Abstract
Objective
The current research aimed to investigate the physicochemical and bacteriological quality status of the Kalte River in Wolaita Sodo Town, southern Ethiopia.
Methods
A total of 42 water samples were collected using sterile glass bottles from three different river sites: Damota (upstream), Kera (midstream), and Gututo (downstream). All the water samples were examined for the presence of heterotrophic bacteria, total coliform and fecal coliform using direct plate count method and membrane filtration method. Standard methods suggested by American water works association were used to analysis the physicochemical parameters of the water samples.
Results
The results revealed that the total heterotrophic bacteria, total coliform, and fecal coliform count ranged from 8.9 to 12.6 × 104 cfu/ml, 7.5–11.3 × 102 cfu/ml and 5.7–9.7 × 104 cfu/ml, respectively. The bacterial count results indicated that the river water crossed the WHO-recommended limit of potable water. Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella, and Shigella species were the common bacterial pathogens isolated from river water samples. The results of the physicochemical analysis revealed that some of the parameters Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), and turbidity exceeded the maximum permissible limits of WHO and other parameters were below the WHO permissible limits.
Conclusion
Therefore, the presence of bacterial pathogens, fecal coliform indicators, and some physicochemical parameters of the Kalte River exceeding the recommended limits may expose users of the river water to the risk of infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-024-06854-0.
Keywords: Bacteriological analysis, Kalte River, Physicochemical analysis, Water quality, Coliform, Wolaita Sodo Town
Introduction
Rivers are one of the most abundant and readily available sources of fresh water for all living things, including humans [16]. Unfortunately, most of the rivers are polluted by different factors, such as the disposal of untreated industrial waste, sewage waste, and the overabundance of human activities, which affect the physio-chemical and bacteriological characteristics of the river [4].
Using contaminated drinking water and having poor sanitation are the major causes of these diarrhea cases. In poor nations, diarrhea mortality and morbidity are exacerbated by a lack of good water and sanitation. Over 800 million people are affected in Africa and Asia where access to clean water is a serious issue [18]. In Ethiopia, with a population of 75 million, more than half of the population has no access to safe water and it is estimated that approximately 35 million people do not have access to sanitation services [10]. In Ethiopia, more than 60% of transmissible diseases are due to unsafe and inadequate water supplies and poor hygiene and sanitation practices [15].
The Kalte River is one of several rivers in Ethiopia that receives solid and liquid wastes from domestic settlements, and hence understanding the status of the water quality is necessary by monitoring the physico-chemical and bacteriological analyses that enable us to understand the health risks associated with Kalte River water. However, no previous studies have investigated the physicochemical and bacteriological parameters that can indicate the quality of the water of Kalte River. Therefore, this study fills this gap by investigating the bacteriological quality analysis and physico-chemical parameters to understand the status of the water quality of the Kalte River.
Materials and methods
Description of the study area
The study was conducted on the Kalte River in Sodo Town, southern Ethiopia (Fig. 1). It is located 340 km and 160 km away from Addis Ababa and Hawassa, respectively [20]. The location of the study area is in Markato Sub-city, Fana Kebele, Sodo Town, and entirely relies on Kalte Rivers. The Kalte River drains from the highland areas of Damota Mountain, with main small drainage systems in that the middle part of the catchments drains from Kalte womba to Sorfela locality.
Fig. 1.
Map of Study areas
(Source: GIS Ethiopia, 2016)
Sampling sites and sample collection
In total, forty-two (42) samples were collected from three sampling sites (Damota/Upstream, Kera/Midstream, and Gututo/Downstream). The physical parameters like temperature, turbidity, total dissolved solids, conductivity and pH were analysed onsite using portable thermometer, turbidity and pH meters. At each sampling station, fourteen (14) water samples [1] were purposively collected for bacteriological and physicochemical analysis using clean sterile containers (Fig. S1). All samples were appropriately labeled and stored in an ice box for transport to the Post Graduate Microbiology Laboratory at Wolaita Sodo University and analyzed within 6 h of sample collection according to WHO guidelines for sample collection [35].
Enumeration of total heterotrophic bacteria
The heterotrophic plate count was carried out using plate count agar as described by Aliyu et al. [4]. The number of colonies forming units was counted, and the values were multiplied by the dilution factor to obtain the actual microbial levels (Fig. S2).
Enumeration of fecal coliform and total coliform
A volume of 0.1 ml of serial diluted sample was aseptically transferred to the center of a prepared Eosin Methylene Blue (E.M.B) Agar and incubated. Lactose-fermenting colonies (Fig. S2) formed were counted as fecal coliform in cfu/ml, and the value was multiplied by the dilution factor to obtain the actual level of the bacteria in each of the collected water samples [4]. The membrane filtration technique was employed in the evaluation of the total coliform count. The membrane filter with 47 mm diameter, with 0.45 μm pore size (Merck Millipore, USA) was used to filter the river water samples according to Akubuenyi et al. [3]. After filtration, transfer the membrane filter and rolled over the surface of a Petri dish containing Endo Agar Base. Colonies that were dark red, mucoid, had a dark center, or (more typically) produced a metallic sheen were counted (Fig. S2) and counted as described by Forster and Pinedo, [12].
Isolation and identification of selected bacteria from river water samples
A volume of one milliliter (1 ml) of river water sample was mixed with 9 ml of peptone water as pre-enrichment and incubated at 37 °C for 24 h and then streaked on different selective media such as Endo agar base for E. coli, mannitol salt agar for S. aureus, Salmonella-Shigella agar for Salmonella sp. and Shigella sp. and CLED agar for P. aeruginosa [4, 21]. All isolated bacterial cultures were identified by several standard biochemical tests referred with Bergey’s Manual of Determinative Bacteriology [9].
Physico-chemical analysis of river water samples
In this study, the water physicochemical parameters were determined following the Standard Methods for the Examination of Water and Wastewaters [7].
Data analysis
The results were analyzed using Statistical Package for Social Sciences (SPSS) software version 21 and Microsoft Office Excel. The data obtained were subjected to analysis for means, standard deviation, and significance between the means at the 5% probability level. The data were analyzed using single factor analysis of variance (ANOVA); thereafter, individual means were compared using post-hoc comparisons and were assessed using Dunnett’s T3.
Results and discussion
Bacteriological analysis of the Kalte River of Wolaita Sodo
The study results in the Table 1 indicated that all samples were collected from the rivers severely contaminated with total heterotrophic, total coliform, and fecal coliform bacteria and exceeded the standard guidelines for drinking water quality set by the WHO [36]. This could be attributed to the discharge of domestic and agricultural wastes as well as human excreta/wastes into the Kalte River. According to a report by Izah and Angaye [17], domestic waste and sewage are discharged into most surface water by communities aligning rivers. The present study is in line with the reports of Sebsibe et al. [28], Hailu [15], Gadiso et al. [13], Birtukan et al. [8], and Amenu et al. [5] which indicated that all water samples collected were positive for total coliforms and fecal coliforms. The findings of this study are also comparable to the reports of Akubuenyi et al. [3], Seiyaboh et al. [30], Seiyaboh and Kolawole [29], and Omoigberale et al. [24].
Table 1.
Quantitative analysis of bacterial contamination in the Kalte River of Wolaita Sodo Town
| Sample site | Total heterotrophic (CFU/ml) | Fecal coliform (CFU/ml) | Total coliform (CFU/ml) |
|---|---|---|---|
| Damota | 8.9 ± 0.7 × 104 | 5.7 ± 1.1 × 104 | 7.5 ± 0.78 × 102 |
| Kera | 12.6 ± 0.78 × 104 | 9.7 ± 0.99 × 104 | 11.3 ± 0.84 × 102 |
| Gututo | 10.9 ± 0.71 × 104 | 8.1 ± 0.68 × 104 | 9.6 ± 0.76 × 102 |
Data are expressed as the mean ± standard deviation; the mean difference is significant at the 0.05 level (P < 0.05)
Isolation and identification of bacterial pathogens from water samples of the Kalte River
In the present study, five bacterial pathogens i.e., E. coli, Salmonella species, Shigella species, S. aureus, and P. aeruginosa were isolated from river water samples collected from different sites of the Kalte River of Wolaita Sodo by using selective media. All the abovementioned bacterial pathogens were selected as suspected forms from selective media based on the color of colony morphology (Table 2, Table S1).
Table 2.
Isolation of bacterial pathogens based on colony morphology using selective media
| Isolates | Selective Agar | Colony morphology |
|---|---|---|
| Escherichia coli | Endo Agar Base | Pink to a rose-red, green metallic sheen -Lactose Fermenter |
| Salmonella species | Salmonella Shigella Agar | Colonies with large black centers |
| Shigella species | Salmonella Shigella Agar | Colorless colonies |
| Staphylococcus aureus | Mannitol salt agar (MSA) | Golden yellow colonies with yellow color zone around the colonies |
| Pseudomonas aeruginosa | CLED agar | Bluish Green colonies with typical mattered surface and rough periphery |
Colony morphology is one of the important preliminary examinations to isolate appropriate bacterial cultures. Similarly, a study conducted by Krishna Moorthy and Subramaniyan, [21] revealed the isolation and identification of common bacterial pathogens using selective media from well water samples. All the bacterial cultures isolated based on colony morphological characteristics were further confirmed by biochemical characterization using different biochemical tests. The biochemical test results are presented in the form of tables (Tables 3, 4).
Table 3.
Identification of bacterial pathogens isolated from water samples of Kalte River, Wolaita Sodo Town
| S. No | Name of test | Response of E. coli | Response of Salmonella spp. | Response of Shigella spp. |
|---|---|---|---|---|
| 1 | Gram’s staining | Gram-negative, Rods | Gram-negative, Rods | Gram-negative Rods |
| 2 | Motility | Positive | Positive | Negative |
| 3 | Indole production | Positive | Negative | Negative |
| 4 | Sulfide production | Negative | Positive | Negative |
| 5 | Methyl red | Positive | Positive | Positive |
| 6 | Urease production | Negative | Negative | Negative |
| 7 | Citrate utilization | Negative | Positive | Negative |
| 8 | Catalase test | Positive | Positive | Positive |
| 9 | Coagulase test | Negative | Negative | Negative |
Table 4.
Identification of bacterial pathogens isolated from water samples of Kalte River, Wolaita Sodo Town
| S. no | Name of test | Response of P. aeruginosa | Response of S. aureus |
|---|---|---|---|
| 1 | Gram’s staining | Gram-negative, Rods | Gram-positive, Cocci |
| 2 | Motility | Positive | Negative |
| 3 | Indole production | Negative | Negative |
| 4 | Sulphide production | Negative | Negative |
| 5 | Methyl red | Negative | Positive |
| 6 | Urease production | Negative | Positive |
| 7 | Citrate utilization | Positive | Positive |
| 8 | Catalase test | Positive | Positive |
| 9 | Coagulase test | Negative | Positive |
Prevalence of common bacterial pathogens in the water samples collected from the Kalte River
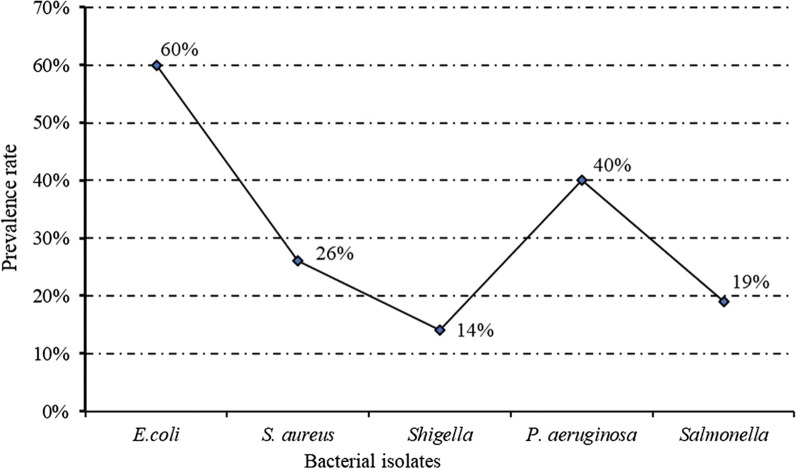
According to this study, the dominant organism among the isolates was E. coli, which was found to be positive in 25 water samples with a 60% prevalence, followed by P. aeruginosa, which was present in 21 water samples with a 40% prevalence. The lowest prevalence rate was Shigella spp., with 14% (Fig. 2). Similarly, a study reported by Abhishek et al. [1] reveals that the occurrence of E. coli was 61%, Salmonella 25%, S. aureus 14%, and P. aeruginosa 53% as 22, 9, 5, and 19 samples out of 36 water samples were found contaminated, respectively. In a similar study, the most isolated organisms from water sources included E. coli (22.7%), Salmonella spp. (13.3%), Shigella spp. (19.3%), Proteus spp. (18.5%), Klebsiella spp. (19.3%), and P. aeruginosa (4.2%) [23]. The various bacterial contaminants of water have been reported by Wekulo et al. [34], Akubuenyi et al. [3], Seiyaboh et al. [30] and Seiyaboh and Kolawole [29]. Terfasa et al. [32] reported that bacterial pathogens such as Staphylococcus spp., E. coli, Shigella spp., Bacillus spp., Salmonella spp. frequently found in the Wabe River of Ethiopia. In a related study, Gebrewahd et al. [14] reported the presence of E. coli, Salmonella spp., Shigella spp., Yersinia, Campylobacter, Legionella, and Pseudomonas as the most common isolates in their study from potable water sources. These bacteria are microbes of public health importance. The bacterial isolate mainly belongs to the family Enterobacteriaceae, which is known to consist of several pathogenic bacteria [34]. Salmonella spp. and E. coli are considered food and waterborne pathogens, and E. coli is a good indicator of fecal contamination of water [2].
Fig. 2.
Prevalence rate of human pathogenic bacteria in river water samples collected from the Kalte River of Wolaita Sodo, Ethiopia
Physico-chemical analysis of water samples collected from the Kalte River
The physicochemical analysis results are presented below in Tables 5, 6 and 7. The temperature is one of the most important ecological factors, and is closely related to latitude, altitude, and season [22]. The present study is in line with the reports of Ken-Onukuba et al. [19], Raji et al. [26], Terfasa et al. [32], and Tesfaye et al. [11].
Table 5.
Physical parameters of the river water samples collected from the Kalte River
| Sites | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | pH | Cond. µS/cm | TDS (mg/l) | TA (mg/l) | Turb. (NTU) | TH mg/l CaCO3 | |
| Damota | 15.7 ± 1.2 | 7.67 | 42.75 ± 15.1 | 28.5 ± 11 | 102 | 8 | 90 |
| Kera | 20.9 ± 1.7 | 7.93 | 185.5 ± 9.5 | 125 ± 6.5 | 162 | 10 | 140 |
| Gututo | 22.8 ± 1.8 | 7.95 | 184.3 ± 48.5 | 123 ± 33 | 122 | 8 | 80 |
Temp. water temperature, Cond. Conductivity, TDS total dissolved solids, Turb turbidity, TA total alkalinity, TH total hardness
Table 6.
Chemical oxygen demand (COD) and biological oxygen demand (BOD) of the river water samples collected from the Kalte River
| Sites | BOD mg/l | COD mg/l |
|---|---|---|
| Damota | 42.5 | 53 |
| Kera | 79.7 | 91 |
| Gututo | 59.6 | 82 |
Table 7.
Chemical parameters of the river water samples collected from the Kalte River
| Chemical parameters | Unit | Sample sites | ||
|---|---|---|---|---|
| Damota | Kera | Gututo | ||
| NH4+ | mg/l | 0.61 | 1.53 | 1.21 |
| NO2− | mg/l | 0.15 | 1.31 | 1.31 |
| NO3− | mg/l | 5.0 | 3.9 | 6.3 |
| PO43− | mg/l | 0.18 | 0.31 | 1.95 |
| HCO3− | mg/l | 122 | 250 | 146 |
| Na+ | mg/l | 11.0 | 8.5 | 28.2 |
| K+ | mg/l | 3.1 | 12.0 | 4.0 |
| Ca+ | mg/l | 8 | 28 | 24 |
| Mg+ | mg/l | 17 | 17 | 4.9 |
| Fe | mg/l | 0.56 | 0.42 | 0.33 |
| Cu2+ | mg/l | 0.22 | 0.07 | 0.8 |
| Mn2+ | mg/l | 0.1 | 0.1 | 0.2 |
| Cr6+ | mg/l | 0.02 | 0.03 | 0.02 |
| F− | mg/l | 0.08 | 0.20 | 0.19 |
| Cl− | mg/l | < 10 | < 10 | < 10 |
pH is used to measure the intensity of acidity or alkalinity of water [27]. The pH values of the water samples in the study area ranged from 7.67 to 7.95 (Table 5). The recorded pH value of this study is comparable with other studies reported from different areas, where pH value ranged from 7.2 to 7.3 in the Wabe River of Wolkite town [32], 6.65–6.96 in the Jakara River of northwestern Nigeria [6] and the average value in the Hare River (7.8) and Kulfo River (8.4) in Arbaminch town of southern Ethiopia [33].
The TDS value observed in this study was below the WHO recommended limit (500 mg/l) [36]. The present study is in line with the reports Raj et al. [25] and Teshome et al. [33]. Hardness is a measure of how much calcium and magnesium is present in water [11]. The variations in total hardness may be because of decomposition and mineralization of organic materials [31]. The results of the present study indicate (Table 5) that the value of total hardiness was below the maximum permissible limit of drinking water standards (500 mg/l CaCO3) [36]. The result obtained in the present study is comparable with other studies reported by Tesfaye et al. [11], Raji et al. [26] and Ken-Onukuba et al. [19].
The values of BOD and COD observed in this study are presented in Table 6. The results of the present study when compared to the results of other similar studies of Ken-Onukuba et al. [19] and Tesfaye et al. [11], the COD and BOD records of Kalte River in Sodo Town were high. The higher BOD and COD in Kalte River might be due to the high nutrient concentration from domestic wastes and the presence of more organic load from urban settlements and slaughterhouse wastewater. High values of BOD and COD indicate that the Kalte River is unfit for human consumption.
Conclusion
The present study indicated the detrimental impacts of anthropogenic activities on the water quality of the Kalte River. Slaughterhouse effluents, the wheat flour milling industry effluent, domestic sewage, wastes from cloth washing close to the river, and agricultural input are the major environmental pollutants deteriorating the river water quality. The pollutants increased bacterial contamination and significantly changed the physicochemical quality parameters of the Kalte River. The anthropogenic impact resulted in moderate and heavy pollution of Damota and Gututo sites, and Kera station, respectively.
The bacteriological profile of the river confirmed the presence of S. aureus, E. coli, Salmonella, and Shigella species together with the opportunistic P. aeruginosa. The high load of indicator organisms in the water samples of the study area suggested that the Kalte River water is polluted at a moderate level of contamination with faecal coliforms that potentially can threaten anyone consuming the water. The physicochemical parameters such as turbidity, BOD, and COD were above the maximum permissible limits of potable water at all sites. In conclusion, the Kalte River of Wolaita Sodo is polluted with agricultural, domestic, and industrial wastes as well as fecal contamination, which makes river water unsafe for human consumption and poses a risk of infection. Therefore, the government authority and regulatory body should take necessary intervention action to regulate domestic and industrial waste disposal as well as proper public awareness on the health and socioeconomic risks of microbial (fecal) contaminants is deemed necessary to maintain the quality and status of the Kalte River.
Limitations
Due to lack of finance, the sampling strategy were restricted only in three different sites of the Kalte River. However, it was better to sample more sites of river. Since, this study conducted within April to June in dry season, it was not able to see Kalte River by seasonal variation in terms of water quality. This study focused on only limited to five different bacterial pathogens, due to lack of microbiological media and chemicals.
Supplementary Information
Author contributions
KRISHNA MOORTHY SIVALINGAM: Contributed to conceptualization, Sample collection, laboratory work, writing original draft; ISRAEL SOLOMON: Contributed to Sample collections, laboratory work, writing original draft; EYOB CHUKALO: Review and editing.
Funding
The authors declare that no funds, grants, or other support were received for the research work and during the preparation of this manuscript.
Data availability
The author declares that all available data are reported in this paper.
Declarations
Declarations
All authors have read, understand, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the instructions for authors.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abhishek C, Pankaj G, Ajit V. Microbiological evaluation of drinking water sold by roadside vendors of Delhi, India. Appl Water Sci. 2015;7:1635–1644. doi: 10.1007/s13201-015-0315-x. [DOI] [Google Scholar]
- 2.Aishvarya N, Malviya MK, Tambe A, Sati P, Dhakar K, Pandey A. Bacteriological assessment of river Jataganga located in Indian Himalaya, with reference to physicochemical and seasonal variations under anthropogenic pressure: a case study. J Environ Microbiol. 2018;1(1):10–16. [Google Scholar]
- 3.Akubuenyi FC, Otu J, Nyong R. Bacteriological quality and antibiogram of isolates obtained from Creek Town River, Odukpani LGA, and Cross River State, Nigeria. Asian J Environ Ecol. 2018;8(2):1–11. doi: 10.9734/AJEE/2018/44505. [DOI] [Google Scholar]
- 4.Aliyu A, Ibrahim YK, Oyi RA. Bacteriological Assessment of river Lavun, Bida Niger State, Nigeria. J Pharm Res Dev Pract. 2016;1(1):34–46. [Google Scholar]
- 5.Amenu D, Menkir S, Gobena T. Assessing the bacteriological quality of drinking water from sources to household water samples of the rural communities of Dire Dawa Administrative Council, Eastern Ethiopia. Sci Technol Arts Res J. 2013;2(3):126–133. doi: 10.4314/star.v2i3.98750. [DOI] [Google Scholar]
- 6.Amoo AO, Zakari AW, Ijanu EM, Adeleye AO, Amoo NB. Physicochemical and Bacteriological Assessment of Surface Water Quality: a case study of Jakara River, North-western Nigeria. Int J Appl Res Technol. 2017;6(9):65–74. [Google Scholar]
- 7.APHA. Standard Methods for the Examination of Water and Wastewaters (21st ed.). American water works Association (AWWA), water pollution control Federation (WPCF) and American Public Health Association (APHA). Washington DC, USA. 2005.
- 8.Birtukan G, Feleke M, Berhanu A. Physico-chemical and bacteriological quality of water from different sources in Gondar town. World Appl Sci J. 2014;32(9):1800–1807. doi: 10.5829/idosi.wasj.2014.32.09.1003. [DOI] [Google Scholar]
- 9.Breed RS, Murray EGD, Smith NR. Bergey’s manual of determinative bacteriology. 7. Baltimore: The Williams & Wilkins Company; 1957. [Google Scholar]
- 10.Edessa N, Geritu N, Mulugeta K. Microbiological assessment of drinking water with reference to diarrheagenic bacterial pathogens in Shashemane Rural District, Ethiopia. Afr J Microbiol Res. 2017;11(6):254–263. doi: 10.5897/AJMR2016.8362. [DOI] [Google Scholar]
- 11.Tesfaye E, Berhe A, Terle D. Physicochemical analysis of Baro River Water and Other Potable Water of Gambella Town, Ethiopia. Int J Adv Res. 2018;6(12):628–637. doi: 10.21474/IJAR01/8182. [DOI] [Google Scholar]
- 12.Forster B, Pinedo CA. Bacteriological examination of waters: membrane filtration protocol. Am Soc Microbiol. 2015;1–15.
- 13.Gadiso MC, Degefu T, Belay Z. Assessment of bacteriological and physico-chemical quality of drinking water in Munesa Woreda, Arsi Zone, Oromia, Ethiopia. Ethiop J Sci Sustain Dev. 2020;7(2):42–49. doi: 10.20372/ejssdastu:v7.i2.2020.207. [DOI] [Google Scholar]
- 14.Gebrewahd A, Adhanom G, Gebremichail G, et al. Bacteriological quality and associated risk factors of drinking water in Eastern zone, Tigrai, Ethiopia, 2019. Trop Dis Travel Med Vaccines. 2020;6(1):1–7. doi: 10.1186/s40794-020-00116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hailu A. Assessment of some bacteriological quality of streams of Upper Awash River, Central Ethiopia. J Nat Sci Res. 2020;10(9):1–9. [Google Scholar]
- 16.Hamdi KM, Lihan S, Hamdan N, Guan TM. Water quality assessment and the prevalence of antibiotic-resistant bacteria from a recreational river in Kuching, Sarawak, Malaysia. J Sustain Sci Manag. 2022;17(5):37–59. doi: 10.46754/jssm.2022.05.004. [DOI] [Google Scholar]
- 17.Izah SC, Angaye TC. Ecology of Human Schistosomiasis intermediate host and Plant Molluscicides used for control: a review. Sky J Biochem Res. 2016;5(6):075–082. [Google Scholar]
- 18.Karamage F, Zhang C, Ndayisaba F, et al. The need for awareness of drinking water loss reduction for sustainable water resource management in Rwanda. J Geosci Environ Protection. 2016;4:74–87. doi: 10.4236/gep.2016.410005. [DOI] [Google Scholar]
- 19.Ken-Onukuba DN, Okeke OC, Amadi CC, et al. Water quality assessment of Ekulu and Asata Rivers in Enugu Area, Southeastern Nigeria, using physico-chemical and bacteriological parameters. J Environ Earth Sci. 2021;11(4):1–23. [Google Scholar]
- 20.Kerse BL. Factors affecting the adoption of soil and water conservation practices in the case of Damota watershed, Wolaita zone, Southern, Ethiopia. Int J Agric Sci Res. 2018;7(1):001–009. [Google Scholar]
- 21.Krishna Moorthy S, Subramaniyan V. Isolation and identification of multiple drug resistant bacterial pathogens from well water samples in and around Wolaita Sodo Town, Southern Ethiopia. J Drug Delivery Ther. 2021;11(3):70–78. doi: 10.22270/jddt.v11i3.4794. [DOI] [Google Scholar]
- 22.Masese FO, Muchiri M, Raburu PO. Macroinvertebrate assemblages as biological indicators of water quality in the Moiben River, Kenya. Afr J Aquat Sci. 2009;34:15–26. doi: 10.2989/AJAS.2009.34.1.2.727. [DOI] [Google Scholar]
- 23.Oluyege JO, Dada AC, Odeyemi AT. Incidence of multiple antibiotics-resistant Gram-negative bacteria isolated from surface and underground water sources in south southwestern region of Nigeria. Water Sci Technol. 2009;59:1929–1936. doi: 10.2166/wst.2009.219. [DOI] [PubMed] [Google Scholar]
- 24.Omoigberale MN, Isibor JO, Izegaegbe JI, Iyamu MI. Seasonal variation in the bacteriological quality of Ebutte River in Ehor community, Edo state, Nigeria. Am J Res Commun. 2013;1(7):59–69. [Google Scholar]
- 25.Raj Kumar B, Sharma GD. Seasonal bacteriological analysis of Barak River, Assam, India. Appl Water Sci. 2013;3:625–630. doi: 10.1007/s13201-013-0120-3. [DOI] [Google Scholar]
- 26.Raji M, Adeleye AO, Amoo FK, Saadatu AY, Isah SF, Amoo AO. Water quality assessment: bacteriological and physicochemical evidence from River Ngalda, Northeast Nigeria. Nigerian Res J Chem Sci. 2021;9(2):1–12. [Google Scholar]
- 27.Saxena U, Saxena S. Correlation study on physico-chemical parameters and quality assessment of ground water of Bassi Tehsil of District Jaipur, Rajasthan, India. S GV U Int J Env Sci Technol. 2015;1(1):78–91. [Google Scholar]
- 28.Sebsibe I, Degaga B, Feye G, Tekle T. Bacteriological and physical quality of Fiche drinking water from households and reservoirs, Oromia, Ethiopia. Water Pract Technol. 2021;16(3):1–11. doi: 10.2166/wpt.2021.043. [DOI] [Google Scholar]
- 29.Seiyaboh E, Kolawole E. Diversity and levels of bacteriological contamination in Orashi River, Mbiama Community, River State, Nigeria. J Adv Microbiol. 2017;4(3):1–6. doi: 10.9734/JAMB/2017/34671. [DOI] [Google Scholar]
- 30.Seiyaboh EI, Izah SC, Bokolo JE. Bacteriological quality of water from river nun at Amassoma Axises, Niger Delta, Nigeria. ASIO J Microbiol Food Sci Biotechnol Innov. 2017;3(1):22–26. [Google Scholar]
- 31.Sekhar P. Bacteriological characteristics of spring water in Ambo Town, West Shoa Zone, Oromia Region, Ethiopia. Int J Environ Agric Res. 2020;6(11):1–9. [Google Scholar]
- 32.Terfasa F, Daba AT, Pandian M. Assessments of the total heterotrophic bacterial population density from Wabe River, south-central Ethiopia. Int J Innov Pharm Sci Res. 2019;7(01):1–13. [Google Scholar]
- 33.Teshome BB, Yesigat A, Habte T. Assessment of selected physico-chemical parameters of different water sources quality. Appl J Environ Sci. 2020;6(2):149–159. [Google Scholar]
- 34.Wekulo KJ, Musyimi MD, Netondo WG. Microbial characterization and identification, and portability of River Kuywa Water, Bungoma, Kenya. Int J Biomol Biomed. 2020;11(2):1–11. [Google Scholar]
- 35.WHO. Technical Support Document for Ontario Drinking Water Standards, Objectives, and Guidelines. Ministry of the Environment. 2003.
- 36.WHO (World Health Organization). Acceptability aspects: Taste, odor, and appearance. Guidelines for Drinking-water Quality. 2011;38(3); 219–230.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The author declares that all available data are reported in this paper.