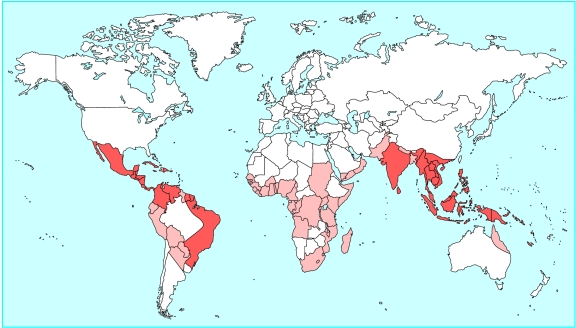
Dengue viruses, single stranded RNA viruses of the family Flaviviridae, are the most common cause of arboviral disease in the world. They are found virtually throughout the tropics (fig 1) and cause an estimated 50-100 million illnesses annually, including 250 000-500 000 cases of dengue haemorrhagic fever—a severe manifestation of dengue—and 24 000 deaths.1–3 More than two fifths of the world's population (2.5 billion) live in areas potentially at risk for dengue.1 Because travellers to endemic areas are also at risk, healthcare providers should have an understanding of the spectrum of infection, how to diagnose it, and what the appropriate treatment is.
Figure 1.
Geographical distribution of dengue (light shading) and dengue fever plus dengue haemorrhagic fever (dark shading)
Come then, let us play at unawares
And see who wins in this sly game of bluff
Man or mosquito
D H Lawrence, The Mosquito
Summary points
Dengue is the most common cause of arboviral disease
The disease is more prevalent now than at any other time, and its prevalence is expected to increase
A severe manifestation of dengue is dengue haemorrhagic fever, which is more common after a secondary infection with dengue virus
Dengue is a relatively common cause of fever in travellers to the tropics, but severe disease is rare
A cost effective vaccine is needed for the prevention and control of dengue
Methods
Our review was prepared from literature on dengue up to 15 April 2002. We searched Medline (for all English articles using the keyword “dengue”), comprehensive textbooks, the Cochrane Library, the internet, and our own files.
Epidemiology
Four dengue virus serotypes are recognised. Infection with one serotype is thought to produce lifelong immunity to that serotype but only a few months immunity to the others.1,4 Humans and mosquitoes are the principal hosts of dengue virus; the mosquito remains infected for life, but the viruses are only known to cause illness in humans. In forest and enzootic cycles in Africa and Asia the virus is probably sustained through vertical (transovarial) transmission in the mosquito, with periodic amplification in non-human primates.3,5 The virus is transmitted by bites from Aedes mosquito.
The principal vector of dengue, Aedes aegypti, is found worldwide between latitudes 35°N and 35°S (fig 2). It is an efficient vector for several reasons: it is highly susceptible to dengue virus; it feeds preferentially on human blood6; it thrives in close proximity to humans; it is a daytime feeder; its bite is almost imperceptible; and it is a restless mosquito as the slightest movement interrupts feeding, thus several people may be bitten in a short period for one blood meal.7,8 Unlike most mosquitoes, A aegypti takes more than one blood meal during a gonotropic cycle—that is, before the eggs are laid.8 In many areas, dengue epidemics occur during the warm, humid, rainy seasons, which favour abundant mosquitoes and shorten the extrinsic incubation period.4,9,10
Figure 2.
Aedes aegypti taking blood meal
In the past 60 years the incidence, distribution, and clinical severity of dengue has increased dramatically. Population growth in the tropics provides many susceptible hosts. Uncontrolled urbanisation leads to inadequate management of water and waste, providing a range of large water stores and disposable, non-biodegradable containers that become habitats for the larvae. Few control programmes are effective against the mosquitoes.11 In addition, air travel has enabled infected humans to import viruses. These factors can change a region from non-endemic (no virus present) to hypoendemic (one serotype present) to hyperendemic (multiple serotypes present).3
In South East Asia the mean number of annual cases of dengue haemorrhagic fever has increased from below 10 000 in the 1950s and 60s to over 200 000 in the 90s. The same pattern is now unfolding in the Americas. In the 80s there were 15 000 cases of dengue haemorrhagic fever, in the 90s there were 56 000 cases, and in 2001 alone there were 15 000 cases.12,13 The numbers of reported cases of imported dengue in countries outside the tropics have also been increasing.14–16 Nevertheless, dengue is often asymptomatic or causes a non-specific febrile illness, and dengue is not a reportable disease in most countries.17 Thus, despite the proliferation of reported cases, it is generally accepted that the incidence of infection and disease is largely under-reported.
Pathology
Dengue haemorrhagic fever is distinguished from dengue by the presence of increased vascular permeability, not by the presence of haemorrhage.1 Patients with dengue may have severe haemorrhage without meeting WHO criteria for dengue haemorrhagic fever (see bmj.com). In these cases the pathogenesis probably derives from thrombocytopenia or a consumptive coagulopathy, not from the vascular leak syndrome seen in dengue haemorrhagic fever.18 Dengue haemorrhagic fever may (grades III and IV) or may not (grades I and II) include clinical shock, referred to as dengue shock syndrome (see bmj.com).1
Dengue virus antigen has been found in a variety of tissues, predominately the liver and reticuloendothelial system.1,10 Viral replication is thought to occur primarily in the macrophages, although dendritic cells (Langerhans cells) in the skin may be an early target of infection.19 As with yellow fever, focal central necrosis has been found in the liver of patients who have died of dengue.4,20 Autopsies of patients who died of dengue haemorrhagic fever show diffuse petechial haemorrhages in most organs and serous effusions of pericardial, peritoneal, and pleural spaces. Dengue virus (by isolation and reverse transcription-polymerase chain reaction) and antibody (including IgM) have been identified in the cerebrospinal fluid, but direct involvement of dengue virus in neuronal damage is controversial.4,21–23 More studies on this are needed.
All four serotypes have been associated with dengue haemorrhagic fever. Variations in virus strains within and between the four serotypes may influence disease severity. Secondary infections (particularly with serotype 2) are more likely to result in severe disease and dengue haemorrhagic fever.24,25 This is explained by the theory of antibody dependent enhancement,26 whereby cross reactive but non-neutralising antibodies from a previous infection bind to the new infecting serotype and facilitate virus entry into cells resulting in higher peak viral titres. In primary and secondary infections, higher viral titres are associated with more severe disease.25 Higher titres may result in an amplified cascade of cytokines and complement activation causing endothelial dysfunction, platelet destruction, and consumption of coagulation factors, which result in plasma leakage and haemorrhagic manifestations.27,28
Clinical features and diagnosis
The clinical features of dengue vary with the age of the patient and, in addition to clinically inapparent infections, can be classified into five presentations: non-specific febrile illness, classic dengue, dengue haemorrhagic fever, dengue haemorrhagic fever with dengue shock syndrome, and other unusual syndromes such as encephalopathy and fulminant liver failure.4,29
Young children with dengue often have an undifferentiated febrile illness with a maculopapular rash. Upper respiratory infections, especially pharyngitis, are common. Most infections in children under 15 years are asymptomatic or minimally symptomatic; a study of schoolchildren in Thailand found only 13% of those infected missed more than one day of school because of illness.17
Classic dengue is more commonly seen among older children, adolescents, and adults. They are less likely to be asymptomatic.30 Dengue is abrupt in onset, typically with high fever accompanied by severe headache, incapacitating myalgias and arthralgias, nausea and vomiting, and rash (box B1). Rash, typically macular or maculopapular, often becoming confluent and sparing small islands of normal skin, has been reported in over half of infected people.31 Other signs and symptoms include flushed facies, sore throat, cough, cutaneous hyperaesthesia, and taste aberrations.4 Recovery may be prolonged and include depression.1,15
Box 1.
Diagnosis of dengue fever and dengue haemorrhagic fever
- Haemorrhagic manifestations (shown by positive tourniquet test, petechiae, ecchymoses or purpura, or bleeding from mucosa, gastrointestinal tract, injection sites, or other locations)
- Platelet count <100 000/mm3
- Objective evidence of plasma leakage due to increased vascular permeability shown by either fluctuation of packed cell volume ⩾20% during the course of illness and recovery or clinical signs of plasma leakage such as pleural effusion, ascites, or hypoproteinaemia
- Pulse pressure <20 mm Hg or
- Hypotension (defined as systolic pressure <80 mm Hg for those aged <5 years or <90 mm Hg for those ⩾5)
- Supportive serology on single serum sample: titre ⩾1280 with haemagglutination inhibition test, comparable IgG titre with enzyme linked immunosorbent assay, or positive for IgM antibody test
- Occurrence at same location and time as confirmed cases of dengue fever
- Isolation of dengue virus from serum or autopsy samples
- Fourfold or greater increase in serum IgG (by haemagglutination inhibition test) or increase in IgM antibody specific to dengue virus
- Detection of dengue virus in tissue, serum, or cerebrospinal fluid by immunohistochemistry, immunofluorescence, or enzyme linked immunosorbent assay
- Detection of dengue virus genomic sequences by reverse transcription-polymerase chain reaction
Dengue haemorrhagic fever is primarily a disease of children under 15 years in hyperendemic areas. Black populations may be at decreased risk.32 The disease is characterised by increased capillary permeability and haemostatic changes (see box B1). If major plasma leakage occurs, it usually develops 24 hours before to 24 hours after defervescence. Patients may develop effusions and ascites with a variable amount of bleeding. Enlargement and tenderness of the liver has been reported in up to 40% of patients.4 Mortality can be as high as 10-20% (over 40% if shock occurs) without early appropriate treatment, but it is as low as 0.2% in hospitals with staff experienced in the disease. Warning signs that dengue shock syndrome is impending include sustained abdominal pain, persistent vomiting, change in level of consciousness (irritability or somnolence), a sudden change from fever to hypothermia, and a sudden decrease in platelet count.4,9
Rare presentations of infection include severe haemorrhage, jaundice, parotitis, and cardiomyopathy. Unusual neurological presentations include mononeuropathies, polyneuropathies, encephalitis, and transverse myelitis.22 Guillain-Barré syndrome has been associated with dengue.33 Encephalopathy occurs occasionally and may result from cerebral oedema, cerebral haemorrhage, liver failure, or electrolyte imbalances.
Laboratory findings commonly associated with dengue include neutropenia, lymphocytosis, increased concentration of liver enzymes, and thrombocytopenia. Diagnosis can be confirmed with several laboratory tests; most often the haemagglutination inhibition test and IgG or IgM enzyme immunoassays (see box B1).1,3 The non-specific presentation and course of infection underscore the importance of laboratory testing for confirmation of cause. Several diseases should be considered in the differential diagnosis (box B2).
Box 2.
Differential diagnosis of dengue
- Arboviruses
- Viral diseases
- Bacterial diseases
- Parasitic diseases
Travellers
Travellers may unwittingly be infected with dengue virus because transmission is maintained even between epidemics; malaria should be ruled out in those returning with symptoms from an endemic area.34 In Australia and Germany up to 8% of travellers returning with febrile illnesses were found to have dengue.35–37 Because the incubation period can vary from 3 to 14 days (typically between 5 and 7 days) and viraemia can persist up to 12 days (typically 4 to 5 days),9,25 dengue can be ruled out if symptoms begin more than 2 to 3 weeks after the patient has left an endemic area or if the fever lasts more than two weeks.9 Nevertheless, dengue haemorrhagic fever and dengue shock syndrome are rare in travellers; those with a history of dengue should be advised to protect themselves well from mosquitoes when travelling to endemic areas.
Treatment
No specific therapeutic agents exist for dengue; steroids, antivirals, or carbazochrome (which decreases capillary permeability) have no proven role. In patients without shock, oral hydration should be started early. Paracetamol (aspirin and other non-steroidal anti-inflammatory drugs should be avoided owing to the increased risk for Reye's syndrome and haemorrhage) can be used for fever and analgesia. Assessment of the patient's condition includes packed cell volume, platelet count, liver function tests, prothrombin time, partial thromboplastin time, electrolytes, and blood gas analysis. The patient's clinical condition should be monitored until at least 24 hours after defervesence because of the risk of shock.1
Patients with signs of severe dehydration or haemorrhage or those who cannot be monitored or return quickly if symptoms worsen should be admitted to hospital. Invasive procedures should be considered carefully because of the risk of haemorrhage. The choice of crystalloid or colloid solutions in dengue shock syndrome is under debate.38 No studies have found a difference in clinically significant outcomes, but they do show that appropriate volume repletion is effective in dengue shock syndrome and that lactated Ringer's solution is no better (perhaps worse) than normal saline.38 Larger scale studies may be needed if important clinical differences between colloids and crystalloids are to be found (including the possibility that dextran may worsen bleeding complications).
Prevention
Current prevention of dengue must focus on the mosquito. Aedes aegypti is difficult to control owing to its intimacy with humans. The mosquito readily finds habitats for its larvae in water storage containers and domestic rubbish. Environmental methods for control include the management of water supplies and storage to prevent breeding, the management of solid waste, and the modification of larval habitats created by humans. Vietnam has had some success in eradicating larvae from water storage containers by using the predacious copepod, Mesocyclops spp.39 Mosquito bites may be avoided by removing stagnant sources of water or by using protective clothing, repellants, larvicides (especially for containers that cannot be eliminated), and, in cases of epidemics, insecticides. Insect repellants should be used in the early morning and late afternoon when Aedes mosquitoes are most active.
The lack of a dengue animal model has been an obstacle in vaccine development. Also, safety and efficacy tests on dengue vaccines must consider antibody dependent enhancement, thus necessitating the development of a tetravalent vaccine. Live attenuated tetravalent vaccines are being evaluated in phase 2 trials. New approaches to vaccine development being studied include infectious clone DNA and naked DNA vaccines that may offer simpler and cheaper methods of manufacturing in conjunction with the possibility of greater stability and safety.
It may still be many years before a vaccine is available. The factors contributing to the increased incidence of dengue and emergence of dengue haemorrhagic fever are intensifying. Until the Aedes mosquito can be effectively controlled or a cost effective vaccine developed, dengue can be expected to continue to escalate.
Additional educational resources
Useful websites
Centers for Disease Control and Prevention (www.cdc.gov/ncidod/dvbid/dengue/)—home page for dengue fever. Includes a new section on information for healthcare providers
World Health Organization (www.who.int/ctd/dengue/)—information on status of dengue and its control, with links to WHO documents and relevant publications
Pan American Health Organization (www.paho.org)—website contains presentation of 63 slides related to dengue
Useful books
Gubler DJ, Kuno G, eds. Dengue and dengue haemorrhagic fever. New York: CAB, 1997
Innis BL. Dengue and dengue haemorrhagic fever. In: Porterfield JS, ed. Kass handbook of infectious diseases: exotic virus infections. London: Chapman and Hall, 1995:103-46
Information for patients
Centers for Disease Control and Prevention (www.cdc.gov/travel/)—page specifically dealing with diseases that can affect traveller
World Health Organization (www.who.int/inf-fs/en/fact117.html)—fact sheet on dengue, with links to information on countries where outbreaks have occurred. Describes prevention and control
Supplementary Material
Acknowledgments
Figure 2 was provided by Ed Rowton. The opinions contained herein are those of the authors and should not be construed as representing the official policies of the Department of the Army or the Department of Defense.
Footnotes
Competing interests: None declared.
Criteria for dengue fever and dengue haemorrhagic fever appear on bmj.com
References
- 1.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd edn. Geneva: WHO; 1997. [Google Scholar]
- 2.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Innis BL. Dengue and dengue hemorrhagic fever. In: Porterfield JS, editor. Kass handbook of infectious diseases: exotic virus infections. London: Chapman and Hall Medical; 1995. pp. 103–146. [Google Scholar]
- 5.Rico-Hesse R. Molecular evolution and distribution of dengue viruses type 1 and 2 in nature. Virology. 1990;174:479–493. doi: 10.1016/0042-6822(90)90102-w. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Gubler DJ, Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv Virus Research. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 8.Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, Vaughn DW. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 9.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 10.McBride WJ, Bielefeldt-Ohmann H. Dengue viral infections; pathogenesis and epidemiology. Microbes Infect. 2000;2:1041–1050. doi: 10.1016/s1286-4579(00)01258-2. [DOI] [PubMed] [Google Scholar]
- 11.Gubler DJ. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up? Charles Franklin Craig Lecture. Am J Trop Med Hyg. 1989;40:571–578. doi: 10.4269/ajtmh.1989.40.571. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. National Center for Infectious Diseases, Division of Vector-Borne Infectious Diseases. Dengue fever home page www.cdc.gov/ncidod/dvbid/dengue/slideset/index.htm (accessed 3 Apr 2002).
- 13. World Health Organization. Dengue and dengue haemorrhagic fever. Fact sheet No 117 www.who.int/inf-fs/en/fact117.html (accessed 17 Apr 2002).
- 14.Kurane I, Takasaki T, Yamada K. Trends in flavivirus infections in Japan. Emerg Infect Dis. 2000;6:569–571. doi: 10.3201/eid0606.000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigau-Perez JG, Gubler DJ, Vorndam AV, Clark GG. Dengue: a literature review and case study of travelers from the United States, 1986-1994. J Travel Med. 1997;4:65–71. doi: 10.1111/j.1708-8305.1997.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 16.From the Centers for Disease Control and Prevention. Imported dengue—United States, 1997 and 1998. JAMA. 2000;283:1953–1954. [PubMed] [Google Scholar]
- 17.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurti C, Kalayanarooj S, Cutting MA, Peat RA, Rothwell SW, Reid TJ, et al. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Hyg. 2001;65:840–847. doi: 10.4269/ajtmh.2001.65.840. [DOI] [PubMed] [Google Scholar]
- 19.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, et al. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 20.Rosen L. Comments on the epidemiology, pathogenesis, and control of dengue. Med Trop. 1999;59:495–498. [PubMed] [Google Scholar]
- 21.Lum LC, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 22.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 23.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848–851. doi: 10.4269/ajtmh.2001.65.848. [DOI] [PubMed] [Google Scholar]
- 24.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan NJ. Antibody-mediated enhancement of viral disease. Curr Top Microbiol Immunol. 2001;260:145–169. doi: 10.1007/978-3-662-05783-4_8. [DOI] [PubMed] [Google Scholar]
- 27.Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomed Sci. 2001;8:377–388. doi: 10.1007/BF02255946. [DOI] [PubMed] [Google Scholar]
- 28.Kurane I, Takasaki T. Dengue fever and dengue haemorrhagic fever: challenges of controlling an enemy still at large. Rev Med Virol. 2001;11:301–311. doi: 10.1002/rmv.324. [DOI] [PubMed] [Google Scholar]
- 29.Isturiz RE, Gubler DJ, Brea del Castillo J. Dengue and dengue hemorrhagic fever in Latin America and the Caribbean. Infect Dis Clin North Am. 2000;14:121–140. doi: 10.1016/s0891-5520(05)70221-x. [DOI] [PubMed] [Google Scholar]
- 30.Sharp TW, Wallace MR, Hayes CG, Sanchez JL, DeFraites RF, Arthur RR, et al. Dengue fever in US troops during Operation Restore Hope, Somalia, 1992-1993. Am J Trop Med Hyg. 1995;53:89–94. [PubMed] [Google Scholar]
- 31.Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117–122. doi: 10.1016/0738-081x(89)90034-5. [DOI] [PubMed] [Google Scholar]
- 32.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, et al. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 33.Gaultier C, Angibaud G, Laille M, Lacassin F. [Probable Miller Fisher syndrome during dengue fever type 2] Rev Neurol (Paris) 2000;156:169–171. [PubMed] [Google Scholar]
- 34.Magill AJ. Fever in the returned traveler. Infect Dis Clin North Am. 1998;12:445–469. doi: 10.1016/s0891-5520(05)70013-1. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien D, Tobin S, Brown GV, Torresi J. Fever in returned travelers: review of hospital admissions for a 3-year period. Clin Infect Dis. 2001;33:603–609. doi: 10.1086/322602. [DOI] [PubMed] [Google Scholar]
- 36.Jelinek T. Dengue fever in international travelers. Clin Infect Dis. 2000;31:144–147. doi: 10.1086/313889. [DOI] [PubMed] [Google Scholar]
- 37.Potasman I, Srugo I, Schwartz E. Dengue seroconversion among Israeli travelers to tropical countries. Emerg Infect Dis. 1999;5:824–827. doi: 10.3201/eid0506.990615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nhan NT, Cao XT, Kneen R, Wills B, Nguyen VM, Nguyen TQ, et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis. 2001;32:204–213. doi: 10.1086/318479. [DOI] [PubMed] [Google Scholar]
- 39.Nam VS, Yen NT, Holynska M, Reid JW, Kay BH. National progress in dengue vector control in Vietnam: survey for Mesocyclops (Copepoda), Micronecta (Corixidae), and fish as biological control agents. Am J Trop Med Hyg. 2000;62:5–10. doi: 10.4269/ajtmh.2000.62.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.