Abstract
We have constructed a chimeric yellow fever/dengue (YF/DEN) virus, which expresses the premembrane (prM) and envelope (E) genes from DEN type 2 (DEN-2) virus in a YF virus (YFV-17D) genetic background. Immunization of BALB/c mice with this chimeric virus induced a CD8 T-cell response specific for the DEN-2 virus prM and E proteins. This response protected YF/DEN virus-immunized mice against lethal dengue encephalitis. Control mice immunized with the parental YFV-17D were not protected against DEN-2 virus challenge, indicating that protection was mediated by the DEN-2 virus prM- and E-specific immune responses. YF/DEN vaccine-primed CD8 T cells expanded and were efficiently recruited into the central nervous systems of DEN-2 virus challenged mice. At 5 days after challenge, 3 to 4% of CD8 T cells in the spleen were specific for the prM and E proteins, and 34% of CD8 T cells in the central nervous system recognized these proteins. Depletion of either CD4 or CD8 T cells, or both, strongly reduced the protective efficacy of the YF/DEN virus, stressing the key role of the antiviral T-cell response.
Dengue (DEN) virus infection is a global public health problem, with recurring epidemics in tropical and subtropical regions of Asia, Africa, and the Americas (15). It is estimated that 100 million cases of dengue fever and 250,000 cases of the more severe dengue hemorrhagic fever occur annually on a worldwide basis (24). The incidence of dengue has increased dramatically during the past 30 years, and a vaccine is urgently needed.
Dengue virus is a member of the genus Flavivirus of the family Flaviviridae. This family comprises more than 70 RNA viruses, many of which are important human pathogens (in addition to DEN virus, the genus includes such pathogens as yellow fever virus [YFV], Japanese encephalitis virus [JEV], and tick-borne encephalitis [TBE] virus). There are four serotypes of DEN virus, and all four are human pathogens. The clinical outcomes of DEN virus infection can vary from asymptomatic infection to mild febrile dengue fever to severe and life-threatening dengue hemorrhagic fever (DHF)-dengue shock syndrome. The pathogenesis of DHF is not well understood, but a striking feature is that most cases of DHF occur in individuals who have previously been infected with a heterologous serotype (8, 15). The concept of antibody-dependent enhancement of DEN virus infection of monocytes was proposed to explain this condition (8, 11). In support of this hypothesis, it was shown that DEN viruses replicate to higher titers in human monocytes in the presence of cross-reactive, nonneutralizing antibodies in vitro (8, 9, 11, 12). It is believed that this is due to more efficient infection of monocytes by virus-antibody complexes binding to the Fcγ receptors present on these cells (8, 11, 12). Although the antibody-dependent enhancement phenomenon is well documented in vitro, its importance in vivo remains to be determined. Nevertheless, the potential role of cross-reactive, nonneutralizing antibodies in DHF has obvious implications for vaccine design: any effective dengue vaccine should induce neutralizing antibodies and/or T-cell immunity against all four serotypes.
The paradigm for flavivirus vaccines is the YFV-17D vaccine strain (28). The YFV-17D vaccine strain is one of the most effective and safest vaccines available and induces long-lasting immunity (1, 21). These properties make the 17D virus very attractive as a live carrier vaccine. The potential of YFV-17D as a vaccine vector for other flaviviruses was highlighted by the observation that the premembrane (prM) and envelope (E) proteins are interchangeable among different flaviviruses. This was first demonstrated by Pletnev and coworkers, who showed that a chimeric virus containing the prM and E genes from TBE virus (19) or Langat virus (20) and the capsid and nonstructural genes from DEN type 4 (DEN-4) virus grew to high titers in tissue culture and protected mice against lethal TBE or Langat virus challenge. More recently, it was shown by Chambers and colleagues that similar chimeras could be made based on the YFV-17D genome (4). Chimeric viruses that carried the prM and E genes from JEV in a YFV-17D genetic background induced JEV-specific neutralizing antibodies and provided excellent protection against lethal JEV challenge in mice (6) and in rhesus monkeys (16, 17). Here, we have used the same approach to construct chimeric DEN-2/YF viruses. We found that chimeric viruses expressing the DEN-2 prM and E genes in a YFV-17D background were immunogenic and induced solid protection against lethal DEN virus challenge in BALB/c mice. The chimeric virus induced a DEN-2 prM- and E-specific CD8 T-cell response and neutralizing antibodies. We found that the prM- and E-specific CD8 T-cell response was predominantly directed against an Ld-restricted epitope in the E protein (25). The population of prM- and E-specific CD8 T cells expanded after the challenge, indicating that vaccine-primed T cells are efficiently recruited by the challenge virus.
MATERIALS AND METHODS
Mice.
Three-week-old female BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, Main).
Peptides.
The E protein residues 331 to 339 (SPCKIPFEI; E331) were synthesized at the Biopolymer Synthesis and Analysis Facility at the California Institute of Technology. The composition of the peptide was ascertained by mass spectrometry and high-performance liquid chromatography HPLC analysis.
Cells and virus.
BHK-21, SW-13, and Vero cells were propagated in minimal essential medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, antibiotics, and nonessential amino acids. Virus titers were determined on BHK-21 cells (YFV-17D and the chimeric YF/DEN virus) or Vero cells (DEN virus), and virus stocks (YFV-17D and YF/DEN) were grown in SW-13 cells. A20 cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics.
Construction of plasmids.
A chimeric YF/DEN-2 virus genome was constructed using fusion PCR, following a protocol described by Yao et al. (31). The proofreading enzyme Pfu polymerase (Stratagene) was used for all amplifications. The oligonucleotide primer sequences are shown in Table 1. The prM and E sequences from DEN-2 virus strain PR-159 S1 were amplified from cDNA clone C8 (7) using primers GB7 and GB8. The GB7 and -8 PCR product was fused to a 5′-terminal YFV-17D fragment amplified from pYF5′3′IV (23) with primers GB5 and GB6. The fusion product was amplified with GB5 and GB8 and cloned into NheI-NsiI-cut pYF5′3′IV, yielding pYD5′3′. The chimeric pYFM5.2/DEN plasmid (pYDM) was made by amplifying the DEN virus E protein cDNA with primers GB1 and GB2 and amplifying a 3′-terminal YFV DNA with primers GB3 and GB4. The fusion DNA was amplified with primers GB1 and GB4 and cloned into NsiI-MluI-digested pYFM5.2, yielding pYDM. The sequences of all PCR-derived DNA fragments were verified by automated sequence analysis.
TABLE 1.
Oligonucleotides used in PCR mutagenesis
| Oligonucleotide | Sequence |
|---|---|
| GB1 | GCTGCCCGGAGCAGACAC |
| GB3 | TTATGGTGCAGGCCGATCAAGGATGCGC |
| GB4 | CCGTTCACCGCTGCACCC |
| GB5 | CCTGTCGGGTTTCGCCAC |
| GB6 | GTGTGGTCAGATGAAATCCACCCGTCATCAA |
| GB7 | TTGATGACGGGTGGATTTCATCTGACCACAC |
| GB8 | GTCCATTCTCAGCCTGCAC |
| GB9 | GTCCGGCCTACTCAATGCGCTGCATAGG |
| GB10 | CCTATGCAGCGCATTGAGTAGGCCGGAC |
Transcription and transfection.
In vitro ligation of the pYF5′3′IV and pYFM5.2 plasmids and the chimeric plasmids (pYD5′3′ and pYDM) was done as described previously using NsiI- and AatII-digested DNA fragments (Boehringer Mannheim) (23). Transcription of in vitro-ligated YFV and YF/DEN virus cDNAs was done according to established protocols. RNA transcripts were transfected into SW-13 cells using Lipofectin (Gibco), as described previously (5). Stocks of YFV-17D and YF/DEN virus were obtained by infecting fresh monolayers of SW-13 cells with the supernatants from transfected cells.
Immunoprecipitation.
SW-13 cells were infected with YFV-17D or with the chimeric YF/DEN virus and were metabolically labeled using Express-35S protein-labeling mix (NEN) for 2 h at 16 h postinfection. Viral proteins were immunoprecipitated with hyperimmune sera against YFV-17D (American Type Culture Collection) or DEN-2 virus (American Type Culture Collection) or with a polyclonal antiserum against the YFV-17D E protein (29). Cell lysis and immunoprecipitations were done according to standard protocols. Protein samples were analyzed on 12% polyacrylamide gels (Bio-Rad).
Immunization and challenge of mice.
Three-week-old BALB/c mice were immunized by subcutaneous (s.c.) injection of 5 × 105 PFU of YFV-17D or chimeric YF/DEN virus or were mock immunized with phosphate-buffered saline (PBS). The mice were challenged 2 weeks later by intracerebral (i.c.) injection of 1.5 × 104 PFU (100 50% lethal doses [LD50]) of mouse-adapted, neurovirulent DEN-2 virus, strain New Guinea C (NGC) (provided by Kenneth Eckels, Walter Reed Army Institute for Research, Rockville, Md.) (10, 26). The LD50 of the DEN-2 NGC stock was determined by the method described by Reed and Muench (22).
Depletion of CD4 and CD8 T cells.
To deplete T-cell subsets, immunized mice were injected intraperitoneally with monoclonal antibodies (MAbs) against CD4 (GK1.5) or CD8 (Lyt 2.43). CD8 T cells were depleted by injecting 0.5 ml (0.5 mg) of Lyt 2.43 MAb 2 days before challenge. CD4 T cells were depleted by injecting 0.5 ml (0.5 mg) of GK1.5 MAb 2 days before challenge and on the day of challenge. Depletion of CD4 and/or CD8 T cells was monitored 3 days after challenge by staining peripheral blood mononuclear cells obtained by retro-orbital bleeding with fluorescein isothiocyanate-conjugated anti-CD4 (clone GK1.5) and R-phycoerythrin-conjugated anti-CD8 MAbs (clone 53-6.7) (Pharmingen) and analyzing the cells by flow cytometry. Both regimens resulted in >99% depletion of the relevant subset.
Isolation of lymphocytes from mouse brain.
Isolation of lymphocytes from the brains of naive or DEN-2 virus-infected mice was done as described previously (14). Briefly, mouse brains were homogenized using a Tenbroeck homogenizer and lymphocytes were isolated by centrifugation over a Percoll cushion.
Intracellular cytokine staining.
A20 stimulator cells were infected with a recombinant vaccinia virus expressing the DEN-2 virus prM and E proteins (provided by Ching-Juh Lai, National Institutes of Health, Bethesda, Md.) (3) or the NS3 protein (provided by Margo Brinton, Georgia State University) (32) at a multiplicity of infection of 1 and were used at 8 h postinfection. A20 cells (2 × 105), either vaccinia virus infected, uninfected, or peptide coated (E331-339; 0.1 μg/ml) were incubated with 8 × 105 splenocytes for 6 h in the presence of brefeldin A (PharMingen). The cells were first surface stained with fluorescein isothiocyanate-conjugated anti-CD4 (clone GK1.5) and R-phycoerythrin-conjugated anti-CD8 (clone 53-6.7) MAbs (PharMingen). Following fixation and permeabilization (Cytofix/Cytoperm kit; PharMingen), intracellular cytokine staining was done using an allophycocyanin-conjugated MAb against gamma interferon (IFN-γ) (clone XMG1.2; PharMingen). Samples were analyzed with a Becton-Dickinson FACScalibur flow cytometer.
Plaque reduction neutralization assay.
Sera from immunized or challenged mice were obtained by retro-orbital bleeding. After inactivation (30 min; 56°C), the sera were diluted in minimal essential medium containing 0.25% bovine serum albumin and guinea pig serum complement and were incubated with 50 to 100 PFU of DEN-2 virus (DEN-2 strain 16681; provided to us by Robert Putnak, Walter Reed Army Institute for Research) for 30 min at 37°C. Fifty percent plaque reduction neutralization titers (PRNT50), defined as the reciprocal of the dilution in which the number of plaques is reduced by 50% compared with the controls, were determined by plaque assay on Vero cells.
RESULTS
Construction and characterization of chimeric YF/DEN viruses.
A chimeric flavivirus (YF/DEN) was constructed in which the prM and E genes from YFV-17D were replaced by their DEN-2 virus counterparts (Fig. 1A). The DEN-2 virus prM and E genes were derived from the PR-159 S1 candidate vaccine strain (7). We used the YFV-17D cDNA clones, from which infectious RNA can be produced (23), to construct the chimeric YF/DEN virus genome. Full-length YFV-17D and YF/DEN virus RNAs were transcribed from the in vitro-ligated YFV-17D and YF/DEN virus plasmids, respectively, and were transfected into SW-13 cells. Virus viability was assessed by plaque titration of progeny virus and by immunofluorescence analysis of the transfected cells (data not shown). On average, titers of the chimeric YF/DEN virus were threefold lower than the titer of the parental YF-17D virus (1.1 × 107 versus 3.7 × 107 PFU/ml for YF/DEN virus and YFV-17D, respectively).
FIG. 1.
(A) Schematic representation of the genome organization of the chimeric YF/DEN virus. The DEN-2 prM and E genes are indicated by a cross-hatched bar, and YFV-17D genes are indicated by open bars. (B) Expression of DEN-2 prM and E genes. SW-13 cells were infected with YFV-17D or with the chimeric YF/DEN virus and were metabolically labeled at 16 h postinfection. The radiolabeled proteins were immunoprecipitated and analyzed on a 12% polyacrylamide gel. Positions of radiolabeled molecular size markers (in kilodaltons) are on the right.
To demonstrate that the DEN-2 virus prM and E genes were correctly expressed in the YFV-17D background, SW-13 cells were infected with the chimeric YF/DEN virus or with YFV-17D and were metabolically labeled for 2 h at 16 h postinfection. Radiolabeled viral proteins were immunoprecipitated from infected-cell lysates using antisera against YFV-17D, DEN-2 virus, and the YFV-17D E protein (Fig. 1B). The results clearly show that the chimeric YF/DEN virus expresses the DEN-2 virus prM and E proteins instead of the YFV-17D prM and E proteins.
Immunogenicity of the chimeric YF/DEN virus.
To evaluate the immunogenicity of the chimeric virus, 3-week-old BALB/c mice were immunized by s.c. injection of 5 × 105 PFU of YF/DEN virus. Control mice were immunized with 5 × 105 PFU of YFV-17D or with PBS. We analyzed both T-cell responses and neutralizing-antibody titers following immunization. DEN-2 virus-specific T-cell responses were measured using an intracellular IFN-γ staining assay in which T cells that produce IFN-γ in response to antigen are quantitated by flow cytometry. In this assay, splenocytes are incubated with antigen in the presence of the secretion inhibitor brefeldin A to allow detection of intracellular cytokine. To provide the antigenic stimulus, we used either BALB/c syngeneic B cells (A20 cells) infected with a recombinant vaccinia virus expressing the DEN-2 virus prM and E proteins or A20 cells coated with a peptide encompassing an Ld-restricted epitope in the E protein (residues 331 to 339; SPCKIPFEI [25]).
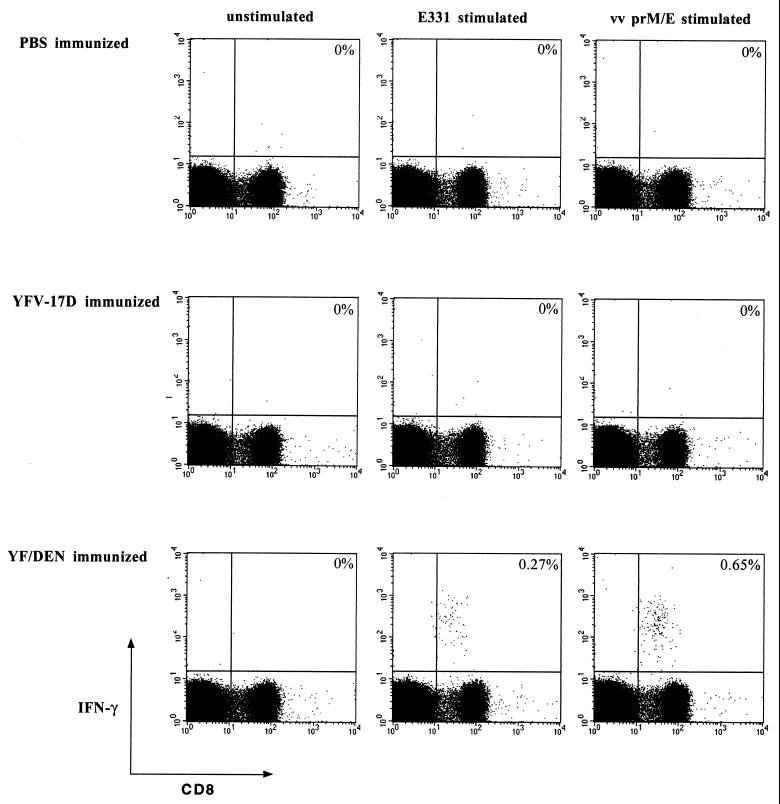
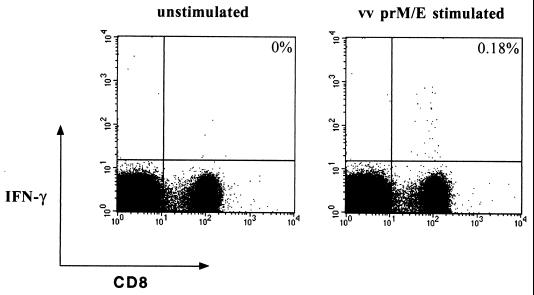
Splenocytes from immunized and control mice were harvested 14 days postimmunization and were mixed with recombinant vaccinia virus-infected or peptide-coated (E331-339) A20 cells. As a control, we used uninfected A20 cells. After 6 h of stimulation, the spleen cells were surface stained with MAbs against CD4 and CD8 and were then permeabilized to visualize the production of IFN-γ in response to antigen. Analysis by flow cytometry clearly demonstrated that immunization with the chimeric virus induced a prM- and E-specific CD8 T-cell response. We found that 1 of 200 CD8 T cells recognized prM- and E at 14 days postimmunization (3.0 × 104 specific cells per spleen) (Fig. 2). Of these T cells, approximately 50% were specific for the E331-339 epitope (Fig. 2). No DEN-2 prM and E responses were detected in mice immunized with PBS or with YFV-17D (Fig. 2). To obtain an estimate of the size of the prM- and E-specific long-term memory pool, we also analyzed the DEN-2 prM- and E-specific responses at 100 days postinfection. We found that prM- and E-specific memory CD8 T cells were readily detectable at a frequency of 1/700 CD8 T cells (6.8 × 103 per spleen) 100 days after vaccination (Fig. 3).
FIG. 2.
PrM- and E-specific and E331-epitope-specific CD8 T-cell responses in YF/DEN virus-immunized mice. Mice were immunized by s.c. inoculation with 5 × 105 PFU of YF/DEN virus or YFV-17D or were mock immunized with PBS, and the frequencies of prM- and E-specific CD8 T cells were measured 14 days postimmunization by intracellular IFN-γ staining. The frequencies of specific cells are shown as percentages of total CD8 T cells. The results shown are from individual mice and differ slightly from the average frequencies presented in the text. vv, vaccinia virus.
FIG. 3.
Long-term prM- and E-specific immunity. Mice were immunized by s.c. inoculation with 5 × 105 PFU of YF/DEN virus, and the frequencies of prM- and E-specific CD8 T cells were measured 100 days postimmunization by intracellular IFN-γ staining. The frequencies of specific cells are shown as percentages of total CD8 T cells. The results shown are from individual mice and differ slightly from the average frequencies presented in the text. vv, vaccinia virus.
Antibody responses were measured by titrating DEN-2-neutralizing antibodies in the serum at 2 and 12 weeks after immunization. Neutralizing-antibody titers are expressed as 50% plaque reduction neutralization titers. Although we could not detect neutralizing antibodies in any of the immunized mice 2 weeks after immunization, we did observe antibody titers 10 weeks later (PRNT50 = 19). YF/DEN virus-immunized mice that received a YF/DEN virus booster immunization (5 × 105 PFU, s.c., 12 days after priming) had high titers of neutralizing antibodies against DEN-2 virus (PRNT50 = 422, 34 days after the booster immunization). No neutralizing antibodies directed against DEN-2 virus could be detected in PBS- or YFV-17D-immunized mice at any time.
Challenge of immunized mice.
The efficacy of the YF/DEN vaccine was evaluated by analyzing its capacity to induce protective immunity against lethal challenge with DEN-2 virus. To test this, 3-week-old BALB/c mice were immunized with YF/DEN virus. Control mice were immunized with YFV-17D (5 × 105 PFU, s.c.) or with PBS. YF/DEN virus-immunized and control mice were challenged by intracerebral (i.c.) injection of 1.5 × 104 PFU (100 LD50) of DEN-2 virus 2 weeks later. As shown in Fig. 4, immunization with the chimeric YF/DEN virus induced solid protection against the DEN-2 virus challenge: all of the mice survived the infection without showing signs of disease. Survival after DEN-2 virus challenge was not due to slower kinetics, since all these mice survived for more than 6 months after the challenge. The few surviving mice in the control groups (Fig. 4) displayed clear signs of malaise, and their average weights at day 12 postchallenge were lower than those of the YF/DEN virus-immunized mice (14.2 [n = 4] versus 17.8 g [n = 7]).
FIG. 4.
Survival of YF/DEN virus-immunized and control mice after DEN-2 virus challenge.
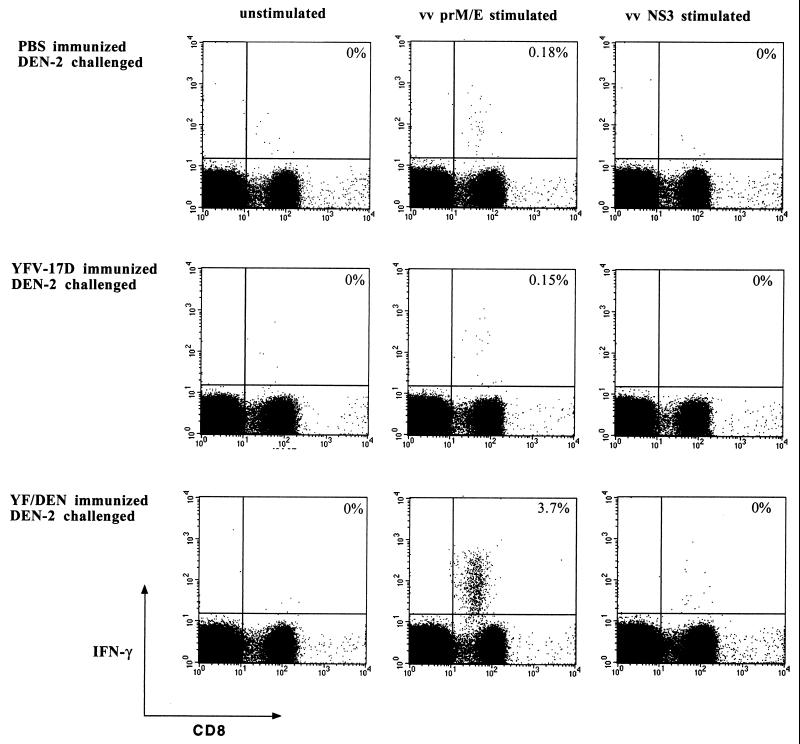
The role of vaccine-primed immune responses in mediating protective immunity was assessed by quantitating T-cell and antibody responses after the DEN-2 virus challenge. Analysis of prM- and E-specific T-cell responses after the DEN-2 challenge revealed a strong, prM- and E-specific CD8 T-cell response in the spleens and brains of YF/DEN virus-immunized and DEN-2 virus-challenged mice. At day 5 after challenge, prM- and E-specific CD8 T cells (1 of 30 CD8 T cells; 2.2 × 105 per spleen) were readily detected in the spleens of these mice (Fig. 5). We found no responses after stimulation of the spleen cells with A20 cells infected with a vaccinia virus recombinant expressing the DEN-2 virus NS3 protein (Fig. 5). The DEN-2 virus NS3 protein was not encoded by the chimeric YF/DEN virus used for immunization, and potential NS3-specific responses after the challenge would therefore represent primary responses. Thus, the absence of DEN-2 virus NS3-specific responses after the challenge suggests that the vaccine-primed (secondary) prM- and E-specific T-cell response was dominant. This experiment (i.e., using the vaccinia virus recombinant expressing NS3) also serves as a control for the prM and E specificity of the response, since it clearly shows that we do not observe responses against vaccinia virus proteins.
FIG. 5.
PrM- and E-specific CD8 T-cell responses in the spleens of DEN-2 virus-challenged mice. The results shown are from individual mice and differ slightly from the average frequencies presented in the text. YF/DEN virus-immunized and control mice were challenged by i.c. inoculation with 1.5 × 104 PFU of DEN-2 virus. The frequencies of prM- and E-specific CD8 T cells in the spleen and in the brain were measured 5 days postchallenge by intracellular IFN-γ staining. The frequencies of specific cells are shown as percentages of total CD8 T cells. vv, vaccinia virus.
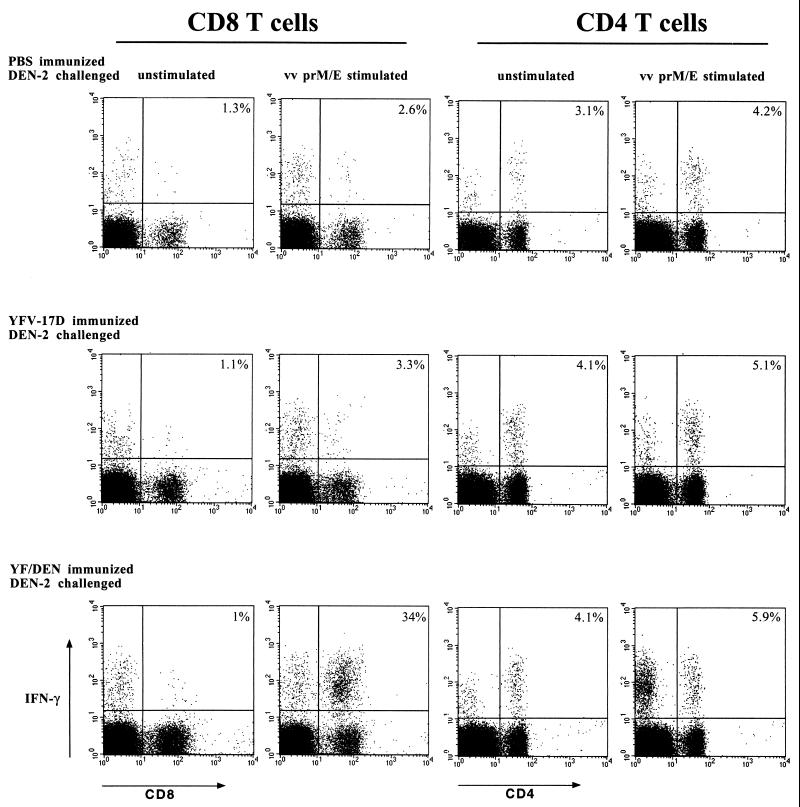
We found that the prM- and E-specific CD8 T cells efficiently infiltrated the central nervous system (CNS): at day 5 after challenge, one of three CD8 T cells in the brain recognized prM and E (Fig. 6, left panel). PrM- and E-specific responses in the challenged control mice (i.e., YFV-17D- or PBS-immunized) were detectable, both in the spleens and in the brains, but were very weak (Fig. 6, left panel). Our data also revealed a CD4 T-cell response in the brains of DEN-2 virus-infected mice (Fig. 6, right panel). The A20 stimulator cells express both major histocompatibility complex class I and II molecules and could therefore be expected to stimulate both CD4 and CD8 T cells. Puzzlingly, however, this response was measured after stimulation with both infected and uninfected A20 cells. There was a small increase after stimulation with the infected cells (Fig. 6, right panel). The most likely explanation is that viral antigen is copurified during the isolation of brain lymphocytes and that this antigen is capable of stimulating the CD4 T cells. Viral antigen could be endocytosed by the A20 cells (or other antigen-presenting cells) and presented through the major histocompatibility complex class II pathway. Adding even more antigen (in the form of vaccinia virus-infected A20 cells) would then result in only a minor increase. Note that CD4 T-cell responses are measured in all mice, even in YFV-17D- or PBS-immunized animals, suggesting that these are primary responses induced by the DEN-2 virus infection in the CNS.
FIG. 6.
CD4 and CD8 T-cell responses in the brains of DEN-2 virus-challenged mice. The results shown are from individual mice and differ slightly from the average frequencies presented in the text. YF/DEN virus-immunized and control mice were challenged by i.c. inoculation of 1.5 × 104 PFU of DEN-2 virus. The frequencies of responding CD4 and CD8 T cells in the brains of the challenged mice were measured 5 days postchallenge by intracellular IFN-γ staining. The frequencies of responding cells are shown as percentages of total CD4 or CD8 T cells. vv, vaccinia virus.
The neutralizing-antibody titers were similar in the challenged mice 8 days after infection, regardless of the initial immunization protocol (PRNT50 = 23, 30, and 33 for PBS-, YFV-17D, and YF/DEN virus-immunized mice, respectively). Thus, these antibodies appear to be induced by the DEN-2 virus challenge, and the role of the YF/DEN vaccine in inducing these antibodies is unclear. In mice that survived the DEN-2 virus challenge (after YF/DEN virus immunization), we found that the neutralizing-antibody titer had increased by 56 days postchallenge (PRNT50 = 77).
Requirements of protective immunity.
To address the roles of CD4 and CD8 T cells in providing protective immunity against DEN-2 virus challenge, we depleted CD4 and/or CD8 T cells in YF/DEN virus-immunized mice at 12 days after immunization by intraperitoneal injection of anti-CD4 and/or anti-CD8 MAbs. The antibody treatments resulted in >99% depletion of the relevant subset(s) (data not shown). Mice in which CD4 T cells, CD8 T cells, or both CD4 and CD8 T cells had been depleted were challenged with DEN-2 virus 2 days later. As positive and negative controls, we used untreated YF/DEN virus- and PBS-immunized mice, respectively. As shown in Fig. 7, in vivo depletion of either T cell subset (or both) abrogated protection against lethal DEN-2 virus challenge. The impact of CD4 T-cell depletion appears to be less than that of depletion of CD8 T cells or depletion of both subsets. These results demonstrate that both CD4 and CD8 T cells are required to provide protective immunity against lethal DEN-2 virus challenge in the CNS.
FIG. 7.
Survival of DEN-2 virus-challenged mice after immunization with YF/DEN virus, followed by depletion of CD4 and/or CD8 T cells. The control groups included PBS- and YF/DEN virus-immunized mice which were not treated with the depleting antibodies. The surviving mice survived even beyond the latest time point.
DISCUSSION
In this study we describe a chimeric YF/DEN virus that induces both neutralizing antibodies and an antiviral CD8 T-cell response against the DEN-2 virus prM and E proteins in BALB/c mice. We found that a single Ld-restricted epitope in the E protein (residues 331 to 339; SPCKIPFEI) (25) represents a major target of this CD8 T-cell response. The frequencies of DEN virus-specific T cells that we have measured are higher than has previously been appreciated. At 5 days postchallenge, 1 of 30 CD8 T cells in the spleen and 1 of 3 in the CNS are specific for the viral prM and E proteins, and most of this response is directed against the E331-339 epitope. Immunization with the chimeric virus provided solid protection against lethal DEN virus challenge. In contrast, mice immunized with the parental YFV-17D were not protected, demonstrating that putative flavivirus cross-reactive responses do not play a significant role in protection. The DEN-2 virus challenge induced a strong prM- and E-specific CD8 T-cell response only in the YF/DEN virus-immunized mice, indicating that vaccine-primed prM and E T cells are recruited efficiently. These prM- and E-specific CD8 T cells efficiently infiltrated the CNS. This recruitment of vaccine-primed CD8 T cells after challenge in YF/DEN virus-immunized mice indicates that these cells may play a major role in controlling DEN-2 virus infection. The in vivo depletion experiments confirmed the key role of CD8 T cells and also demonstrated that the CD4 T-cell response is important: depletion of either subset reduced protective immunity. A requirement for both CD4 and CD8 T cells to control infections in the CNS has also been shown for JEV (18) and mouse hepatitis virus (27).
The strategy of creating chimeric flaviviruses has also yielded promising results in the case of YFV/JEV chimeras (6, 17), DEN-4/TBE virus chimeras (19, 20), and intertypic DEN virus chimeras (2). In these previous reports, the two parameters analyzed were induction of neutralizing antibodies and protective immunity. In the present study, we not only demonstrate that the chimeric virus provides excellent protective immunity, but we also show the frequencies and epitope specificity of the CD8 T cells that are induced by the vaccine.
Immunization with our chimeric YF/DEN virus also induced neutralizing antibodies, albeit at low titers. DEN-2 virus-specific neutralizing-antibody titers were below the detection limit at 2 weeks postimmunization but could be detected at 12 weeks after immunization or after a booster immunization. Thus, the chimeric virus was clearly immunogenic in terms of antibody responses. The weak antibody response after the first immunization is reminiscent of findings reported by Guirakhoo et al. (6). These authors showed that YFV-neutralizing antibodies were induced by YFV-17D only when mice had been immunized with low doses of virus. At higher doses (>25,000 PFU), neutralizing antibody titers decreased dramatically. Since we used an even higher dose of virus for immunization (5 × 105 PFU), it seems possible that we are observing the same phenomenon. The experience with the YFV-17D vaccine, which induces strong and long-lasting antibody responses in humans (21) but weaker responses in mice (6), suggests that our chimeric virus may also be more immunogenic in humans than in mice.
Induction of neutralizing antibodies in humans will be a highly desirable feature of a DEN virus vaccine, given the association between cross-reactive, nonneutralizing antibodies and DHF. To avoid a situation in which the vaccine induces protective immunity against one DEN virus serotype but primes the immunized individual for DHF after infection by a different serotype, it will be important that a candidate DEN virus vaccine induce neutralizing antibodies and T-cell responses against all four serotypes simultaneously. Using our strategy, this can be accomplished by constructing chimeras for all four serotypes and using these to create a tetravalent vaccine. Such a tetravalent vaccine would consist of a cocktail of the four chimeric viruses. The advantage of our approach, compared to using a cocktail of attenuated DEN viruses, is that the genetic backbone (i.e., YFV-17D) will be the same for all four serotypes. Although the chimeric YF/DEN viruses are likely to be attenuated (because the DEN virus- and YFV-derived proteins are not optimally adapted to each other), the precise level of attenuation will depend on the source of the DEN virus prM and E proteins, which will give us a wide choice in modifying the level of attenuation. The recent discovery that development of DHF is strain specific (30) and may be correlated with specific amino acid sequences in the E protein (13) is therefore of critical importance for vaccine design, since it identifies the sequences that should probably be avoided.
In summary, both our results and those reported recently for JEV (6, 16, 17) indicate that the strategy of creating chimeric flaviviruses opens new avenues for flavivirus vaccine development. A particularly promising observation is that the chimeric YFV/JEV virus appears to be safer than the YFV-17D vaccine in nonhuman primates (16).
ACKNOWLEDGMENTS
We thank Charles M. Rice for providing the YFV-17D plasmids, Kenneth H. Eckels for providing the DEN-2 NGC virus, J. Robert Putnak for providing the reagents for neutralizing plaque assays, and Ching-Juh Lai and Margo Brinton for providing the prM- and E- and NS3-expressing vaccinia virus recombinants, respectively. We also thank Thomas P. Monath, Stephen A. Stohlman, Conni C. Bergmann, Jeroen Corver, and Laurie E. Harrington for stimulating discussions and Edith Lenches for technical assistance.
This work was supported by NIH grants AI20612 to J.H.S. and R21AI44724 to R.A.
ADDENDUM IN PROOF
Recently, Guirakhoo and coworkers published an article (F. Guirakhoo, R. Weltzin, T. J. Chambers, Z.-X. Zhang, K. Soike, M. Ratterree, J. Arroyo, K. Georgakopoulos, J. Catalan, and T. P. Monath, J. Virol. 74:5477–5485, 2000) that showed that a chimeric YF/DEN-2 virus is immunogenic and provides protective immunity in nonhuman primates. We believe that their data complement our results and that the combined data emphasize the potential of chimeric YF/DEN viruses as vaccine candidates.
REFERENCES
- 1.Barrett A. Yellow fever vaccines. Biologicals. 1997;25:17–25. doi: 10.1006/biol.1997.0056. [DOI] [PubMed] [Google Scholar]
- 2.Bray M, Lai C-J. Construction of intertypic chimeric dengue viruses by substitution of structural protein genes. Proc Natl Acad Sci USA. 1991;88:10342–10346. doi: 10.1073/pnas.88.22.10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Lai C J. Dengue virus premembrane and membrane proteins elicit a protective immune response. Virology. 1991;185:505–508. doi: 10.1016/0042-6822(91)90809-p. [DOI] [PubMed] [Google Scholar]
- 4.Chambers T J, Nestorowicz A, Mason P W, Eckels K H, Rice C M. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol. 1999;73:3095–3101. doi: 10.1128/jvi.73.4.3095-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grakoui A R, Levis R, Raju R, Huang H V, Rice C M. A cis-acting mutation in the Sindbis virus junction region which affects subgenomic RNA synthesis. J Virol. 1989;63:5216–5227. doi: 10.1128/jvi.63.12.5216-5227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guirakhoo F, Zhang Z-X, Chamber T J, Delagrave S, Arroyo J, Barrett A D T, Monath T P. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 1999;257:363–372. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- 7.Hahn Y S, Galler R, Hunkapiller T, Dalrymple J M, Strauss J H, Strauss E G. Nucleotide sequence of Dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988;162:167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- 8.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 9.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage, a pathogenic cascade. Rev Infect Dis. 1989;11:S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman B M, Summers P L, Dubois D R, Eckels K H. Monoclonal antibodies against dengue-2 virus E-glycoprotein protect mice against lethal dengue virus infection. Am J Trop Med Hyg. 1987;36:427–434. doi: 10.4269/ajtmh.1987.36.427. [DOI] [PubMed] [Google Scholar]
- 11.Kliks S. Antibody-enhanced infection of monocytes as the pathogenic mechanism for severe dengue illness. AIDS Res Hum Retrovir. 1990;6:993–998. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 12.Kliks S C, Nisalak A, Brandt W E, Wahl L, Burke D S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 13.Leitmeyer K C, Vaughn D W, Watts D M, Salas R, Villalobos de Chacon I, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marten N W, Stohlman S A, Smith-Begolka W, Miller S D, Dimicali E, Yao Q, Stohl S, Goverman J, Bergmann C C. Selection of CD8 T cells with highly focused specificity during viral persistence in the central nervous system. J Immunol. 1999;162:3905–3914. [PubMed] [Google Scholar]
- 15.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Raven Publishers; 1996. pp. 961–1024. [Google Scholar]
- 16.Monath T P, Levenbrook I, Soike K, Zhang Z-X, Ratterree M, Draper K, Barrett A D T, Nickols R, Weltzin R, Arroyo J, Guirakhoo F. Chimeric yellow fever virus 17D-Japanese encephalitis vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol. 2000;74:1742–1751. doi: 10.1128/jvi.74.4.1742-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monath T P, Soike K, Levenbrook I, Zhang Z-X, Arroyo J, Delagrave S, Myers G, Barrett A D T, Shope R E, Chambers T J, Guirakhoo F. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax™) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine. 1999;17:1869–1882. doi: 10.1016/s0264-410x(98)00487-3. [DOI] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Ravi V, Manjunath R. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirements for L3T4+ T cells. J Gen Virol. 1996;77:705–714. doi: 10.1099/0022-1317-77-4-705. [DOI] [PubMed] [Google Scholar]
- 19.Pletnev A G, Bray M, Huggins J, Lai C-J. Construction and characterization of chimeric tick-borne encephalitis/dengue type 4 viruses. Proc Natl Acad Sci USA. 1992;89:10532–10536. doi: 10.1073/pnas.89.21.10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pletnev A G, Men R. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc Natl Acad Sci USA. 1998;95:1746–1751. doi: 10.1073/pnas.95.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poland J D, Calisher C H, Monath T P, et al. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull W H O. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 22.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 23.Rice C M, Grakoui A, Galler R, Chambers T J. Transcription of infectious Yellow Fever RNA from full-length cDNA templates by in vitro ligation. New Biol. 1989;1:285–296. [PubMed] [Google Scholar]
- 24.Rigau-Pérez J G, Clark G G, Gubler D J, Reiter P, Sanders E J, Vorndam A V. Dengue and dengue hemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 25.Rothman A L, Kurane I, Ennis F A. Multiple specificities in the murine CD4(+) and CD8(+) T-cell response to dengue virus. J Virol. 1996;70:6540–6546. doi: 10.1128/jvi.70.10.6540-6546.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlesinger J J, Brandiss M W, Walsh E E. Protection of mice against dengue-2 encephalitis by immunization with the dengue-2 virus nonstructural glycoprotein NS1. J Gen Virol. 1987;68:853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- 27.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4 T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 28.Theiler M, Smith H H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Most R G, Corver J, Strauss J H. Mutagenesis of the RGD motif in the yellow fever virus 17D envelope protein. Virology. 1999;265:83–95. doi: 10.1006/viro.1999.0026. [DOI] [PubMed] [Google Scholar]
- 30.Watts D M, Porter K R, Putvatana P, Vasquez B, Calampa C, Hayes C G, Halstead S B. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 31.Yao J S, Strauss E G, Strauss J H. Interactions between PE2, E1, and 6K required for assembly of alphaviruses studied with chimeric viruses. J Virol. 1996;70:7910–7920. doi: 10.1128/jvi.70.11.7910-7920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng L, Kurane I, Okamoto Y, Ennis F A, Brinton M A. Identification of amino acids involved in recognition by dengue virus NS3-specific, HLA-DR15-restricted cytotoxic CD4+ T-cell clones. J Virol. 1996;70:3108–3117. doi: 10.1128/jvi.70.5.3108-3117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]