Abstract
The baboon endogenous retrovirus (BaEV) belongs to a large, widely dispersed interference group that includes the RD114 feline endogenous virus and primate type D retroviruses. Recently, we and another laboratory independently cloned a human receptor for these viruses and identified it as the human sodium-dependent neutral amino acid transporter type 2 (hASCT2). Interestingly, mouse and rat cells are efficiently infected by BaEV but only become susceptible to RD114 and type D retroviruses if the cells are pretreated with tunicamycin, an inhibitor of protein N-linked glycosylation. To investigate this host range difference, we cloned and analyzed NIH Swiss mouse ASCT2 (mASCT2). Surprisingly, mASCT2 did not mediate BaEV infection, which implied that mouse cells might have an alternative receptor for this virus. In addition, elimination of the two N-linked oligosaccharides from mASCT2 by mutagenesis, as substantiated by protein N-glycosidase F digestions and Western immunoblotting, did not enable it to function as a receptor for RD114 or type D retroviruses. Based on these results, we found that the related ASCT1 transporters of humans and mice are efficient receptors for BaEV but are relatively inactive for RD114 and type D retroviruses. Furthermore, elimination of the two N-linked oligosaccharides from extracellular loop 2 of mASCT1 by mutagenesis enabled it to function as an efficient receptor for RD114 and type D retroviruses. Thus, we infer that the tunicamycin-dependent infection of mouse cells by RD114 and type D retroviruses is caused by deglycosylation of mASCT1, which unmasks previously buried sites for viral interactions. In contrast, BaEV efficiently employs the glycosylated forms of mASCT1 that occur normally in untreated mouse cells.
The largest and most widely dispersed interference group of retroviruses includes the RD114 feline endogenous virus, baboon endogenous virus (BaEV), human endogenous virus type W (HERV-W), type D primate retroviruses, and avian reticuloendotheliosis viruses (REV) (4, 12, 31). Sequence comparisons have suggested that RD114 and avian REV were probably derived from rare cross-species transmissions (zoonoses) of retroviruses from primates to cats and birds, respectively (2, 11, 12, 17, 37). Recently, we and another laboratory independently isolated human cDNA clones that encode a receptor for these viruses (28, 32), and we identified this receptor as the previously reported human sodium-dependent neutral amino acid transporter type 2 (hASCT2) (15). ASCT2 has a broad specificity for neutral amino acids and is a member of a glutamate transporter superfamily (30).
Although these viruses cross interfere in many cells and use ASCT2 as a common receptor, they do not have identical host ranges. For example, BaEV efficiently infects rat and mouse cells, whereas these cells become susceptible to RD114 and primate type D retroviruses only after pretreatment with tunicamycin, an inhibitor of protein N-linked glycosylation (18, 29). Similarly, tunicamycin overcomes the natural resistances of Chinese hamster ovary (CHO) cells to several retroviruses (25, 26), of Mus dunni fibroblasts to the Moloney strain of ecotropic murine leukemia virus (10), and of some human cells to human immunodeficiency virus type 2 (34). To interpret these results, we initially hypothesized that the ASCT2 transporters of mice and rats might function as receptors for BaEV but remain inactive for RD114 and primate type D retroviruses due to masking by a mechanism that requires protein N-linked glycosylation. However, as described below, we found that mouse ASCT2 (mASCT2) is inactive as a receptor for BaEV and that removal of its N-linked oligosaccharides does not enable it to function as a receptor for RD114 or primate type D retroviruses. Furthermore, we present evidence that the neutral amino acid transporter ASCT1, which is 57% identical to ASCT2, is an efficient receptor in humans and mice for BaEV but not for RD114 or primate type D viruses. Moreover, mASCT1 functions as a strong receptor for RD114 and type D retroviruses when its N-linked oligosaccharides are eliminated by mutagenesis.
MATERIALS AND METHODS
Mice, cell lines, viruses, and plasmids.
NIH Swiss inbred NFS/N mice were obtained from Jackson Laboratory, Bar Harbor, Maine. NIH 3T3, Rat208F, and HeLa cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS). HEK293T cells were grown in Dulbecco modified Eagle high-glucose medium supplemented with 10% FBS. CHO cells were grown in α-modified minimal essential medium supplemented with 10% FBS.
LacZ(RD114) was produced by TELCeB6/RDF-7 helper-free packaging cells (8). LacZ(BaEV) was rescued by infection of mink Mv-1-Lu cells harboring a lacZ vector (33) with a replication-competent BaEV stock. LacZ(SRV-2) was produced from TELCeB6 cells infected with a replication-competent simian retrovirus type 2 (SRV-2) stock (32). RD114 replication-competent virus stock was produced in chronically infected TE671 human cells (kindly provided by Yasuhiro Takeuchi, The Institute of Cancer Research, London, United Kingdom).
The cDNA encoding hASCT1 (POG2.hASCT1 prokaryotic vector, kindly provided by Michael P. Kavanaugh, Vollum Institute, Oregon Health Sciences University) was cloned into pcDNA 3.1 eukaryotic expression vector (Invitrogen, Carlsbad, Calif.).
Receptor nomenclature and cDNA cloning.
We employ the common names ASCT1 and ASCT2 for the receptor proteins. The standard nomenclature for their genes is SLC1A4 and SLC1A5, respectively (OMIM database, The National Center for Biotechnology Information, National Institutes of Health; http://www.ncbi.nlm.nih.gov/entrez). mASCT2 and mASCT1 receptor cDNAs were isolated by reverse transcription-PCR amplification with total RNA. Total RNA was prepared from NIH 3T3 cells with RNeasy Midi kit (Qiagen, Valencia, Calif.), whereas total RNA from mouse kidney tissue, isolated from NIH Swiss mice, was prepared by the cesium chloride method (7). The 1.668-kb mASCT2 cDNA was amplified by using primers complementary to the 5′ and 3′ ends of the mASCT2 coding region (36) (upstream primer, 5′-TAAAGCTTATGGCAGTGGATCCCCCT-3′ containing a HindIII restriction site [underlined sequence]; downstream primer, 5′-CCGAATTCTCACATGACAGATTCCTT-3′ containing an EcoRI restriction site [underlined sequence]). The 1.599-kb mASCT1 cDNA was amplified by using primers complementary to the 5′ and 3′ ends of the hASCT1 coding region (upstream primer, 5′-AAAAGCTTATGGAGAAGAGCGGCGAGACC-3′ containing a HindIII restriction site [underlined sequence]; downstream primer, 5′-AACTCGAGTCACAGCACTGACTCCTTGGA-3′ containing an XhoI restriction site [underlined sequence]). The PCR products were subsequently cloned into the pCDNA3.1V5His-TOPO mammalian expression vector (Invitrogen) and sequenced by the Microbiology and Molecular Immunology Core Facility on the PE/ABD 377 sequencer using dye terminator cycle-sequencing chemistry (Applied Biosystems, Foster City, Calif.).
Transfection and infection assays.
CHO cells expressing ASCT2 and ASCT1 receptors were generated by transient transfection of the corresponding cDNAs using SuperFect Transfection Reagent (Qiagen). The transient transfectants were challenged with either LacZ(RD114) or LacZ(BaEV) pseudotype virus, with overnight incubations with virus beginning 24 h after transfection. Infected cells were stained by treating them with 0.25% glutaraldehyde and were assayed for β-galactosidase activity with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) as a substrate (21). The blue CFU were counted, and titers of infection were expressed as numbers of CFU per milliliter of virus supernatant.
l-[3H]alanine transport.
The l-[3H]alanine transport assay was performed in HEK293T cells transiently expressing ASCT receptors. The initial rate of l-[3H]alanine uptake was analyzed 48 h after the transfections were begun, as previously described (38).
Mutagenesis.
mASCT2 and mASCT1 receptor residues were mutated by PCR mutagenesis using two complementary mutagenic primers containing the targeted point mutation and a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Plasmid DNA from three independent clones was sequenced to confirm the mutations. DNA sequence was determined as described above. The mutants were designated by the parental receptor name followed by the mutated amino acid followed by the residue number and the new amino acid.
Immunoblot analyses.
HEK293T cells expressing ASCT2 receptors were generated by transient transfection of the corresponding cDNAs using SuperFect Transfection Reagent (Qiagen). The cell lysates were prepared 48 h after the transfections were begun. Briefly, the cells were washed with cold phosphate-buffered saline, scraped off the culture dishes, centrifuged at 200 × g and 4°C for 5 min, resuspended in lysis buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, protease inhibitor cocktail), and incubated on ice for 30 min, and cell debris and nuclei were removed by centrifugation of samples at 15,000 × g at 4°C for 10 min. Five micrograms of total protein cell lysate, untreated or treated with N-glycosidase F (2 h; 37°C), was processed for SDS-polyacrylamide gel electrophoresis. After the transfer of proteins to nitrocellulose filters, immunostaining was performed in PBS with 5% milk powder and 0.1% Tween 20. The blots were probed with anti-myc tag monoclonal antibody 9E10 (Sigma) and developed by using a horseradish peroxidase-conjugated goat anti-mouse antibody (Southern Biotechnology Associates, Inc.) and an enhanced-chemiluminescence kit (NEN Life Research Products, Boston, Mass.).
Interference assays.
Interference assays were performed as follows. Control HeLa and HEK293T cells, as well as HeLa.RD114 and HEK293T.RD114 (infected with RD114 replication-competent virus) cells, were transfected with ASCT receptor cDNAs using SuperFect Transfection Reagent. After 24 h, the transfected cells were tested for susceptibility to infections with LacZ(RD114) and LacZ(BaEV) as outlined above.
Nucleotide sequence accession numbers.
The cDNA nucleotide sequences have been assigned GenBank accession numbers as follows: mASCT1, AF246129; mASCT2, AF246130.
RESULTS
The mASCT2 transporter and its nonglycosylated mutants do not function as retroviral receptors.
Previously, we cloned the cell surface receptor for RD114 by transducing a human cDNA library into murine NIH 3T3 fibroblasts and by isolating cell clones that became susceptible to infection by an RD114 pseudotyped virus that encodes a dominant selectable gene for resistance to puromycin (32). Consistent with this cloning strategy, NIH 3T3 cells are almost completely resistant to RD114. However, in agreement with a previous report (18), these cells and other cells from mice or rats are highly susceptible to BaEV. Moreover, these rodent cells become susceptible to RD114 and to SRV-2 infections after they are pretreated with tunicamycin (Table 1).
TABLE 1.
Tunicamycin treatment of rodent cells enables their infection by RD114 and simian type D retrovirusesa
| Cell line | Titer of LacZ pseudotype (CFU/ml)
|
|||||
|---|---|---|---|---|---|---|
| RD114
|
SRV-2
|
BaEV
|
||||
| − Tunic | + Tunic | − Tunic | + Tunic | − Tunic | + Tunic | |
| NIH 3T3 | 7 | 4.8 × 104 | 21 | 2.2 × 103 | 9.4 × 103 | 2.6 × 104 |
| Rat208F | 1.4 × 102 | 1.8 × 105 | − | − | 1.4 × 105 | 1.2 × 105 |
Mouse NIH 3T3 and Rat208F cells were tested for their susceptibilities to LacZ(RD114), LacZ(SRV-2), and LacZ(BaEV) pseudotype viruses before treatment with tunicamycin (−Tunic) and after treatment with 125 ng of tunicamycin/ml (+Tunic). The titers are averages of two experiments. Titers of SRV-2 on Rat208F cells were not determined, as indicated by the dashes.
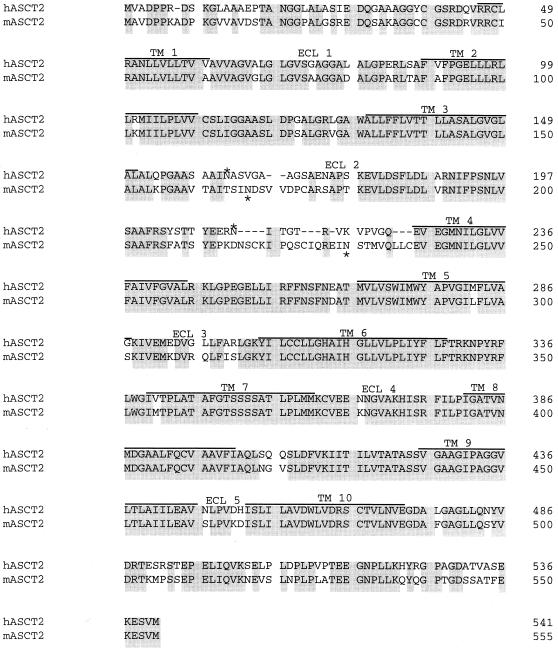
To study the basis for this host range difference between RD114 and BaEV, we isolated RNA from NIH 3T3 cells and used reverse transcription-PCR to isolate a cDNA for mASCT2 from this source. The deduced amino acid sequence of this mASCT2 is compared in Fig. 1 with the previously described hASCT2 sequence. The mASCT2 protein contains 555 amino acids and is 81% identical to hASCT2, which contains only 541 amino acids. Interestingly, most of the differences between these proteins are concentrated in a hypervariable presumptive extracellular-loop 2 (ECL2) region that contains insertions and deletions and substantial shifts in the positions of NX(S/T) consensus sites for N-linked glycosylation. In a related investigation, we have found that a mouse-human ASCT2 chimera containing only the human ECL2 region is an active receptor for RD114, whereas the reciprocal chimera is inactive (M. Marin, C. S. Tailor, and D. Kabat, unpublished data).
FIG. 1.
Amino acid sequence comparison of hASCT2 with mASCT2. hASCT2 and mASCT2 have 81% sequence identity. Common amino acids are shaded. The 10 hydrophobic potential membrane-spanning domains (TM) are shown as lines over the amino acid sequence and are identified by the numbers 1 to 10. The putative ECL regions are indicated by the numbers 1 to 5. Deletions in sequences are indicated by dashes. The numbers at the right of the sequences correspond to the position of the last amino acid shown. Potential N-glycosylation sites are indicated by asterisks.
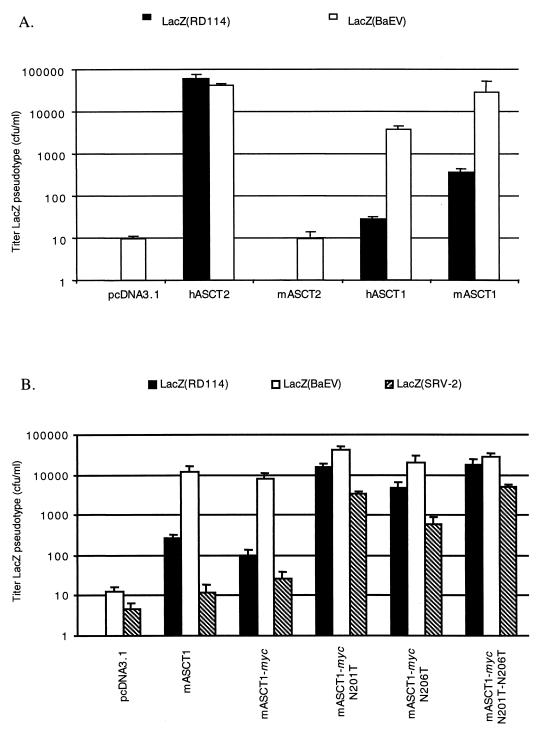
Consistent with these sequence relationships, expression of mASCT2 in human HEK293T cells resulted in a substantial increase in the rate of uptake of l-[3H]alanine (Table 2), confirming the presence of this transporter on the cell surfaces. Surprisingly, however, expression of mASCT2 in CHO cells did not confer susceptibility to BaEV (Fig. 2A). This implies that the receptor for BaEV in NIH 3T3 fibroblasts may not be mASCT2.
TABLE 2.
l-[3H]alanine uptake mediated by wild-type and mutant ASCT1 and ASCT2 transporters
| Expt | Exogenous receptor assayeda | l-[3H]alanine uptake (nmol/min/mg of protein)a |
|---|---|---|
| 1b | pcDNA3.1 | 25.9 ± 8.9 |
| mASCT2 | 56.3 ± 6.5 | |
| mASCT2.N167H | 42.9 ± 8.9 | |
| mASCT2.N230H | 41.2 ± 4.5 | |
| mASCT2.N167H-N230H | 39.6 ± 7.8 | |
| 2 | pcDNA3.1 | 13.8 ± 2.4 |
| hASCT1 | 20.3 ± 4.6 | |
| mASCT1 | 21.1 ± 0.8 | |
| hASCT2 | 22.1 ± 2.9 | |
| mASCT2 | 24.0 ± 1.8 |
The initial rates of l-[3H]alanine transport were measured in HEK293T cells transiently transfected with either the empty vector (pcDNA3.1) or the receptor expression vectors. Uptake of l-[3H]alanine was measured for 1 min at 37°C in medium containing sodium. The data are presented as mean uptake ± standard deviation of quadriplicate measurements.
In experiment 1, the wild-type and mutant mASCT2 proteins were myc tagged.
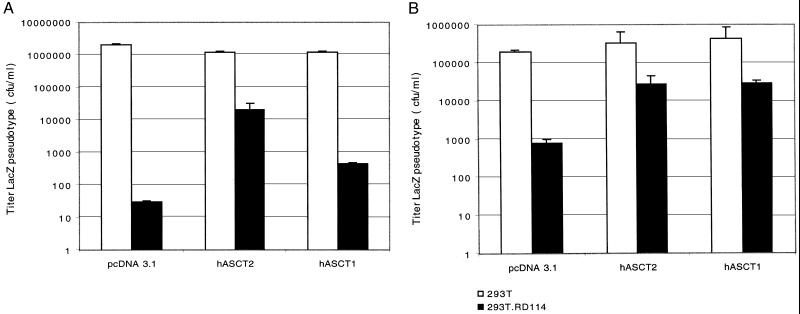
FIG. 2.
Mediation of infections by ASCT1 and ASCT2 receptors and mASCT1 mutants. Infectivity assays were done with CHO cells after transient transfection of expression vectors. Infections with LacZ pseudotype viruses were initiated 24 h after the transfections were begun. The titers are averages of three independent experiments + standard errors. (A) Infections for ASCT1 and ASCT2 receptors; (B) infections for mASCT1 N-glycosylation mutant receptors.
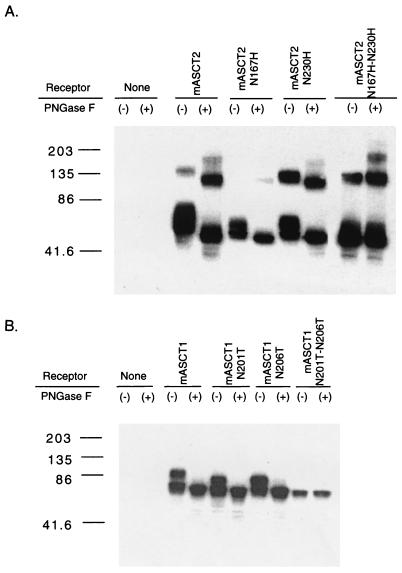
As indicated above, the critical ECL2 hypervariable region of the human and mouse ASCT2 proteins contains their only NX(S/T) consensus sites for potential N-linked glycosylation (Fig. 1). However, the two potential glycosylation sites in each of these proteins occur in different positions. To determine whether N-linked glycosylation of mASCT2 at positions N167 or N230 might be responsible for the tunicamycin dependency of RD114 infections in NIH 3T3 cells, we added a myc epitope tag at the carboxyl terminus of this protein, and we analyzed it by Western immunoblotting in the presence and absence of incubation with protein N-glycanase F (PNGase F). In addition, we constructed and analyzed the mASCT2 mutants N167H, N230H, and the double mutant by site-directed mutagenesis. As shown in Table 2 (experiment 1), expression of the wild-type and mutant myc-tagged mASCT2 proteins in HEK293T cells caused enhanced uptake of l-[3H]alanine, indicating that they were all active transporters that were expressed on cell surfaces. Figure 3A shows a Western immunoblot analysis of these proteins. Treatment of the wild-type mASCT2 protein with PNGaseF caused an increase in its electrophoretic mobility consistent with the expected Mr of 60,000 for the unglycosylated protein. In addition, the single mASCT2 mutants N167H and N230H both had faster electrophoretic mobilities than the undigested wild-type glycoprotein, and their mobilities were further increased to the apparent Mr of 60,000 by PNGase F digestions. In contrast, the double mutant had the same Mr of 60,000 as the deglycosylated wild-type protein and was unaffected by PNGaseF. These results strongly indicate that mASCT2 is glycosylated at both N167 and N230.
FIG. 3.
Western blot analysis of cell lysates prepared from transiently transfected HEK293T cells with myc-tagged mASCT2, mASCT1, and their N-glycosylated mutants expression vectors. The cell lysates were prepared 48 h after the transfections were begun, as described in Materials and Methods. Five micrograms of total protein cell lysate untreated (−) or treated (+) with N-glycosidase F was processed for SDS-polyacrylamide gel electrophoresis. (A) myc-tagged mASCT2 and its three N-glycosylated mutants, mASCT2-N167H-N230H, and -N167H-N230H expression vectors; (B) myc-tagged mASCT1 and its three N-glycosylated mutants, mASCT1-N201T, -N206T, and -N201T-N206T expression vectors.
An interesting result of this analysis is the potential dimeric structure of mASCT2. As shown in Fig. 3A, a component with an approximate Mr of 140,000 was present in the cell lysate that contained wild-type mASCT2, and this component was converted to an apparent Mr of 120,000 by digestion with PNGase F. Similar results were obtained with the single mASCT2 mutants, whereas the double mutant had an apparent Mr of approximately 120,000 and was unaffected by PNGase F. The hypothesis that mASCT2 may be a dimer or higher oligomer is compatible with recent evidence concerning other members of this transporter family (M. P. Kavanaugh, personal communication).
We then analyzed the three mASCT2 mutants N167H, N230H, and the double mutant for possible function as receptors for the LacZ(RD114) or LacZ(SRV-2) retroviruses. The results were negative, indicating that these hemiglycosylated and unglycosylated mASCT2 proteins are not receptors for these viruses (data not shown).
Human and mouse ASCT1 transporters are receptors for BaEV.
The failure of mASCT2 to function as a receptor for BaEV (Fig. 2A) suggested that mouse and rat cells might contain an alternative receptor for the virus. Consequently, we tested several other members of this transporter family, including hASCT1 (ca. 57% identity to hASCT2) and the human glutamate transporters hEAAT1 and hEAAT2. This initial screen indicated significant BaEV receptor activity only for hASCT1. Therefore, we isolated an ASCT1 cDNA clone from NIH Swiss mouse kidney tissue (mASCT1) in order to characterize its receptor properties. Figure 4 shows a comparison of the deduced amino acid sequences of hASCT1 with this mASCT1. Interestingly, the ECL2 regions of these ASCT1 proteins are much more closely related than the corresponding regions of the mouse and human ASCT2 proteins (Fig. 1) (see Discussion). As shown in Table 2 (experiment 2), expression of mASCT1 and hASCT1 in HEK293T cells caused enhanced cellular uptake of l-[3H]alanine, indicating the presence of these transporters on cell surfaces.
FIG. 4.
Amino acid sequence comparison of hASCT1 with mASCT1. hASCT1 and mASCT1 have 90% identity. The 10 hydrophobic potential membrane-spanning domains (TM) are shown as lines over the amino acid sequence and are identified by the numbers 1 to 10. The putative ECL regions are indicated by the numbers 1 to 5. The numbers at the right of the sequences correspond to the position of the last amino acid shown. Potential N-glycosylation sites are indicated by asterisks.
We then tested the retroviral-receptor functions of hASCT1 and mASCT1 by expressing them in CHO cells and assaying for infections by LacZ(RD114) and LacZ(BaEV). As shown in Fig. 2A, hASCT1 conferred strong susceptibility to infection by BaEV and weak susceptibility to RD114 pseudotyped viruses. Similar results were obtained with mASCT1. Thus, relative to the comparable titers of these virus preparations in CHO cells that expressed hASCT2, both the human and mouse ASCT1 transporters mediated BaEV infections approximately 100 times more efficiently than RD114 infections. However, mASCT1 appeared to be reproducibly approximately 10 times more active as a receptor for both viruses than hASCT1. Neither ASCT1 protein was active as a receptor for the LacZ(SRV-2) virus (results not shown).
Nonglycosylated mutants of mASCT1 function as efficient receptors for RD114 and simian type D viruses.
NX(S/T) consensus sites for potential N-linked glycosylation are present at positions N201 and N206 in the ECL2 region of mASCT1 (Fig. 4). To determine whether N-linked glycosylation at these positions might be responsible for the tunicamycin dependency of RD114 and simian type D infections in NIH 3T3 cells, we added a myc epitope tag at the carboxy terminus of mASCT1 and we constructed the mutants N201T, N206T, and the double mutant by site-directed mutagenesis of this myc-tagged mASCT1 clone. We then expressed these wild-type and mutant forms in CHO cells and analyzed the cells for susceptibilities to LacZ(RD114) and LacZ(SRV-2). As shown in Fig. 2B, the addition of a myc epitope tag at the carboxyl terminus of mASCT1 did not affect its function as an efficient receptor for BaEV. Moreover, all three mutants mediated LacZ(BaEV) infection with the same efficiency as wild-type mASCT1, indicating that they were all present on cell surfaces. More importantly, all three mutants functioned as dramatically improved receptors for LacZ(RD114) and LacZ(SRV-2) pseudotype retroviruses. Fig. 3B shows a Western immunoblot analysis of these proteins in transfected HEK293T cells. Treatment of the wild-type mASCT1 protein with PNGaseF caused an increase in its electrophoretic mobility consistent with the expected Mr of 60,000 for the unglycosylated protein. In addition, the single mASCT1 mutants N201T and N206T both had slightly faster electrophoretic mobilities than the undigested wild-type glycoprotein, and their mobilities were further increased to the apparent Mr of 60,000 by PNGase F digestions. In contrast, the double mutant had the same size as the deglycosylated wild-type protein and was unaffected by PNGase F. These results strongly indicate that mASCT1 is glycosylated at both N201 and N206.
Interference assays.
Previous studies demonstrated that RD114, BaEV, and type D primate retroviruses occur in a common interference group (31), in agreement with evidence that they can all use hASCT2 as a receptor (28, 32). However, our current results suggest that BaEV can use hASCT1 and mASCT1 and that these receptors can be used by RD114 only at a 100-fold-lower efficiency. This evidence is not in conflict with the previous interference data, because many cell lines and tissues lack ASCT1 (35) and because hASCT1 is a relatively weak receptor for BaEV compared with hASCT2 or mASCT1 (Fig. 2). In any case, we would expect that RD114 might not completely interfere with BaEV infections in cells that coexpress hASCT2 and hASCT1.
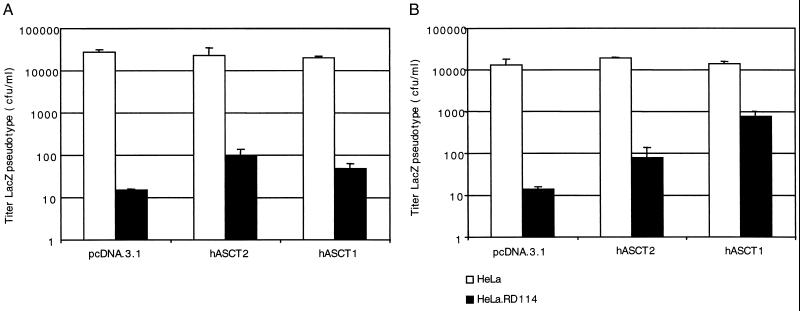
To test this hypothesis, we used two human cell lines, HeLa cells that contain hASCT2 but not hASCT1 (35) and HEK293T cells that contain small amounts of both hASCT2 and hASCT1 (24). As shown in Fig. 5, infection of HeLa cells with RD114 strongly interfered with superinfections of LacZ(RD114) and LacZ(BaEV), consistent with the presence in these cells of only hASCT2. In contrast, in HEK293T cells the interference was much stronger for LacZ(RD114) than for LacZ(BaEV), consistent with the presence of hASCT1 (Fig. 6). In addition, we transiently transfected these cell lines with hASCT1 and hASCT2 expression vectors. As indicated in Fig. 5 and 6, overexpression of hASCT2 significantly diminished but did not eliminate the interference caused by RD114. However, overexpression of hASCT1 selectively alleviated the interference with BaEV superinfections. These interferences were not completely eliminated, presumably because RD114 can weakly interact with hASCT1 (Fig. 2) and because only approximately 10 to 20% of the cells expressed the transiently transfected expression vectors. In other studies, we have found that expression of mASCT1 also causes a strong selective abrogation of RD114-mediated interference with BaEV superinfections (results not shown).
FIG. 5.
Studies of interference using hASCT2 and hASCT1 receptors. The assays were done using control HeLa cells or HeLa.RD114 productively infected with RD114 replication-competent virus. These cells were transiently transfected with expression vectors for the hASCT2 and hASCT1 receptors. Infections with the LacZ pseudotype viruses were initiated 24 h after the transfections were begun. The titers are averages of three independent experiments + standard errors. (A) Infections of the LacZ(RD114) virus: (B) infections of the LacZ(BaEV) virus.
FIG. 6.
Studies of interference using hASCT2 and hASCT1 receptors. The assays were done using control HEK293T cells or HEK293T.RD114 productively infected with RD114 replication-competent virus. The cells were transiently transfected with expression vectors for the hASCT2 and hASCT1 receptors. Infections with the LacZ pseudotype viruses were initiated 24 h after the transfections were begun. The titers are averages of three independent experiments + standard errors. (A) Infections of the LacZ(RD114) virus; (B) infections of the LacZ(BaEV) virus.
DISCUSSION
An important conclusion of this work is that members of the widely dispersed RD114/BaEV/HERV-W/primate type D/avian REV family of retroviruses have become adapted in some cases to promiscuous use of both ASCT1 and ASCT2 as receptors for cellular infections. These receptors, which are only approximately 57% identical in sequence, are differentially expressed in cells and are both Na+-dependent transporters for an overlapping but nonidentical set of neutral amino acids (1, 28, 32). For example, glutamine is transported by ASCT2 but not by ASCT1 (1, 5, 15, 36). Recent evidence also suggests that ASCT1 and ASCT2 function as exchangers to balance the pools of intracellular neutral amino acids rather than in the net flux of amino acids, and that they have an associated Cl− channel activity (5, 39). A previous study suggested that hASCT2 transport function was partially down-modulated by intracellular expression of the RD114 envelope glycoprotein, but the mechanisms were not investigated (28). In this context, it is interesting that glutamine can be a limiting nutrient for lymphocyte function (6, 16) and that primate type D retroviruses cause severe immunodeficiencies (9, 14, 22, 23). An important consequence of the promiscuous use of ASCT1 and ASCT2 by BaEV is its increased host range, as indicated previously (18, 29) and confirmed in this investigation. This promiscuity would very likely also broaden the cell and tissue tropism of the virus in infected animals.
Interestingly, ASCT1 is used with very different efficiencies by members of this interference group of retroviruses. Indeed, our studies suggest that human and mouse ASCT1 proteins are used approximately 100 times more efficiently by BaEV than by RD114 and are almost completely inactive in mediating infections of primate type D retroviruses (Fig. 2). In addition, mASCT1 appears to be reproducibly more active as a viral receptor than hASCT1. These differences could be used in chimera and mutagenesis studies to identify amino acids in the receptors and in the viral envelope glycoproteins that are important for infections. These results also raise the possibility that retroviruses that do not use ASCT2 and that have been classified in other interference groups might use ASCT1 or other members of this transporter superfamily exclusively as their receptors.
We emphasize that ASCT2 is the common receptor for this interference group of retroviruses and that ASCT1 functions as an auxiliary receptor for only certain members of the group. In accordance with the dominance of ASCT2 as a receptor, it is intriguing that its ECL2 sequence, which is critical for its receptor function (M. Marin, C. S. Tailor, and D. Kabat, unpublished data), is extremely hypervariable (Fig. 1), whereas the corresponding ECL2 region of ASCT1 has been much more highly conserved during mammalian evolution (Fig. 4). These results are consistent with the hypothesis that ECL2 of ASCT2 has been an exceptionally important focal point for virus-host coevolution in mammals. In this context, it also seems likely that viruses such as BaEV that have become adapted to promiscuous use of different receptors would be more resistant to fitness losses caused by mutations in a particular receptor. This may be one reason extant retroviruses have often evolved to utilize receptors that are members of multigene families (12).
We have also identified the mASCT2 protein by Western immunoblotting and have demonstrated that it contains two N-linked oligosaccharides in ECL2, one at N167 and the other at N230 (Fig. 3A). This strongly indicates that the presumptive ECL2 sequence is indeed on the extracellular surface. The N167 sequence NDS is notable because acidic residues in the X position of NX(S/T) consensus sequences were previously associated with inefficient glycosylation (19), which is not apparent in this case. In addition, immunofluorescence microscopy studies have indicated that the carboxyl-terminal myc tag epitope used for this project is cytosolic (results not shown), in agreement with our topological model (Fig. 1).
Similarly, our results strongly imply that N-linked oligosaccharides occur at positions N201 and N206 in the ECL2 region of mASCT1 (Fig. 3B). In this case, however, the two glycosylation sites are tightly clustered at positions that are highly conserved throughout mammalian evolution (Fig. 4). The glycosylations of these sites in mASCT1 may have been incomplete in the HEK293T cells, as suggested by the occurrence of approximately 60,000-Mr components in the cell extracts that were untreated with PNGase F (Fig. 3B). Moreover, we believe that there may be interference between these two closely situated N201 and N206 glycosylation sites, in the efficiencies of glycosylation and/or in the processing of the oligosaccharides, as implied by the apparent absence of doubly glycosylated larger components in the extracts that contained wild-type mASCT1. We have not determined whether similar patterns of N-linked glycosylation would also occur in other cells, such as CHO, that were used for our infectivity assays. However, it is notable that the structures of oligosaccharides and the efficiencies of N-linked glycosylations are often highly dependent on the cell type and on the local protein environment (27). Additional studies will be needed to investigate these issues.
The resistance of mouse and rat cells to RD114 and to primate type D retroviruses can be abrogated by treatments with tunicamycin (18) (Table 1). Based on previous investigations of related virus systems, it is clear that such effects of tunicamycin could have several causes (3, 10, 13, 20). One possibility is that N-linked glycosylation might directly mask a site on the receptor that is essential for infection (10). Evidently, this is not the case for mASCT2, which was inactive as a receptor even when its N-linked oligosaccharides were eliminated by mutagenesis. In contrast, our results support this interpretation in the case of mASCT1. As shown in Fig. 2B, removal of the mASCT1 glycosylation sites at N201 and/or N206 caused approximately 100-fold increases in the infectivities of RD114 and SRV-2 without significantly enhancing the infectivity of BaEV. Based on these results, we propose that N-linked glycosylations at N201 and N206 of mASCT1 can mask a site in ECL2 that is critical for interaction with RD114 and SRV-2 but is less important or irrelevant for BaEV. Considered together, our evidence suggests that host range control in this family of retroviruses is caused by evolutionary changes in ASCT2, by promiscuous use by some viruses of the related receptor ASCT1, and by N-linked glycosylation of a virus binding site in ASCT1.
ACKNOWLEDGMENTS
This research was supported by NIH grants CA25810 and CA83835 from the National Cancer Institute.
We are grateful to our colleagues Susan L. Kozak, Emily Platt, Navid Madani, Shawn Kuhmann, Vadivel Ganapathy, and Michael P. Kavanaugh for suggestions and encouragement.
REFERENCES
- 1.Arriza J L, Kavanaugh M P, Fairman W A, Wu Y N, Murdoch G H, North R A, Amara S G. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 2.Barbacid M, Hunter E, Aaronson S A. Avian reticuloendotheliosis viruses: evolutionary linkage with mammalian type C retroviruses. J Virol. 1979;30:508–514. doi: 10.1128/jvi.30.2.508-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassin R H, Ruscetti S, Ali I, Haapala D K, Rein A. Normal DBA/2 mouse cells synthesize a glycoprotein which interferes with MCF virus infection. Virology. 1982;123:139–151. doi: 10.1016/0042-6822(82)90301-4. [DOI] [PubMed] [Google Scholar]
- 4.Blond J L, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset F L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broer A, Wagner C, Lang F, Broer S. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem J. 2000;346:705–710. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang W K, Yang K D, Shaio M F. Effect of glutamine on Th1 and Th2 cytokine responses of human peripheral blood mononuclear cells. Clin Immunol. 1999;93:294–301. doi: 10.1006/clim.1999.4788. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 8.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel M D, King N W, Letvin N L, Hunt R D, Sehgal P K, Desrosiers R C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984;223:602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- 10.Eiden M V, Farrell K, Wilson C A. Glycosylation-dependent inactivation of the ecotropic murine leukemia virus receptor. J Virol. 1994;68:626–631. doi: 10.1128/jvi.68.2.626-631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautier R, Jiang A, Rousseau V, Dornburg R, Jaffredo T. Avian reticuloendotheliosis virus strain A and spleen necrosis virus do not infect human cells. J Virol. 2000;74:518–522. doi: 10.1128/jvi.74.1.518-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter E. Viral entry and receptors. In: Coffin J, Hughes S, Varmus H, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 13.Ikeda H, Sugimura H. Fv-4 resistance gene: a truncated endogenous murine leukemia virus with ecotropic interference properties. J Virol. 1989;63:5405–5412. doi: 10.1128/jvi.63.12.5405-5412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen E M, Zelljadt I, Chopra H C, Mason M M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970;30:2388–2393. [PubMed] [Google Scholar]
- 15.Kekuda R, Prasad P D, Fei Y J, Torres-Zamorano V, Sinha S, Yang-Feng T L, Leibach F H, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 16.Kew S, Wells S M, Yaqoob P, Wallace F A, Miles E A, Calder P C. Dietary glutamine enhances murine T-lymphocyte responsiveness. J Nutr. 1999;129:1524–1531. doi: 10.1093/jn/129.8.1524. [DOI] [PubMed] [Google Scholar]
- 17.Koo H M, Gu J, Varela-Echavarria A, Ron Y, Dougherty J P. Reticuloendotheliosis type C and primate type D oncoretroviruses are members of the same receptor interference group. J Virol. 1992;66:3448–3454. doi: 10.1128/jvi.66.6.3448-3454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo H M, Parthasarathi S, Ron Y, Dougherty J P. Pseudotyped REV/SRV retroviruses reveal restrictions to infection and host range within members of the same receptor interference group. Virology. 1994;205:345–351. doi: 10.1006/viro.1994.1651. [DOI] [PubMed] [Google Scholar]
- 19.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 20.Lyu M S, Nihrane A, Kozak C A. Receptor-mediated interference mechanism responsible for resistance to polytropic leukemia viruses in Mus castaneus. J Virol. 1999;73:3733–3736. doi: 10.1128/jvi.73.5.3733-3736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGregor G R, Mogg A E, Burke J F, Caskey C T. Histochemical staining of clonal mammalian cell lines expressing E. coli beta galactosidase indicates heterogeneous expression of the bacterial gene. Somat Cell Mol Genet. 1987;13:253–265. doi: 10.1007/BF01535207. [DOI] [PubMed] [Google Scholar]
- 22.Marx P A, Bryant M L, Osborn K G, Maul D H, Lerche N W, Lowenstine L J, Kluge J D, Zaiss C P, Henrickson R V, Shiigi S M, et al. Isolation of a new serotype of simian acquired immune deficiency syndrome type D retrovirus from Celebes black macaques (Macaca nigra) with immune deficiency and retroperitoneal fibromatosis. J Virol. 1985;56:571–578. doi: 10.1128/jvi.56.2.571-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marx P A, Maul D H, Osborn K G, Lerche N W, Moody P, Lowenstine L J, Henrickson R V, Arthur L O, Gilden R V, Gravell M, et al. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984;223:1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- 24.Matthews J C, Aslanian A M, McDonald K K, Yang W, Malandro M S, Novak D A, Kilberg M S. An expression system for mammalian amino acid transporters using a stably maintained episomal vector. Anal Biochem. 1997;254:208–214. doi: 10.1006/abio.1997.2432. [DOI] [PubMed] [Google Scholar]
- 25.Miller D G, Miller A D. Inhibitors of retrovirus infection are secreted by several hamster cell lines and are also present in hamster sera. J Virol. 1993;67:5346–5352. doi: 10.1128/jvi.67.9.5346-5352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D G, Miller A D. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J Virol. 1992;66:78–84. doi: 10.1128/jvi.66.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rademacher T W, Parekh R B, Dwek R A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 28.Rasko J E, Battini J L, Gottschalk R J, Mazo I, Miller A D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnitzer T J, Weiss R A, Zavada J. Pseudotypes of vesicular stomatitis virus with the envelope properties of mammalian and primate retroviruses. J Virol. 1977;23:449–454. doi: 10.1128/jvi.23.3.449-454.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slotboom D J, Konings W N, Lolkema J S. Structural features of the glutamate transporter family. Microbiol Mol Biol Rev. 1999;63:293–307. doi: 10.1128/mmbr.63.2.293-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 32.Tailor C S, Nouri A, Zhao Y, Takeuchi Y, Kabat D. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J Virol. 1999;73:4470–4474. doi: 10.1128/jvi.73.5.4470-4474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi Y, Cosset F L, Lachmann P J, Okada H, Weiss R A, Collins M K. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol. 1994;68:8001–8007. doi: 10.1128/jvi.68.12.8001-8007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talbot S J, Weiss R A, Schulz T F. Reduced glycosylation of human cell lines increases susceptibility to CD4-independent infection by human immunodeficiency virus type 2 (LAV-2/B) J Virol. 1995;69:3399–3406. doi: 10.1128/jvi.69.6.3399-3406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamarappoo B K, McDonald K K, Kilberg M S. Expressed human hippocampal ASCT1 amino acid transporter exhibits a pH-dependent change in substrate specificity. Biochim Biophys Acta. 1996;1279:131–136. doi: 10.1016/0005-2736(95)00259-6. [DOI] [PubMed] [Google Scholar]
- 36.Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 37.van der Kuyl A C, Dekker J T, Goudsmit J. Discovery of a new endogenous type C retrovirus (FcEV) in cats: evidence for RD-114 being an FcEV(Gag-Pol)/baboon endogenous virus BaEV(Env) recombinant. J Virol. 1999;73:7994–8002. doi: 10.1128/jvi.73.10.7994-8002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Dechant E, Kavanaugh M, North R A, Kabat D. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J Biol Chem. 1992;267:23617–23624. [PubMed] [Google Scholar]
- 39.Zerangue N, Kavanaugh M P. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J Biol Chem. 1996;271:27991–27994. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]