Abstract
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and represents a significant global health burden. Management of HCC can be challenging due to multiple factors, including variable expectations for treatment outcomes. Several treatment options are available, each with specific eligibility and ineligibility criteria, and are provided by a multidisciplinary team of specialists. Radiologists should be aware of the types of treatment options available, as well as the criteria guiding the development of individualized treatment plans. This awareness enables radiologists to contribute effectively to patient-centered multidisciplinary tumor boards for HCC and play a central role in reassessing care plans when the treatment response is deemed inadequate.
This comprehensive review aims to equip radiologists with an overview of HCC staging systems, treatment options, and eligibility criteria. The review also discusses the significance of imaging in HCC diagnosis, treatment planning, and monitoring treatment response. Furthermore, we highlight the crucial branch points in the treatment decision-making process that depend on radiological interpretation.
Keywords: Liver imaging, Hepatocellular carcinoma, Liver cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third most common cause of cancer-related death worldwide[1]. HCC is most frequently seen in people with underlying liver disease, such as hepatitis B or cirrhosis caused by hepatitis C infection, alcohol abuse, or steatotic liver disease[2]. Diagnosis of this malignancy is typically made by imaging but can be confirmed by a biopsy when needed. Several treatment options are available, including surgical resection, liver transplantation (LT), ablation therapy, radiation therapy, targeted or systemic chemotherapy, and immunotherapy[3]. The choice of treatment depends on the stage and size of the tumor, as well as the overall health of the patient, and each treatment option has specific eligibility and ineligibility criteria[4].
Radiologists play a vital role in the multidisciplinary management of HCC. A dedicated hepatobiliary radiologist can provide expert opinion and accurate interpretation of liver imaging studies, which are crucial for guiding treatment and management decisions. Radiologists must be aware of the types of treatment options available and the criteria by which the treatment plan is developed in order to be a valuable member of a patient-centered multidisciplinary tumor board for HCC[5–7]. This review aims to provide radiologists with a comprehensive overview of HCC treatment options and management algorithms and to discuss the most important questions that the radiologist needs to answer when helping to guide management decisions of the multidisciplinary liver tumor (MDLT) board.
Overview of HCC staging systems
Several staging systems are used to classify and stage HCC. The American Joint Committee on Cancer (AJCC) utilizes the TNM staging system to quantify the size and spread of the tumor[8]. However, for treatment decisions, the most widely used classification system for treatment of HCC is the Barcelona Clinic Liver Cancer (BCLC) staging system. This system is based on the size and number of tumors, the presence of symptoms, the patient’s performance status, and the liver functional status as determined by the Child-Pugh classification system[4]. The Child-Pugh classification is determined by the patient’s bilirubin and albumin levels, the prothrombin time, and the presence of ascites and/or encephalopathy[9]. The BCLC staging system classifies HCC into five stages: 0, A, B, C, and D. Stage 0 is early-stage HCC that is considered curable, whereas stages C and D are considered advanced-stage HCC with poor prognosis (Table 1)[4]. None of these staging systems are mutually exclusive, and multiple systems are used together to provide a comprehensive assessment of the patient’s overall condition in the context of cirrhosis and treatment options that can have significant side effects. The most common treatments are liver-directed therapies and surgical options (Figure 1).
Table 1:
Summary of Barcelona Clinic Liver Cancer staging and treatment recommendations.
| Stage | Description | Treatment Recommendations |
|---|---|---|
| 0 | Very early stage: Single tumor ≤2 cm | Resection, liver transplantation, or ablation |
| A | Early stage: Single tumor >2 cm or ≤3 nodules ≤3 cm | Resection, liver transplantation, or ablation |
| B | Intermediate stage: Multinodular without vascular invasion or extrahepatic spread | Transarterial chemoembolization (TACE) or other locoregional therapies |
| C | Advanced stage: Presence of vascular invasion, extrahepatic spread, or cancer-related symptoms | Systemic therapy (e.g., sorafenib, lenvatinib, and regorafenib) |
| D | Terminal stage: Severe liver dysfunction or poor performance status | Supportive care |
Figure 1:
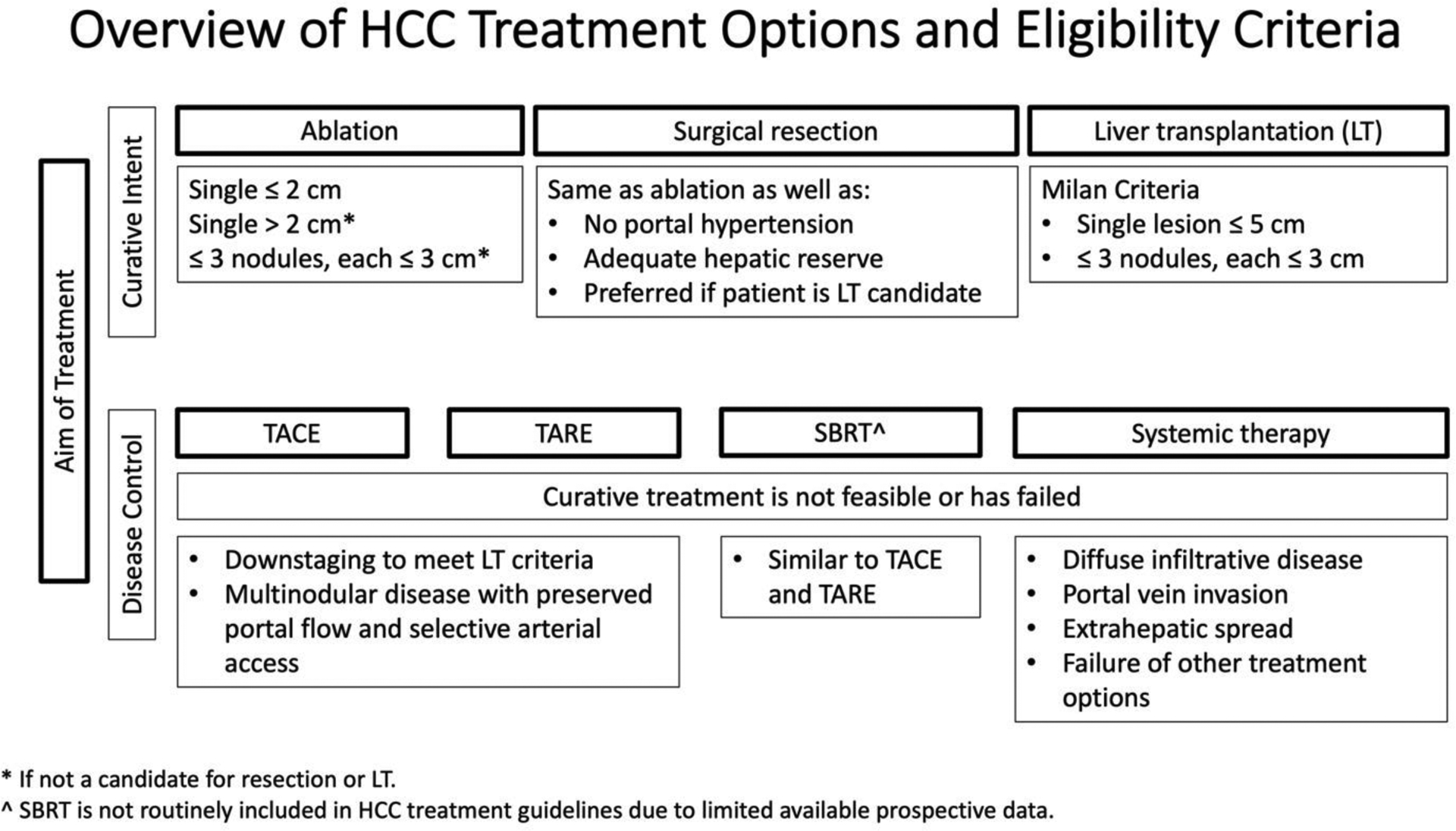
Summary of HCC treatment options and eligibility criteria based on the Barcelona Clinic Liver Criteria 2022.
Serum Alpha-fetoprotein (AFP) level is the most widely used tumor marker for HCC[10]. Patients with high AFP levels (≥400 ng/mL) were found to have a poorer prognosis[11]. Higher pretreatment AFP level was found to predict tumor recurrence after hepatic resection or liver transplantation as well as predict prognosis and survival with locoregional therapies[12, 13].
Overview of HCC treatment options
A). Percutaneous ablative therapies.
Ablation destroys tumor cells by raising the temperature within the targeted tissue high enough to cause coagulative necrosis and subsequent tumor eradication (radiofrequency ablation and microwave ablation) or by cooling the tissue to the point of creating an ice-ball with a freeze-thaw-freeze cycle to achieve tumor necrosis (cryoablation)[14]. Ablation is the treatment of choice for patients with single HCC tumors ≤ 2 cm and preserved liver function (e.g., Child-Pugh class A) and is considered a curative treatment[4]. In lesions >2 cm, radiofrequency ablation is less effective and has an increased risk of recurrence[4]. Microwave ablation usually achieves more tumor necrosis than radiofrequency ablation and can be successful in lesions up to 4 cm[15]. Ablation can be done through percutaneous or laparoscopic approaches. Laparoscopic ablation is advantageous for treating tumors that are not accessible percutaneously due to factors like targeting window constraints, hepatic surface location, subdiaphragmatic locations, proximity to important extra-hepatic structures, or multiplicity[16].
Other, less common, ablative techniques include high-intensity focused ultrasound, laser therapy, and irreversible electroporation.
B). Surgical treatment
Surgical resection is another option for solitary HCC. Although radiofrequency ablation and resection offer comparable survival benefits for HCC tumors ≤2 cm[17, 18], resection has the advantage in larger solitary tumors. It also provides an additional benefit if subsequent LT is an option, as it allows for the study of lesion pathology[19]. If the pathologic features indicate an increased risk of recurrence, this may induce consideration for future LT. Surgical resection carries a significant risk of increased morbidity and mortality in patients with portal hypertension, and LT should be considered as an alternative option because it provides better medium- and long-term survival[20]. Sufficient postoperative healthy liver volume is also an important consideration when planning for resection; typically, a well-perfused liver volume remnant that is at least 40% of the original liver is needed to avoid post-resection liver failure.
Liver transplantation should always be considered in patients with HCC, regardless of disease stage, due to the high risk of recurrence[4, 21]. However, several clinical and patient-dependent factors determine eligibility for LT. The Milan criteria define eligibility for transplantation as a single lesion smaller than 5 cm or up to 3 lesions, none larger than 3 cm, without macrovascular invasion/tumor thrombus[22]. Access to liver transplantation is crucial in patients with early HCC and severe liver dysfunction, as it is the treatment option of choice if they meet LT enlistment criteria. The model for end-stage liver diseases (MELD) score system is commonly used for organ allocation in patients undergoing LT as it determines the disease severity based on laboratory parameters to allocate donor organs to the sickest patient first [23]. Patients with HCC commonly have a relatively low MELD score at the time of diagnosis which underestimates their urgency for transplantation before tumor progression beyond the criteria of LT. However, MELD exception points can be granted to some HCC patients based on their tumor stage to improve their access to the LT donor pool[23].
If patients are ineligible for LT due to non-HCC factors such as poor general condition or alcohol/substance use disorder, their disease is staged as terminal (BCLC D) because of their poor predicted survival[4].
C). Locoregional therapies.
Trans-arterial chemoembolization (TACE) involves the selective delivery of chemotherapeutic agents into the hepatic artery supplying the tumor, followed by embolization to block the blood flow. This dual approach starves the tumor of oxygen and nutrients while simultaneously delivering a high concentration of chemotherapy, leading to targeted tumor cell death and sparing the surrounding healthy liver tissue. TACE is commonly used to treat multifocal HCC to prevent tumor progression when the LT wait time exceeds 6 months or to downstage the tumor if the patient is ineligible for LT [4, 24]. Post-embolization syndrome, characterized by fever, abdominal pain, nausea, and vomiting, is the most frequent side effect, affecting 35–100% of patients undergoing TACE. Possible complications include liver failure, injury to the biliary tree or hepatic artery, and infection[25]. Because of the increased risk of adverse effects and decreased survival after TACE in patients with poor liver function, a bilirubin level of <2 mg and no fluid retention requiring diuretic therapy are prerequisites for this treatment option [24]. Contraindications include but are not limited to decompensated cirrhosis, malignant ascites, portal vein thrombosis, extrahepatic metastasis, and hypovascular tumor based on imaging appearance.
Trans-arterial radioembolization (TARE) involves intra-arterial administration of radioactive microbeads containing β-emitting yttrium-90 (Y90) isotopes directly to the tumor via the hepatic artery branches[26]. TARE can be used as bridging therapy before liver transplantation, to facilitate resection (with radiation lobectomy), or as an alternative to ablation[27, 28]. TARE has demonstrated survival durations comparable to those with TACE, but with a notably longer time to progression (>26 months compared to 6.8 months for TACE)[29]. Additionally, TARE presents fewer complications, which is crucial for patients awaiting transplantation [29]. In comparison to TACE, TARE has proven to be more effective in downstaging the disease [27, 28]. Optimal results can be achieved in patients with Child-Pugh class A disease with solitary peripheral tumors measuring less than 8 cm[30]. Additionally, Y90 microspheres have a minimal embolic effect and are, thus, considered safe in patients with portal vein invasion. In fact, there is no difference in risk of liver failure between patients with main portal vein invasion, those with branch portal vein invasion, or those with no portal vein invasion[26]. Over the past 20 years, complications from TARE have decreased due to improved patient selection, radioembolic devices, and advancements in angiography, including cone-beam CT. TARE can be performed as an outpatient procedure, with fatigue being the most common side effect. Major complications, such as gastrointestinal ulceration and radiation-induced issues, are rare, while radioembolization-induced liver disease and perihepatic fluid or pleural effusions are mainly seen in patients with limited hepatic reserve. Biliary necrosis, biloma, and hepatic abscess occur more frequently in the treatment of metastatic liver disease than in HCC[31].
Stereotactic body radiation therapy (SBRT) involves the delivery of high-dose radiation in a targeted manner, with the radiation dose quickly decreasing outward from the center of the targeted zone[32]. This results in sparing the surrounding non-tumorous liver tissue while effectively treating the tumor. SBRT is usually administered in 3–5 doses and has a low risk of radiation-induced liver disease. Patients with moderately good liver function are ideal candidates for SBRT, as they have a lower risk of hepatic decompensation compared to those with advanced cirrhosis[33]. SBRT is not yet included in the Barcelona Conference guidelines but is recommended as a treatment option for non-operable HCC in the National Comprehensive Cancer Center guidelines[34]. SBRT can be used as definitive, palliative, or bridge-to-transplantation therapy for HCC[32]. Although SBRT was historically indicated for patients ineligible for surgical resection or other localized treatments, its use has increased as clinical trials have shown SBRT to be effective in providing local tumor control for HCCs; however, SBRT has variable outcomes depending on the patient’s liver function, and data on long-term follow-up are lacking[32]. SBRT can treat tumors not suitable for percutaneous ablation and in patients with portal vein tumor thrombosis.
Irreversible electroporation (IRE) is an emerging technology that addresses challenges posed by some tumor locations, which current ablation methods struggle with. Thermal ablative methods depend on delivering heat to tissues, presenting challenges related to the size and location of their application[35]. Research on livers treated with RFA shows that successful tumor necrosis drops below 50% when tumors are near vessels larger than 3mm due to the heat sink effect[36]. Moreover, full ablation becomes challenging for lesions near the gallbladder, in subcapsular locations, or close to crucial structures like major bile ducts, portal vein, and hepatic veins[35]. Unlike traditional methods that use heat, IRE uses short electrical pulses to form permanent pores in cell membranes, causing cell death. A significant advantage of IRE is that it preserves the extracellular matrix, maintaining the integrity of nearby structures like bile ducts and blood vessels[35]. Numerous studies have demonstrated its safety and effectiveness in HCC ablation[35, 37, 38].
D). Systemic therapy.
Systemic therapies in the form of molecularly targeted and immune checkpoint therapies, is usually reserved for advanced-stage HCC. Patients with end-stage liver disease or major cancer-related symptoms who are ineligible for LT due to HCC burden or non-HCC-related factors are often candidates for systemic therapy, as well[4].
Figures 2 and 3 provide a simplified decision-making algorithm. However, real-world HCC treatment planning can be more complex, with multiple clinical and nonclinical factors that may change the treatment of choice for each individual patient. Due to the varied treatment options provided by multiple specialists, patient-centered treatment plans are best developed by a multidisciplinary tumor board comprising all of the relevant specialists.
Figure 2:
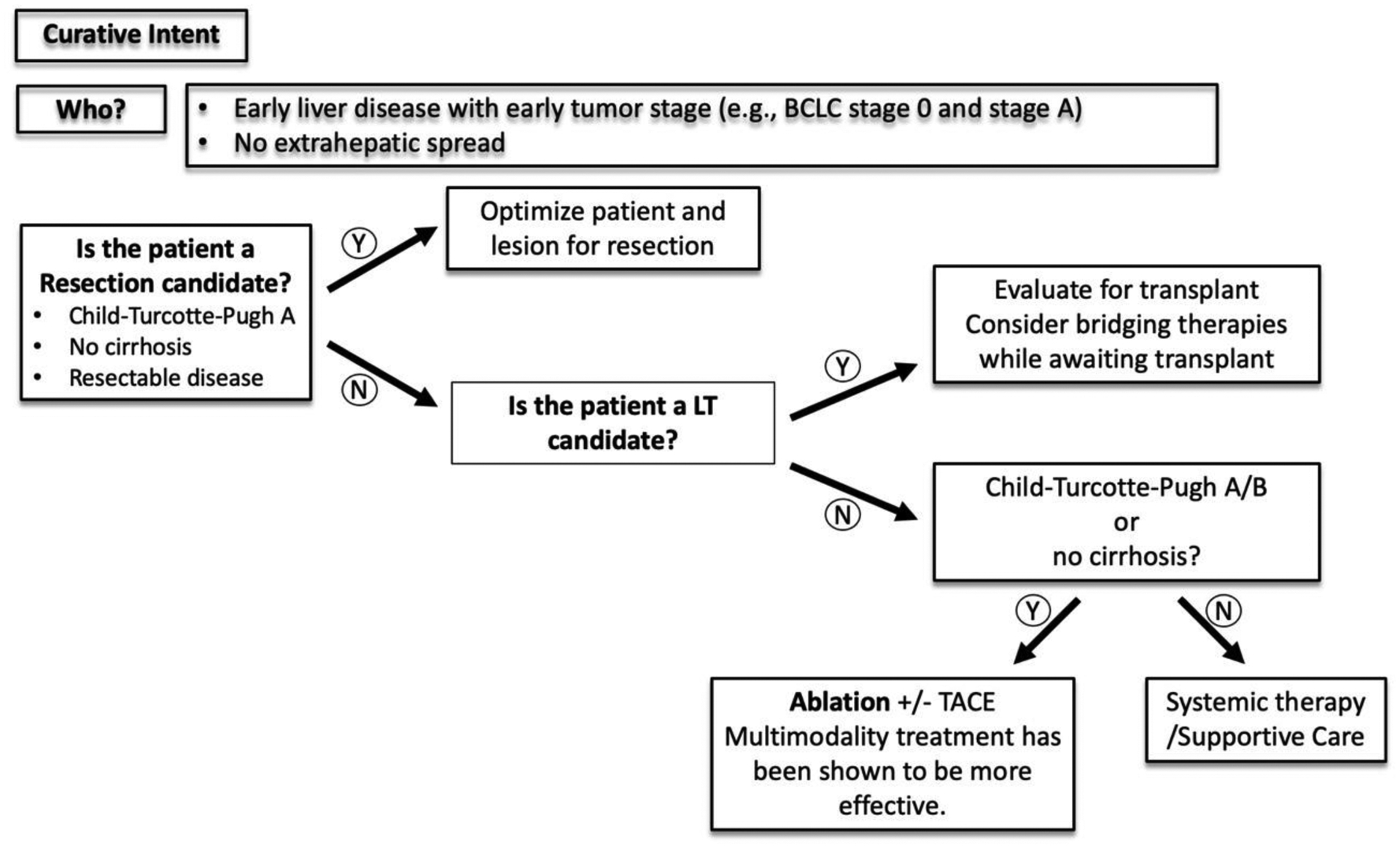
A simplified decision-making algorithm for HCC management with curative intent.
Figure 3:
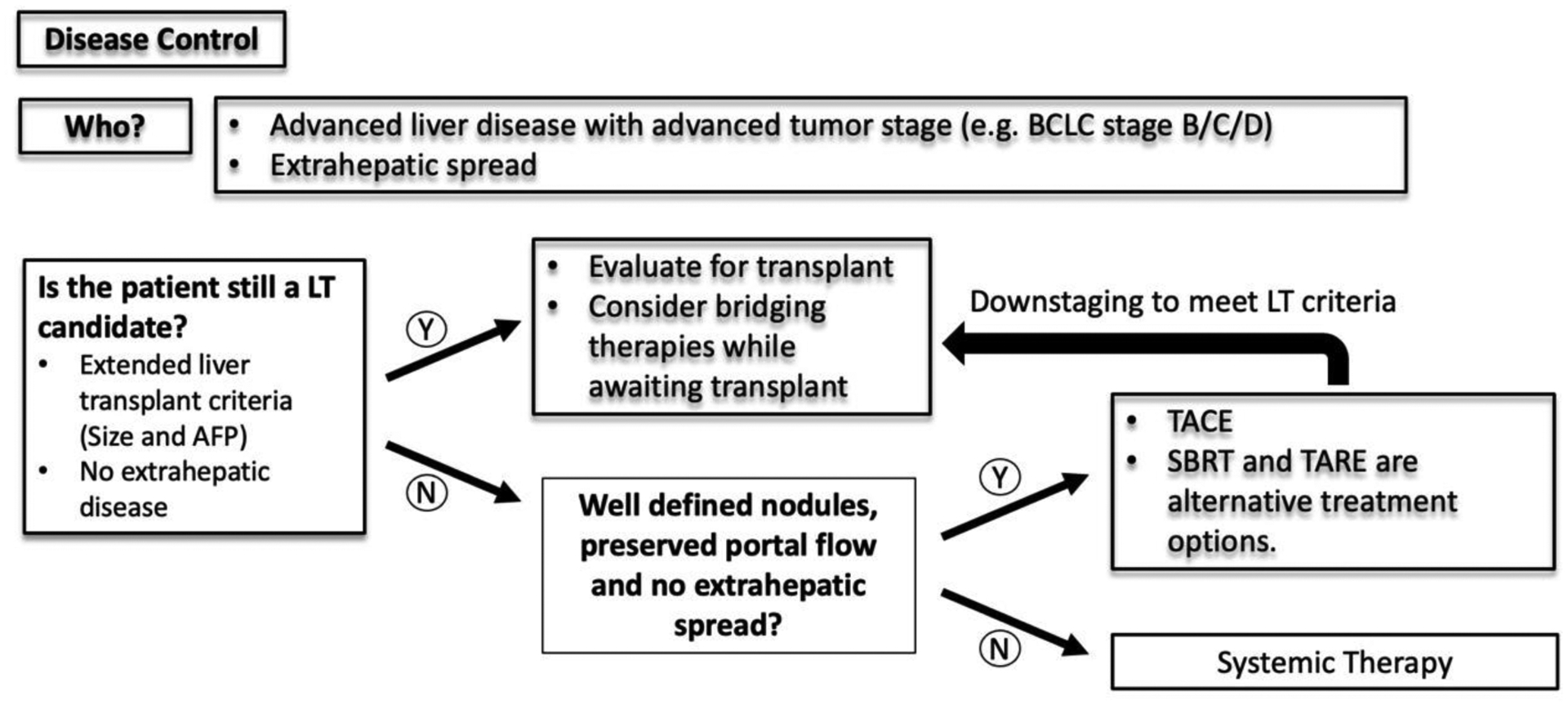
A simplified decision-making algorithm for HCC management for disease control.
Role of radiologists in MDLT boards
The accurate interpretation of liver imaging studies by a radiologist is crucial in guiding treatment and management decisions for patients with HCC. In this section, we explore the key information that imaging can provide and the questions that the clinical multidisciplinary tumor board team seeks to answer.
1. Does the patient have HCC?
Depending on the modality used, imaging features diagnostic for HCC can vary. Ultrasound is mostly used for HCC screening in patients who have cirrhosis or are at risk for HCC [39]. HCCs have a variable appearance on ultrasound but typically are seen as hypoechoic lesions with well-defined margins. Lesions found by screening ultrasound must be further characterized by diagnostic computed tomography (CT), magnetic resonance imaging (MRI), or contrast-enhanced ultrasound (CEUS). In a multiphase contrast-enhanced CT/MRI study, HCC typically appears as arterially enhancing, with non-rim arterial hyperenhancement and washout in the portal venous or delayed phases[40]. Similarly, on CEUS, HCC will typically appear as a hyper-enhancing lesion in the early arterial phase but with mild washout that occurs later and is less significant than that seen with metastases or cholangiocarcinoma [41].
The Liver Imaging Reporting and Data System (LI-RADS) is a standardized system of imaging criteria and reporting developed by radiologists in conjunction with surgeon and gastroenterologist stakeholders, endorsed by the American College of Radiology (ACR), for the diagnosis of HCC. LI-RADS criteria incorporate both morphological and functional imaging features to classify liver lesions into five categories: LR-1 (benign), LR-2 (probably benign), LR-3 (indeterminate), LR-4 (probably HCC), and LR-5 (almost definitely HCC)[40]. The diagnosis of HCC based on imaging requires non-rim, arterial-phase hyperenhancement (APHE) with one or more of the following major features, depending on lesion size: delayed washout, enhancing capsule, and threshold growth[40] (Table 2). Threshold growth is defined as a ≥ 50% size increase in ≤ 6 months[40]. Abdominal MRI offers the use of optional ancillary findings that can upgrade an observation to LR-4 and possibly trigger more aggressive surveillance or biopsy after discussion at a multidisciplinary case conference, but it cannot be used to definitively categorize a lesion as LR-5. An important note is that the LI-RADS algorithm only applies to patients with cirrhosis due to specific etiologies and patients with chronic hepatitis B viral infection with or without cirrhosis. It is not applicable to patients with cirrhosis due to congenital hepatic fibrosis or vascular disorders (such as Budd-Chiari) or to patients post-Fontan surgery[40]. Although CEUS is not currently recognized by the Organ Procurement and Transplantation Network (OPTN) for HCC diagnosis, it is considered adequate by the ACR LI-RADS and the European Association for the Study of the Liver in patients who are ineligible for liver transplantation[41, 42] (Figure 4).
Table 2:
LI-RADS version 2018 CT/MRI diagnostic table
| Arterial hyperenhancement (APHE) | No APHE | Non-rim APHE | ||||
|---|---|---|---|---|---|---|
| Observation size (mm) | < 20 | ≥ 20 | <10 | 10–19 | ≥20 | |
No. of additional major features:
|
None | LR-3 | LR-3 | LR-3 | LR-3 | LR-4 |
| One | LR-3 | LR-4 | LR-4 | LR-4/5* | LR-5 | |
| ≥ Two | LR-4 | LR-4 | LR-4 | LR-5 | LR-5 | |
Observations in this cell are categorized based on one additional major feature:
- LR-4 if enhancing capsule.
- LR-5 if non-peripheral washout or threshold growth.
Figure 4:
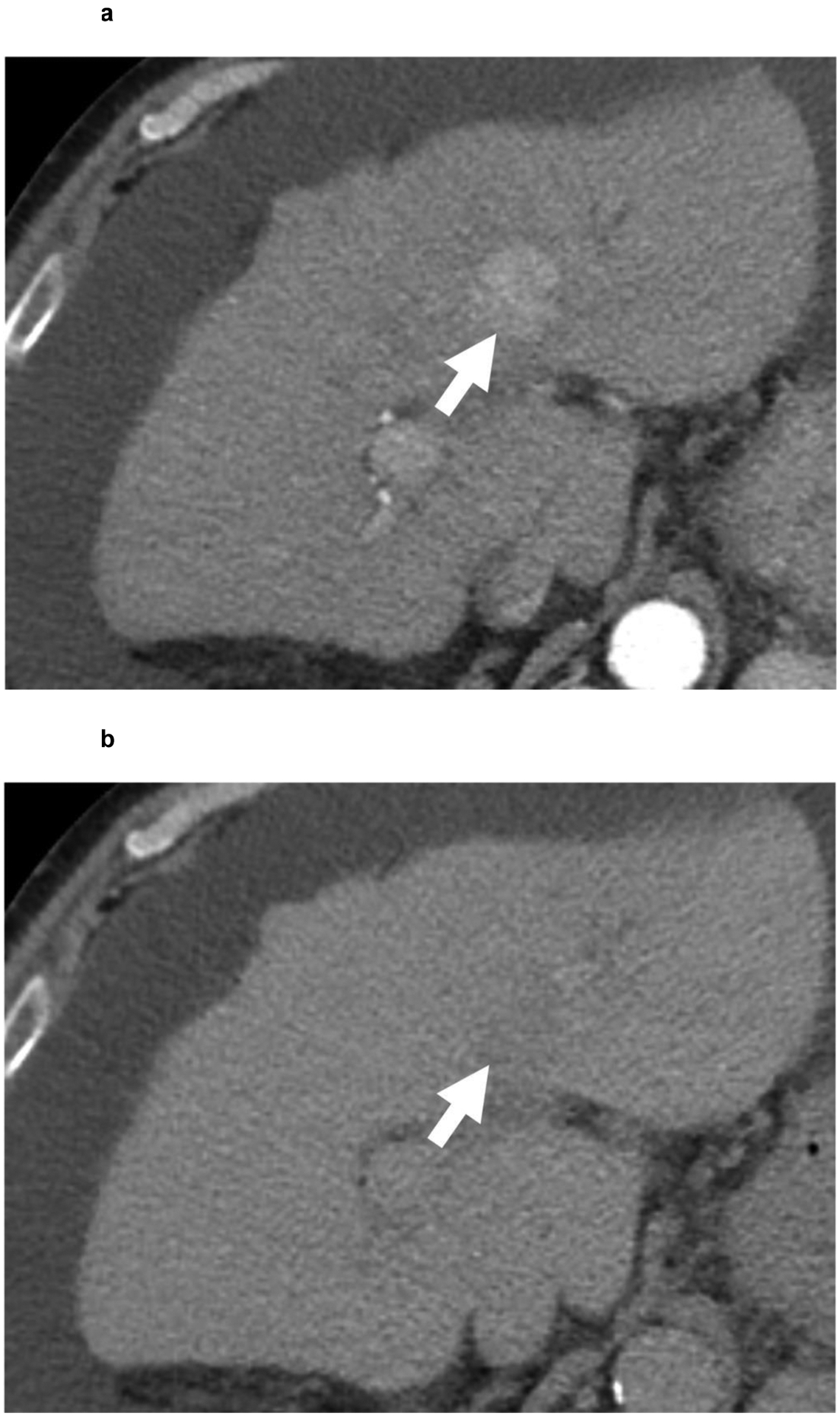
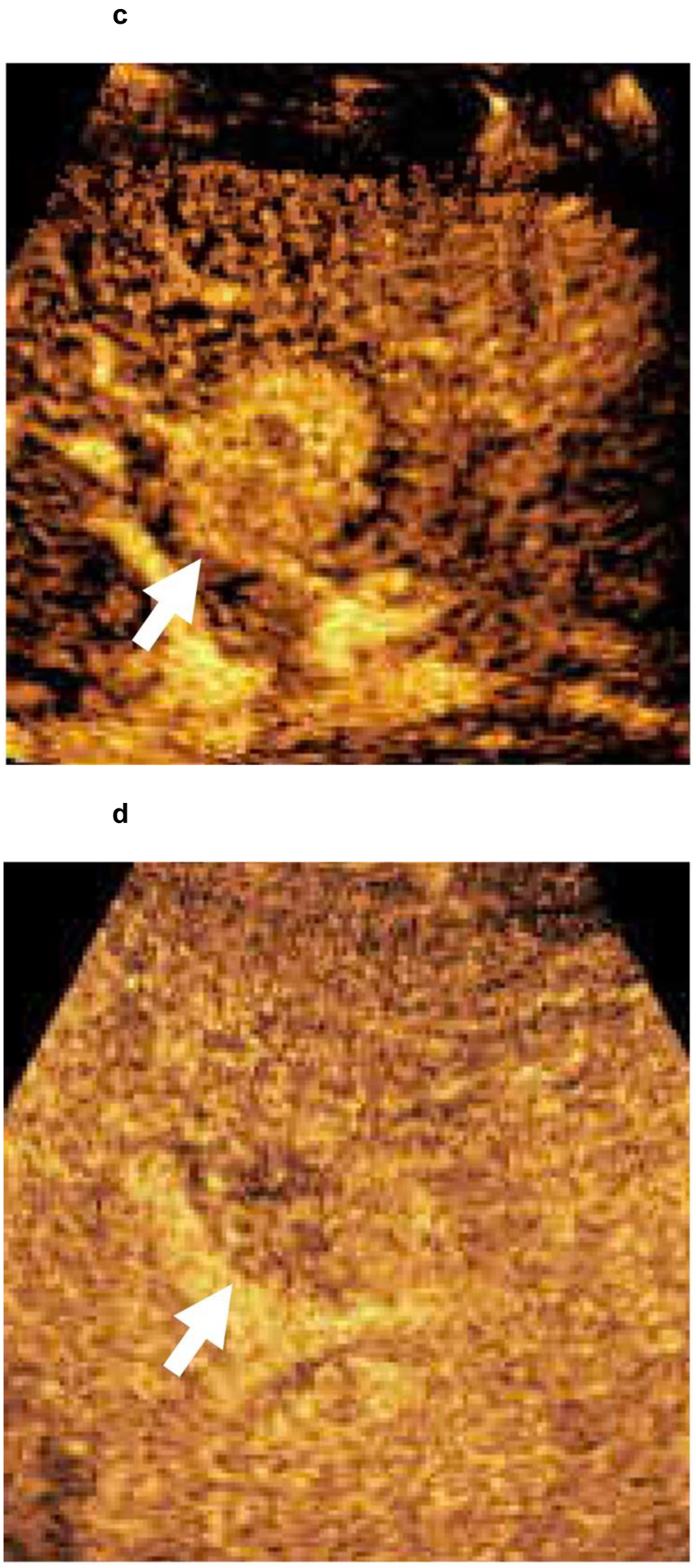
67-year-old patient with a 3.0 cm segment 2 LI-RADS 4 observation (white arrow) showing (a) APHE and (b) questionable washout in multiphase CT examination. Given the risks associated with liver biopsy in the patient, contrast-enhanced ultrasound confirmed a LI-RADS 5 segment 2 observation with (c) APHE and (d) faint washout. HCC diagnosis by CEUS is not approved for liver transplantation purposes. However, as the patient was not considered for liver transplantation because of non-HCC factors, a biopsy was not performed.
The imaging diagnosis of liver observations rests on a diagnostic technique that includes multiphasic exams of the late hepatic arterial phase, portal venous phase, and a 3- to 5-minute delayed phase. The LI-RADS algorithm offers guidance and recommendations for the technical optimization of diagnostic liver imaging[43]. When the diagnosis is established by imaging, biopsy confirmation is unnecessary[40]. While some studies investigate the value of routine biopsy of HCC for morphological and molecular subtyping, the added information is irrelevant in the current HCC staging system and does not change patient management algorithms [44]. Biopsies are usually reserved for indeterminate lesions in patients at high risk for HCC, lesions that are malignant but not HCC-specific, tumor-in-vein lesions, and cases where the LI-RADS algorithm cannot be used[40].
2. What is the liver tumor burden?
Both BCLC staging and the Milan criteria for liver transplantation are highly dependent on hepatic tumor burden[4, 22]. A single HCC tumor ≤ 2 cm is considered very early stage (BCLC 0) and curable with ablation or resection. Early-stage disease (BCLC A) is a single HCC tumor > 2 cm or three lesions that are ≤ 3 cm each. Ablation, resection, and/or liver transplantation are the main treatment options for early-stage HCC[4]. However, a single HCC tumor > 5 cm or an HCC tumor >3 cm in patients with more than one lesion falls outside the Milan criteria and is considered ineligible for LT without disease downstaging[22].
Diffuse or multifocal disease is intermediate-stage disease (BCLC B) and may benefit from liver-directed therapies or systemic treatment. A subset of these patients may be eligible for LT through extended liver transplant criteria[45].
Because lesion size is a major criterion for staging and LT eligibility, accurate measurement is crucial. Generally, any imaging phase or MRI sequence showing clear tumor margins may be used for measurement. However, because lesions may appear larger than they actually are in the late hepatic arterial phase owing to shunting, it should be used for measurement only if lesion margins are unclear on other sequences [46]. Measuring diffusion-weighted images should also be avoided because of the distortion artifacts often seen on this sequence[47].
3. Is the portal vein patent? Is there any variant arterial anatomy?
Portal vein patency plays a critical role in HCC management planning. Portal vein tumor invasion is considered a sign of advanced-stage disease (BCLC C)[4]. Imaging can usually diagnose portal vein thrombosis, which can be bland thrombus or HCC tumor thrombus[48]. The presence of neovascularity within the thrombus, portal vein expansion, and direct extension of the thrombus from the HCC are suggestive of a portal vein tumor thrombus[49]. Portal vein tumor thrombus limits treatment options for HCC and is associated with poor prognosis [50]. According to the BCLC, the recommended treatment for HCC with portal vein invasion (BCLC C) is palliative management with systemic therapy. Surgical resection has shown promising results in select cases, but the prognosis is still modest [51, 52]. Other treatment options for portal vein tumor thrombus include TARE and SBRT[25, 53]. In patients with second-order branch portal vein tumor thrombosis, TACE/TARE may be considered, and LT may be possible after downstaging to meet liver transplantation criteria[24].
Variant hepatic artery anatomy can also have an impact on the treatment of HCC. In most patients, a single common hepatic artery arises from the celiac trunk and then branches into right and left hepatic arteries, each supplying the corresponding hepatic lobe[54]. However, variant arterial supply to the liver can be present in up to 45% of the population. An accessory or replaced hepatic artery is an additional vessel that supplies blood to the liver and most commonly arises from the superior mesenteric artery (accessory right hepatic artery), the left gastric artery (accessory left hepatic artery), celiac trunk, or aorta[54]. The right or left hepatic arteries are called “replaced” arteries when they arise from a different origin than the common hepatic artery. The presence of an accessory or replaced hepatic artery may impact HCC treatment planning in the setting of surgical resection and TACE[54]. Hepatic artery anatomy is usually more clearly demonstrated on CT, but a good-quality MRI may be sufficient.
4. Is there cirrhosis or signs of portal hypertension? What is the necessary residual liver volume after surgery?
Imaging features of cirrhosis and signs of portal hypertension, such as ascites, portosystemic shunts/varices, and splenomegaly, are strong indicators of advanced chronic liver disease. In patients with increased portal venous pressure and/or severe liver dysfunction, surgical resection may not be a viable option [20].
For patients who are candidates for surgery, the amount of residual liver volume is crucial to prevent post-surgical liver failure. The volume of liver needed post-operatively is affected by the degree of cirrhosis or fatty liver[55]. CT hepatic segmentation can provide accurate measurements for surgical planning, allowing surgeons to determine the amount of healthy liver tissue (future liver remnant) that will remain after surgery [56, 57]. Techniques such as portal vein embolization or radiation lobectomy may be used to induce hypertrophy of the remaining liver volume before surgery, reducing the risk of post-surgical complications[55].
5. Is there any metastatic disease?
Up to 14% of HCC patients present with metastatic disease at the time of initial diagnosis [58]. Regional and distant metastases are the most prevalent causes of death in HCC patients. The lungs are the most frequent metastatic site, representing one-third of cases, followed by the peritoneum and bones[59]. The presence of metastatic disease defines advanced-stage HCC (BCLC C). Management is palliative with systemic therapy[4]. To determine whether metastatic disease is present, CT of the chest, bone scans, or additional imaging studies are frequently part of the MDLT board discussion (Figure 5).
Figure 5:
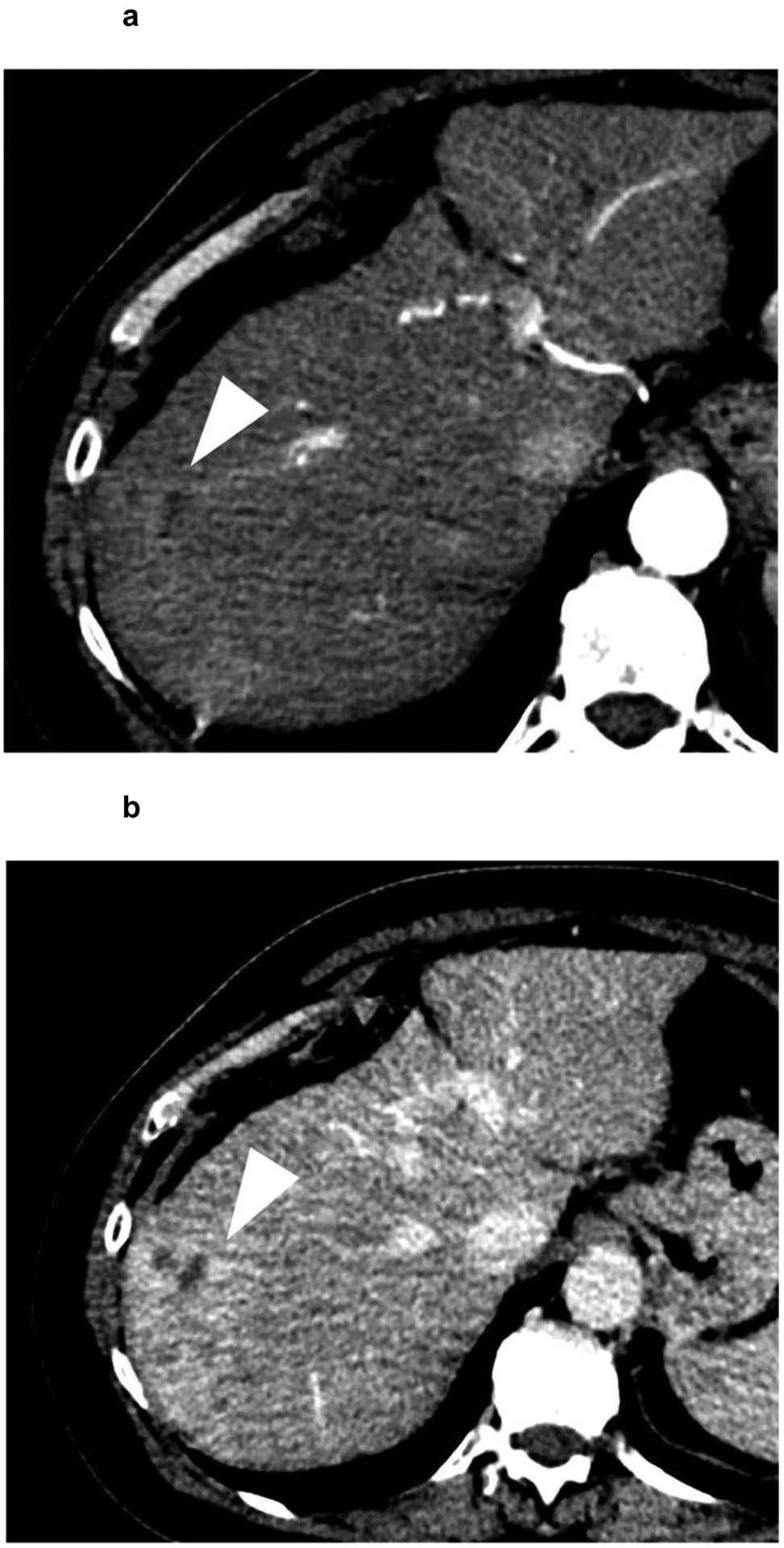
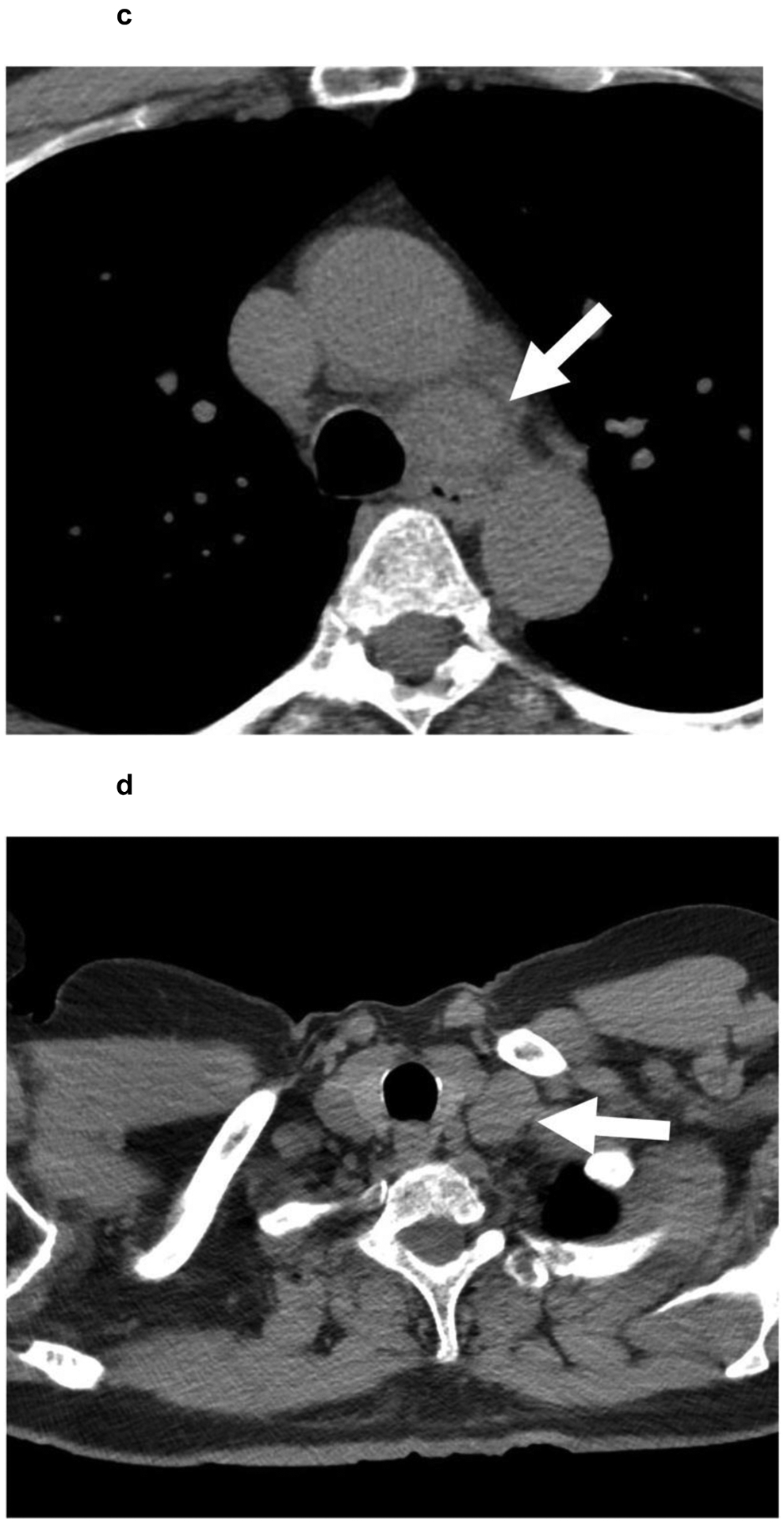
A 62-year-old patient with (a,b) a 7.4 cm segment 7 infiltrative LR-M observation (white arrowhead) showing (a) APHE and (b) persistent delayed heterogeneous enhancement. Biopsy revealed HCC. (c,d) Noncontrast CT of the chest reveals enlarged (c) mediastinal and (d) supraclavicular lymph nodes (white arrows). The patient was referred to systemic therapy.
6. What is the treatment response?
Evaluating treatment response in patients with HCC is important for monitoring the effectiveness of treatment and guiding clinical decision-making throughout the course of the disease. Assessment of treatment response after liver-directed therapies can be challenging due to the different mechanisms of action in each of the therapies. The goal of post-treatment imaging is to 1) identify any residual or recurrent viable tumor and/or presence of new tumors; 2) recognize expected post-treatment changes, which vary depending on the type of therapy and 3) detect any treatment-related complications.
The presence of post-treatment persistent nodular arterial enhancement after liver-directed therapies is generally suggestive of viable tumor (similar to pretreatment HCC features)[60, 61]. However, the interpretation of post-treatment imaging findings should consider the type of locoregional therapy and the time since the intervention. For example, in ablation, a successfully treated tumor will show no residual enhancement within the tumor bed, with a treatment zone that is at least 5–10 mm larger than the original tumor size[60, 62]. After transcatheter therapy with chemoembolization, the tumor should also show no enhancement in any post-contrast phase. The treated tumor frequently appears hyperdense on non-contrast CT due to the uptake of the hyperdense ethiodized oils (such as lipiodol or ethiodol) commonly used as binding agents in TACE[60, 62].
Identifying post-treatment response after radiation-based therapies (TARE, SBRT), on the other hand, is more challenging because these treatments do not cause immediate cell death, and often arterial phase hyperenhancement of the treated tumor can persist for up to one year despite the lack of viable tumor. The post-treatment changes after radiation therapy take time to evolve, and often, a decrease in tumor size and enhancement may not be evident until 6 months after treatment or even later, with fibrosis and shrinkage of the tumor and surrounding parenchyma progressing slowly over time[63] (Figure 6).
Figure 6:
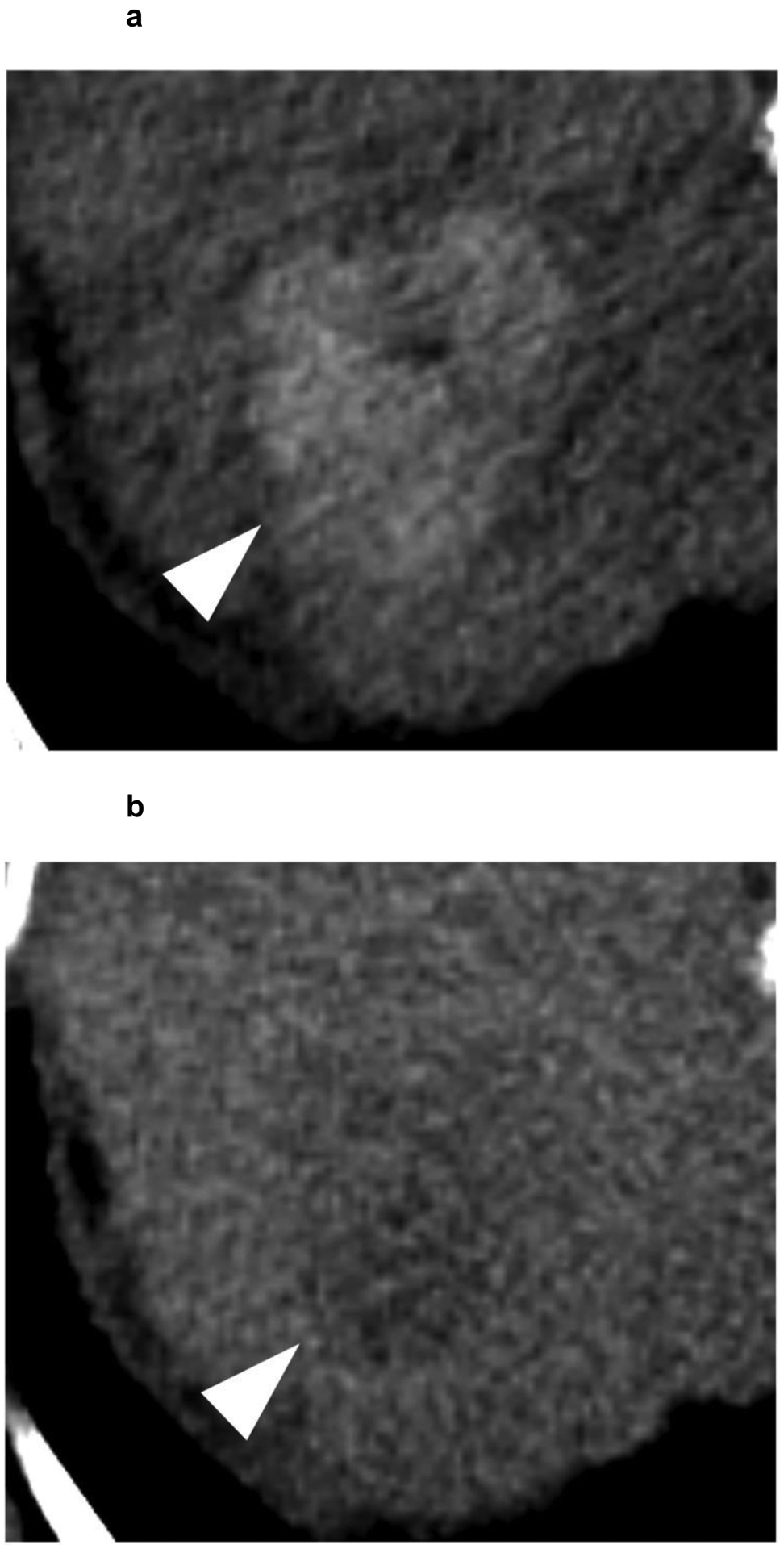
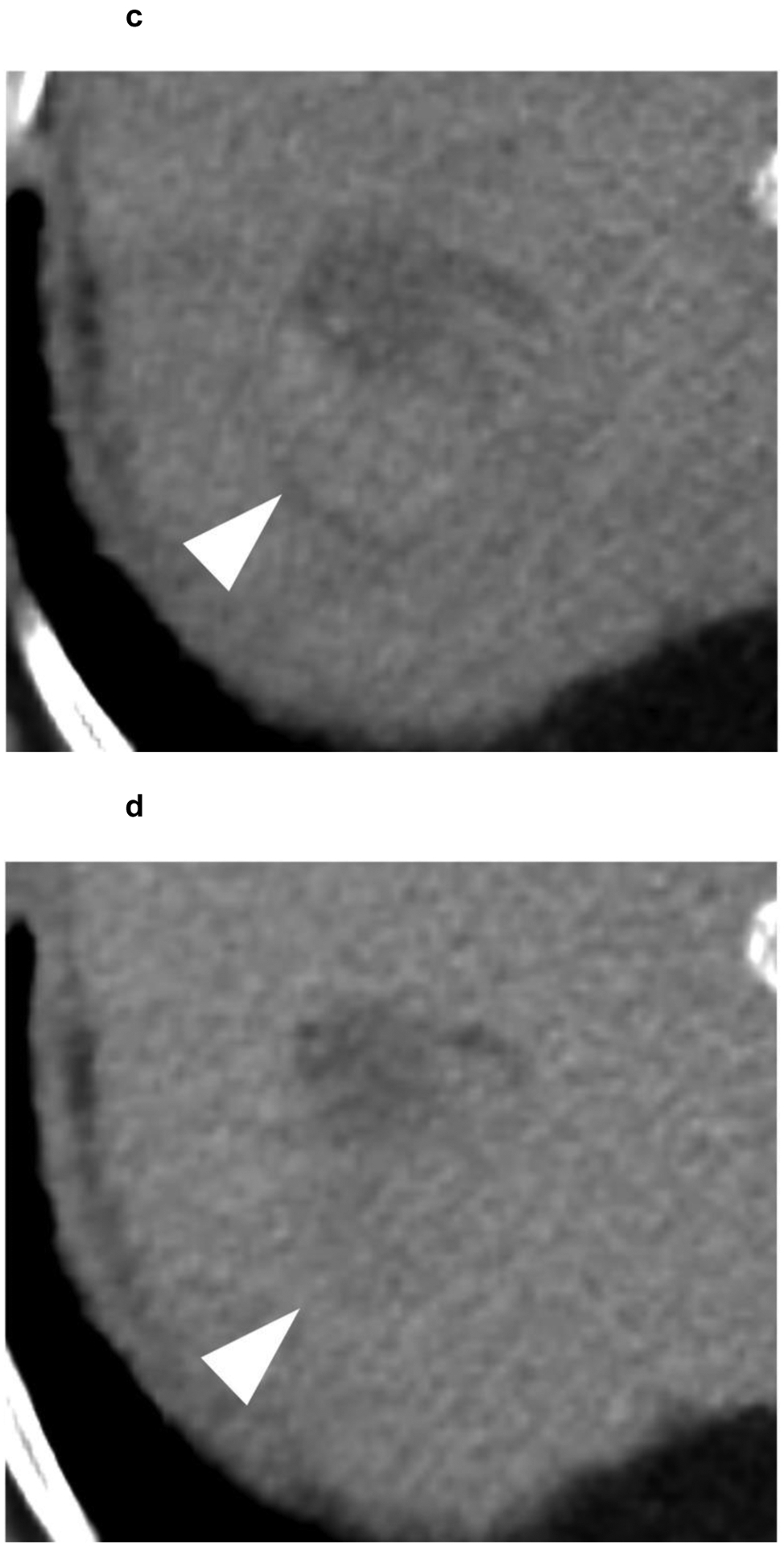
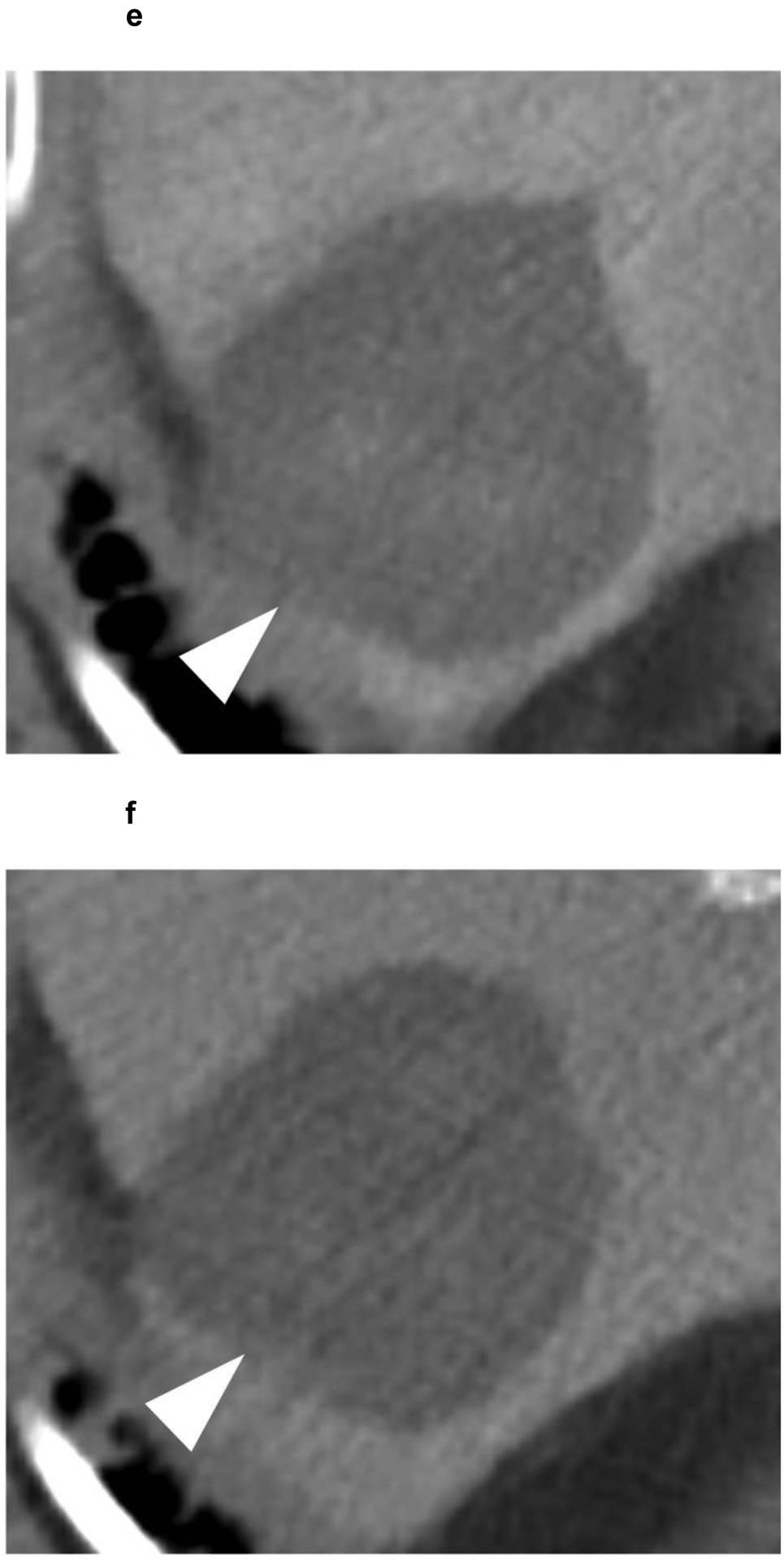
A 63-year-old patient presents with a 4.1 cm segment 7 LI-RADS 5 observation (white arrowhead) with (a) APHE and (b) washout appearance. A CT performed 3 months after SBRT shows (c) residual enhancement with (d) equivocal washout (LI-RADS TR equivocal). (e,f) The arterial and delayed phases from CT performed 7 months after SBRT reveal resolution of the suspicious enhancement (LI-RADS TR non-viable). No intervening additional treatment was performed after the SBRT or during the follow-up period.
The LI-RADS treatment response (LR-TR) algorithms help assess treatment response after locoregional treatment of HCC. LR-TR has the advantage of being specific to HCC and is based on changes in the size, number, and characteristics of the liver lesions on imaging studies before and after treatment. While LR-TR is still evolving as more data become available, its use is still beneficial for clinical workflow.
Within the LR-TR algorithm, a treated tumor may be assigned one of four categories: viable, non-viable, equivocal, and non-evaluable[61]. The viable category is used when imaging suggests a viable tumor, whether residual or recurrent disease. The non-viable category is used when imaging suggests the lack of tumor, but this does not correspond to complete necrosis on histopathology. The equivocal category is used when the tumor does not show changes that can definitively be characterized as viable or non-viable. Studies have shown that lesions in the equivocal category often demonstrate viable microscopic tumor by histopathology[61]. The true clinical implications of this are not yet known, and more studies are needed to determine appropriate management. The radiologist should understand these limitations to help the MDLT board team determine the appropriate management, which could include continued imaging follow-up, additional imaging, biopsy, or intervention.
The non-evaluable category is used when imaging is degraded such that an accurate assessment cannot be made [61]. The radiologist can help guide the multidisciplinary team to the most appropriate study if repeat imaging is needed.
The modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria can also be used for treatment response assessment of HCC and classifies response as complete response, partial response, stable disease, or progressive disease based on changes in the size of the tumor’s viable enhancing component. However, this criteria does not account for the varying appearances on imaging that may occur due to the different types of liver-directed therapies [64].
Optimized MDLT board of the future
Further research into hepatocarcinogenesis leading to HCC may improve our understanding of the characteristic imaging features of HCC, potentially enabling radiologists to accurately identify high-risk hepatic lesions and precancerous conditions. Imaging can offer a comprehensive view of a tumor, in contrast to biopsy, and can potentially predict pathologic factors such as tumor grade and subtype[65]. Imaging features like larger size, disrupted capsules, and low apparent diffusion coefficient can correlate with a worse tumor grade[65–68]. Similarly, non-smooth tumor margins or capsule disruption in gadextate-enhanced MRI, have been linked to the presence of microvascular invasion which is a major risk factor for survival outcomes and intrahepatic metastasis after resection in patients with HCC[69, 70]. Conversely, certain imaging features are associated with a favorable prognosis. For instance, the presence of fat deposition indicates a well-differentiated HCC, which generally carries a good prognosis[71].
In addition, advanced HCC treatments are constantly evolving, and current studies are exploring the potential benefits of combination therapies, such as combined locoregional and systemic treatment or combined drug therapy[72, 73]. In the setting of combination therapies, it is important for radiologists to have a thorough understanding of staging classifications, relative and absolute contraindications, and the imaging appearance of treatment response [26, 32].
Footnotes
Statements and Declarations: None
References
- 1.Konyn P, Ahmed A, and Kim D, Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol, 2021. 15(11): p. 1295–1307. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L and El-Serag HB, Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology, 2019. 156(2): p. 477–491 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayuso C, et al. , Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol, 2018. 101: p. 72–81. [DOI] [PubMed] [Google Scholar]
- 4.Reig M, et al. , BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol, 2022. 76(3): p. 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amorim J, et al. , Critical review of HCC imaging in the multidisciplinary setting: treatment allocation and evaluation of response. Abdom Radiol (NY), 2020. 45(10): p. 3119–3128. [DOI] [PubMed] [Google Scholar]
- 6.Sinn DH, et al. , Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One, 2019. 14(1): p. e0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy-Bhangle AS, et al. , Role of the radiologist at HCC multidisciplinary conference and use of the LR-TR algorithm for improving workflow. Abdom Radiol (NY), 2021. 46(8): p. 3558–3564. [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, et al. , The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin, 2017. 67(2): p. 93–99. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner R, et al. , Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology, 2003. 124(1): p. 91–6. [DOI] [PubMed] [Google Scholar]
- 10.Galle PR, et al. , Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int, 2019. 39(12): p. 2214–2229. [DOI] [PubMed] [Google Scholar]
- 11.Tangkijvanich P, et al. , Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol, 2000. 31(4): p. 302–8. [DOI] [PubMed] [Google Scholar]
- 12.Ikai I, et al. , Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer, 2004. 101(4): p. 796–802. [DOI] [PubMed] [Google Scholar]
- 13.Mehta N, et al. , Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant, 2018. 18(5): p. 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusceddu C, et al. , The Increasing Role of CT-Guided Cryoablation for the Treatment of Liver Cancer: A Single-Center Report. Cancers (Basel), 2022. 14(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Fan YC, and Wang K, Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore), 2020. 99(43): p. e22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musick JR, et al. , Laparoscopic microwave ablation versus percutaneous microwave ablation of hepatic malignancies: Efficacy and recurrence-free survival outcomes in patients. Surgery, 2023. 173(3): p. 598–602. [DOI] [PubMed] [Google Scholar]
- 17.Cucchetti A, et al. , Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol, 2013. 59(2): p. 300–7. [DOI] [PubMed] [Google Scholar]
- 18.Cho YK, et al. , Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology, 2010. 51(4): p. 1284–90. [DOI] [PubMed] [Google Scholar]
- 19.Fuks D, et al. , Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology, 2012. 55(1): p. 132–40. [DOI] [PubMed] [Google Scholar]
- 20.Berzigotti A, et al. , Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology, 2015. 61(2): p. 526–36. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer-Fabrega J, et al. , Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology, 2016. 63(3): p. 839–49. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, et al. , Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med, 1996. 334(11): p. 693–9. [DOI] [PubMed] [Google Scholar]
- 23.Machicao VI, Model for End-Stage Liver Disease-Sodium Score: The Evolution in the Prioritization of Liver Transplantation. Clin Liver Dis, 2017. 21(2): p. 275–287. [DOI] [PubMed] [Google Scholar]
- 24.Kloeckner R, Galle PR, and Bruix J, Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology, 2021. 73 Suppl 1: p. 137–149. [DOI] [PubMed] [Google Scholar]
- 25.Couri T and Pillai A, Goals and targets for personalized therapy for HCC. Hepatol Int, 2019. 13(2): p. 125–137. [DOI] [PubMed] [Google Scholar]
- 26.Guiu B, et al. , TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc Intervent Radiol, 2022. 45(11): p. 1599–1607. [DOI] [PubMed] [Google Scholar]
- 27.Lewandowski RJ, et al. , A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant, 2009. 9(8): p. 1920–8. [DOI] [PubMed] [Google Scholar]
- 28.Gabr A, et al. , Liver Transplantation Following Yttrium-90 Radioembolization: 15-Year Experience in 207-Patient Cohort. Hepatology, 2021. 73(3): p. 998–1010. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, et al. , Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology, 2016. 151(6): p. 1155–1163 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabr A, et al. , Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging, 2021. 48(2): p. 580–583. [DOI] [PubMed] [Google Scholar]
- 31.Riaz A, Awais R, and Salem R, Side effects of yttrium-90 radioembolization. Front Oncol, 2014. 4: p. 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shampain KL, et al. , SBRT for HCC: Overview of technique and treatment response assessment. Abdom Radiol (NY), 2021. 46(8): p. 3615–3624. [DOI] [PubMed] [Google Scholar]
- 33.Lasley FD, et al. , Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1–2 trial of stereotactic body radiation therapy. Pract Radiat Oncol, 2015. 5(5): p. e443–e449. [DOI] [PubMed] [Google Scholar]
- 34.Benson AB, et al. , Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2021. 19(5): p. 541–565. [DOI] [PubMed] [Google Scholar]
- 35.Cannon R, et al. , Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol, 2013. 107(5): p. 544–9. [DOI] [PubMed] [Google Scholar]
- 36.Lu DS, et al. , Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology, 2005. 234(3): p. 954–60. [DOI] [PubMed] [Google Scholar]
- 37.Bhutiani N, et al. , Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child-Pugh B (7/8) hepatocellular carcinoma (HCC). HPB (Oxford), 2016. 18(7): p. 593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou YW, et al. , The latest research progress on minimally invasive treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int, 2023. 22(1): p. 54–63. [DOI] [PubMed] [Google Scholar]
- 39.Harris PS, et al. , Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol, 2019. 25(13): p. 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chernyak V, et al. , Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology, 2018. 289(3): p. 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenbrey JR, et al. , Contrast-enhanced ultrasound (CEUS) in HCC diagnosis and assessment of tumor response to locoregional therapies. Abdom Radiol (NY), 2021. 46(8): p. 3579–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartolotta TV, et al. , Contrast-enhanced ultrasound of hepatocellular carcinoma: where do we stand? Ultrasonography, 2019. 38(3): p. 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kambadakone AR, et al. , LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom Radiol (NY), 2018. 43(1): p. 56–74. [DOI] [PubMed] [Google Scholar]
- 44.Di Tommaso L, et al. , Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol, 2019. 25(40): p. 6041–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toniutto P, et al. , Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Comprehensive Review. J Clin Med, 2021. 10(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seuss CR, et al. , Comparison of MRI pulse sequences for prediction of size of hepatocellular carcinoma at explant evaluation. AJR Am J Roentgenol, 2014. 203(2): p. 300–5. [DOI] [PubMed] [Google Scholar]
- 47.Dietrich O, et al. , Technical aspects of MR diffusion imaging of the body. European Journal of Radiology, 2010. 76(3): p. 314–322. [DOI] [PubMed] [Google Scholar]
- 48.Jha RC, Khera SS, and Kalaria AD, Portal Vein Thrombosis: Imaging the Spectrum of Disease With an Emphasis on MRI Features. AJR Am J Roentgenol, 2018. 211(1): p. 14–24. [DOI] [PubMed] [Google Scholar]
- 49.Sherman CB, et al. , Distinguishing Tumor From Bland Portal Vein Thrombus in Liver Transplant Candidates With Hepatocellular Carcinoma: the A-VENA Criteria. Liver Transpl, 2019. 25(2): p. 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawagishi N, HCC with portal vein tumor thrombosis: how to manage? Hepatol Int, 2020. 14(5): p. 609–611. [DOI] [PubMed] [Google Scholar]
- 51.Hatano E, et al. , Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci, 2018. 25(9): p. 395–402. [DOI] [PubMed] [Google Scholar]
- 52.Kokudo T, et al. , Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol, 2016. 65(5): p. 938–943. [DOI] [PubMed] [Google Scholar]
- 53.Lau WY, et al. , Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology, 2013. 84(5): p. 311–8. [DOI] [PubMed] [Google Scholar]
- 54.Xu YC, Yang F, and Fu DL, Clinical significance of variant hepatic artery in pancreatic resection: A comprehensive review. World J Gastroenterol, 2022. 28(19): p. 2057–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Entezari P, et al. , Promoting Surgical Resection through Future Liver Remnant Hypertrophy. Radiographics, 2022. 42(7): p. 2166–2183. [DOI] [PubMed] [Google Scholar]
- 56.Lian D, et al. , CT volumetry helps predict prognosis of large hepatocellular carcinoma after resection. Clin Radiol, 2022. 77(8): p. e599–e605. [DOI] [PubMed] [Google Scholar]
- 57.Lim MC, et al. , CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol, 2014. 69(9): p. 887–95. [DOI] [PubMed] [Google Scholar]
- 58.Forner A, Llovet JM, and Bruix J, Hepatocellular carcinoma. Lancet, 2012. 379(9822): p. 1245–55. [DOI] [PubMed] [Google Scholar]
- 59.Uka K, et al. , Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol, 2007. 13(3): p. 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kielar A, et al. , Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY), 2018. 43(1): p. 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ram R, et al. , LI-RADS treatment response lexicon: review, refresh and resolve with emerging data. Abdom Radiol (NY), 2021. 46(8): p. 3549–3557. [DOI] [PubMed] [Google Scholar]
- 62.Aslam A, et al. , Hepatocellular carcinoma Liver Imaging Reporting and Data Systems treatment response assessment: Lessons learned and future directions. World J Hepatol, 2020. 12(10): p. 738–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendiratta-Lala M, et al. , Natural history of hepatocellular carcinoma after stereotactic body radiation therapy. Abdom Radiol (NY), 2020. 45(11): p. 3698–3708. [DOI] [PubMed] [Google Scholar]
- 64.Llovet JM and Lencioni R, mRECIST for HCC: Performance and novel refinements. J Hepatol, 2020. 72(2): p. 288–306. [DOI] [PubMed] [Google Scholar]
- 65.Ronot M, et al. , Imaging to Predict Prognosis in Hepatocellular Carcinoma: Current and Future Perspectives. Radiology, 2023. 307(3): p. e221429. [DOI] [PubMed] [Google Scholar]
- 66.Li X, et al. , Correlations between the minimum and mean apparent diffusion coefficient values of hepatocellular carcinoma and tumor grade. J Magn Reson Imaging, 2016. 44(6): p. 1442–1447. [DOI] [PubMed] [Google Scholar]
- 67.Witjes CD, et al. , Histological differentiation grade and microvascular invasion of hepatocellular carcinoma predicted by dynamic contrast-enhanced MRI. J Magn Reson Imaging, 2012. 36(3): p. 641–7. [DOI] [PubMed] [Google Scholar]
- 68.Ocal O, et al. , Prognostic value of baseline imaging and clinical features in patients with advanced hepatocellular carcinoma. Br J Cancer, 2022. 126(2): p. 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W, et al. , The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep, 2021. 11(1): p. 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chong HH, et al. , Multi-scale and multi-parametric radiomics of gadoxetate disodium-enhanced MRI predicts microvascular invasion and outcome in patients with solitary hepatocellular carcinoma </= 5 cm. Eur Radiol, 2021. 31(7): p. 4824–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calderaro J, et al. , Molecular and histological correlations in liver cancer. J Hepatol, 2019. 71(3): p. 616–630. [DOI] [PubMed] [Google Scholar]
- 72.Kim GH, et al. , Emerging Trends in the Treatment of Advanced Hepatocellular Carcinoma: A Radiological Perspective. Korean J Radiol, 2021. 22(11): p. 1822–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Llovet JM, et al. , Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol, 2021. 18(5): p. 293–313. [DOI] [PubMed] [Google Scholar]


