeding risk after PCI and proposed a standardized definition of high bleeding risk.22 Several studies concluded that the ARC-HBR definition successfully identifies patients at high risk of bleeding after PCI. 23-27 Ueki et al,23 Cao et al,24 and Nakamura et al26 reported C statistics of 0.69, 0.68, and 0.68, respectively. The present study, conducted on the same database as the Doomun et al27 study on the ARC-HBR definition, calculated the database’s C statistic for successful prediction of high risk of bleeding per the ARC-HBR definition, which had not been previously reported, and obtained a value of 0.73. The performance of the ARC-HBR definition in predicting major bleeding was superior to that of both the HAS-BLED (P = .02) and PARIS (P = .01) risk scores. The comparison, however, is inherently limited by the fact that the ARC-HBR score is binary, while the 3 scores studied in this investigation have 3 categories each.
Taken together, all 3 scores demonstrated statistically significant predictive ability for bleeding events, especially major bleeding events. Despite being originally developed for patients with AF, the HEMORR2HAGES score showed the best accuracy in predicting bleeding events after PCI. The ARC-HBR score outperformed both HAS-BLED and PARIS scores in predicting major bleeding events.
Limitations
The current study was limited in size, and its conclusions should be interpreted as hypothesis generating. This is not a prospective assessment of the HAS-BLED, HEMORR2HAGES, and PARIS risk scores, and the retrospective nature of the study raises the issue of unmeasured bias as well as incomplete data collection, though patients with incomplete medical records were excluded from the study. One should be cautious in extrapolating the current results as this was a single-center study with homogenous practices among its operators, a preponderant use of the femoral approach in PCI, and frequent use of prasugrel as the P2Y12 inhibitor for DAPT. Data on DAPT adherence after discharge were not available, and future research will be important for understanding how these factors influence bleeding rates in patients with high bleeding risk. Another limitation is that some criteria were binary for simplification purposes, but the risk for bleeding and ischemic events was a continuum. Finally, because treatment influences the risk of bleeding, the combined addition of each antithrombotic agent incrementally increased the risk of bleeding. The final choice of treatment duration was at the physicians’ discretion. Though the patient population’s initial antithrombotic regimens are known, the duration of the antithrombotic regimens was not recorded and may have influenced bleeding events during clinical follow-up. This final limitation does not alter the manuscript’s main message, however, which is a comparison of prognostic scores in a population treated in accordance with currently published recommendations.
Conclusion
HAS-BLED, HEMORR2HAGES, and PARIS risk scores demonstrated a statistically significant ability to predict bleeding events after PCI, especially major bleeding events, during a 2-year clinical follow-up. High bleeding risk was also associated with high ischemic risk and increased mortality.
Supplementary Material
Abbreviations and Acronyms
- AF
atrial fibrillation
- ARC-HBR
Academic Research Consortium for High Bleeding Risk
- AUC
area under the curve
- BARC
Bleeding Academic Research Consortium
- DAPT
dual antiplatelet therapy
- HAS-BLED
Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol
- HEMORR2HAGES
Hepatic or Renal Disease, Ethanol Abuse, Malignancy, Older Age, Reduced Platelet Count or Function, Re-Bleeding, Hypertension, Anemia, Genetic Factors, Excessive Fall Risk and Stroke
- OAC
oral anticoagulant
- PARIS
Patterns of non-Adherence to Anti-Platelet Regimen in Stented Patients
- PCI
percutaneous coronary intervention
- POCE
patient-oriented composite end point
Article Information
Open Access: © 2024 The Authors. Published by The Texas Heart Institute®. This is an Open Access article under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC, https://creativecommons.org/licenses/by-nc/4.0/), which permits use and distribution in any medium, provided the original work is properly cited, and the use is noncommercial.
Author Contributions: D.D. and I.D. contributed to the work equally, participating in the acquisition of data, conducting the analysis, interpreting the data for the work, and drafting the manuscript. S.S. and S.P. substantially participated in the analysis and interpretation of data for the work, made critical revisions to the manuscript, provided final approval of the version to be published, and agreed to be held accountable for all aspects of the work by ensuring that questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved. D.A., S.T.C., T.H., J.J.G., J.C.S., and M.T. substantially participated in the acquisition, analysis, and interpretation of data for the work as well as in the drafting of the work and its critical revision. They also provided final approval of the version to be published and agreed to be held accountable for all aspects of the work by ensuring that questions related to its accuracy and integrity were appropriately investigated and resolved. S.C. conceived the study, secured funding for it, and substantially participated in the acquisition and interpretation of data for the work as well as in the initial drafting of the work. All authors have made substantial contributions to (1) the conception and design of the study, (2) data acquisition and data analysis and interpretation, (3) the writing of the paper and critical review for important intellectual content, and (5) final approval of the version to be submitted.
Conflict of Interest/Disclosure: The authors report no financial relationships or conflicts of interest regarding the content herein. The Hospital and University Fribourg received institutional funding (<$50,000 each) from Abbott Cardiovascular; Bayer; Biosensors International Group, Ltd; Biotronik; Boston Scientific; Cordis; Medtronic; MicroPort; and Novartis during the study.
Funding/Support: The trial was an investigator-initiated study supported by an unrestricted grant from the Fonds Scientifique Cardiovasculaire (Fribourg, Switzerland).
Acknowledgments: We would like to express our gratitude to our research nurses for their dedication and valuable contributions to patient follow-up and to database records.
References
- 1.Mauri L, Kereiakes DJ, Yeh RW, et al. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–2166. doi: 10.1056/NEJMoa1409312. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh RW, Kereiakes DJ, Steg PG, et al. DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65(20):2211–2221. doi: 10.1016/j.jacc.2015.03.003. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg P, Galper BZ, Cohen DJ, Yeh RW, Mauri L. Balancing the risks of bleeding and stent thrombosis: a decision analytic model to compare durations of dual antiplatelet therapy after drug-eluting stents. Am Heart J. 2015;169(2):222–233.e5. doi: 10.1016/j.ahj.2014.11.002. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. doi: [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123–e155. doi: 10.1161/CIR.0000000000000404. doi: [DOI] [PubMed] [Google Scholar]
- 6.Dangas GD, Claessen BE, Mehran R, et al. Development and validation of a stent thrombosis risk score in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2012;5(11):1097–1105. doi: 10.1016/j.jcin.2012.07.012. doi: [DOI] [PubMed] [Google Scholar]
- 7.Palmerini T, Genereux P, Caixeta A, et al. A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: the ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage Strategy—Percutaneous Coronary Intervention) risk score. JACC Cardiovasc Interv. 2012;5(11):1108–1116. doi: 10.1016/j.jcin.2012.07.011. doi: [DOI] [PubMed] [Google Scholar]
- 8.Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67(19):2224–2234. doi: 10.1016/j.jacc.2016.02.064. doi: [DOI] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. doi: [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. doi: [DOI] [PubMed] [Google Scholar]
- 11.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. doi: [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. doi: [DOI] [PubMed] [Google Scholar]
- 13.Puurunen MK, Kiviniemi T, Schlitt A, et al. HADS2, CHA2DS2-VASc and HAS-BLED as predictors of outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention. Thromb Res. 2014;133(4):560–566. doi: 10.1016/j.thromres.2014.01.007. doi: [DOI] [PubMed] [Google Scholar]
- 14.Yoshida R, Ishii H, Morishima I, et al. Performance of HAS-BLED, ORBIT, PRECISE-DAPT, and PARIS risk score for predicting long-term bleeding events in patients taking an oral anticoagulant undergoing percutaneous coronary intervention. J Cardiol. 2019;73(6):479–487. doi: 10.1016/j.jjcc.2018.10.013. doi: [DOI] [PubMed] [Google Scholar]
- 15.Chen TY, Chung WJ, Lee CH, et al. Evaluation of bleeding risk in patients with acute myocardial infarction undergoing transradial percutaneous coronary intervention. Int Heart J. 2019;60(3):577–585. doi: 10.1536/ihj.18-377. doi: [DOI] [PubMed] [Google Scholar]
- 16.Yildirim E, Uku O, Bilen MN, Secen O. Performance of HAS-BLED and CRUSADE risk scores for the prediction of haemorrhagic events in patients with stable coronary artery disease. Cardiovasc J Afr. 2019;30(4):198–202. doi: 10.5830/CVJA-2019-014. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa F, Tijssen JG, Ariotti S, et al. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc. 2015;4(12):e002524. doi: 10.1161/JAHA.115.002524. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konishi H, Miyauchi K, Tsuboi S, et al. Impact of the HAS-BLED score on long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2015;116(4):527–531. doi: 10.1016/j.amjcard.2015.05.015. doi: [DOI] [PubMed] [Google Scholar]
- 19.Raposeiras-Roubín S, Caneiro Queija B, D’Ascenzo F, et al. Usefulness of the PARIS score to evaluate the ischemic-hemorrhagic net benefit with ticagrelor and prasugrel after an acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2019;72(3):215–223. doi: 10.1016/j.rec.2018.06.004. doi: [DOI] [PubMed] [Google Scholar]
- 20.Montalto C, Crimi G, Morici N, et al. Bleeding risk prediction in elderly patients managed invasively for acute coronary syndromes: external validation of the PRECISEDAPT and PARIS risk scores. Int J Cardiol. 2021;328:22–28. doi: 10.1016/j.ijcard.2020.11.065. doi: [DOI] [PubMed] [Google Scholar]
- 21.Bianco M, D’Ascenzo F, Raposeiras Roubin S, et al. Comparative external validation of the PRECISE-DAPT and PARIS risk scores in 4424 acute coronary syndrome patients treated with prasugrel or ticagrelor. Int J Cardiol. 2020;301:200–206. doi: 10.1016/j.ijcard.2019.11.132. doi: [DOI] [PubMed] [Google Scholar]
- 22.Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40(31):2632–2653. doi: 10.1093/eurheartj/ehz372. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueki Y, Bär S, Losdat S, et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARCHBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16(5):371–379. doi: 10.4244/EIJ-D-20-00052. doi: [DOI] [PubMed] [Google Scholar]
- 24.Cao D, Mehran R, Dangas G, et al. Validation of the Academic Research Consortium high bleeding risk definition in contemporary PCI patients. J Am Coll Cardiol. 2020;75(21):2711–2722. doi: 10.1016/j.jacc.2020.03.070. doi: [DOI] [PubMed] [Google Scholar]
- 25.Natsuaki M, Morimoto T, Shiomi H, et al. Application of the Academic Research Consortium High Bleeding Risk criteria in an all-comers registry of percutaneous coronary intervention. Circ Cardiovasc Interv. 2019;12(11):e008307. doi: 10.1161/CIRCINTERVENTIONS.119.008307. doi: [DOI] [PubMed] [Google Scholar]
- 26.Nakamura M, Kadota K, Nakao K, et al. High bleeding risk and clinical outcomes in East Asian patients undergoing percutaneous coronary intervention: the PENDULUM registry. EuroIntervention. 2021;16(14):1154–1162. doi: 10.4244/EIJ-D-20-00345. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doomun D, Doomun I, Schukraft S, et al. Ischemic and bleeding outcomes according to the Academic Research Consortium high bleeding risk criteria in all comers treated by percutaneous coronary interventions. Front Cardiovasc Med. 2021;8:620354–620354. doi: 10.3389/fcvm.2021.620354. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


