Abstract
Activins are a subgroup of the TGFβ superfamily of growth and differentiation factors, dimeric in nature and consisting of two inhibin beta subunits linked via a disulfide bridge. Canonical activin signaling occurs through Smad2/3, with negative feedback initiated by Smad6/7 following signal transduction, which binds activin type I receptor preventing phosphorylation of Smad2/3 and activation of downstream signaling. In addition to Smad6/7, other inhibitors of activin signaling have been identified as well, including inhibins (dimers of an inhibin alpha and beta subunit), BAMBI, Cripto, follistatin, and follistatin-like 3 (fstl3). To date, activins A, B, AB, C, and E have been identified and isolated in mammals, with activin A and B having the most characterization of biological activity. Activin A has been implicated as a regulator of several important functions of liver biology, including hepatocyte proliferation and apoptosis, ECM production, and liver regeneration; the role of other subunits of activin in liver physiology are less understood. There is mounting data to suggest a link between dysregulation of activins contributing to various hepatic diseases such as inflammation, fibrosis, and hepatocellular carcinoma, and emerging studies demonstrating the protective and regenerative effects of inhibiting activins in mouse models of liver disease. Due to their importance in liver biology, activins demonstrate utility as a therapeutic target for the treatment of hepatic diseases such as cirrhosis, NASH, NAFLD, and HCC; further research regarding activins may provide diagnostic or therapeutic opportunity for those suffering from various liver diseases. This review will summarize the current findings of the role of activin A, B, C, and E in liver physiology.
Keywords: Activin, Inhibin, Follistatin, Transforming growth factor β, Liver fibrosis, NASH, NAFLD
1. Introduction/Structure and Classification of Activins
Activin was originally discovered and isolated from porcine follicular fluid in the 1980s, and in contrast to inhibin which is known to suppress follicle-stimulating hormone (FSH)[1], demonstrated the ability to enhance gonadotropin-releasing hormone (GnRH)-mediated secretion of FSH[2,3]. Due to their ability to potently activate production of FSH, the term “activins” was coined to describe these proteins. Activins are members of the TGFβ superfamily of growth and differentiation factors[4] and are formed via the covalent dimerization of two β subunits of inhibin[5]. These activin subunits are produced as precursor polypeptides with an NH2-terminal prodomain comprised of 250-350 residues and a COOH-terminal mature domain[4]. This precursor polypeptide is cleaved to release the biologically active mature domain by furin-like proteases[6]. In humans and other mammals, four subunits of activin have been identified: βA, βB, βC and βE [7]. Activin A (βA/βA), activin B (βB/βB), activin AB (βA/βB), activin C (βC/βC), and activin E (βE/βE) have been isolated in mammals, with activins A, B, and AB having the most well-defined biological activity as of yet[2,3,8-10].
2. Activin Receptors and Mechanisms of Signaling
Activin has both a type I and type II receptor which each contain a short extracellular domain that binds ligand, and a larger intracellular serine/threonine kinase domain[11]. The type II receptor (ActRIIA or ActRIIB) is constitutively active, and will dimerize and bind activin first[11]. Afterwards, the ligand-bound type II receptor complex will recruit a type I receptor (ActRI) each and activate them via phosphorylation of the membrane-proximal glycine- and serine-rich sequence (GS) region[12]. The type I receptor, termed activin receptor-like kinase (ALK), has 7 different variants (ALK1-ALK7) identified by homology cloning[13]. ALK4 has been described as the dominant type I receptor used for activin signaling[14], with activins A, B, and AB showing the ability to activate a combination of ActRIIA and ALK4 with various levels of potency[15], although activin AB and B (but not A) have been shown to activate ActRIIA and ALK7 as well[15,16]. Following ligand binding, receptors are internalized[17]; the Smad (suppressor of mothers against decapentaplegic) anchor for receptor activation (SARA) is a zinc double finger FYVE domain (derived from the names of the first four proteins recognized to contain this domain: Fab1p, YOTB, Vac1p, and EEA1[18]) containing protein present in the early endosome which is internalized along with the hetero-tetrameric receptor complex via clathrin-mediated endocytosis[19]. SARA then will recruit a receptor-regulated Smad (R-Smad), orienting the R-Smad so that the serine residue on its C-terminus can bind the catalytic L45 region of the type I receptor[20]. The type I receptor will then phosphorylate this serine residue of the R-Smad, inducing a conformational change which allows dissociation of the R-Smad from the receptor complex and SARA[21]. Activins have been shown to signal through the R-Smads Smad 2 and Smad3[22]. The dissociated, phosphorylated R-Smad then binds the common mediator Smad4 and this complex translocates to the nucleus to regulate expression of target genes[23] such as KLF10, IL11, ANGPTL4, and others[24]. Following activation, inhibitory Smad6/7 induced by the activated Smad complex initiates a negative feedback mechanism[25,26], forming a complex with type 1 activin receptor, thereby preventing Smad 2 and 3 phosphorylation and inhibiting activation of the downstream pathway[27]. Whereas Smad6 has been shown to preferentially inhibit Smad signaling of type 1 receptors ALK3 and ALK6[28], Smad7 has been shown to interact with all activated type 1 receptors[29]
3. Inhibition of Activin Signaling
In addition to negative feedback initiation of SMAD6/7, multiple proteins show inhibitory activity of activins to bind to membrane receptor. This section will specifically focus on Inhibins, BAMBI (bone morphogenetic protein (BMP) and activin membrane-bound inhibitor), Cripto, follistatin, and follistatin-like proteins as negative regulators of activin signalling. Inhibin A (a heterodimer comprised of an α and βA subunit) binds the α subunit to the type III TGF-β receptor betaglycan, and the β subunit competitively binds to the type II receptor[4], preventing the ability to recruit type I receptor, effectively inhibiting activin signaling[30]. For inhibin B, TGFβ receptor type III-like (TGFBR3L) has been identified as the co-receptor required for signaling instead of betaglycan[31]. BAMBI is a transmembrane psuedoreceptor structurally similar to type I receptors, except that it lacks the intracellular kinase domain [32]. BAMBI inhibits signaling through its intracellular domain, which is similar to the homodimerization interface of type 1 receptor, and blocks the formation of receptor complexes[33]. Cripto, a member of the epidermal growth factor/Cripto-1/FRL-1/Cryptic family (EGF-CFC), is required for nodal signaling and can form a complex with activins and type 2 receptor, preventing recruitment of ALK4 and thereby preventing downstream activin signaling[34]. Follistatin is an important regulatory molecule of activins, and is able to form a complex with activins to prevent receptor binding and block signaling[35]. This is accomplished when two follistatin molecules bind to one activin dimer, blocking a third of its residues and receptor binding sites[36]. Follistatin has been demonstrated to bind to activins A, B, AB, and E[37,38]. In addition to follistatin, there also exists a follistatin-like protein known as follistatin-like 3 (fstl3) encoded by follistatin-related gene (FLRG), which is also able to bind to TGFβ family proteins, but only contains two of the three follistatin domains[39].
4. Activins in Liver Biology
Of all the activins covered in this review (activin A, B, C, and E), activin A is the most well-characterized to-date and is involved in the regulation of various biological functions. Mice lacking activin βA do not develop whiskers, incisors, or mandibular molar teeth[40,41]. In addition, these mice have defective secondary palates, which results in death within 24 hours following birth due to impaired ability to suckle[41]. In the liver, activin A is involved with the regulation of several important functions such as hepatocyte proliferation and apoptosis, ECM production, and liver regeneration[42]. In human hepatoma HLF cells, activin βA antisense oligonucleotides induce cell proliferation, implying a regulatory effect of endogenous activin A on growth inhibition[43]. Activin A has been shown to inhibit mitogen-induced DNA synthesis as well as induce apoptosis in vivo and in vitro[44-46]. Indeed, mRNA expression of activin βA has been shown to be significantly decreased shortly following partial hepatectomy in rat liver which returns to and eventually exceeds expression[47]. However, activin βA expression may have a more complex dynamic during the regenerative phase in the liver, as expression of activin βA has been shown to be increased 12 hours following hepatectomy as well[48]. In addition, inhibin α-deficient mice display a cachexic phenotype as a result of hepatocyte destruction, with 10-fold elevated serum levels of activin A, again suggesting a regulatory effect of activin A on hepatocyte growth[49]. Activin A has also been implicated in both pro- and anti- inflammatory effects and the progression of fibrosis in the liver - activin A induction occurs quickly following systemic inflammation, and has been shown to inhibit the acute phase reaction as well as antagonize interleukin 6 effects[7]. Liver-specific overexpression of activin A via adenoassociated virus in low density lipoprotein (LDL) receptor-deficient mice on Western diet reduced inflammation, total and LDL cholesterol, hematopoietic stem cell expansion, liver steatosis, and fat accumulation[50].Activin A has been shown to activate macrophages[51,52], and activin βA expression is elevated following activation of hepatic stellate cells (HSC)[53]. Addition of activin A to HSC and hepatocyte co-cultures increases secretion of collagen, α-smooth muscle actin and fibronectin, as well as connective tissue growth factor[54,55]. In rat models of liver fibrosis and cirrhosis, activin βA expression has been shown to be increased[56-59], and circulating activin A levels are increased in patients suffering from acute liver failure, hepatitis infection, alcohol-induced cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH)[60-66].
In regard to liver biology, much less is known about the other activin β subunits compared to βA. The function of activin B in the liver is poorly understood[67]. Mice deficient in inhibin βB show defective eyelid fusion during embryonic development, leading to eye lesions, and mutant females are incapable of reproduction as their offspring suffer perinatal lethality[68]. Expression of βB subunit of activin is low in rodent liver[9], but is detectable by immunohistochemistry at low levels in hepatocytes of normal rat livers and in connective tissue septa in fibrotic livers[56]. In humans, however, βA and βB transcripts are expressed at similar levels in liver[7]. Compared to activin A and AB, activin B does not inhibit DNA synthesis in primary rat hepatocytes[69]. However, ectopic expression of the preferred activin B type I receptor, ALK7[15], has been shown to induce apoptosis in hepatoma cells[70]. Expression of activin βB mRNA has been shown to be highly upregulated in stellate cells of rat livers following CCL4 administration[71] and following exposure to peroxisome proliferator di-n-butyl phthalate[72]. Additionally, in a model of lipopolysaccharide (LPS)-induced liver inflammation in mice βB subunit expression levels are significantly increased, but not inhibin α or βA subunit, and detectable within endothelial cells and Kupffer cells[73]. In the same study, activin B induced phosphorylation of Smad2/3, and increased expression of connective tissue growth factor as well. In patients with liver fibrosis, circulating and hepatic levels of activin B are significantly increased compared to healthy controls[74].
The role of βC in the liver is not well-defined at this point. Activin C has been demonstrated to signal through ALK7, with lower affinity for the cognate type II receptor than activin A or B, and is resistant to neutralization via follistatin[75]. βC knockout mice develop normally and show normal liver function and regenerative capability[76]. Partial hepatectomy results in a transient down-regulation of βC subunit expression[77], and in hepatoma cell lines βC expression is lower than in normal liver tissue[78]. Ectopic βC expression reduces cell number in both human and rat hepatocytes via apoptosis induction in hepatocytes[78] and decreases DNA synthesis in mouse liver[79]. However, βC expression is increased in rat liver following administration of CCL4 in a model of cirrhosis[80] as well as following administration of peroxisome proliferator di-n-butyl phthalate[72]. Additionally, activin C treatment has been demonstrated to increase DNA synthesis in a mouse liver cell line and in primary rat hepatocytes[81] and adenovirus-mediated overexpression of βC was shown to increase liver regeneration in rats following partial hepatectomy[82]. A plausible explanation for the growth stimulation effects of βC may be that by utilizing βA to produce AC heterodimers decreases the available pool of βA units for production of activin A[83].
Like βC knockout mice, βE knockout animals develop normally and do not show impaired liver function or regenerative ability[76]. However, the data for activin βE is much more consistent compared to βC[42]. Activin βE negatively regulates cellular growth in human hepatoma cell lines HepG2 and Hep3B via overexpression[78], as well as in immortalized mouse hepatocytes[81]. Transient overexpression in mice of βE results in inhibited regenerative DNA synthesis in the liver[79]. Following partial hepatectomy, βE levels increase rapidly and nearly return to basal levels 48 hours following hepatectomy[76]. Following LPS stimulation, βE expression in the liver is significantly increased, suggesting a role during liver inflammation[84]. Additionally, βE expression in the liver follows a diurnal pattern based on feeding – rats have low liver βE expression during the light phase, increasing until the beginning of the dark phase, which returns to low levels of expression when allowed food or remains high if fasted during the dark phase[42]. βA expression is reversed compared to βE expression in this scenario as well. Overexpression of hepatic βE in rodents activates thermogenesis and improves insulin sensitivity, and activin E treatment in cultured brown adipocytes stimulates expression of Ucp1[85]. In HepG2 cells, treatment with insulin resulted in upregulation of βE expression, and expression of βE mRNA was upregulated in the liver of diet-induced obese mice, suggesting a role for activin E in glucose metabolism[86]. In human, βE expression positively correlates with insulin resistance and body mass index, and silencing βE (via siRNA) in db/db mice resulted in reduced body weight gain driven by reduced fat versus lean mass[87]. Loss of function variants in the βE gene were associated with lower waist-to-hip ratio adjusted for body mass index, as well as in variants in ACVR1C, further suggesting a connection between activins and fat distribution[88]. Additionally, heterozygous protein-truncating mutations in βE gene were found to be associated with favorable fat distribution, improved metabolic profile and protection from type 2 diabetes[89].
5. Activins as a Potential Therapeutic Target in Liver Diseases
There is mounting evidence showing dysregulated expression of activins in various hepatic diseases such as inflammation, fibrosis, and hepatocellular carcinoma, which suggests the utility of activin signaling as a therapeutic target in various liver diseases. Follistatin administered to CCl4-treated rats reduced the formation of fibrosis in the liver and attenuated apoptosis of hepatocytes[53]. In addition, we have demonstrated that inhibition of activin A or B via neutralizing antibodies attenuated liver fibrosis and improved liver function in CCl4-treated mice, both in preventative and therapeutic modalities with established disease, and that combination treatment of both antibodies had additive benefits[74]. Adenovirus-mediated knockdown of activin A receptor type 2A attenuated immune-mediated hepatic fibrosis induced by chronic concanavalin A administration in mice and inhibited interleukin-17-induced activation of primary hepatic stellate cells as well[90]. Indeed, in our hands, inhibition of activin A or B via neutralizing antibodies in our acute concanavalin A-induced liver injury model protected hepatocytes, improved liver function, and significantly reduced circulating cytokines[91]. Additionally, we have demonstrated that inhibition of activin A and B in a bile duct ligation model of liver fibrosis improved liver function and reduced fibrosis, and combination inhibition of both A and B had additive benefits as well as reducing hepatic and systemic inflammatory cytokine production in this model[92]. In addition to the anti-inflammatory and anti-fibrotic effects of neutralization of activin A and B, the effects of activin E on energy expenditure and insulin sensitivity suggest a potential for therapeutic benefit in obesity and other metabolism-associated disorders as well[85,93].
6. Conclusion
The activin axis plays an important role in the maintenance of liver architecture and cellular homeostasis and has been implicated in the pathogenesis of hepatic diseases such as liver failure, inflammation, fibrosis, and hepatocellular carcinoma. Abnormal expression of activins and/or follistatin is present in many different liver diseases and has been shown to contribute to inflammatory and fibrotic conditions in the liver[7]. Further research is necessary to elucidate the function of activin B in the liver, and to identify the molecular interaction of βC and βE subunits with cell surface receptors and/or secreted proteins to ascertain their biological activities. Activins represent a promising target for the treatment of various hepatic diseases, and further research and learning regarding activins and follistatins may provide better diagnostic or therapeutic opportunity for those suffering from various liver-related diseases.
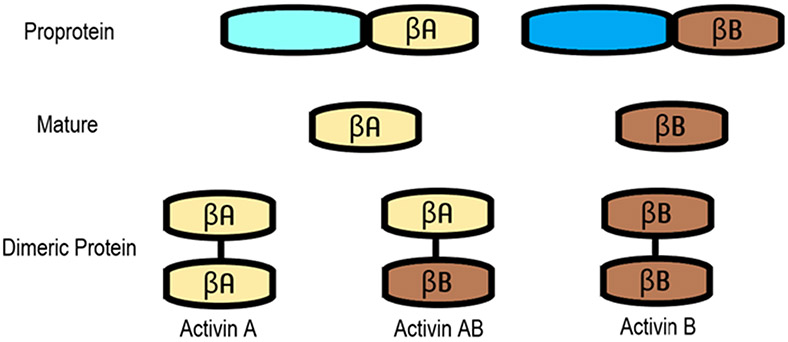
Figure 1. Structure of dimeric mature activin proteins.
β subunits are produced as proproteins containing a prodomain and the mature domain. Furin-like proteases cleave the proprotein into the biologically active mature protein, and activins are produced from dimers containing two β subunits linked via disulphide bridges. The structures of activin A, B, and AB are depicted here as homodimers or heterodimers.
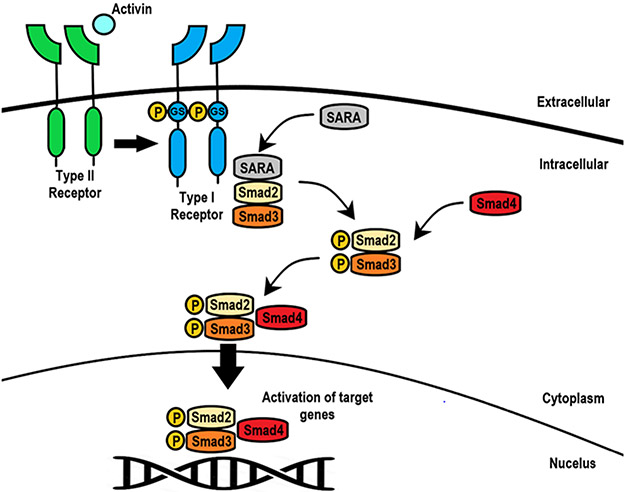
Figure 2. Activin receptor signaling pathway.
Activin will bind to two type II receptors, which then recruits and phosphorylates two type I receptors. The receptors are internalized, and then SARA recruits Smad2/3 which binds to and is phosphorylated by the type I receptor. Smad2/3 then dissociates from the receptor complex and SARA and binds the common mediator Smad4; this complex then translocates to the nucleus to regulate gene expression.
Acknowledgements
This work was supported by the Translational Research Pilot Grant from Indiana University, the Lilly Graduate Research Advanced Degree program, and by a grant from the National Institutes of Health (grant number 1R01DK117076).
Abbreviations:
- ActRI
Activin type 1 receptor
- ActRIIA/B
Activin type 2 receptor
- ALK
Activin receptor-like kinase
- BAMBI
Bone morphogenetic protein and activin membrane-bound inhibitor
- BMP
Bone morphogenetic protein
- ECM
Extracellular matrix
- FLRG
Follistatin-related gene
- FSH
Follicle-stimulating hormone
- fstl3
Follistatin-like 3
- GnRH
Gonadotropin-releasing hormone
- GS
Glycine- and serine-rich sequence
- HCC
Hepatocellular carcinoma
- HSC
Hepatic stellate cells
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharide
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- R-Smad
Receptor-regulated Smad
- SARA
Smad anchor for receptor activation
- Smad
Suppressor of mothers against decapentaplegic
- TGFβ
Transforming growth factor beta
- TGFBR3L
TGFβ receptor type III-like
Footnotes
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work
References
- [1].Burger HG, Evidence for a negative feedback role of inhibin in follicle stimulating hormone regulation in women, Hum. Reprod 8 (1993) 129–132. 10.1093/HUMREP/8.SUPPL_2.129. [DOI] [PubMed] [Google Scholar]
- [2].Vale W, Rivier J, Vaughan J, Mcclintock R, Corrigan A, Woo W, Karr D, Spiess J, Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid, Nat. 1986 3216072. 321 (1986) 776–779. 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- [3].Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R, Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin, Nat. 1986 3216072. 321 (1986) 779–782. 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- [4].Bloise E, Ciarmela P, Dela Cruz C, Luisi S, Petraglia F, Reis FM, Activin A in mammalian physiology, Physiol. Rev 99 (2019) 739–780. 10.1152/physrev.00002.2018. [DOI] [PubMed] [Google Scholar]
- [5].Schmierer B, Hill CS, TGFβ–SMAD signal transduction: molecular specificity and functional flexibility, Nat. Rev. Mol. Cell Biol 8 (2007) 970–982. 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- [6].Wang X, Fischer G, Hyvönen M, Structure and activation of pro-activin A, Nat. Commun 7 (2016) 1–11. 10.1038/ncomms12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kreidl E, Oztürk D, Metzner T, Berger W, Grusch M, Activins and follistatins: Emerging roles in liver physiology and cancer, World J. Hepatol 1 (2009) 17. 10.4254/wjh.v1.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Walton KL, Makanji Y, Harrison CA, New insights into the mechanisms of activin action and inhibition, Mol. Cell. Endocrinol 359 (2012) 2–12. 10.1016/j.mce.2011.06.030. [DOI] [PubMed] [Google Scholar]
- [9].Vejda S, Cranfield M, Peter B, Mellor SL, Groome N, Schulte-Hermann R, Rossmanith W, Expression and dimerization of the rat activin subunits betaC and betaE: evidence for the ormation of novel activin dimers., J. Mol. Endocrinol 28 (2002) 137–48. http://www.ncbi.nlm.nih.gov/pubmed/11932210. [DOI] [PubMed] [Google Scholar]
- [10].Mason AJ, Berkemeier LM, Schmelzer CH, Schwall RH, Activin B: Precursor Sequences, Genomic Structure and in Vitro Activities, Mol. Endocrinol 3 (1989) 1352–1358. 10.1210/MEND-3-9-1352. [DOI] [PubMed] [Google Scholar]
- [11].Thompson TB, Woodruff TK, Jardetzky TS, Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions., EMBO J. 22 (2003) 1555–66. 10.1093/emboj/cdg156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Massagué J, Blain SW, Lo RS, TGFbeta signaling in growth control, cancer, and heritable disorders., Cell. 103 (2000) 295–309. http://www.ncbi.nlm.nih.gov/pubmed/11057902. [DOI] [PubMed] [Google Scholar]
- [13].ten Dijke P, Ichijo H, Franzén P, et al. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 8(10) (1993) 2879–2887. https://pubmed.ncbi.nlm.nih.gov/8397373/. [PubMed] [Google Scholar]
- [14].Pangas SA, Woodruff TK, Activin signal transduction pathways, Trends Endocrinol. Metab 11 (2000) 309–314. 10.1016/S1043-2760(00)00294-0. [DOI] [PubMed] [Google Scholar]
- [15].Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H, Activin isoforms signal through type I receptor serine/threonine kinase ALK7, Mol. Cell. Endocrinol 220 (2004) 59–65. 10.1016/j.mce.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [16].Bernard DJ, Lee KB, Santos MM, Activin B can signal through both ALK4 and ALK7 in gonadotrope cells, Reprod. Biol. Endocrinol 4 (2006) 52. 10.1186/1477-7827-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doré JJE, Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB, Mechanisms of Transforming Growth Factor-β Receptor Endocytosis and Intracellular Sorting Differ between Fibroblasts and Epithelial Cells, Mol. Biol. Cell 12 (2001) 675–684. 10.1091/mbc.12.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stenmark H, Aasland R, Toh BH, D’Arrigo A, Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger, J. Biol. Chem 271 (1996) 24048–24054. 10.1074/JBC.271.39.24048. [DOI] [PubMed] [Google Scholar]
- [19].Runyan CE, Schnaper HW, Poncelet A-C, The role of internalization in transforming growth factor beta1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells., J. Biol. Chem. 280 (2005) 8300–8. 10.1074/jbc.M407939200. [DOI] [PubMed] [Google Scholar]
- [20].Moustakas A, Smad signalling network., J. Cell Sci 115 (2002) 3355–6. http://www.ncbi.nlm.nih.gov/pubmed/12154066. [DOI] [PubMed] [Google Scholar]
- [21].Souchelnytskyi S, Rönnstrand L, Heldin CH, ten Dijke P, Phosphorylation of Smad signaling proteins by receptor serine/threonine kinases., Methods Mol. Biol 124 (2001) 107–20. http://www.ncbi.nlm.nih.gov/pubmed/11100470. [DOI] [PubMed] [Google Scholar]
- [22].Harrison CA, Gray PC, Fischer WH, Donaldson C, Choe S, Vale W, An Activin Mutant with Disrupted ALK4 Binding Blocks Signaling via Type II Receptors, J. Biol. Chem 279 (2004) 28036–28044. 10.1074/jbc.M402782200. [DOI] [PubMed] [Google Scholar]
- [23].Wang S-Y, Tai G-X, Zhang P-Y, Mu D-P, Zhang X-J, Liu Z-H, Inhibitory effect of activin A on activation of lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells, Cytokine. 42 (2008) 85–91. 10.1016/j.cyto.2008.01.010. [DOI] [PubMed] [Google Scholar]
- [24].Baroncelli M, Drabek K, Eijken M, van der Eerden BCJ, van de Peppel J, van Leeuwen JPTM, Two-day-treatment of Activin-A leads to transient change in SV-HFO osteoblast gene expression and reduction in matrix mineralization, J. Cell. Physiol 235 (2020) 4865–4877. 10.1002/JCP.29365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Massagué J, Seoane J, Wotton D, Smad transcription factors, Genes Dev. 19 (2005) 2783–2810. 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- [26].Miyazawa K, Miyazono K, Regulation of TGF-β Family Signaling by Inhibitory Smads, Cold Spring Harb. Perspect. Biol 9 (2017). 10.1101/CSHPERSPECT.A022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yan X, Liu Z, Chen Y, Regulation of TGF-β signaling by Smad7, Acta Biochim. Biophys. Sin. (Shanghai) 41 (2009) 263–272. 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K, Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors, J. Biol. Chem 282 (2007) 20603–20611. 10.1074/JBC.M702100200. [DOI] [PubMed] [Google Scholar]
- [29].Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K, The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling, J. Cell Biol 155 (2001) 1017–1027. 10.1083/JCB.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Makanji Y, Zhu J, Mishra R, Holmquist C, Wong WPS, Schwartz NB, Mayo KE, Woodruff TK, Inhibin at 90: From discovery to clinical application, a historical review, Endocr. Rev 35 (2014) 747–794. 10.1210/er.2014-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brule E, Wang Y, Li Y, Lin YF, Zhou X, Ongaro L, Alonso CAI, Buddle ERS, Schneyer AL, Byeon CH, Hinck CS, Mendelev N, Russell JP, Cowan M, Boehm U, Ruf-Zamojski F, Zamojski M, Andoniadou CL, Sealfon SC, Harrison CA, Walton KL, Hinck AP, Bernard DJ, TGFBR3L is an inhibin B co-receptor that regulates female fertility, Sci. Adv 7 (2021) 4391. 10.1126/SCIADV.ABL4391/SUPPL_FILE/SCIADV.ABL4391_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tramullas M, Lantero A, Díaz Á, Morchón N, Merino D, Villar A, Buscher D, Merino R, Hurlé JM, Izpisúa-Belmonte JC, Hurlé MA, BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-β family in pain modulation, J. Neurosci 30 (2010) 1502–1511. 10.1523/JNEUROSCI.2584-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massagué J, Niehrs C, Silencing of TGF-beta signalling by the pseudoreceptor BAMBI, Nature. 401 (1999) 480–485. 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- [34].Gray PC, Harrison CA, Vale W, Cripto forms a complex with activin and type II activin receptors and can block activin signaling, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 5193–5198. 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Winter JP, Ten Dijke P, De Vries CJM, Van Achterberg TAE, Sugino H, De Waele P, Huylebroeck D, Verschueren K, Van AJM Den Eijnden-Van Raaij, Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors, Mol. Cell. Endocrinol 116 (1996) 105–114. 10.1016/0303-7207(95)03705-5. [DOI] [PubMed] [Google Scholar]
- [36].Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS, The structure of the follistatin: Activin complex reveals antagonism of both type I and type II receptor binding, Dev. Cell 9 (2005) 535–543. 10.1016/j.devcel.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [37].Schneyer A, Schoen A, Quigg A, Sidis Y, Differential binding and neutralization of activins A and B by follistatin and follistatin like-3 (FSTL-3/FSRP/FLRG), Endocrinology. 144 (2003) 1671–1674. 10.1210/en.2002-0203. [DOI] [PubMed] [Google Scholar]
- [38].Hashimoto O, Tsuchida K, Ushiro Y, Hosoi Y, Hoshi N, Sugino H, Hasegawa Y, cDNA cloning and expression of human activin βE subunit, Mol. Cell. Endocrinol 194 (2002) 117–122. 10.1016/S0303-7207(02)00157-0. [DOI] [PubMed] [Google Scholar]
- [39].Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H, Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-β family, J. Biol. Chem 275 (2000) 40788–40796. 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- [40].Matzuk M, Kumar T, Vassalli A et al. Functional analysis of activins during mammalian development. Nature 374 (1995) 354–356. 10.1038/374354a0 [DOI] [PubMed] [Google Scholar]
- [41].Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT, Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition, Genes Dev. 12 (1998) 2636–2649. 10.1101/GAD.12.16.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodgarkia-Dara C, Vejda S, Erlach N, Losert A, Bursch W, Berger W, Schulte-Hermann R, Grusch M, The activin axis in liver biology and disease, Mutat. Res. Mutat. Res 613 (2006) 123–137. 10.1016/j.mrrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [43].Takabe K, Lebrun JJ, Nagashima Y, Ichikawa Y, Mitsuhashi M, Momiyama N, Ishikawa T, Shimada H, Vale WW, Interruption of activin A autocrine regulation by antisense oligodeoxynucleotides accelerates liver tumor cell proliferation, Endocrinology. 140 (1999) 3125–3132. 10.1210/endo.140.7.6767. [DOI] [PubMed] [Google Scholar]
- [44].Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA, Induction of apoptosis in the murine liver with recombinant human activin a, Hepatology. 20 (1994) 854–862. 10.1002/hep.1840200413. [DOI] [PubMed] [Google Scholar]
- [45].Schwall R, Activin induces cell death in hepatocytes in vivo and in vitro, Hepatology. 18 (1993) 347–356. 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- [46].Yasuda H, Mine T, Shibata H, Eto Y, Hasegawa Y, Takeuchi T, Asano S, Kojima I, Activin A: An autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes, J. Clin. Invest 92 (1993) 1491–1496. 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gold EJ, Zhang X, Wheatley AM, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Fleming JS, βA- and βC-activin, follistatin, activin receptor mRNA and βC-activin peptide expression during rat liver regeneration, J. Mol. Endocrinol 34 (2005) 505–515. 10.1677/jme.1.01657. [DOI] [PubMed] [Google Scholar]
- [48].Date M, Matsuzaki K, Matsushita M, Tahashi Y, Sakitani K, Inoue K, Differential regulation of activin A for hepatocyte growth and fibronectin synthesis in rat liver injury, J. Hepatol 32 (2000) 251–260. 10.1016/S0168-8278(00)80070-7. [DOI] [PubMed] [Google Scholar]
- [49].Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A, Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 8817–8821. 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu H, Hallauer Hastings M, Kitchen R, Xiao C, Baldovino Guerra JR, Kuznetsov A, Rosenzweig A, Beneficial Effects of Moderate Hepatic Activin A Expression on Metabolic Pathways, Inflammation, and Atherosclerosis, Arterioscler. Thromb. Vasc. Biol 43 (2023) 330–349. 10.1161/ATVBAHA.122.318138. [DOI] [PubMed] [Google Scholar]
- [51].Nüsing RM, Barsig J, Induction of prostanoid, nitric oxide, and cytokine formation in rat bone marrow derived macrophages by activin A, Br. J. Pharmacol 127 (1999) 919–926. 10.1038/sj.bjp.0702626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang X, Li Y, Tai G, Xu G, Zhang P, Yang Y, Lao F, Liu Z, Effects of Activin A on the Activities of the Mouse Peritoneal Macrophages, Cell Mol Immunol. 2(1) (2005) 63–67. [PubMed] [Google Scholar]
- [53].Patella S, Phillips DJ, Tchongue J, De Kretser DM, Sievert W, Follistatin attenuates early liver fibrosis: Effects on hepatic stellate cell activation and hepatocyte apoptosis, Am. J. Physiol. - Gastrointest. Liver Physiol 290 (2006). 10.1152/ajpgi.00080.2005. [DOI] [PubMed] [Google Scholar]
- [54].Ding ZY, Jin GN, Wang W, Sun YM, Chen WX, Chen L, Liang HF, Datta PK, Zhang MZ, Zhang B, Chen XP, Activin a-smad signaling mediates connective tissue growth factor synthesis in liver progenitor cells, Int. J. Mol. Sci 17 (2016). 10.3390/ijms17030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wada W, Kuwano H, Hasegawa Y, Kojima I, The dependence of transforming growth factor-β-induced collagen production on autocrine factor activin A in hepatic stellate cells, Endocrinology. 145 (2004) 2753–2759. 10.1210/en.2003-1663. [DOI] [PubMed] [Google Scholar]
- [56].De Bleser PJ, Niki T, Xu G, Rogiers V, Geerts A, Localization and cellular sources of activins in normal and fibrotic rat liver, Hepatology. 26 (1997) 905–912. 10.1002/hep.510260416. [DOI] [PubMed] [Google Scholar]
- [57].Sugiyama M, Ichida T, Sato T, Ishikawa T, Matsuda Y, Asakura H, Expression of Activin A is increased in cirrhotic and fibrotic rat livers, Gastroenterology. 114 (1998) 550–558. 10.1016/S0016-5085(98)70539-6. [DOI] [PubMed] [Google Scholar]
- [58].Huang X, Li DG, Wang ZR, Wei HS, Cheng JL, Zhan YT, Zhou X, Xu QF, Li X, Lu HM, Expression changes of activin A in the development of hepatic fibrosis, World J. Gastroenterol 7 (2001) 37–41. 10.3748/wjg.v7.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gold EJ, Francis RJB, Zimmermann A, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Wheatley AM, Fleming JS, Changes in activin and activin receptor subunit expression in rat liver during the development of CCl4-induced cirrhosis., Mol. Cell. Endocrinol 201 (2003) 143–53. http://www.ncbi.nlm.nih.gov/pubmed/12706302. [DOI] [PubMed] [Google Scholar]
- [60].Hughes RD, Evans LW, Activin A and follistatin in acute liver failure, Eur. J. Gastroenterol. Hepatol 15 (2003) 127–131. 10.1097/00042737-200302000-00004. [DOI] [PubMed] [Google Scholar]
- [61].DE LIN S, KAWAKAMI T, USHIO A, SATO A, SATO S-I, IWAI M, ENDO R, TAKIKAWA Y, SUZUKI K, Ratio of circulating follistatin and activin A reflects the severity of acute liver injury and prognosis in patients with acute liver failure, J. Gastroenterol. Hepatol 21 (2006) 374–380. 10.1111/j.1440-1746.2005.04036.x. [DOI] [PubMed] [Google Scholar]
- [62].Elsammak MY, Amin GM, Khalil GM, Ragab WS, Abaza MM, Possible contribution of serum activin A and IGF-1 in the development of hepatocellular carcinoma in Egyptian patients suffering from combined hepatitis C virus infection and hepatic schistosomiasis, Clin. Biochem 39 (2006) 623–629. 10.1016/j.clinbiochem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- [63].Pirisi M, Fabris C, Luisi S, Santuz M, Toniutto P, Vitulli D, Federico E, Del Forno M, Mattiuzzo M, Branca B, Petraglia F, Evaluation of circulating activin-A as a serum marker of hepatocellular carcinoma, Cancer Detect. Prev 24 (2000) 150–155. http://europepmc.org/article/MED/10917135. [PubMed] [Google Scholar]
- [64].Patella S, Phillips DJ, De Kretser DM, Evans LW, Groome NP, Sievert W, Characterization of serum activin-A and follistatin and their relation to virological and histological determinants in chronic viral hepatitis, J. Hepatol 34 (2001) 576–583. 10.1016/S0168-8278(00)00029-5. [DOI] [PubMed] [Google Scholar]
- [65].Yuen MF, Norris S, Evans LW, Langley PG, Hughes RD, Transforming growth factor-beta 1, activin and follistatin in patients with hepatocellular carcinoma and patients with alcoholic cirrhosis, Scand. J. Gastroenterol 37 (2002) 233–238. 10.1080/003655202753416939. [DOI] [PubMed] [Google Scholar]
- [66].Yndestad A, Haukeland JW, Dahl TB, Bjøro K, Gladhaug IP, Berge C, Damås JK, Haaland T, Løberg EM, Linnestad P, Birkeland K, Konopski Z, Halvorsen B, Berge RK, Aukrust P, A Complex Role of Activin A in Non-Alcoholic Fatty Liver Disease, Am. J. Gastroenterol 104 (2009) 2196–2205. 10.1038/ajg.2009.318. [DOI] [PubMed] [Google Scholar]
- [67].Namwanje M, Brown CW, Activins and Inhibins: Roles in Development, Physiology, and Disease, Cold Spring Harb. Perspect. Biol 8 (2016) a021881. 10.1101/cshperspect.a021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vassalli A, Matzuk MM, Gardner HAR, Lee KF, Jaenisch R, Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction., Genes Dev. 8 (1994) 414–427. 10.1101/GAD.8.4.414. [DOI] [PubMed] [Google Scholar]
- [69].Niimi S, Horikawa M, Seki T, Ariga T, Kobayashi T, Hayakawa T, Effect of activins AB and B on DNA synthesis stimulated by epidermal growth factor in primary cultured rat hepatocytes, Biol. Pharm. Bull 25 (2002) 437–440. 10.1248/bpb.25.437. [DOI] [PubMed] [Google Scholar]
- [70].Kim BC, Van Gelder H, Kim TA, Lee HJ, Baik KG, Chun HH, Lee DA, Choi KS, Kim SJ, Activin receptor-like kinase-7 induces apoptosis through activation of MAPKs in a Smad3-dependent mechanism in hepatoma cells, J. Biol. Chem 279 (2004) 28458–28465. 10.1074/jbc.M313277200. [DOI] [PubMed] [Google Scholar]
- [71].Heymann F, Hamesch K, Weiskirchen R, Tacke F, The concanavalin A model of acute hepatitis in mice, Lab. Anim 49 (2015) 12–20. 10.1177/0023677215572841. [DOI] [PubMed] [Google Scholar]
- [72].Kobayashi T, Niimi S, Fukuoka M, Hayakawa T, Regulation of inhibin beta chains and follistatin mRNA levels during rat hepatocyte growth induced by the peroxisome proliferator di-n-butyl phthalate., Biol. Pharm. Bull 25 (2002) 1214–6. http://www.ncbi.nlm.nih.gov/pubmed/12230121. [DOI] [PubMed] [Google Scholar]
- [73].Kanamori Y, Sugiyama M, Hashimoto O, Murakami M, Matsui T, Funaba M, Regulation of hepcidin expression by inflammation-induced activin B., Sci. Rep 6 (2016) 38702. 10.1038/srep38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang Y, Hamang M, | Culver Alexander, Jiang H, Yanum J, Garcia V, Lee J, White E, | Kusumanchi Praveen, Chalasani N, | Liangpunsakul Suthat, Yaden BC, Dai G, Activin B promotes the initiation and progression of liver fibrosis, Hepatol. Commun 6 (2022) 2812–2826. 10.1002/hep4.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Goebel EJ, Ongaro L, Kappes EC, Vestal K, Belcheva E, Castonguay R, Kumar R, Bernard DJ, Thompson TB, The orphan ligand, activin C, signals through activin receptor-like kinase 7, Elife. 11 (2022). 10.7554/ELIFE.78197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lau AL, Kumar TR, Nishimori K, Bonadio J, Matzuk MM, Activin βC and βE Genes Are Not Essential for Mouse Liver Growth, Differentiation, and Regeneration, Mol. Cell. Biol 20 (2000) 6127–6137. 10.1128/mcb.20.16.6127-6137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Esquela AF, Zimmers TA, Koniaris LG, Sitzmann JV, Lee SJ, Transient down-regulation of inhibin-βC expression following partial hepatectomy, Biochem. Biophys. Res. Commun 235 (1997) 553–556. 10.1006/bbrc.1997.6850. [DOI] [PubMed] [Google Scholar]
- [78].Vejda S, Expression of activins C and E induces apoptosis in human and rat hepatoma cells, Carcinogenesis. 24 (2003) 1801–1809. 10.1093/carcin/bgg154. [DOI] [PubMed] [Google Scholar]
- [79].Chabicovsky M, Herkner K, Rossmanith W, Overexpression of Activin βC or Activin βE in the Mouse Liver Inhibits Regenerative Deoxyribonucleic Acid Synthesis of Hepatic Cells, Endocrinology. 144 (2003) 3497–3504. 10.1210/en.2003-0388. [DOI] [PubMed] [Google Scholar]
- [80].Gold EJ, Francis RJB, Zimmermann A, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Wheatley AM, Fleming JS, Changes in activin and activin receptor subunit expression in rat liver during the development of CCl4-induced cirrhosis, Mol. Cell. Endocrinol 201 (2003) 143–153. 10.1016/S0303-7207(02)00417-3. [DOI] [PubMed] [Google Scholar]
- [81].Wada W, Maeshima A, Zhang YQ, Hasegawa Y, Kuwano H, Kojima I, Assessment of the function of the βC-subunit of activin in cultured hepatocytes, Am. J. Physiol. - Endocrinol. Metab 287 (2004). 10.1152/ajpendo.00390.2003. [DOI] [PubMed] [Google Scholar]
- [82].Wada W, Medina J, Hasegawa Y, Kuwano H, Kojima I, Adenovirus-mediated overexpression of the activin βc subunit accelerates liver regeneration in partially hepatectomized rats, J. Hepatol 43 (2005) 823–828. 10.1016/j.jhep.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [83].Rodgarkia-Dara C, Vejda S, Erlach N, Losert A, Bursch W, Berger W, Schulte-Hermann R, Grusch M, The activin axis in liver biology and disease, Mutat. Res. - Rev. Mutat. Res 613 (2006) 123–137. 10.1016/j.mrrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [84].O’Bryan MK, Sebire KL, Gerdprasert O, Hedger MP, Hearn MTW, De Kretser DM, Cloning and regulation of the rat activin β(E) subunit, in: J. Mol. Endocrinol., Society for Endocrinology, 2000: pp. 409–418. 10.1677/jme.0.0240409. [DOI] [PubMed] [Google Scholar]
- [85].Sekiyama K, Ushiro Y, Kurisaki A, Funaba M, Hashimoto O, Activin E enhances insulin sensitivity and thermogenesis by activating brown/beige adipocytes, J. Vet. Med. Sci 81 (2019) 646. 10.1292/JVMS.19-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hashimoto O, Sekiyama K, Matsuo T, Hasegawa Y, Implication of activin E in glucose metabolism: Transcriptional regulation of the inhibin/activin βE subunit gene in the liver, Life Sci. 85 (2009) 534–540. 10.1016/J.LFS.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [87].Sugiyama M, Kikuchi A, Misu H, Igawa H, Ashihara M, Kushima Y, Honda K, Suzuki Y, Kawabe Y, Kaneko S, Takamura T, Inhibin βE (INHBE) is a possible insulin resistance-associated hepatokine identified by comprehensive gene expression analysis in human liver biopsy samples., PLoS One. 13 (2018) e0194798–e0194798. 10.1371/JOURNAL.PONE.0194798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Deaton AM, Dubey A, Ward LD, Dornbos P, Flannick J, Yee E, Ticau S, Noetzli L, Parker MM, Hoffing RA, Willis C, Plekan ME, Holleman AM, Hinkle G, Fitzgerald K, Vaishnaw AK, Nioi P, Rare loss of function variants in the hepatokine gene INHBE protect from abdominal obesity, Nat. Commun 2022 131. 13 (2022) 1–12. 10.1038/s41467-022-31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Akbari P, Sosina OA, Bovijn J, Landheer K, Nielsen JB, Kim M, Aykul S, De T, Haas ME, Hindy G, Lin N, Dinsmore IR, Luo JZ, Hectors S, Geraghty B, Germino M, Panagis L, Parasoglou P, Walls JR, Halasz G, Atwal GS, Della Gatta G, Jones M, LeBlanc MG, Still CD, Carey DJ, Giontella A, Orho-Melander M, Berumen J, Kuri-Morales P, Alegre-Díaz J, Torres JM, Emberson JR, Collins R, Rader DJ, Zambrowicz B, Murphy AJ, Balasubramanian S, Overton JD, Reid JG, Shuldiner AR, Cantor M, Abecasis GR, Ferreira MAR, Sleeman MW, Gusarova V, Altarejos J, Harris C, Economides AN, Idone V, Karalis K, Della Gatta G, Mirshahi T, Yancopoulos GD, Melander O, Marchini J, Tapia-Conyer R, Locke AE, Baras A, Verweij N, Lotta LA, Multiancestry exome sequencing reveals INHBE mutations associated with favorable fat distribution and protection from diabetes, Nat. Commun 2022 131. 13 (2022) 1–17. 10.1038/s41467-022-32398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhang H, Ju B, Nie Y, Song B, Xu Y, Gao P, Adenovirus-mediated knockdown of activin A receptor type 2A attenuates immune-induced hepatic fibrosis in mice and inhibits interleukin-17-induced activation of primary hepatic stellate cells, Int. J. Mol. Med 42 (2018) 279–289. 10.3892/ijmm.2018.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hamang MJ, THE ROLES OF ACTIVIN A AND B IN LIVER INFLAMMATION AND FIBROSIS, Purdue University Graduate School, 2019. 10.25394/PGS.8049611.V1. [DOI] [Google Scholar]
- [92].Wang Y, ACTIVIN B PROMOTES HEPATIC FIBROGENESIS, Purdue University Graduate School, 2019. 10.25394/PGS.8979728.V1. [DOI] [Google Scholar]
- [93].Hashimoto O, Funaba M, Sekiyama K, Doi S, Shindo D, Satoh R, Itoi H, Oiwa H, Morita M, Suzuki C, Sugiyama M, Yamakawa N, Takada H, Matsumura S, Inoue K, Oyadomari S, Sugino H, Kurisaki A, Activin E Controls Energy Homeostasis in Both Brown and White Adipose Tissues as a Hepatokine, Cell Rep. 25 (2018) 1193–1203. 10.1016/J.CELREP.2018.10.008. [DOI] [PubMed] [Google Scholar]