Abstract
Background:
Chronic granulomatous disease (CGD) is a rare disorder normally diagnosed in infancy.
Case presentation:
A 27-year-old man admitted with non-specific symptoms of CGD first underwent endoscopy, and colonoscopy procedures as primary evaluation of clinical presentation. Eighteen months after the first admission, he was referred to the emergency department for hematemesis, and critical situations, such as a severe anemic with Hgb= 2.6 mg/dl. As a result of this specific clinical presentation, urgent emergency treatment was performed, and endoscopic examination revealed ulcers and abnormalities in the duodenal bulb and jejunum. Other imaging procedures, such as sonography, and abdominal CT scans, showed splenomegaly. He underwent splenectomy, and after that, endoscopic treatment with balloon TTS dilation was scheduled, but this procedure failed. So, we decided to do a gastro-jujenostomy that alleviated the clinical symptoms. After nine months, he was referred to GOO, and endoscopic evaluation showed giant ulceration with severe stricture in the duodenum, and a polyp in the jejunostomy. Finally, Based on clinical presentation and pathologic evidence of biopsies, the patient approached CGD as the final diagnosis.
Conclusion:
Step-by-step, rule out of different highly suspicious diseases may result in a definite CGD diagnosis, and rapid management of these patients may increase the chance of survival.
Key Words: Chronic granulomatous disease, Gastric outlet obstruction, Gastrointestinal endoscopy
Introduction
Chronic granulomatous disease (CGD) is a life-threatening inherited disorder with challenging clinical presentations. The recurrent fungal, and bacterial infections alongside prevalent granulomatous tissue response may determine it (1).
This event may occur at any age stage; however, the overwhelming majority of patients are diagnosed at primary ages (2). Early life expectancy has been substantially increased due to the faster diagnosis and more effective treatment of this disease. However, thousands of fatalities were attributed to this disease within the last three decades (3). We tend to describe a young patient with a staggering progression of the chronic granulomatous disease's life-threatening gastrointestinal manifestation.
Case report
A 27-year-old male patient was admitted to the emergency department with severe abdominal pain, nausea, and vomiting. Prior to admission, he had stomach discomfort for a minimum of seven days, and the symptoms had intensified during the prior two days. Upon admission, the patient's hemodynamic values were within acceptable levels, as indicated by his clinical presentation. He had a blood pressure of 115/69 mmHg, a heart rate of 100 beats/minute, and a respiratory rate of 19 breaths/minute, and he had no evidence related to fever with a tympanic temperature of 37.2 °C. Oxygen saturation was 96% at rest with a finger pulse oximeter on the index finger.
The physical examination showed normal findings except mild epigastric tenderness. Moreover, there was no gastrointestinal bleeding, diarrhea, or constipation history. The laboratory findings showed a significant decrease in Hgb=7.2 mg/dl, with MCV=70. Other findings showed normal values in normal values in liver function tests, and pancreatic enzymes. The creatinine and BUN were in the normal ranges. The stool exam and culture were normal. The fecal calprotectin level was 700 micrograms/gram. He was referred to the imaging department, and an abdominopelvic CT scan with intravenous contrast was done. All findings of this imaging procedure were reported to be normal.
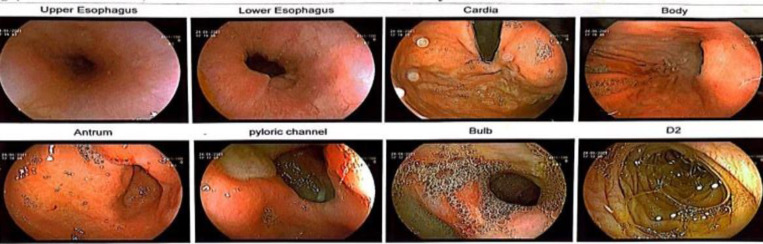
The patient was hospitalized in terms of significant anemic situation, and clinical presentation. So, the patient was referred to the gastrointestinal tract endoscopy. The results indicated a typical esophagus and cardia layout. The stomach assessment revealed the presence of erythema and bile reflux in the body, as well as mucosal ulcers extending from the pyloric channel to the bulb of the antrum. Thus, circumferential ulceration with stricture formation with the mucosal fissuring was seen in the duodenum (Figure 1). So, we decided to take a biopsy from these regions.
Figure 1.
The endoscopic findings in the first evaluation of the patient’s clinical presentation.
The pathologic assay showed that erosive mucosa infiltrated with chronic inflammation (lymphoplasmocytic) within lamina properia, revealing cryptitis, stromal fibrosis, edema, hemorrhage, and lymphocyte aggregation. Furthermore, h-pylori was negative. On the other hand, in the duodenal mucosa, there was no prominent villi atrophy, and intraepithelial lymphocytosis.
Based on these findings, we approached the patient with three differential diagnoses: 1) Chronic active erosive gastritis. 2) Ulcerative duodenitis may be in terms of Crohn's disease, although definite findings, such as granuloma and crypt abscess are not seen in the specimen, and 3) Non-specific inflammation with the ulcer.
Based on the identified differential diagnoses and the presence of anemia, a colonoscopy was performed. The findings indicated that the rectum was normal. The sigmoid was normal, and some bloody secretion without any lesion was seen. The only significant finding in this procedure was the presence of two bloody erosions in the transverse colon. Based on these findings, we considered focal active colitis. The pathologic findings from bloody erosion areas showed non-specific inflammation with non-destructive colitis.
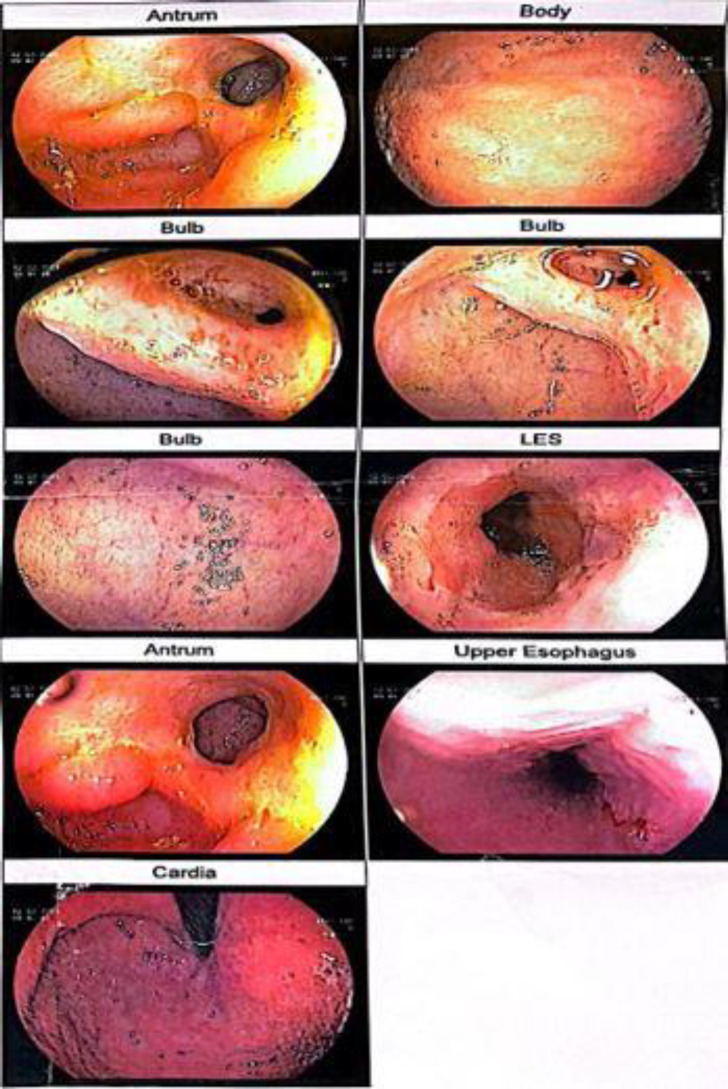
After ruling out the infectious causes, such as amebiasis infection, clostridium difficile, and HIV, and based on this evidence, Crohn's disease was managed as a highly suspicious diagnosis. The patient was considered an inflammatory bowel disease, and the treatment started with this approach. After seven days, he was discharged with an acceptable clinical situation. We advised him to refer to gastro-enterology clinic for follow-up and treatment of IBD after one month. Nonetheless, his medical condition was overlooked, and he was not referred for further diagnostic and therapeutic procedures. Eighteen months later, he was referred to the emergency department for hematemesis, fever (Temperature=38.6°C), and severe abdominal pain. The critical finding in his laboratory hematologic test was Hgb=2.6 gr/dl. The patient was conscious with a blood pressure=90/44 mmHg, tachypnea (RR 26 brpm), and tachycardia (HR=110 bpm). The abdominal physical examination showed epigastric tenderness without rigidity and guarding. He was emergently under packed RBC (four units), and fresh frozen plasma (two units) transfusion. Therefore, he was rehydrated with crystalloid fluids. After transferring him to the intensive care unit, a sepsis evaluation was conducted. Immediately following emergency medical intervention and stabilization of the vital signs, he was transferred to the endoscopic department. The patient underwent an endoscopy. This study showed some ulcers and deformities at the duodenal bulb and jejunum (Figure 2). At this time, the gastric outlet obstruction (GOO) was considered. The microscopic findings in the specimen from this area showed that the duodenal mucosal section had focal ulcers, and infiltration of lymph-mononuclear inflammatory cells, and neutrophils without increasing lymphocytes was seen. These findings were consistent with the duodenal ulcers with reactive epithelial changes.
Figure 2.
There were some ulcers on the duodenal bulb and jejunum.
Then, he underwent GI-bleeding standard treatment, and abdominal sonography was done. This study revealed hypo-echo regions in the superior and inferior borders of the spleen. Thus, free fluid's non-significant volume was in the abdominal cavity. He underwent an abdominal CT scan with and without contrast was done. There was a thickened wall hypo-dense area with the size 88*15 mm in the greater curvature of the stomach that is considered a collection mass. Hence, other masses with the size of 19*31 mm and 12*50 mm were seen in the subcapsular region in the superior and inferior borders of the spleen, respectively. The splenomegaly was another significant finding in this assessment, with a size of 173 mm in diameter. Besides splenomegaly, multiple hypodense regions with cortical development are considered splenic infarct. Also, multiple porta-hepatis were seen.
Based on CT scan findings related to the spleen infarction, he was scheduled for splenectomy. After this surgery, he was closely monitored in the ICU. After stabilizing the critical situation, the endoscopic treatment with balloon TTS dilation was done (The tract was dilated with balloon TTS size 18-20). This procedure failed, so we decided to do a gastro-jujenostomy.
At this time, clinical manifestations were alleviated, and after that, fortunately, he was transferred to gastro-entrology ward. During this time, he was prevented from disease treatment and discharged after six days by personal-consent protocol.
The biopsy pathologic evaluation showed chronic active gastritis and granulation.
With GOO, he was readmitted to our facility after nine months had passed. During the endoscopic examination, a substantial ulceration accompanied by a severe stricture was observed in the initial segment of the duodenum, while a polyp was detected in the jejunostomy (Figure 3). Then, multiple biopsies were taken.
Figure 3.
Deformity and fibrosis were seen in pylorus. Moreover, the endoscopic evaluation showed ulceration with severe stricture in the first 20 cm section of the duodenum.
Considering clinical and para-clinical findings, another diagnosis was considered. So, the immunologic consultation is essential. Based on the immunologist's order, the laboratory findings showed an increased alkaline phosphatase level (899 IU/L) and a critically increasing CRP level (95 mg/dl). Other laboratory results include: WBC= 18.48*103, Platelets=496*103, Serum albumin=2.1 g/dl, and Ferritin=480 micg/L. Immunologic assay consisting of ANA, Anti dsDNA, P-ANCA, and C-ANCA was negative.
The macroscopic and microscopic pathologic assay proved that the granulation tissue. Thus, the microbiologic assay showed candida spp is the fungi responsible for this disorder. According to this evidence, after immunology and infection disease specialty consultation, the patient candidate received interferon-gamma (IFN-γ), Co-trimoxazole, and Voriconazole 15mg per kg. Nevertheless, using IFN-γ was not prescribed based on the poor evidence and the fact that this agent can deteriorate the patient's situation.
So, the patient approached with CGD, and treatment was continuous based on this final diagnosis. Furthermore, the patient is under close observation in the intensive care unit using a reverse isolation strategy, as stated by the immunologist.
Discussion
Chronic granulomatous disease (CGD) is an immunodeficiency caused by defects in any of the five subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (4). Individuals diagnosed with CGD have a higher likelihood of experiencing severe infections caused by various bacteria and fungi. These infections may lead to inflammation and have significant negative impacts on both the patient's health and their chances of survival (5).Although vast majority of patients with CGD are diagnosed in childhood, the adult ones tend to become increasingly debilitated to death with advancing age (6).
GI symptoms in terms of CGD occur in the first decade of life. So, it seems that managing adults with this disease is challenging and has grave critical risks in treatment (7). Clinical manifestations of CGD are different, ranging from mild, moderate, and severe forms (8).
These manifestations depend on the disease agent and may be presented in mild form, such as mouth ulcers, pneumonia, lung, inguinal, and perianal abscesses, or severe lethal respiratory infections. However, the most common clinical features in Iranian patients with CGD were hyper-gamma-globulinemia, hepatomegaly, and splenomegaly (9-10). According to other research, individuals with CGD are more likely to have inflammatory symptoms, and the gastrointestinal (GI) tract is the organ most often affected, accounting for 33–60% of cases (11). GI manifestations may precede the diagnosis of CGD and the development of infectious complications. On the other hand, evidence suggests that CGD should be considered in all patients with early-onset inflammatory bowel disease (IBD) (12).
However, GI symptoms are generally non-specific and may be presented only with abdominal pain or nausea, vomiting, and failure to thrive (13). Furthermore, besides the IBD, the hepatic involvement in these patients not only could not be neglected but also should be considered a significant complication (14).
Additional research revealed that individuals with CGD may have increasing liver symptoms, including splenic sequestration, hepatosplenomegaly, non-cirrhotic portal hypertension, and nodular regenerative hyperplasia. Therefore, thrombocytopenia secondary to splenic sequestration strongly predicts mortality (15-17). Our patient was admitted with a non-specific manifestation of CGD. The GI tract endoscopy and imaging findings proved highly suspicious disorders related to inflammatory bowel disease. Also, the splenomegaly may be in terms of the progressive trend of CGD, but other life-threatening clinical presentations, such as thrombocytopenia, did not occur with the surgical approach and splenectomy.
Predominant medical management is based on wide-spectrum antibiotics, and antifungal prophylaxis in patients with CGD, known as a conventional approach (18). In the meantime, evidence-based findings revealed that the prophylactic use of IFN-γ remains debated. Many studies showed a clear benefit of IFN-γ prophylaxis with a decrease in the number and severity of infections in CGD patients (18).
On the other hand, others claimed that the rate of infected patients had not altered much as a result of preventive use of IFN-γ, and they came to the conclusion that there was insufficient data to support the treatment of CGD patients in this way (18). The evidenced-based approach to our patient was using wide-spectrum antibiotics that alleviated the symptoms of the disease. It may be that IFN-γ can effectively manage this case. However, it seems that using this agent results in the severity of the manifestations due to the presentation of multi-organ failure in the last trial, where we achieved the definitive diagnosis of CGD.
Our patient had some evidence related to CGD as a rare differential diagnosis of IBD. Although clinical presentation was not specific, emphasizing the IBD causes and step-by-step rule out of different highly suspicious diseases may result in a definite CGD diagnosis.
Acknowledgment
The authors thank the patient and his guardians who participated in this study. We would like to thank the gastroenterology department staff at Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interests
The authors have no conflicts of interest to declare.
References
- 1.Rawat A, Vignesh P, Sudhakar M, Sharma M, Suri D, Jindal A, et al. Clinical, immunological, and molecular profile of chronic granulomatous disease: a multi-centric study of 236 patients from India. Front Immunol. 2021;12:625320. doi: 10.3389/fimmu.2021.625320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, et al. Chronic granulomatous disease: The European experience. PLoS One. 2009;4:5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderman L, Brown J, Chaudhury S, O'Gorman M, Fuleihan R, Khanolkar A, et al. Co-occurring X-linked agammaglobulinemia and X-linked chronic granulomatous disease: two isolated pathogenic variants in one patient. Biomedicines. 2023;11:959. doi: 10.3390/biomedicines11030959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold DE, Heimall JR. A review of chronic granulomatous disease. Adv Ther. 2017;34:2543–2557. doi: 10.1007/s12325-017-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho NHH, Patel S, Pathak P. A fatal case of chronic granulomatous disease in a young man. Cureus. 2023;15:40266. doi: 10.7759/cureus.40266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra K, Folkmanaitė M, Stockdale L, Shathish V, Ishibashi S, Bergin R, et al. Duox is the primary NADPH oxidase responsible for ROS production during adult caudal fin regeneration in zebrafish. iScience. 2023;26:106147. doi: 10.1016/j.isci.2023.106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abd Elaziz D, El Hawary R, Meshaal S, Alkady R, Lotfy S, Eldash A, et al. Chronic granulomatous disease: a cohort of 173 patients-10-years single center experience from Egypt. J Clin Immunol. 2023:11. doi: 10.1007/s10875-023-01541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köker MY, Camcıoğlu Y, van Leeuwen K, Kılıç SŞ, Barlan I, Yılmaz M, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132:1156–1163. doi: 10.1016/j.jaci.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Mortaz E, Azempour E, Mansouri D, Tabarsi P, Ghazi M, Koenderman L, et al. Common infections and target organs associated with chronic granulomatous disease in Iran. Int Arch Allergy Immunol. 2019;179:62–73. doi: 10.1159/000496181. [DOI] [PubMed] [Google Scholar]
- 10.Movahedi M, Aghamohammadi A, Rezaei N, Shahnavaz N, Jandaghi AB, Farhoudi A, et al. Chronic granulomatous disease: a clinical survey of 41 patients from the Iranian primary immunodeficiency registry. Int Arch Allergy Immunol. 2004;134:253–9. doi: 10.1159/000078774. [DOI] [PubMed] [Google Scholar]
- 11.Magnani A, Brosselin P, Beauté J, de Vergnes N, Mouy R, Debré M, et al. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol. 2014;134:655–662. doi: 10.1016/j.jaci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Vuyyuru SK, Kedia S, Sahu P, Ahuja V. Immune-mediated inflammatory diseases of the gastrointestinal tract: Beyond Crohn's disease and ulcerative colitis. JGH Open. 2022;6:100–111. doi: 10.1002/jgh3.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villanacci V, Reggiani-Bonetti L, Salviato T, Leoncini G, Cadei M, Albarello L, et al. Histopathology of IBD Colitis A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD) Pathologica. 2021;113:39–53. doi: 10.32074/1591-951X-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes-Santos CJ, Koh C, Rai A, Sacco K, Marciano BE, Kleiner DE, et al. Nodular regenerative hyperplasia in X-linked agammaglobulinemia: an underestimated and severe complication. J Allergy Clin Immunol. 2022;149:400–409. doi: 10.1016/j.jaci.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiGiacomo DV, Shay JE, Crotty R, Yang N, Bloom P, Corey K, et al. Liver stiffness by transient elastography correlates with degree of portal hypertension in common variable immunodeficiency patients with nodular regenerative hyperplasia. Front Immunol. 2022;13:864550. doi: 10.3389/fimmu.2022.864550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Maruyama H, Arora A, Sharma P, Anikhindi SA, Bansal N, et al. Diagnostic accuracy of transient elastography in diagnosing clinically significant portal hypertension in patients with chronic liver disease: a systematic review and meta-analysis. J Med Ultrason (2001) 2022;49:333–346. doi: 10.1007/s10396-022-01239-x. [DOI] [PubMed] [Google Scholar]
- 17.Fuss IJ, Friend J, Yang Z, He JP, Hooda L, Boyer J, et al. Nodular regenerative hyperplasia in common variable immunodeficiency. J Clin Immunol. 2013;33:748–58. doi: 10.1007/s10875-013-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugo Reyes SO, González Garay A, González Bobadilla NY, Rivera Lizárraga DA, Madrigal Paz AC, Medina-Torres EA, et al. Efficacy and safety of interferon-gamma in chronic granulomatous disease: a systematic review and meta-analysis. J Clin Immunol. 2023;43:578–584. doi: 10.1007/s10875-022-01391-6. [DOI] [PubMed] [Google Scholar]