Abstract
In a previous study we identified the subpopulations of thymus cells that were infected by the lymphomagenic MCF13 murine leukemia virus (MLV) (F. K. Yoshimura, T. Wang, and M. Cankovic, J. Virol. 73:4890–4898, 1999) and observed an effect on thymus size by virus infection. In this report we describe our results which demonstrate that MCF13 MLV infection of thymuses reduced the number of T lymphocytes in this organ. Histological examination showed diffuse lymphocyte depletion, which was most striking in the CD4+ CD8+ lymphocyte-enriched cortical zone. Consistent with this, flow cytometric analysis showed that the lymphocytes which were depleted were predominantly the immature CD3− CD4+ CD8+ and CD3+ CD4+ CD8+ cells. A comparison of the percentages of live, apoptotic, and dead cells of the gp70+ and gp70− thymic lymphocytes suggested that this effect on thymus cellularity is a result of virus infection. Studies of the survival of thymic T lymphocytes in culture showed that cells from MCF13 MLV-inoculated mice underwent greater apoptosis and death than cells from control animals. Assays for apoptosis included 7-amino-actinomycin D staining, DNA fragmentation, and cleavage of caspase-3 and poly(ADP-ribose) polymerase proenzymes. Our results suggest that apoptosis of thymic lymphocytes by virus infection is an important step in the early stages of MCF13 MLV tumorigenesis.
Murine leukemia viruses (MLVs) with simple genomes require a relatively long latency for the development of thymic lymphoma. In AKR mice, thymic tumors begin to arise when the animals are about 6 to 7 months old (6, 29a). It is thought that one of the reasons for this long latency in AKR mice is the requirement for the generation of recombinant MLVs, i.e., mink cell focus-forming (MCF) viruses, which are the proximal etiologic agent of disease (6, 34). The oncogenic class I MCF MLVs are recombinant retroviruses with an ecotropic MLV backbone and nonecotropic viral sequences at the N-terminal portion of the SU protein and the C terminus of TM and the U3 region of the long terminal repeat (12, 28, 51). Class I MCF viruses are detectable in the thymus of AKR mice beginning at about 4 to 5 months of age (12, 47). Compared with the time course for the appearance of spontaneous thymic tumors in AKR mice, MCF13 inoculation into AKR neonates results in accelerated leukemogenesis which begins at about 10 weeks postinoculation (54). Although extensive studies have examined the later events of MCF MLV tumorigenesis (8, 9, 36), less work has been done to study the earlier preleukemic period. It has been observed that efficient virus replication in the thymus during the preleukemic period is essential for thymus development (21, 34, 35). We have recently reported that the sequences between the enhancer and promoter (DEN) in the long terminal repeat of the MCF13 MLV regulate virus replication in the thymus during the early stages of lymphomagenesis (59).
As a part of this study we identified the thymic subpopulations which were infected by MCF13 MLV. We observed that the subpopulation in which the highest level of virus replication occurred during the preleukemic period was the immature CD3+ CD4+ CD8+ (59). These cells accounted for 70 to 80% of virus-infected cells. The subpopulation with the next highest percentage (10 to 18%) of virus-infected cells was CD3− CD4+ CD8+, which are the precursors to the CD3+ CD4+ CD8+ cells (14, 41, 44). The effect of MLV infection on CD4+ CD8+ cells has also been observed for the SL3-3 virus, which produces thymic lymphoma similar to MCF13 (18, 27). It has been shown that the normal development of these cells is affected during the maturation of CD4− CD8− cells when they were isolated from SL3-3 virus-inoculated mice and either cultured with fetal thymic stroma in vitro or inoculated intrathymically into mice (16, 17). The idea that thymic lymphocytes which have a CD4+ CD8+ phenotype may be the major targets for cellular transformation is further supported by the detection of this phenotype for cells present in the majority of MLV-induced thymic tumors by ourselves and other investigators (18, 26, 59).
The CD4+ CD8+ thymic subpopulation in which MCF13 MLV replication predominantly occurs (59) is the same subset in which both positive and negative selection of thymic T lymphocytes normally occurs (14, 41). It has been observed that the negative selection process involves the apoptosis of CD4+ CD8+ cells which recognize self-antigens. Because in our previous study we noticed that one of the effects of MCF13 MLV infection was the reduction of thymus size, we explored the effect of virus replication on the dynamic cellular processes of this organ, and particularly on the CD4+ CD8+ subpopulation during the early stages of tumorigenesis. This report summarizes the results of this study.
MATERIALS AND METHODS
Virus inoculation and isolation of thymic lymphocytes.
Neonatal AKR mice were injected intraperitoneally with 50 μl of inoculum containing 106 infectious units (IFUs) of MCF13 MLV. Control mice were inoculated with tissue culture medium. Mice were euthanized by CO2 inhalation and cervical dislocation, and thymuses were removed into cold RPMI 1640 containing 2% inactivated fetal calf serum (IFCS). Single-cell suspensions were made by pressing the thymus tissue through a wire mesh into RPMI plus 2% IFCS. Cells were washed once with medium without serum. The viability of thymic lymphocytes in suspension, which was determined by trypan blue dye exclusion, was routinely greater than 90%.
In vitro culture of thymic cells.
Single-cell suspensions of thymic lymphocytes were placed into 75-cm2 tissue culture flask with RPMI 1640 containing 10% IFCS and 100 μg of penicillin-streptomycin per ml at a density of 107 cells per ml. Cells were harvested at 6, 24, and 48 h after incubation. Cell viability was determined at each time point.
Thymus histology.
Thymuses from euthanized MCF13-inoculated and control mice were rapidly removed and immediately fixed overnight in 10% formalin. After dehydration in graded ethanol and xylene, the tissues were embedded in paraffin. Serial 5-μm sections were prepared, and alternate sections were stained by Giemsa or hematoxylin and eosin stains.
Staining of thymic cells.
Thymic lymphocytes (106) were washed twice with phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.02% sodium azide. Washed cells were incubated with 100 μl of monoclonal antibody (MAb) 514 on ice for 30 min, after which time 100 μl of the secondary antibody (fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G [IgG]) at a 1:100 dilution was added. After being washed twice with PBS, the cells were resuspended in 100 μl of PBS containing hamster anti-CD3 antibody conjugated to phycoerythrin, rat anti-CD4 antibody conjugated to Cy-Chrome, rat anti-CD8 antibody conjugated to biotin (PharMingen), and NeutraLite avidin conjugated to Cascade blue (Molecular Probes, Eugene, Oreg.) at dilutions previously determined by titration assays. Washed cells were resuspended in 0.7 ml of 0.5% paraformaldehyde and analyzed by flow cytometry.
Flow cytometric analysis.
Prior to analysis, dead cells were gated out based on forward versus side scatter dot plots. Flow cytometry was performed on a FACS Vantage flow cytometer equipped with an HP 9000 computer running the LYSYS II software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Fluorescein isothiocyanate, phycoerythrin, and Cy-Chrome were excited with an ILT 5500A argon ion laser (Ion Laser Technology, Salt Lake City, Utah). Cascade blue was excited with 50 mW of all lines of UV light (351 to 365 nm wavelength). Electronic compensation for spectral overlap of the fluorochromes was performed with single-color control samples prepared with the test samples. All data presented were based on analysis of 2 × 104 cells with the Paint-A-Gate software (Becton Dickinson). Analysis gates were set on isotype controls.
7-AAD staining.
Staining was performed with 0.0625 μg of 7-amino-actinomycin D (7-AAD) PharMingen added to 105 cells in 100 μl of PBS. Cells were stained for 15 min at room temperature (RT) in the dark, then brought to a final volume of 500 μl of PBS. Samples were run within 1 h on the FACScan. Unstained cells were used for a negative control. Data on 2 × 104 cells were analyzed using the CELLQuest software. Scattergrams were generated by combining forward light scatter with 7-AAD fluorescence. Regions of clear-cut populations having negative, dim, and bright fluorescence were selected.
DNA fragmentation assay.
Cellular DNA was extracted from 2 × 107 cells using the Apoptotic DNA Ladder kit (Boehringer Mannheim). Cells were lysed in 200 μl of lysis buffer (6 M guanidine-HCl, 10 mM Tris-HCl, 10 mM urea, 20% Triton X-100 [pH 4.4]) and incubated for 10 min at RT. Samples subsequently were mixed with 100 μl of isopropanol, filtered by centrifugation for 1 min at 8,000 rpm in an Eppendorf centrifuge, and washed twice. DNA was eluted into 100 μl of 10 mM Tris-HCl (pH 8.5) buffer, and 1.5 μg of DNA was electrophoresed through a 1.5% agarose gel for 1.5 h at 100 V in 1× TBE (81 mM Tris base, 81 mM boric acid, 1.8 mM EDTA). DNA was visualized by staining with ethidium bromide and examination under UV light.
Caspase-3 assay.
Cells (2 × 107) were collected at each time point, washed twice with PBS, and lysed in 200 μl of 50 mM Tris-HCl (pH 7.5) containing 0.03% Nonidet and 1 mM dithiothreitol. Nuclei were removed by centrifugation (1,200 × g) for 5 min, and 50 μl of the cytosolic fraction was incubated with 40 μM DEVD-amc–10 mM HEPES (pH 7.5)–50 mM NaCl–2.5 mM dithiothreitol in a total volume of 200 μl for 120 min at 37°C. Coumarin fluorescence, released by caspase cleavage of DEVD-amc, was measured using 360-nm excitation and 415-nm emission wavelengths. A charge-coupled device (Instaspec IV; Oriel, Stratford, Conn.) fitted with a monochromator was used to measure the fluorescence emission spectrum. The system was initially calibrated with known levels of aminomethylcoumarin. Protein amounts were measured using the bicinchoninic acid protein assay (Pierce). Units of enzyme activity are expressed as picomoles of substrate hydrolyzed per microgram of protein per hour.
PARP cleavage assay.
Cells (2 × 107) were washed twice with PBS, lysed with buffer containing 2% sodium dodecyl sulfate (SDS), 0.5 M Tris-HCl (pH 6.8), and 20% glycerol and subsequently boiled for 5 min. Equal amounts of protein were loaded on a 8% polyacrylamide gel and separated by electrophoresis at 120 V for 2 h. Following transfer, nitrocellulose membranes were reacted with a poly(ADP-ribose) polymerase (PARP)-specific primary antibody (C-2-10; BIOMOL Research Laboratory) for 1 h. After being washed three times with 1× TTBS (0.2% Tween 20, 0.136 M NaCl, 2.7 mM KCl, 25 mM Tris-HCl [pH 7.4]; Sigma), the membrane was incubated with horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary antibody for another hour at RT. Antigen was detected using the ECL detection system (Pierce) according to the manufacturer's instructions. Membranes were exposed to Kodak X-ray film.
RESULTS
Depletion of thymic T lymphocytes by MLV infection.
In our studies of the pathogenic properties of the MCF13 MLV, we examined the effect of virus infection on thymus size. We detected a significant decrease in thymus weight at 8 weeks after MCF13 MLV inoculation of neonatal AKR/J mice. The mean weight of thymuses from 23 mice at 8 weeks postinoculation (p.i.) was 110.3 ± 20.4 mg compared with a mean weight of 128.2 ± 19.5 mg for 19 age-matched control thymuses. The difference in thymus weights for MCF13-inoculated and uninoculated mice was statistically significant, with a P value of 0.003 as determined by Student's t test. We picked 8 weeks to examine thymus weight because at this time point we had previously observed a maximum of 58% of virus-infected cells in the thymus before thymic lymphoma first began to appear at 10 weeks p.i. (54, 59).
To determine whether the difference in thymic weights was due to a difference in cellularity, we performed a longitudinal study of the effect of MCF13 MLV infection on thymic cell number. We started at 3 weeks p.i. to assess the total number of thymic cells because in a previous study we observed that this was the earliest time after virus inoculation when we could reproducibly detect virus-infected thymic lymphocytes (59). By detection of the MCF13 envelope glycoprotein (gp70) by flow cytometry, we observed that 8% of thymic lymphocytes were infected by virus at 3 weeks p.i. (59). As shown in Table 1, there was no difference in cell number for thymuses from MCF13-inoculated mice compared with control animals at 3 and 4 weeks p.i. However, at later times after virus inoculation, i.e., 6 and 8 weeks p.i., we observed a statistically significant decrease in the number of lymphocytes isolated from thymuses from virus-inoculated mice compared with control animals. We previously observed that at 6 weeks p.i., virus-infected (gp70+) cells represented 52% of thymic lymphocytes, and at 8 weeks p.i. this number increased slightly to 58% (59). Thus, a decrease in thymus size and cell number correlated with an increase in the number of MCF13 MLV-infected cells present in the thymus.
TABLE 1.
Thymus cell numbera
| Wk p.i. | Mean no. of lymphocytes (108) ± SD
|
|
|---|---|---|
| Control | MCF13 MLV | |
| 3 | 3.8 ± 0.9 (7) | 4.0 ± 1.2 (9) |
| 4 | 4.6 ± 1.1 (10) | 4.0 ± 1.0 (10) |
| 6 | 3.9 ± 1.0 (11) | 2.5 ± 1.1b (9) |
| 8 | 4.0 ± 1.1 (10) | 2.2 ± 0.5b (11) |
Mean values and standard deviations were calculated from the number of total lymphocytes from thymuses isolated from mice inoculated with either medium (control) or 106 IFU of MCF13 MLV. Numbers in parentheses are the number of mice examined at each time point.
P ≤ 0.006 compared to control at the same time point, as determined by Student's t test.
Morphological lymphocyte depletion of thymic lymphocytes produced by MCF13 MLV infection.
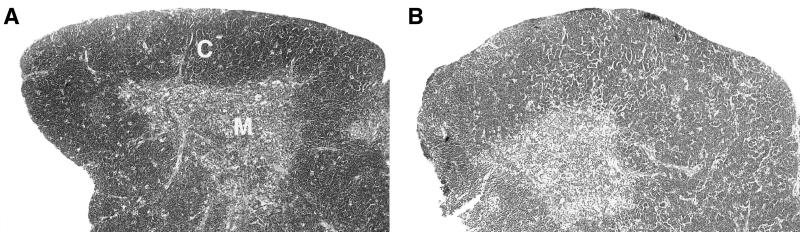
Because we detected a decrease in thymus cellularity due to MCF13 MLV infection of this organ, we examined thymic sections at 8 weeks p.i. by histological staining (Fig. 1). Sections from MCF13-inoculated mice showed an overall decrease in lymphocyte numbers throughout the thymus relative to control animals. At low magnification (e.g., Fig. 1), this is apparent as a decrease in the intensity of hematoxylin staining for the virus-infected thymus. This decrease in cellularity was most pronounced in the CD4+ CD8+-rich cortical regions.
FIG. 1.
Histological comparison of thymuses from MCF13-inoculated and control mice. (A) The thymus of an 8-week-old control mouse shows a thick zone of densely staining cortex (C) and a small zone of lightly staining medulla (M). (B) Lymphocyte depletion of the thymus is detectable 8 weeks after inoculation of MCF13 MLV.
Identification of thymic subpopulations which are depleted by MCF13 MLV infection.
The thymus comprises a number of different subpopulations of T lymphocytes, which have been identified by their differential expression of the CD3, CD4, and CD8 cell surface antigens (14, 41, 44). To identify the subpopulation which was reduced in number in thymuses from mice inoculated with MCF13 MLV, we performed flow cytometric analysis of thymic lymphocytes stained with anti-CD3, anti-CD4, and anti-CD8 fluorescent-labeled antibodies. We examined the five predominant subpopulations in the thymus detectable with these antibodies, as presented in Table 2. These subpopulations were the immature CD3− CD4− CD8−, CD3− CD4+ CD8+, and CD3+ CD4+ CD8+ cells and the more mature CD3+ CD4+ CD8− and CD3+ CD4− CD8+ cells. Although other investigators have identified additional subsets of thymic cells, i.e., CD3− CD4+ CD8−, CD3− CD4− CD8+, and CD3+ CD4− CD8− (14, 23, 24, 44), their numbers were too few to be reproducibly detectable and hence they were not included in our analysis.
TABLE 2.
Number of cells in each thymic subpopulationa
| Wk p.i. | Mice | Mean no. of cells (107) ± SD
|
||||
|---|---|---|---|---|---|---|
| CD3− CD4− CD8− | CD3− CD4+ CD8+ | CD3+ CD4+ CD8+ | CD3+ CD4− CD8+ | CD3+ CD4+ CD8− | ||
| 3 | Control | 1.2 ± 0.4 | 5.4 ± 1.8 | 27.2 ± 7.0 | 0.4 ± 0.2 | 2.4 ± 0.5 |
| MCF13 | 1.4 ± 0.6 | 5.6 ± 1.7 | 28.8 ± 11.3 | 0.5 ± 0.1 | 2.5 ± 0.8 | |
| 4 | Control | 1.7 ± 0.6 | 6.1 ± 2.0 | 29.0 ± 6.5 | 0.5 ± 0.2 | 3.1 ± 1.0 |
| MCF13 | 2.2 ± 1.0 | 4.4 ± 2.0 | 26.8 ± 9.2 | 0.4 ± 0.2 | 3.1 ± 1.5 | |
| 6 | Control | 2.2 ± 0.8 | 5.7 ± 1.8 | 23.4 ± 7.0 | 0.5 ± 0.2 | 2.8 ± 0.8 |
| MCF13 | 1.8 ± 0.7 | 3.9 ± 1.5b | 12.4 ± 3.6b | 0.8 ± 0.3 | 1.9 ± 0.5 | |
| 8 | Control | 2.4 ± 0.9 | 5.8 ± 1.5 | 28.1 ± 8.5 | 0.5 ± 0.2 | 2.8 ± 1.0 |
| MCF13 | 2.7 ± 1.1 | 3.0 ± 0.5b | 12.2 ± 2.3b | 0.7 ± 0.2 | 2.4 ± 0.5 | |
Thymuses from four to eight mice inoculated with either medium (control) or MCF13 MLV were individually analyzed for each time point. Cell numbers for each subpopulation were calculated by multiplying the number of total thymic lymphocytes by the percentage of each subpopulation as determined by flow cytometric analysis (Materials and Methods).
P < 0.05 compared with control, as determined by Student's t test.
The number of T lymphocytes in each of these five subpopulations was determined from 3 to 8 weeks p.i. (Table 2). Similar to our results from examining total thymic lymphocytes as described above, we detected no difference in the numbers of any of the subpopulations of cells isolated from thymuses from MCF13-inoculated mice compared with control mice at 3 and 4 weeks p.i. However, at both 6 and 8 weeks p.i. we observed a statistically significant decrease in the number of lymphocytes present in the CD3− CD4+ CD8+ and CD3+ CD4+ CD8+ subpopulations of thymuses from virus-inoculated mice. We detected no significant changes in the number of cells in the other three subpopulations at any time. The depletion of these two subpopulations correlated with results from a previous study in which we observed that the CD3− CD4+ CD8+ and CD3+ CD4+ CD8+ subpopulations had the greatest number of virus-infected thymic cells at 6 and 8 weeks p.i. (59). At 6 weeks p.i., gp70+ cells constituted 43% of the CD3− CD4+ CD8+ cells and 51% of the CD3+ CD4+ CD8+ cells. At 8 weeks p.i., gp70+ cells accounted for more than 50% of both subpopulations. Thus, the reduction in cell number in these two subpopulations is mainly responsible for the decrease in total thymic cell numbers due to MCF13 MLV infection.
Thymic lymphocyte depletion involves apoptosis.
To better understand the basis for the reduction in thymic cell number after MCF13 MLV infection, we assessed the percentages of live, apoptotic, and dead cells in thymuses at approximately 2 to 3 and 8 to 9 weeks after virus inoculation. These two time points were selected because they corresponded to times when we detected either no difference in total thymus cell number (3 weeks) or a significant decrease in cell number (8 weeks) as described above (Table 1).
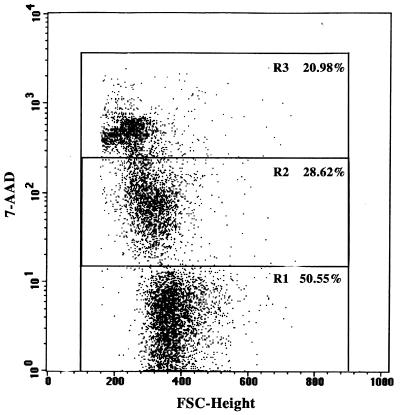
Isolated thymic lymphocytes were treated with 7-AAD to delineate live, apoptotic, and late apoptotic/dead cells by flow cytometry. 7-AAD is a fluorescent DNA-binding agent which others have shown is able to identify early apoptotic cells (7-AAD dim), which retain membrane integrity, from late apoptotic/dead cells (7-AAD bright), for which intact membrane permeability is altered in contrast to necrotic cells, which have lost membrane integrity (37, 43). Live cells are 7-AAD negative. The ability of this reagent to discriminate among these cell populations is thought to be due to differential transport of the dye due to alterations in the membranes of apoptotic and dead cells (43). In Fig. 2 is shown an example of a dot plot depicting the three populations among thymic lymphocytes.
FIG. 2.
Detection of live, apoptotic, and dead cells by 7-AAD staining and flow cytometry. Thymic lymphocytes isolated from the thymus of a 2-week-old AKR/J mouse and placed in culture for 24 h were stained with 7-AAD. Stained cells (2 × 104) were analyzed by a FACScan flow cytometer. The dot plot was generated with the CELLQuest software. Selected regions are live (R1), apoptotic (R2), and late apoptotic/dead (R3) cells. The percentage of cells in each population is indicated.
We first examined thymic lymphocytes from mice at 2 weeks p.i. For these cells we detected no difference among the percentages of live, apoptotic, and late apoptotic/dead cells between thymic lymphocytes freshly isolated from MCF13 MLV-inoculated and control mice (0 h values in Table 3). Although the brightly staining population consists of a mixture of both late apoptotic and dead cells, we refer to this population simply as dead in Table 3. Because 7-AAD does not differentiate between late apoptotic and dead cells in the brightly staining population, we also calculated the sum of the percentages of apoptotic and dead cells in Table 3 to obtain an approximate number of total apoptotic cells which were in both early and late apoptosis. At 8 weeks p.i., however, we detected statistically significant differences for the live, dead, and sum of apoptotic and dead cell populations between freshly isolated thymic lymphocytes from MCF13-inoculated mice and those from control mice (0 h values). For thymic lymphocytes from MCF13-inoculated mice at 8 weeks p.i., we detected a decrease in the percentage of live cells and increases in the percentages of dead and apoptotic and dead cells compared with those from age-matched control mice.
TABLE 3.
Percentages of live, apoptotic, and dead thymic lymphocytesa
| Wk p.i. | Time in culture (h) | Mice | Mean % of cells ± SD
|
|||
|---|---|---|---|---|---|---|
| Live | Apoptotic | Dead | Apoptotic + dead | |||
| 2 | 0 | Control | 89.2 ± 4.8 | 4.0 ± 3.4 | 6.7 ± 3.5 | 10.6 ± 4.8 |
| MCF13 | 87.5 ± 6.5 | 6.5 ± 4.0 | 6.0 ± 3.0 | 12.5 ± 6.5 | ||
| 24 | Control | 66.8 ± 10.3 | 14.6 ± 6.4 | 18.8 ± 8.0 | 33.3 ± 10.4 | |
| MCF13 | 61.7 ± 11.2 | 16.7 ± 8.5 | 22.0 ± 4.0 | 38.7 ± 11.4 | ||
| 48 | Control | 43.7 ± 8.3 | 38.6 ± 7.0 | 17.5 ± 4.8 | 56.0 ± 8.4 | |
| MCF13 | 40.8 ± 8.5 | 26.9 ± 8.0 | 32.4 ± 7.5b | 59.3 ± 8.7 | ||
| 8 | 0 | Control | 90.5 ± 4.4 | 4.9 ± 3.0 | 4.6 ± 2.4 | 9.5 ± 4.4 |
| MCF13 | 84.4 ± 6.1b | 7.1 ± 4.4 | 8.6 ± 4.6b | 15.8 ± 5.7b | ||
| 24 | Control | 64.1 ± 10.9 | 12.8 ± 4.6 | 23.2 ± 9.7 | 35.9 ± 11.0 | |
| MCF13 | 41.2 ± 16.2b | 18.8 ± 8.5b | 40.0 ± 15.7b | 58.8 ± 16.0b | ||
| 48 | Control | 40.6 ± 11.9 | 15.8 ± 3.5 | 43.8 ± 12.9 | 59.6 ± 11.9 | |
| MCF13 | 26.9 ± 11.6b | 21.5 ± 8.2b | 51.7 ± 15.8 | 73.2 ± 11.5b | ||
Thymic cells (3 × 108 to 4 × 108) pooled from three control or MCF13-inoculated mice were placed in culture at 107 per ml for 0, 24, and 48 h before analysis. A total of 105 thymic cells were stained with 7-AAD at each time point. Mice were sacrificed at 2 and 8 weeks after inoculation with either tissue culture medium (control) or 106 IFU of MCF13 MLV. Mean percentages and standard deviations of live, apoptotic, and dead thymic lymphocytes in culture were determined by 7-AAD staining and flow cytometric analysis as described in Materials and Methods. Mean values represent the result of 6 to 11 independent experiments.
P ≤ 0.015 compared with percentages of thymic cells from mice inoculated with tissue culture medium, as determined by Student's t test.
It has been observed that thymic lymphocytes undergo spontaneous apoptosis when placed in culture (39, 53). To determine whether MCF13 infection has an effect on the survival of thymic lymphocytes in vitro, we assessed the percentages of live, apoptotic, and dead cells that were placed in culture for 24 and 48 h. We did not detect any differences among the three populations between virus-infected and control thymic lymphocytes at 2 weeks p.i. except for dead cells at 48 h (Table 3). In contrast, at 8 weeks p.i. we observed a significant decrease in the percentage of live cells and increases in the percentage of apoptotic or dead cells for thymic lymphocytes from virus-inoculated mice compared with control animals at 0, 24, and 48 h. Although our data showed that thymic cells from control mice did undergo spontaneous apoptosis in culture over time, as observed by others, apoptosis was greater for cells from MCF13-inoculated animals. We also observed that the difference in the percentage of dead cells was greater than that of apoptotic cells between the thymuses from virus-inoculated and control mice. As discussed below, it appears that an increase in apoptosis, which occurs soon after these cells are isolated, contributes to this increase in thymus cell death.
Additional assays for apoptosis of thymic lymphocytes from MCF13-inoculated mice.
We performed additional independent assays for apoptosis to confirm the results from our 7-AAD analysis of freshly isolated thymic lymphocytes as well as those that were placed in culture. To determine whether the increase in apoptosis that we observed for the cultured cells may be due to apoptotic events which occur before 24 h, we included a 6-h time point in these studies. Assays for apoptosis included assessments of DNA fragmentation as well as cleavage of the proenzymes PARP and procaspase-3. All three cellular events are hallmarks of apoptosis in various types of cells, including lymphocytes (1, 7, 11). Again, we analyzed thymic lymphocytes which were isolated from mice at 8 weeks after virus inoculation.
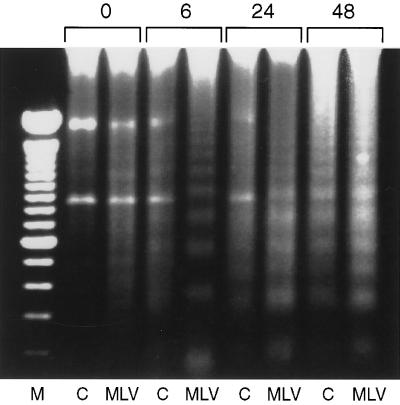
We first examined nuclear DNA degradation by internucleosomal cleavage, which has been shown to occur in thymic lymphocytes undergoing apoptosis (45, 48). Our analysis of total cellular DNA isolated from thymic cells showed an increase in DNA degradation to oligonucleosomal fragments, referred to as fragmentation, for lymphocytes isolated from MCF13 virus-inoculated mice compared with those from control mice (Fig. 3). Our data indicated that although differences in DNA fragmentation were already apparent in freshly isolated thymic cells (0 h), this difference was even greater starting at 6 h after the cells were placed in culture. In addition, we continued to detect significant differences in DNA fragmentation between thymic cells isolated from virus-inoculated and control mice at 24 and 48 h in vitro.
FIG. 3.
DNA fragmentation of thymic lymphocytes. Cellular DNA (1.5 μg) isolated from thymic lymphocytes with the Apoptotic DNA Ladder kit (Boehringer Mannheim) was electrophoresed in each well of a 1.5% agarose gel at 100 V for 1.5 h. DNA was extracted from pooled lymphocytes from thymuses of three mice which were either control (C) or inoculated with MCF13 MLV for 8 weeks (MLV). Thymic cells were placed in culture for 0, 6, 24, and 48 h before DNA extraction. Lane M, 100-bp DNA ladder (Gibco-BRL). Three independent assays which were performed with different pools of thymic lymphocytes yielded similar results.
Additional independent assays, which were performed to detect apoptosis of thymic lymphocytes, included the detection of caspase-3 and PARP proenzyme cleavage. The same pooled thymic cells which were analyzed for DNA fragmentation were also used for these enzymatic assays. It has been observed that cleavage of both procaspase-3 and PARP occurs before DNA fragmentation during cellular apoptosis (20). We first assayed the enzymatic activity of caspase-3, a cysteine protease, by using the DEVD-amc fluorogenic substrate (32). Enzyme activity was measured in five independent experiments performed at different times as described in Materials and Methods. The ratio of caspase-3 activity detected for thymic cells from control versus virus-inoculated mice was then calculated. The mean of these ratios for freshly isolated thymic cells was 3.0, with a range of 1.8 to 4.3. At 6 h in culture, the mean ratio increased to 7.0, with a range of 2.6 to 11.1. The mean ratios of caspase-3 activities for cells placed in culture for 24 and 48 h were 1.1 (range, 0.5 to 1.8) and 0.7 (range, 0.4 to 1.2), respectively. Thus, we detected an increase in caspase-3 activity which peaked at 6 h after culturing for thymic cells isolated from MCF13-inoculated mice compared with cells from control mice. These data are consistent with the observation by others that the activation of caspase-3 is an early event in apoptosis (7).
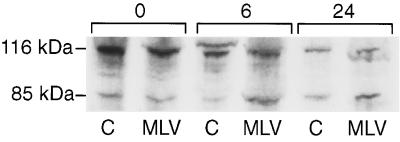
We next assessed the cleavage of the 116-kDa PARP proenzyme into 85- and 31-kDa products (25, 50). The 85-kDa cleavage product contains the catalytic subunit (11). As shown in Fig. 4, we detected a greater amount of the 85-kDa cleavage product for thymic lymphocytes isolated from MCF13-inoculated mice than for those from control mice starting at 6 h of culture. Similar results were obtained for cells in culture for 24 h. Thus, the data from three different assays for apoptosis corroborated our results from the flow cytometric analysis of thymic cells by 7-AAD staining (Tables 1 and 2) and support the idea that MCF-13 MLV infection of thymic lymphocytes induces apoptosis in these cells.
FIG. 4.
Assay of PARP cleavage. Equal amounts of cellular protein were electrophoresed through an SDS–8% polyacrylamide gel for 2 h at 120 V. The uncleaved 116-kDa and 85-kDa cleavage products of PARP were detected by immunoblotting with the C-2-10 antibody (BIOMOL Research Laboratory). Cellular protein was extracted from pooled lymphocytes from thymuses of three mice which were either control (C) or inoculated with MCF13 MLV for 8 weeks (MLV). Thymic cells were placed in culture for 0, 6, and 24 h before protein extraction. This immunoblot is representative of three independent assays performed at different times.
Increase in apoptosis of thymic lymphocytes correlates with MCF13 MLV infection.
To better determine whether MCF13 virus infection of thymic cells affects their ability to undergo cell death caused by apoptosis, we stained cells with 7-AAD and MAb 514 (13), which specifically detects MCF envelope glycoproteins. By flow cytometric analysis we selected cells expressing MCF glycoprotein (gp70+) and those which were negative for glycoprotein expression (gp70−). We then assessed the percentage of live, apoptotic, and dead cells in both of these populations of cells. Because we did not detect any difference between thymic lymphocytes isolated from virus-inoculated and control mice at 2 weeks p.i., we performed this analysis only at 8 weeks p.i. Furthermore, we analyzed only freshly isolated thymic lymphocytes because we observed that cells in culture were sensitive to the additional manipulations required for gp70 detection, which resulted in additional cell death for reasons that are not understood. As shown in Table 4, we observed significant differences between the gp70+ and gp70− cells in all cell populations examined. The percentages of apoptotic and dead cells were significantly higher, as determined by statistical analysis, for the gp70+ cells compared with the gp70− population. We also detected a concomitant decrease in the percentage of live gp70+ cells. Thus, MCF13 MLV infection of thymic lymphocytes leads to an increase in their ability to undergo cell death.
TABLE 4.
Analysis of gp70+ and gp70− thymic cellsa
| Time in culture (h) | gp70 expression | Mean % of gp70+ or gp70− cells ± SD
|
|||
|---|---|---|---|---|---|
| Live | Apoptotic | Dead | Apoptotic + dead | ||
| 0 | − | 93.2 ± 4.3 | 3.0 ± 1.6 | 4.1 ± 3.5 | 7.1 ± 4.4 |
| + | 79.9 ± 8.8b | 5.7 ± 2.8b | 14.6 ± 6.7b | 20.3 ± 8.8b | |
| 24 | − | 73.9 ± 15.4 | 11.0 ± 4.9 | 15.3 ± 13.3 | 26.3 ± 15.4 |
| + | 47.2 ± 21.5b | 9.7 ± 4.3 | 43.2 ± 20.3b | 52.9 ± 21.5b | |
| 48 | − | 46.8 ± 13.3 | 18.3 ± 7.5 | 35.1 ± 18.4 | 53.4 ± 13.1 |
| + | 24.5 ± 17.0b | 16.6 ± 8.0 | 59.0 ± 17.6b | 75.6 ± 17.0b | |
Mean percentages and standard deviations of live, apoptotic, and dead populations of gp70+ and gp70− thymic lymphocytes in culture were determined by 7-AAD staining and flow cytometric analysis as described in Materials and Methods. gp70+ and gp70− cells were selected by the CELLQuest software. Cells were placed in culture as described in Table 3, footnote a. Eleven independent experiments were performed to obtain the mean values. Thymic lymphocytes from either three control or three virus-inoculated mice were pooled for each experiment.
Significant difference, with P ≤ 0.006, as determined by Student's t test.
DISCUSSION
We have shown that MCF13 MLV infection of thymic lymphocytes during the preleukemic period results in an increase in cellular apoptosis as measured by several independent assays. Our data suggest that this increase in apoptosis is responsible for the decrease in thymus cellularity which mainly affects the CD3− CD4+ CD8+ and CD3+ CD4+ CD8+ subpopulations of immature thymic lymphocytes. We have previously shown that these are the same two subpopulations which are predominantly infected by the MCF13 virus (59). A decrease in the percentage of CD4+ CD8+ cells has also been implicated in the development of thymic tumors by the SL3-3 MLV (16, 17). Our detection of an increase in apoptosis and dead cells for freshly isolated gp70+ cells compared with gp70− cells supports the notion that virus infection is directly responsible for cellular apoptosis and subsequent death. The receptor protein for the MCF glycoprotein is closely related to the yeast SYG1 protein (2, 49, 58), which induces transient cell cycle arrest in response to the yeast mating pheromone (46). Whether the mammalian homolog of the SYG1 protein is part of a signal transduction pathway that is involved in the induction of apoptosis of virus-infected cells remains to be determined.
It is interesting that the same subpopulations in the thymus which are depleted by MCF13 MLV infection are those which undergo positive and negative selection (15, 22, 30). The majority of cells in these subpopulations, which together comprise 80 to 90% of total thymic lymphocytes (14, 41), are eliminated by apoptosis within a few days of development in the thymus (29). The CD4+ CD8+ cells are mainly present in the cortical regions of the thymus. Our results of a histological examination of the thymus after MCF13 virus inoculation showed lymphocyte depletion predominantly of the cortical regions, which correlated with our detection of reduced numbers of CD4+ CD8+ cells in virus-infected animals. This observation is corroborated by an earlier study which showed that thymuses from 6-month-old AKR mice undergo cortical involution during the development of spontaneous tumors in these animals (33).
It has been shown that tumorigenesis in the AKR mouse is a complex phenomenon, which also involves a role of the thymic stroma. During the development of both spontaneous and SL3-3 MLV-induced disease, it has been shown that virus infection of thymic stromal cells affects T-lymphocyte maturation (10, 16, 17). Virus infection of the CD4+ CD8+ cells may contribute to an alteration in the interactions of thymic lymphocytes with stromal epithelial cells, thus possibly resulting in abnormal maturation of the virus-infected cells.
Although both the inhibition and induction of apoptosis have been implicated in tumorigenesis, it has been most commonly observed that the development of malignancies is dependent upon an initial inhibition of apoptosis (3, 31, 56). In contrast, we have demonstrated that the development of thymic tumors by MCF MLV infection results in an enhancement of apoptosis during the early stages of lymphomagenesis. Induction of apoptosis as an early event in cellular transformation has been observed for other oncogenic retroviruses as well. Fan and coworkers have shown that the Moloney MLV, which also generates thymic tumors, increased thymic cell apoptosis also during the preleukemic period (4). Their data furthermore demonstrated that the level of cellular apoptosis correlated with viral pathogenicity. Another example includes the subgroups B and D avian leukosis viruses, which cause high levels of cell death during the acute phase of infection (55). This viral cytopathicity has been attributed to the binding of the avian leukosis virus glycoprotein to the receptor molecule CAR1, which is a member of the tumor necrosis factor receptor family (5). Other examples of the induction of apoptosis during cellular transformation include the transformation of pre-B cells in vitro by the Abelson murine leukemia virus (38, 52) and of T cells by the feline leukemia virus subgroup C (40).
For the induction of thymic tumors by MCF13 MLV, we hypothesize that cells which survive the apoptotic crisis induced by virus infection are those which will evolve into a fully malignant cell. It is likely that the progression to a tumor cell requires the inheritance of multiple genetic mutations, some of which result in the inhibition of apoptosis. An example of such an event is proviral insertional mutagenesis of the c-myc protooncogene, which occurs at a significant frequency in thymic tumors induced by various murine leukemia viruses. It has been shown that overexpression of c-myc can rescue lymphocytes from apoptosis (19, 57). Additional genetic mutations involving other cellular proteins which are able to rescue cells from apoptosis, such as members of the Bcl-2 and NF-κB families, may also play a role in the development of MLV-induced thymic tumors. Our detection of an increase in NF-κB in MCF13-induced tumors by 50- to 200-fold compared with normal thymuses would support this idea (unpublished results). It is noteworthy that NF-κB and c-Myc have synergistic roles in inhibiting apoptosis for certain cell types (42). Our results suggest that the induction of apoptosis as an early event in the development of lymphomas may be a common mechanism used by different transforming retroviruses.
ACKNOWLEDGMENTS
We thank Yihua Zhang and Younong Min for excellent technical assistance. Eric Van Buren's help in performing the flow cytometry experiments is greatly appreciated. We also thank Gangyong Li for assistance in the PARP assays and Chris Brantley for assistance in preparing the histological sections.
This work was supported by Public Health Service grants from the National Institutes of Health (CA44166 to F.K.Y., CA64139 to H.-R.C.K., and DK02503 to J.R.T.).
REFERENCES
- 1.Allen P D, Newland A C. Electrophoretic DNA analysis for the detection of apoptosis. Mol Biotechnol. 1998;9:247–251. doi: 10.1007/BF02915798. [DOI] [PubMed] [Google Scholar]
- 2.Battini J L, Rasko J E, Miller A D. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedi A, Pasricha P J, Akhtar A J, Barber J P, Bedi G C, Giardiello F M, Zehnbauer B A, Hamilton S R, Jones R J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- 4.Bonzon C, Fan H. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J Virol. 1999;73:2434–2441. doi: 10.1128/jvi.73.3.2434-2441.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 6.Cloyd M W, Hartley J W, Rowe W P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980;151:542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran L M, Adams J M, Dunn A R, Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984;37:113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- 9.Cuypers H T, Selten G, Quint W, Zijlstra M, Maandag E R, Boelens W, van Wezenbeek P, Melief C, Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984;37:141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- 10.Davey G M, Tucek-Szabo C L, Boyd R L. Characterization of the AKR thymic microenvironment and its influence on thymocyte differentiation and lymphoma development. Leukocyte Res. 1996;20:853–866. doi: 10.1016/0145-2126(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 11.Duriez P J, Shah G M. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- 12.Evans L H, Malik F G. Class II polytropic murine leukemia viruses (MuLVs) of AKR/J mice: possible role in the generation of class I oncogenic polytropic MuLVs. J Virol. 1987;61:1882–1892. doi: 10.1128/jvi.61.6.1882-1892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowlkes B J, Pardoll D M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- 15.Fowlkes B J, Schwartz R H, Pardoll D M. Deletion of self-reactive thymocytes occurs at a CD4+8+ precursor stage. Nature. 1988;334:620–623. doi: 10.1038/334620a0. [DOI] [PubMed] [Google Scholar]
- 16.Hays E F, Bristol G, McDougall S. Mechanisms of thymic lymphomagenesis by the retrovirus SL3-3. Cancer Res. 1990;50:5631s–5635s. [PubMed] [Google Scholar]
- 17.Hays E F, Bristol G C, Lugo J P, Wang X F. Progression to development of lymphoma in the thymus of AKR mice treated neonatally with SL3-3 virus. Exp Hematol. 1989;17:1116–1121. [PubMed] [Google Scholar]
- 18.Hays E F, Bristol G C, McDougall S, Klotz J L, Kronenberg M. Development of lymphoma in the thymus of AKR mice treated with the lymphomagenic virus SL3-3. Cancer Res. 1989;49:4225–4230. [PubMed] [Google Scholar]
- 19.Hermann M, Scholman H J, Marafioti T, Stein H, Schriever F. Differential expression of apoptosis, Bcl-x and c-Myc in normal and malignant lymphoid tissues. Eur J Haematol. 1997;59:20–30. doi: 10.1111/j.1600-0609.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 21.Kawashima K, Ikeda H, Hartley J W, Stockert E, Rowe W P, Old L J. Changes in expression of murine leukemia virus antigens and production of xenotropic virus in the late preleukemic period in AKR mice. Proc Natl Acad Sci USA. 1976;73:4680–4684. doi: 10.1073/pnas.73.12.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 23.Kraft D L, Weissman I L, Waller E K. Differentiation of CD3−4−8− human fetal thymocytes in vivo: characterization of a CD3−4+8− intermediate. J Exp Med. 1993;178:265–277. doi: 10.1084/jem.178.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kydd R, Lundberg K, Vremec D, Harris A, Shortman K. Intermediate steps in thymic positive selection. J Immunol. 1995;155:3806–3814. [PubMed] [Google Scholar]
- 25.Lazebnik Y A, Kaufmann S H, Desnoyers S, Poirier G G, Earnshaw W C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 26.Lazo P A, Klein-Szanto A J P, Tsichlis P N. T-cell lymphoma lines derived from rat thymomas induced by Moloney murine leukemia virus: phenotypic diversity and its implications. J Virol. 1990;64:3948–3959. doi: 10.1128/jvi.64.8.3948-3959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz J, Celander D, Crowther R L, Patarca R, Perkins D W, Haseltine W A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. Nature. 1984;308:467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- 28.Lung M L, Hartley J W, Rowe W P, Hopkins N H. Large RNase T1-resistant oligonucleotides encoding p15E and the U3 region of the long terminal repeat distinguish two biological classes of mink cell focus-forming type C viruses of inbred mice. J Virol. 1983;45:275–290. doi: 10.1128/jvi.45.1.275-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPhee D, Pye J, Shortman K. The differentiation of T lymphocytes. V. Evidence for intrathymic death of most thymocytes. Thymus. 1979;1:151–162. [PubMed] [Google Scholar]
- 29a.Metcalf D. The thymus. Rec Results Cancer Res. 1966;5:1–144. [Google Scholar]
- 30.Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 31.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 33.Nowinski R C, Doyle T. Cellular changes in the thymuses of preleukemic AKR mice: correlation with changes in the expression of murine leukemia viruses. Cell. 1977;12:341–353. doi: 10.1016/0092-8674(77)90110-6. [DOI] [PubMed] [Google Scholar]
- 34.O'Donnell P V, Stockert E, Obata Y, Old L J. Leukemogenic properties of AKR dualtropic (MCF) viruses: amplification of murine leukemia virus-related antigens on thymocytes and acceleration of leukemia development in AKR mice. Virology. 1981;112:548–563. doi: 10.1016/0042-6822(81)90301-9. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell P V, Nowinski R C, Stockert E. Amplified expression of murine leukemia virus (MuLV)-coded antigens on thymocytes and leukemia cells of AKR mice after infection by dualtropic (MCF) MuLV. Virology. 1982;119:450–464. doi: 10.1016/0042-6822(82)90104-0. [DOI] [PubMed] [Google Scholar]
- 36.Peters G. Oncogenes at viral integration sites. Cell Growth Differ. 1990;1:503–510. [PubMed] [Google Scholar]
- 37.Philpott N J, Turner A J, Scopes J, Westby M, Marsh J C, Gordon-Smith E C, Dalgleish A G, Gibson F M. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- 38.Radfar A, Unnikrishnan I, Lee H W, DePinho R A, Rosenberg N. p19(Arf) induces p53-dependent apoptosis during abelson virus-mediated pre-B cell transformation. Proc Natl Acad Sci USA. 1998;95:13194–13199. doi: 10.1073/pnas.95.22.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinner I, Felsner P, Hofer D, Globerson A, Schauenstein K. Characterization of the spontaneous apoptosis of rat thymocytes in vitro. Int Arch Allergy Immunol. 1996;111:230–237. doi: 10.1159/000237372. [DOI] [PubMed] [Google Scholar]
- 40.Rojko J L, Fulton R M, Rezanka L J, Williams L L, Copelan E, Cheney C M, Reichel G S, Neil J C, Mathes L E, Fisher T G, et al. Lymphocytotoxic strains of feline leukemia virus induce apoptosis in feline T4-thymic lymphoma cells. Lab Investig. 1992;66:418–426. [PubMed] [Google Scholar]
- 41.Rothenberg E V. The development of functionally responsive T cells. Adv Immunol. 1992;58:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- 42.Schauer S L, Wang Z, Sonenshein G E, Rothstein T L. Maintenance of nuclear factor-kappa B/Rel and c-myc expression during CD40 ligand rescue of WEHI 231 early B cells from receptor-mediated apoptosis through modulation of I kappa B proteins. J Immunol. 1996;157:81–86. [PubMed] [Google Scholar]
- 43.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 44.Scollay R. T-cell subset relationships in thymocyte development. Curr Opin Immunol. 1991;3:204–209. doi: 10.1016/0952-7915(91)90051-2. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y F, Bissonnette R P, Parfrey N, Szalay M, Kubo R T, Green D R. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146:3340–3346. [PubMed] [Google Scholar]
- 46.Spain B H, Koo D, Ramakrishnan M, Dzudzor B, Colicelli J. Truncated forms of a novel yeast protein suppress the lethality of a G protein alpha subunit deficiency by interacting with the beta subunit. J Biol Chem. 1995;270:25435–25444. doi: 10.1074/jbc.270.43.25435. [DOI] [PubMed] [Google Scholar]
- 47.Stoye J P, Moroni C, Coffin J M. Virological events leading to spontaneous AKR thymomas. J Virol. 1991;65:1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, Okumura K. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur J Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 49.Tailor C S, Nouri A, Lee C G, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tewari M, Quan L T, O'Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 51.Thomas C Y, Coffin J M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982;43:416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thome K C, Radfar A, Rosenberg N. Mutation of Tp53 contributes to the malignant phenotype of Abelson virus-transformed lymphoid cells. J Virol. 1997;71:8149–8156. doi: 10.1128/jvi.71.11.8149-8156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiso M, Gangemi R, Bargellesi Severi A, Pizzolitto S, Fabbi M, Risso A. Spontaneous apoptosis in human thymocytes. Am J Pathol. 1995;147:434–444. [PMC free article] [PubMed] [Google Scholar]
- 54.Tupper J C, Chen H, Hays E F, Bristol G C, Yoshimura F K. Contributions to transcriptional activity and to viral leukemogenicity made by sequences within and downstream of the MCF13 murine leukemia virus enhancer. J Virol. 1992;66:7080–7088. doi: 10.1128/jvi.66.12.7080-7088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weller S K, Temin H M. Cell killing by avian leukosis viruses. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams G T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- 57.Wu M, Yang W, Bellas R E, Schauer S L, FitzGerald M J, Lee H, Sonenshein G E. c-myc promotes survival of WEHI 231 B lymphoma cells from apoptosis. Curr Top Microbiol Immunol. 1997;224:91–101. doi: 10.1007/978-3-642-60801-8_9. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y L, Guo L, Xu S, Holland C A, Kitamura T, Hunter K, Cunningham J M. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura F K, Wang T, Cankovic M. Sequences between the enhancer and promoter in the long terminal repeat affect murine leukemia virus pathogenicity and replication in the thymus. J Virol. 1999;73:4890–4898. doi: 10.1128/jvi.73.6.4890-4898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]