Abstract
The interplay between humans and their microbiome is crucial for various physiological processes, including nutrient absorption, immune defense, and maintaining homeostasis. Microbiome alterations can directly contribute to diseases or heighten their likelihood. This relationship extends beyond humans; microbiota play vital roles in other organisms, including eukaryotic pathogens causing severe diseases. Notably, Wolbachia, a bacterial microbiota, is essential for parasitic worms responsible for lymphatic filariasis and onchocerciasis, devastating human illnesses. Given the lack of rapid cures for these infections and the limitations of current treatments, new drugs are imperative. Here, we disrupt Wolbachia’s symbiosis with pathogens using boron-based compounds targeting an unprecedented Wolbachia enzyme, leucyl-tRNA synthetase (LeuRS), effectively inhibiting its growth. Through a compound demonstrating anti-Wolbachia efficacy in infected cells, we use biophysical experiments and x-ray crystallography to elucidate the mechanism behind Wolbachia LeuRS inhibition. We reveal that these compounds form adenosine-based adducts inhibiting protein synthesis. Overall, our study underscores the potential of disrupting key microbiota to control infections.
An approach to control infection by disrupting pathogen's microbiota is presented.
INTRODUCTION
Understanding the relationship between microbiota and their host species is at the forefront of medical research. There is growing evidence indicating that the interplay between microbiota and their host mammals is crucial for many cellular processes such as the absorption of food nutrients, immune defense, and homeostasis. Alterations of the population and number of microbiota species, the so-called dysbiosis, have been directly linked to the development of human diseases (1, 2). As in humans, bacteria are also crucial for many other eukaryotic species, including pathogens causing major human diseases. However, specific targeting of microbiota species in strong symbiosis with their host pathogens remains an underexploited approach to control infections in humans. A remarkable example of strong symbiosis in pathogens is Wolbachia, a bacterium discovered nearly a century ago (3) that is considered one of the most successful organisms in the biosphere because of its wide colonization among arthropods and nematodes (4). This is due to the strong interdependence between Wolbachia and its hosts on successful reproduction and other symbiotic processes (5–7). Moreover, Wolbachia infects mosquito species that are vectors of disease to humans, and it establishes reproductive parasitism (4). Wolbachia is also an obligate intracellular bacterium vital for the survival and fertility of the parasitic worms causing lymphatic filariasis (LF) and onchocerciasis (Fig. 1A) (8), both devastating diseases that infect 117 million people, mostly in tropical regions. There is no rapid cure for these infections, and the current standards of care, ivermectin (for onchocerciasis) or in combination with albendazole and diethylcarbamazine (for LF), not only are contraindicated for children or women during early pregnancy, but also involve very long treatments that lack efficacy against adult worm parasites (8). Thus, there is an urgent need for faster and more effective drugs to cure these diseases, which could target either the pathogenic worms or, given their strong endosymbiotic relationships, their Wolbachia microbiota (8). The therapeutic benefits of inducing dysbiosis by targeting Wolbachia are supported by the fact that the antibiotics rifampin, tetracycline, and doxycycline can arrest worm development in preclinical models of filariasis (9–14) and by the fact that mosquito populations transmitting diseases can be controlled by targeting their Wolbachian microbiota, leading to incompatible insect reproduction (15–17). However, one of the main challenges in the development of anti-filarial drugs against Wolbachia is the identification of novel drug targets of this microbiota because Wolbachia is an obligate intracellular bacterium. This severely thwarts Wolbachia genetic manipulation outside its hosts, making the search for essential genes by traditional genetic approaches very difficult, and the validation of the putative drug targets and the evaluation of the resistance mechanisms very challenging (4, 5).
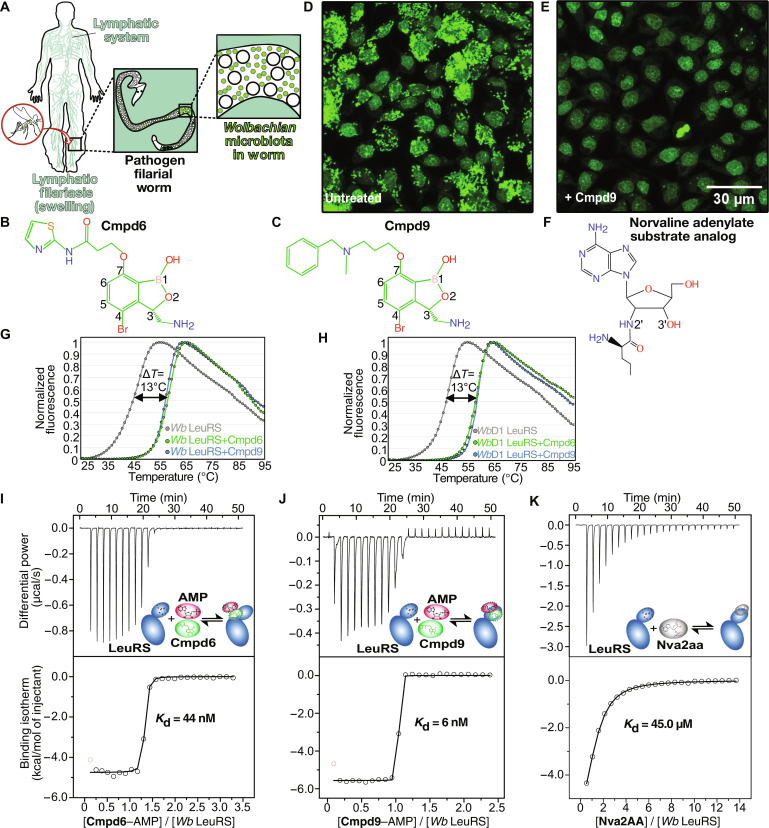
Fig. 1. Benzoxaborole compounds target microbiota Wolbachian LeuRS.
(A) Transmitted by mosquito bites, filarial worm pathogens can reach the lymphatic nodes where they cause severe chronic pain and swelling of tissues. (B, C, and F) Chemical structures of Cmpd6, Cmpd9, and editing substrate analog of norvaline (Nva), respectively. (D) C6/36 insect cells (A. albopictus) stably infected with W. pipientis (wAlbB) were analyzed by fluorescence and high-content image analysis. Single-cell image analysis shows the nucleus (fluorescent large shapes) in the center surrounded by colonizing Wolbachia (small fluorescent dots) in the cytoplasm. (E) Reduction of Wolbachia load in host cells treated with Cmpd9 at 9 μM for 7 days. (G and H) Thermal-shift assays showing the stabilization of Wolbachia LeuRS proteins in the presence of the inhibitors Cmpd6 and Cmpd9. The experiments were performed in triplicates for both the wild-type Wolbachia LeuRS and the construct Wolbachia LeuRS D1 in the presence of AMP. (I, to K) ITC binding experiments showed one order of magnitude higher affinity of Cmpd6/9 in the presence of AMP compared to norvaline posttransfer analog (physiological editing substrate of LeuRS). DSF and ITC experiments were performed in biological and technical triplicates; N = 3.
Here, we report the discovery of potent boron-based compounds that inhibit an unprecedented target of Wolbachia, leucyl-tRNA synthetase (LeuRS), and demonstrate that inhibition of this microbiota target is an efficient approach to arrest the growth of its host. We combine cell-based experiments showing compound efficacy in a Wolbachia-infection model with in vitro experiments by nuclear magnetic resonance (NMR), x-ray crystallography, and other biophysical methods to reveal the molecular basis of the Wolbachia LeuRS inhibition mechanism. Our work shows how targeting the LeuRS protein of pathogen microbiota is a valid approach to control infection, a strategy with potential applications to other human pathogens.
RESULTS
Identification of benzoxaboroles with activity against Wolbachia LeuRS
To identify active compounds against Wolbachia, we carried out a high-throughput screening assay by testing a library of 202 benzoxaborole compounds in a host-cell model of Wolbachia infection (5, 18), which yielded an 18% hit rate. The mosquito-derived cell line C6/36 stably infected with Wolbachia pipientis was treated with each benzoxaborole compound at doses ranging from 10 nM to 5 μM during a short period (7 days) and was used to calculate the half-maximum effective concentration (EC50). The screening in cells was combined with activity experiments (aminoacylation) performed with recombinant full-length Wolbachia LeuRS protein, which were used to calculate the half-maximum inhibitory concentration (IC50) for the most interesting compounds identified in the cell screening. Thus, this yielded compounds with both good anti-Wolbachian in vitro activity and in vivo efficacy, with representative compounds shown in table S1. Cmpd6 and Cmpd9 (Fig. 1, B and C) were the most interesting compounds with IC50 values in the low nanomolar range (table S1). Moreover, the potent in vitro activity of Cmpd9 translated in very good in cellulo efficacy (EC50 = 90 nM) in the Wolbachia infection model (Fig. 1, D and E, and table S1). This may be due to the formation of a very stable inhibition complex by Cmpd9, as judged by the higher half-life (also named reactivation rate) of Cmpd9 (T1/2 = 226 min) compared to other compounds (table S1).
Inhibition of the Wolbachia LeuRS editing site by Cmpd6 and Cmpd9
Next, we sought to identify the target and investigate the inhibition mechanism of these compounds. Previous studies have shown that 3-amino-methyl benzoxaboroles can inhibit the editing site of bacterial LeuRS and form adenosine-based covalent adducts with adenosine monophosphate (AMP), adenosine triphosphate (ATP), and tRNALeu terminal base (19–22). The editing site of LeuRS plays a key role to maintain fidelity in protein translation by hydrolyzing tRNALeu mischarged to noncognate amino acids such as norvaline (23, 24). Thus, we tested whether the benzoxaboroles Cmpd6 and Cmpd9 are inhibitors of Wolbachia LeuRS and whether these compounds compete with the main substrate of the editing site, i.e., norvaline (Fig. 1F). Norvaline is a nonproteinogenic amino acid that can be mischarged to tRNALeu in the LeuRS synthetic site and becomes toxic when incorporated into proteins, if not proofread at the LeuRS editing site (25). We designed constructs of the Wolbachia LeuRS editing domain (fig. S1), which bear the putative drug-binding pocket, for expression and purification of recombinant proteins. Two constructs gave soluble and well-behaving proteins that were used for biophysical and structural studies: a construct encoding for residues 219 to 418, herein named Wolbachia LeuRS; and another construct named Wolbachia LeuRS D1, for which part of an insertion specific to Wolbachia LeuRS (herein denoted as i1) and predicted to be highly disordered was partially deleted (354 to 377) (fig. S1). The folding and the thermal stability of both proteins were studied by differential scanning fluorimetry (DSF) in the absence and presence of compounds. Both proteins were folded, and in the presence of Cmpd6 or Cmpd9 with AMP, the Wolbachian LeuRS constructs exhibited a marked increase of the melting temperature (∆Tm = 13°C) compared to the free proteins (Fig. 1, G and H), suggesting a strong binding of the compounds to the LeuRS protein. Equivalent experiments performed in the presence of Cmpd9 and ATP show a similar increase of the melting temperature, suggesting that ATP and AMP can form similar inhibition adducts with these compounds (fig. S2). To quantitatively assess the affinity of these compounds to Wolbachia LeuRS, isothermal titration calorimetry (ITC) experiments were carried out. In the absence of AMP, Cmpd6 and Cmpd9 did not bind to Wolbachia LeuRS; however, in the presence of AMP, the compounds were potent LeuRS inhibitors, with dissociation constants of 44 and 6 nM, respectively (Fig. 1, I and J). This contrasted with the one order of magnitude weaker affinity (45 μM) of the norvaline adenylate analog (Nva2AA, Fig. 1K), which represents the physiological substrate of the LeuRS editing site. Cmpd9 has stronger affinity than Cmpd6, which arises from a more favorable binding entropy (table S2), likely due to a more optimal conformation by the alkyl-phenyl group of Cmpd9 to bind into the Wolbachia LeuRS drug pocket.
Collectively, our experiments support a LeuRS inhibition mechanism by benzoxaboroles that is dependent on adenosine-based molecules like AMP.
Adenosine-dependent activation mechanism of the prodrugs Cmpd6 and Cmpd9
Benzoxaborole inhibitors of Mycobacterium tuberculosis LeuRS that recently completed phase 2 clinical studies are prodrugs that activate with adenosine to become active (26). To investigate whether our anti-Wolbachian compounds use this activation mechanism, we used NMR spectroscopy to study whether the compounds interact with ATP or AMP in the absence of the protein (fig. S3A and B). We acquired one-dimensional (1D) 1H NMR spectra of free ATP and AMP and of equimolar mixtures of the compounds with AMP or ATP at cellular physiological concentrations (5 mM). The spectra show the interaction of Cmpd6 with AMP/ATP in the absence of the protein, leading to the formation of two covalent adducts (diastereomers), differentiated by the stereochemistry of the boron atom, which we denote as adducts of type “a” (exo-ribose) or “b” (endo-ribose) (fig. S3B and C). Upon adduct formation, the boron atom changes from a neutral sp2 trigonal configuration in the free compounds to a negatively charged sp3 tetrahedral configuration in the adducts (fig. S3C).
To quantitatively assess the formation of the adenosine-based inhibitor adducts, we performed ITC experiments by titrating Cmpd9 into AMP in the absence of protein (fig. S3D). Fitting of the experimental binding isotherm to a one-site binding model gives a Kd of 1.0 mM and shows that the interaction is mainly driven by a favorable enthalpic contribution, as expected for the formation of new bonds between the oxaborole group of the compound and the ribose hydroxyls of adenosine.
Altogether, these experiments show that benzoxaborole inhibitors of Wolbachia LeuRS are prodrugs that, in an enzyme-independent manner, form adducts with adenosine substrates. The adducts then bind with potent nanomolar affinity into the Wolbachia LeuRS editing site and efficiently compete with the physiological substrate norvaline.
Structural basis of the inhibition of Wolbachia LeuRS by benzoxaborole-AMP adducts
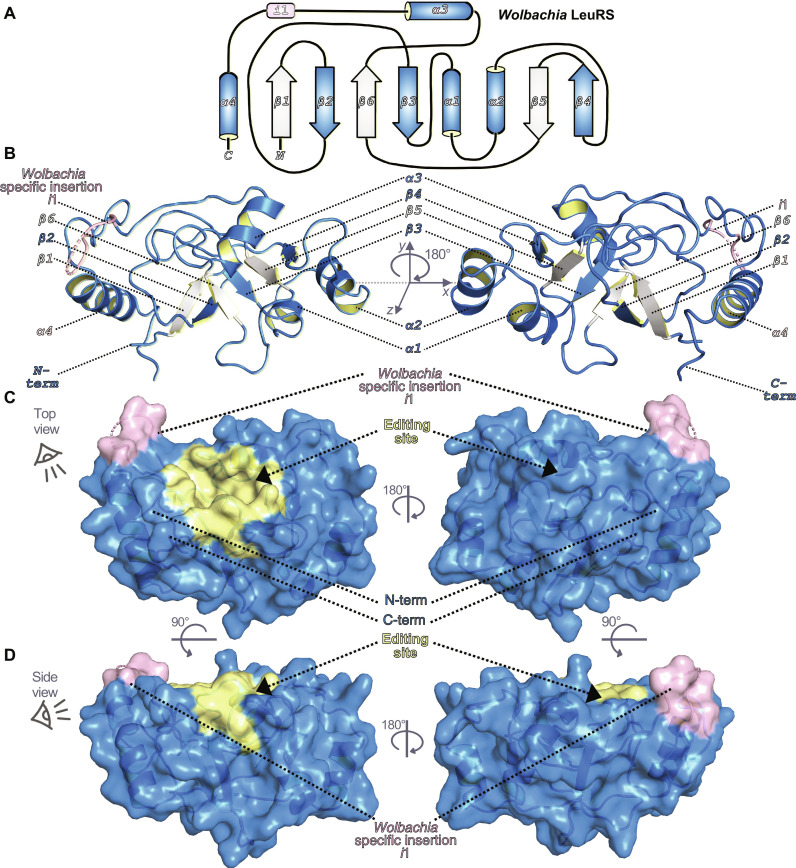
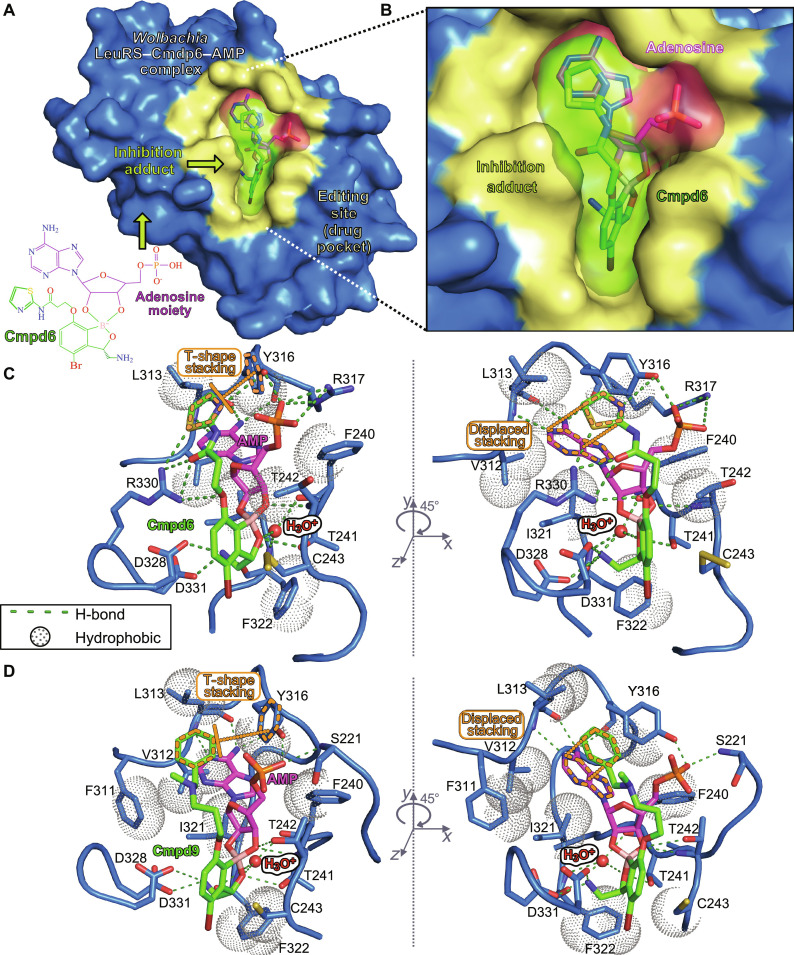
To unveil the structural details of the inhibition mechanism of Wolbachia LeuRS by these compounds, we performed crystallization trials with the protein in the presence and absence of inhibitors and AMP. By using a construct with a shortened, flexible insertion i1, Wolbachia LeuRS D1 (fig. S1), we managed to obtain high-quality crystals of the apo-protein and the complexes with the adenosine adducts formed by Cmpd6 and Cmpd9, which diffracted to 3.0, 1.8, and 2.1 Å, respectively (table S3). The architecture of Wolbachia LeuRS consists of six antiparallel β strands that form an extended β sheet with four inserted α helices, hence adopting a β1-β2-β3-α1-α2-β4-β5-β6-α3-α4 fold (Fig. 2, A to D). Specific features of Wolbachia LeuRS are the very long insertion (i1), which connects helices α3 and α4, and two cysteines (C243 and C324) located in the drug-binding pocket, which are not found in any other bacterial LeuRS species (fig. S1, discussed later) or in human LeuRSs (cytosolic and mitochondrial). The cocrystal structures of Wolbachia LeuRS in complex with Cmpd6 and Cmpd9 show that the LeuRS inhibition mechanism relies on adenosine-based adducts of type “a,” as detected by NMR, which tightly bind into the drug pocket of LeuRS (Fig. 3, A and B, and fig. S4). Docking of diastereomers of type “b” into the Wolbachia LeuRS editing site shows steric clashes of the benzoxaborole within the editing site, indicating that these adducts are not LeuRS inhibitors. Docking of the active adducts of Cmp6/9 into the editing site of human LeuRS shows substantial steric clashes (fig. S5, A to D), which explains the selectivity of this class of compounds toward bacterial LeuRS and hence their low toxicity in humans (20, 21, 26).
Fig. 2. Crystal structure of apo Wolbachia LeuRS.
(A) 2-D architecture of the Wolbachia LeuRS editing domain showing an iterative α-β secondary structure organization. For clarity, α helices are colored blue and β sheets are intermittently colored white/blue. The Wolbachia LeuRS specific insertion i1 is colored pink. (B) Crystal structure of Wolbachia LeuRS D1 editing domain shown in cartoon representation with key secondary elements colored as in (A). (C and D) Semi-transparent surface representations of the Wolbachia LeuRS crystal structure showing a top view rotated by 180° around the y axis and a side view rotated by 45° around the x axis, respectively. The editing site (drug-binding site) is shown in yellow and the insertion i1 is shown in light pink.
Fig. 3. Crystal structures of the Wolbachia LeuRS complexes with AMP-compound adducts.
(A) Overall structure of the Wolbachia LeuRS complex with the adduct AMP-Cmpd6 bound into the editing site. Van der Waals surface representation in blue was used for the protein, with the drug-binding site in yellow. (B) Zoomed-in view showing the inhibition adduct in surface and stick representations, with the AMP group in pink and Cmpd6 in green. (C and D) Main interactions established by the adenosine-drug inhibition adducts formed by Cmpd6 and Cmpd9, respectively. Hydrogen bonds are shown as green dashed lines and hydrophobic interactions are shown as dotted spheres. Key protein residues are shown as blue sticks, the hydroxonium ion is shown as a red sphere, and the compound-AMP adducts are in the same color code as in (B). π-π aromatic stackings of the thiazole/phenyl rings of Cmpd6/9 are highlighted in orange dashed lines. For clarity, panels are rotated by 45° around the y axis.
The potent nanomolar affinities of the two compounds are explained by a combination of multiple polar and hydrophobic interactions established by the inhibition adducts in the editing site (Fig. 3, C and D). Both compounds make equivalent interactions with Wolbachia LeuRS, except for minor differences in the 2-amido-thiazole and amino-methyl phenyl groups of Cmpd6 and Cmpd9, respectively. Specifically, the amino-methyl phenyl of Cmpd9 packs between residues F311, L313, and Y316, and makes several hydrophobic contacts, and at the same time, the phenyl group makes two favorable aromatic π-π interactions, one parallel-displaced with the adenosine ring and one T-shape stacking with Y316 (Fig. 3D). This conformation is further stabilized by hydrogen bonds of the adenosine-phosphate to residues Y316 and S221. In Cmpd6, the 2-amido-thiazole group enables multiple polar contacts to the side chains of R330 and Y316 (Fig. 3C), the latter further being stabilized by interactions of the adenosine-phosphate with Y316 and R317 and an intramolecular interaction within Cmpd6 established by the sulfur atom with the neighboring carbonyl. Furthermore, the oxygen at position 7 hydrogen bonds to the side chain of R330 (Fig. 3C). The amido-thiazole group also packs against the adenosine ring; however, the π-π stacking is not entirely parallel compared to the phenyl of Cmpd9. Other key interactions, shared by the two compounds, are a hydrogen bond by the oxaborole oxygen-1 to T241; and the three hydrogen bonds established by the 3-aminomethyl to F322, D328, and D331. Moreover, a water molecule, likely activated in the form of a hydroxonium cation (H3O+), establishes four hydrogen bonds: three as a donor to the carbonyl of residues F322 and F240 and to the oxygen of the oxaborole group, and one as an acceptor to the amino group of F322 (fig. S6, A and B). This positively charged hydroxonium is likely a leaving water upon adduct formation (water 13 in the structure of the complex with Cmpd9 and water 2 in the complex with Cmpd6) and fits well in a protein cavity formed by residues 239 to 241 and 321 to 322. The hydroxonium ion establishes electrostatic interactions with the negatively charged boron atom of Cmpd6/Cmpd9 and, thus, stabilizes the tetrahedral configuration of the oxaborole moiety adopted in the inhibition adduct (fig. S6C). Altogether, the data show that the hydroxonium-mediated stabilization of the oxaborole-adenosine adduct is important for the Wolbachia LeuRS inhibition mechanism.
Structural rearrangements in Wolbachia LeuRS upon binding of compounds
Comparison of the apo-structure to the complexes of Wolbachia LeuRS shows that the binding of the inhibition adducts of Cmpd6/9 induces several conformational changes (fig. S7). Notably, the regions 267 to 270 and 280 to 285, which are not visible in the apo-structure, respectively adopt α helix and β tongue structures that stabilize the inhibition adduct in the complex (fig. S7A). In addition, the region 313 to 318, which, as judged by the high crystallographic B factors, is highly flexible in the apo-structure, becomes more compact in the complex and makes interactions to the adduct. Specifically, the side chain of R317 shifts 3.5 Å toward the phosphate of the Cmpd6–AMP adduct and stabilizes the aromatic ring of Y316, altogether enabling multiple interactions with the adduct (fig. S7B). Another key change is a 90° rotation of the R330 side chain toward the adduct that gives rise to four hydrogen bonds to the amido-thiazole group (fig. S7C), further stabilizing the inhibition adduct within the Wolbachia LeuRS pocket.
We also note that Cys243, which is a residue specific to Wolbachia LeuRS (arginine in other bacterial LeuRS pathogens) (figs. S1 and S8), forms a disulfide adduct with β-mercaptoethanol (added in our buffer) in the holo-structure with Cmpd6 (Fig. 3C) but not in the unbound protein, suggesting that the binding of the adduct increases the reactivity of the sulfur atom of this cysteine. The sulfur is 3.5 and 3.8 Å away from carbons 5 and 6 of Cmpd6/Cmpd9, respectively; thus, specific 5,6-derivatized compounds have the potential to react covalently with Cys243 (fig. S8, A and B). Another residue unique to Wolbachia LeuRS is Cys324 (figs. S1 and S8). Cys324 does not participate in interactions; however, it is 3.7 Å away from the bromine of Cmpd6/9, suggesting that larger substitutions in position 4 of the core benzoxaborole could enable additional interactions (fig. S8B). In conclusion, the specificities in the Wolbachia LeuRS drug pocket explain the selectivity of these inhibitors, which is an important feature to advance compounds into clinical studies.
Structural basis of the inhibition of LeuRS by oxaborole-tRNALeu complexes
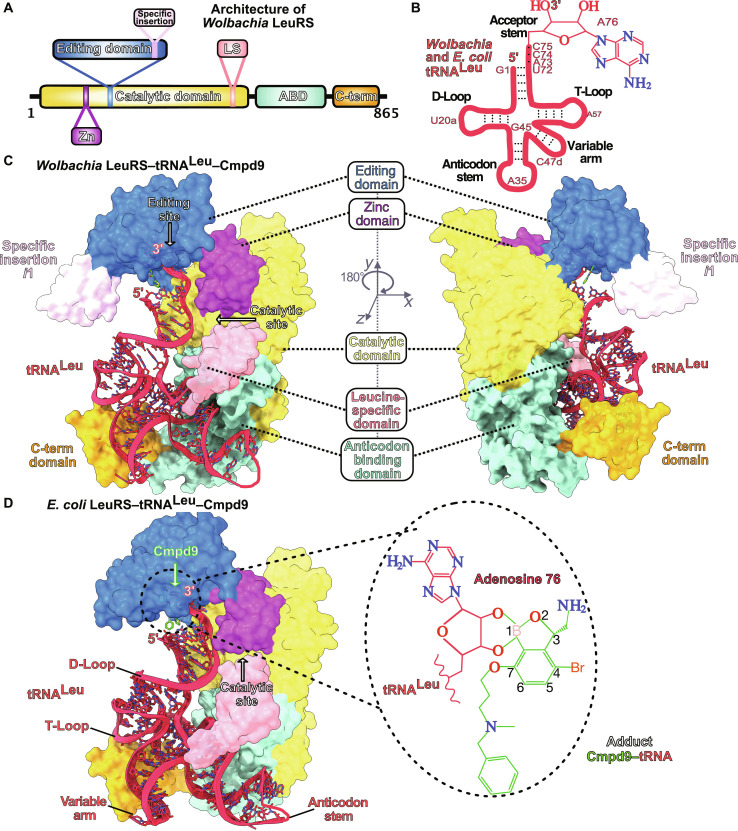
Next, we sought to investigate whether these Wolbachia LeuRS inhibitors can form tRNALeu adducts that bind into the LeuRS editing site as other 3-aminomethyl benzoxaboroles have been observed as adducts with the tRNALeu terminal base (adenosine-76). We expressed and purified full-length Wolbachia LeuRS protein (Wb FL LeuRS); however, extensive crystallization trials did not give crystals. Therefore, based on the high sequence similarity and structural homology of the LeuRS drug-binding pocket between Wolbachia and Escherichia coli as well as the tRNALeu isoacceptors (Fig. 4, A and B, and fig. S9), we produced full-length E. coli LeuRS (Ec LeuRS) and used it to model the structure of full-length Wolbachia LeuRS (Fig. 4C). We succeeded in obtaining high-quality crystals of the Ec LeuRS–tRNALeu complex with Cmpd9, which allowed us to determine its structure at 2.1 Å resolution (Fig. 4D). Both Wolbachia and E. coli LeuRS structures share a common domain architecture, except for an insertion of ~20 residues that is specific to Wolbachia (Fig. 4C and fig. S9). The cocrystal structure of the LeuRS-tRNALeu complex unambiguously shows the adduct formed by Cmpd9 with the last base of the tRNALeu acceptor stem (Ade76) bound into the editing site (Fig. 4D). The position of the adduct formed by the terminal tRNALeu adenosine with Cmpd9 in the complex between full-length LeuRS and tRNALeu and the interactions in the drug pocket are comparable to those observed in the Wolbachia LeuRS editing domain cocrystal structures with AMP-Cmpd9 or AMP-Cmpd6 (fig. S10). The main difference between the adducts formed by tRNALeu terminal adenosine and AMP is the orientation of the phenyl group of Cmpd9 or, by analogy, the amido-thiazole group of Cmpd6. As shown above (Fig. 3, C and D), in the adduct formed with AMP, the phenyl and amido-thiazole groups stack against the adenosine base and enable multiple interactions within the Wolbachia LeuRS drug pocket. However, in the complex of full-length LeuRS with tRNALeu and Cmpd9, the phenyl group is shifted 3.5 Å away from the 3′-tRNALeu due to the intramolecular π-π stacking of the tRNALeu base Ade76 with Cyt75 (fig. S11). This increases the flexibility of the phenyl group in the adduct formed with tRNALeu compared to the AMP adduct (phenyl packed to adenosine), as reflected in the larger crystallographic B factors. Specifically, the phenyl moiety has significantly higher B factors in the tRNALeu adduct (〈B〉 = 59 Å2) compared to the rest of the adduct (〈B〉 = 39 Å2). Conversely, the B factors of the adduct AMP-Cmpd9 are invariably low for all atoms, including the phenyl group. The equivalent is observed for the amido-thiazole group of Cmpd6 in the Wolbachia LeuRS-AMP-Cmpd6 crystal structure.
Fig. 4. Structures of full-length LeuRS complexes with tRNALeu adenosine-compound adducts.
(A) Domain architecture of Wolbachia LeuRS protein. (B) Cloverleaf-like secondary structures of Wolbachia and E. coli tRNAsLeu with highlighted main regions. Key tRNALeu bases are numbered with the last base of the acceptor stem (Ade76) shown as sticks. (C) Wolbachian LeuRS-tRNALeu complex with Cmpd9 built by using as templates the crystal structures of the Wolbachia LeuRS editing domain and of the full-length E. coli LeuRS-tRNALeu-Cmpd9. The LeuRS protein is shown in surface representation, and the different domains are in the same color code as in (A). An insertion that is unique to Wolbachia LeuRS, named specific insertion i1, is shows as light-pink surface. The tRNALeu is shown in sticks/ribbon representation and Cmpd9 is shown as green sticks. (D) Crystal structure of the E. coli LeuRS-tRNALeu-Cmpd9 complex determined at 2.1 Å resolution. Protein and tRNALeu are shown in the same color code as Wolbachia LeuRS complexes. The structure of Cmpd9 is shown as green sticks. The zoomed-in view shows the adduct formed by the tRNALeu terminal adenosine with the oxaborole group of Cmpd9.
Thus, while the overall position of the adducts formed by tRNALeu and AMP with the compounds remains unchanged in the full-length LeuRS and editing domain complexes, the presence of tRNALeu induces a structural rearrangement of the terminal phenyl/amido-thiazole groups to make compatible the binding of the Cmpd9/Cmpd6-tRNALeu inhibition adducts into the LeuRS editing site.
DISCUSSION
The strong symbiosis between parasitic worms and their Wolbachian microbiota, a species that is absent in humans, provides an opportunity to target this bacterium and thus to control human infections such as those caused by filarial pathogens. This is supported by the fact that some antibiotics acting on Wolbachia have shown promising effects in preclinical and clinical studies aiming at developing therapies to treat filarial human diseases (5, 27–29). However, one of the main hurdles for targeting Wolbachia is the identification of new targets. Here, we show how the inhibition of an unprecedented target in Wolbachia by boron-based compounds represents an opportunity to develop therapeutics for the treatment of filarial diseases caused by parasitic worms. We identify potent anti-Wolbachia LeuRS inhibitor prodrugs, one of which has potent nanomolar efficacy in the standard Wolbachian model of infection. Our biophysical and structural analysis provides a detailed view of the inhibition mechanism, which consists of the formation of a covalent adenosine-prodrug adduct in a LeuRS-independent manner. This adduct can be formed with AMP, ATP, or tRNALeu terminal adenosine (Ade76) and binds into the Wolbachia LeuRS editing site with potent nanomolar affinity. Moreover, the positively charged oxaborole group of the inhibition adducts is stabilized by a negatively charged hydroxonium ion that fits well in a cavity of the Wolbachia LeuRS binding site.
The new high-resolution crystal structures presented reveal idiosyncratic features at the drug binding pocket, which rationalize the selectivity of these Wolbachia inhibitors. These features enable the development of narrow-spectrum anti-infectives targeting Wolbachia, a much-heralded property for the development of new antimicrobials with minimized dysbiosis in patients.
In conclusion, LeuRS is a valid drug target of Wolbachia, and besides the recently identified anti-Wolbachian compounds such as flubentylosin, azaquinazoline derivatives, and boron-pleuromutilins (27, 29–31), the benzoxaborole inhibitors revealed here provide a new approach for the treatment of filarial diseases. More generally, this study demonstrates the benefits of selective disruption of bacterial microbiota in strong symbiosis with host pathogens causing disease in humans, a therapeutic approach with vast potential for other infectious diseases. As research continues to unravel the intricacies of the microbiota, we can anticipate transformative breakthroughs that will revolutionize the way we control infectious diseases.
MATERIALS AND METHODS
Efficacy of compounds against Wolbachia in an insect cell infection model
The efficacy of compounds, determined as EC50 against Wolbachia in infected host cells, was determined through the anti-Wolbachia consortium’s (A·WOL) routine screening assay as described previously (18, 27, 29). Briefly, the cell line C6/36 derived from the mosquito vector of disease Aedes albopictus, stably infected with W. pipientis (wAlbB) [C6/36 (wAlbB)], was incubated with the compounds of interest in a dose range from 5 μM to 10 nM. Compounds were incubated for 7 days with 2000 cells per well on a 384-well plate (CellCarrier-384 Ultra, PerkinElmer) in Leibovitz media (Life Technologies) supplemented with 20% fetal bovine serum (FBS, Thermo Fisher Scientific), 2% tryptose phosphate broth (Sigma-Aldrich), and 1% nonessential amino acids (Sigma-Aldrich). The endpoint readout utilized DNA staining of both the host cell nuclei and intracellular Wolbachia (SYTO 11) combined with a high content imaging system (Operetta, PerkinElmer) and analyzed using the associated Harmony software through a cytoplasm texture analysis. Stock solutions at 10 mM of chemical compounds were prepared in dimethyl sulfoxide (DMSO). Chemical compounds were provided by chemical manufactures (ChemPartner and Anacor Pharmaceuticals), and quality control was verified by NMR spectroscopy (1H-NMR) and liquid chromatography coupled to mass spectrometry. Compound purity was higher than 95%. Compound identity and chemical structures are summarized in table S1.
Expression and purification of full-length Wolbachia LeuRS for in vitro activity assays
The full-length Wolbachia LeuRS construct corresponds to residues 1 to 865 of Wolbachia sp. Brugia malayi (Uniprot Q5GS31). It was sub-cloned into a pET-16b vector (Genscript) at the restriction sites Nde I and Bam HI, which contains an N-terminal hexahistidine tag followed by a tobacco etch virus (TEV) cleavage site. The protein was overexpressed in E. coli BL21-CodonPlus (DE3)-RIL grown in terrific broth medium at 37°C until the optical density at 600 nm reached 0.9. Bacerial cell cultures were cooled down to 23°C on a water/ice bath and protein expression was induced with the addition of 1 mM IPTG and then incubated overnight at 23°C. The cells were collected by centrifugation and frozen at −80°C. Cell lysis was carried out by sonication using as purification buffer 20 mM Tris-HCl, pH 8.5, 100 mM NaCl, 5 mM MgCl2, and 20 mM β-mercaptoethanol supplemented with protease inhibitor tablets (Roche). The protein was purified by nickel (Ni-NTA) affinity chromatography (Qiagen). Three washing steps of 50 ml each with buffer were used for Ni affinity chromatography with the same buffer as the purification buffer with the addition of (i) 15 mM imidazole, (ii) 1 M NaCl, and (iii) 15 mM imidazole. The elution buffer was the same as the purification buffer with the addition of 500 mM imidazole. The protein was further purified by size-exclusion chromatography using a HiLoad 200 Superdex column (AKTA) equilibrated in the buffer 20 mM Tris-HCl, pH 8.5, 100 mM NaCl, 5 mM MgCl2, and 5 mM dithiothreitol supplemented with protease inhibitor tablets (Roche). The full-length Wolbachia LeuRS protein was concentrated to 10 mg/ml with a 50-kDa cutoff membrane (Millipore) and flash-frozen in liquid nitrogen before storage at −80°C.
Anti-Wolbachia LeuRS in vitro activity of compounds
The IC50 values were calculated using a similar approach as described earlier (19, 20). Briefly, Wolbachia full-length LeuRS, total E. coli tRNA crude extract (Roche), and compounds were preincubated for 20 min in 50 mM Hepes-KOH (pH 8.0), 30 mM MgCl2, 30 mM KCl, 0.02% (wt/vol) bovine serum albumin, and 1 mM dithiothreitol with 20 μM [14C]leucine (306 mCi/mmol; PerkinElmer) at 30°C. Reactions were started by the addition of 4 mM ATP and, at specific times, tRNA was precipitated by the addition of 10% (w/v) trichloroacetic acid (TCA), recovered by filtration (Millipore Multiscreen; MSHAN4B50), washed with 5% TCA (w/v), and counted by a liquid scintillation counter (Wallac). All reactions were performed in triplicate, and the mean values were used to determine an IC50 using Prism 4 (GraphPad). The reactivation rates (T½) were determined by measuring the time required for Wolbachia LeuRS to recover 50% of its activity. Full-length Wolbachia LeuRS (40 nM) and tRNA and compounds were preincubated for 1 hour at 4°C to inhibit 90% of the enzyme’s activity (approximately 10 times the value of the [IC50]). LeuRS inhibitor complexes were then diluted 200-fold, and enzyme activity was determined at various time intervals, as described before. Compound reactivation rates were determined by fitting the LeuRS activity reactivation curves to a one-phase exponential decay model (GraphPad Prism).
Expression and purification of Wolbachia and E. coli LeuRS samples and in vitro transcription of E. coli tRNALeu
The Wolbachia LeuRS constructs used for crystallization correspond to residues G219-I418 of Wolbachia sp. Brugia malayi (Uniprot Q5GS31), and the Wolbachia LeuRS D1 is the same, but it has a deletion of region I354-N377. It was sub-cloned into a pET-M11b vector (EMBL), which contains an N-terminal hexahistidine tag followed by a TEV cleavage site. The protein was overexpressed in E. coli BL21-CodonPlus (DE3)-RIL grown in lysogeny broth (LB) medium at 37°C until the optical density at 600 nm reached 0.6. Protein expression was induced with the addition of 0.4 mM IPTG and then incubated overnight at 18°C. The cells were collected by centrifugation and frozen at −80°C. Cell lysis was carried out by sonication using as purification buffer 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 5 mM β-mercaptoethanol supplemented with protease inhibitor tablets (Roche). The protein was purified by nickel (Ni-NTA) affinity chromatography (Quieten). The washing buffer used for Ni-NTA chromatography was the same as the purification buffer with the addition of 10 mM imidazole and 1 M NaCl. The elution buffer was the same as the purification buffer with the addition of 400 mM imidazole. The His-tag was cleaved using 1 mg of TEV protein per 100 mg of His-tag protein and dialyzed for 1 hour at 4°C against the purification buffer. The protein was further purified by size-exclusion chromatography using a HiLoad 16/600 Superdex column (Cytiva) equilibrated in the purification buffer. Wolbachia LeuRS D1 protein was concentrated to 15 mg/ml and flash-frozen in liquid nitrogen before storage at −80°C.
The full-length E. coli LeuRS was overexpressed using E. coli BL21-CodonPlus (DE3)-RIL competent cells and purified by Ni-NTA chromatography as previously described (19). The E. coli tRNALeu was produced by in vitro transcription and purified by polyacrylamide gel electrophoresis followed by diethylaminoethyl (DEAE) Sepharose chromatography according to a previously described protocol (19).
Crystallization of protein(s), tRNA, and small-molecule inhibitors
All crystallizations were performed at 20°C by the hanging-drop vapor diffusion method, using 24-microwell plates, by mixing equal volumes of protein solution and reservoir solution. Samples for crystallization were made of Wolbachia LeuRS D1 at 15 mg/ml, AMP at 10 mM, and 1 mM Cmpd6/9, or of Ec LeuRS full-length at 33 μM, tRNALeu at 40 μM, and 1 mM Cmpd6/9. They crystallized respectively in 0.1 M Tris (pH 8.5), 0.2 M lithium sulfate, and 31 to 33% (w/v) polyethylene glycol, molecular weight 4000 (PEG 4000); and in 0.1 M sodium acetate (pH 5.6), 0.2 M sodium chloride, and 16 to 20% (w/v) PEG 6000. The crystals were transferred for a few seconds in the mother liquor containing 22% (v/v) ethylene glycol as cryoprotectant and then frozen in liquid nitrogen. Crystals were diffracted at the European Synchrotron Radiation Facility (ESRF) beamlines ID23-1, ID23-2, ID29, and ID30A-1 (Grenoble, France). Data collection parameters and refinement statistics are summarized in table S3.
Structure determination
Data reduction was carried out by XDS and autoPROC (Global Phasing). Phaser (CCP4 v. 8.0.009) was used to perform molecular replacement using PDB code 3ZJV (20) as a model for the full-length E. coli structures and Streptococcus pneumoniae LeuRS editing domain [PDB: 4K47 (22)] as a model for Wolbachia LeuRS editing domain structures. Manual building of several protein and tRNA regions in Coot (v. 0.9.8.5) (32) was used to complete the final model, and refinement was carried out with Refmac5 (CCP4 v. 8.0.009) (33) and Buster (Global Phasing). Validation of the models was performed with MolProbity (34). Figures were prepared with PyMOL (v2.5.4, Schrödinger), ChimeraX (35), and protein-drug interaction schemes by LigPlot+ (36).
Isothermal titration calorimetry
ITC measurements were performed on a MicroCal iTC200 (GE Healthcare, PA) at 25°C. Wolbachia LeuRS samples were previously dialyzed into the ITC buffer 50 mM Hepes-KOH, pH 7.5, 30 mM KCl, 30 mM MgCl2, and 5 mM β-mercaptoethanol and adjusted to a final concentration of 70 μM. Dialysis buffer was filtered at 0.45 μm and used to dissolve AMP at 10 mM and compounds Cmpd6 and Cmpd9 (ChemPartner Europe) at 1 mM final concentration. A typical ITC experiment consisted of 26 injections of 1.5 μl every 180 s at a stirring speed of 750 rpm. The raw data were processed using MicroCal PEAQ-ITC (v. 1.0.0.1259, Malvern) and Microcal Origin for ITC (v. 7.0). Thermodynamic binding parameters are summarized in table S2.
Thermal shift assays
The thermal shift assays (TSAs) were performed using a qPCR CFX96 (Bio-Rad) with the FRET scan mode. Samples were prepared in a TSA buffer containing 50 mM Hepes (pH 7.5) and 150 mM NaCl. Each well contained 2 μl of samples as prepared for the crystallization trials, 18 μl of TSA buffer, and 20 μl of 10× SYPRO orange (S6650, Thermo Fisher Scientific). The melting curve was obtained by heating samples from 25° to 95°C at a constant rate of 1°C/min.
NMR spectroscopy
NMR samples of AMP, ATP, AMP + Cmpd6, and ATP + Cmpd6 were prepared in 50 mM phosphate buffer, 30 mM KCl, and 30 mM MgCl2 in 99% D2O at pH 7.0 (meter reading at 20°C). In all cases, the concentrations of the samples were 5 mM and mixtures of AMP/ATP with benzoxaboroles were prepared in a 1:1 molar ratio. 1D 1H NMR spectra and 2D 1H-1H Total Correlation Spectroscopy (TOCSY) spectra were acquired for each sample at 25°C on a Bruker spectrometer operating at a 1H frequency of 850 MHz. The TOCSY spectra were recorded with a mixing time of 80 ms and used for assignments of the 1D spectra.
Acknowledgments
We thank the European Synchrotron Radiation Facility and the European Molecular Biology Laboratory in Grenoble for beamtime access and technical support. We thank the CERMAV (CNRS, Grenoble) for access to the ITC instrument. We also acknowledge the SBGrid consortium for technical support with structural biology software (37). We thank Anacor Pharmaceuticals for the provision of compounds to the anti-Wolbachia consortium (A·WOL). The authors are grateful for the support provided by S. Cusack (EMBL, Grenoble) in the initial stages of this work.
Funding: This work was supported by Agence National de la Recherche grant ANR JCJC RC18114CC NovoTargetParasite (to A.P.), Agence National de la Recherche ANR-20-AMRB-0003-01 AntibiOxaborole (to A.P.), and Agence National de la Recherche BoronTrap (to A.P. and M.R.J.). M.R.J. is laureate of the Impulscience program of the Fondation Bettencourt Schueller. This work used the platforms of the Grenoble Instruct-ERIC center (ISBG; UAR 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology (PSB), supported by FRISBI (ANR-10-INBS-0005-02) and GRAL, financed within the University Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (ANR-17-EURE-0003). A·WOL was funded by the Bill & Melinda Gates Foundation grant number 3928 (to M.J.T. and S.A.W.).
Author contributions: Conceptualization: A.P. Investigation: G.H., M.L., R.H.C., K.L.J., E.K.G.M., L.F., J.D.T., S.A.W., M.J.T., M.R.J., and A.P. Methodology: G.H., M.L., R.H.C., K.L.J., E.K.G.M., L.F., J.D.T., S.A.W., M.J.T., M.R.J., and A.P. Supervision: M.R.J., M.J.T., and A.P. Writing—original draft: A.P. Writing—review and editing: all authors. Funding acquisition: M.R.J., M.J.T., S.A.W., and A.P.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The crystal structures of apo-Wolbachia LeuRS, the complex of Wolbachia LeuRS with Cmpd6-AMP, and the complex of Wolbachia LeuRS with Cmpd9-AMP have been deposited in the Protein Data Bank Database in Europe (PDBe) with accession codes 8POQ, 8POR, and 8POS, respectively. The crystal structure of the E. coli LeuRS-tRNALeu complex with Cmpd9 has been deposited in the PDBe with accession code 8POT. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S11
Tables S1 to S3
REFERENCES AND NOTES
- 1.Honda K., Littman D. R., The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Garrett W. S., Cancer and the microbiota. Science 348, 80–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertig M., Wolbach S. B., Studies on rickettsia-like micro-organisms in insects. J. Med. Res. 44, 329–374.7 (1924). [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R., Shropshire J. D., Cross K. L., Leigh B., Mansueto A. J., Stewart V., Bordenstein S. R., Bordenstein S. R., Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 29, 879–893 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston K. L., Hong W. D., Turner J. D., O’Neill P. M., Ward S. A., Taylor M. J., Anti-Wolbachia drugs for filariasis. Trends Parasitol. 37, 1068–1081 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Taylor M. J., Voronin D., Johnston K. L., Ford L., Wolbachia filarial interactions. Cell. Microbiol. 15, 520–526 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Tamarozzi F., Halliday A., Gentil K., Hoerauf A., Pearlman E., Taylor M. J., Onchocerciasis: The role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 24, 459–468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor M. J., Hoerauf A., Bockarie M., Lymphatic filariasis and onchocerciasis. Lancet 376, 1175–1185 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Chirgwin S. R., Coleman S. U., Porthouse K. H., Nowling J. M., Punkosdy G. A., Klei T. R., Removal of Wolbachia from Brugia pahangi is closely linked to worm death and fecundity but does not result in altered lymphatic lesion formation in Mongolian gerbils (Meriones unguiculatus). Infect. Immun. 71, 6986–6994 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma R., Al Jayoussi G., Tyrer H. E., Gamble J., Hayward L., Guimaraes A. F., Davies J., Waterhouse D., Cook D. A. N., Myhill L. J., Clare R. H., Cassidy A., Steven A., Johnston K. L., Ford L., Turner J. D., Ward S. A., Taylor M. J., Minocycline as a re-purposed anti-Wolbachia macrofilaricide: Superiority compared with doxycycline regimens in a murine infection model of human lymphatic filariasis. Sci. Rep. 6, 23458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mand S., Debrah A. Y., Klarmann U., Batsa L., Marfo-Debrekyei Y., Kwarteng A., Specht S., Belda-Domene A., Fimmers R., Taylor M., Adjei O., Hoerauf A., Doxycycline improves filarial lymphedema independent of active filarial infection: A randomized controlled trial. Clin. Infect. Dis. 55, 621–630 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor M. J., Makunde W. H., McGarry H. F., Turner J. D., Mand S., Hoerauf A., Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: A double-blind, randomised placebo-controlled trial. Lancet 365, 2116–2121 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Turner J. D., Mand S., Debrah A. Y., Muehlfeld J., Pfarr K., McGarry H. F., Adjei O., Taylor M. J., Hoerauf A., A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin. Infect. Dis. 42, 1081–1089 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Taylor M. J., Hoerauf A., A new approach to the treatment of filariasis. Curr. Opin. Infect. Dis. 14, 727–731 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Walker T., Johnson P. H., Moreira L. A., Iturbe-Ormaetxe I., Frentiu F. D., McMeniman C. J., Leong Y. S., Dong Y., Axford J., Kriesner P., Lloyd A. L., Ritchie S. A., O’Neill S. L., Hoffmann A. A., The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Zheng X., Zhang D., Li Y., Yang C., Wu Y., Liang X., Liang Y., Pan X., Hu L., Sun Q., Wang X., Wei Y., Zhu J., Qian W., Yan Z., Parker A. G., Gilles J. R. L., Bourtzis K., Bouyer J., Tang M., Zheng B., Yu J., Liu J., Zhuang J., Hu Z., Zhang M., Gong J.-T., Hong X.-Y., Zhang Z., Lin L., Liu Q., Hu Z., Wu Z., Baton L. A., Hoffmann A. A., Xi Z., Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Laven H., Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216, 383–384 (1967). [DOI] [PubMed] [Google Scholar]
- 18.Clare R. H., Cook D. A. N., Johnston K. L., Ford L., Ward S. A., Taylor M. J., Development and validation of a high-throughput anti-Wolbachia whole-cell screen: A route to macrofilaricidal drugs against onchocerciasis and lymphatic filariasis. J. Biomol. Screen. 20, 64–69 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Palencia A., Crépin T., Vu M. T., Lincecum T. L., Martinis S. A., Cusack S., Structural dynamics of the aminoacylation and proofreading functional cycle of bacterial leucyl-tRNA synthetase. Nat. Struct. Mol. Biol. 19, 677–684 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez V., Crépin T., Palencia A., Cusack S., Akama T., Baker S. J., Bu W., Feng L., Freund Y. R., Liu L., Meewan M., Mohan M., Mao W., Rock F. L., Sexton H., Sheoran A., Zhang Y., Zhang Y.-K., Zhou Y., Nieman J. A., Anugula M. R., Keramane E. M., Savariraj K., Reddy D. S., Sharma R., Subedi R., Singh R., O’Leary A., Simon N. L., De Marsh P. L., Mushtaq S., Warner M., Livermore D. M., Alley M. R. K., Plattner J. J., Discovery of a novel class of boron-based antibacterials with activity against gram-negative bacteria. Antimicrob. Agents Chemother. 57, 1394–1403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palencia A., Li X., Bu W., Choi W., Ding C. Z., Easom E. E., Feng L., Hernandez V., Houston P., Liu L., Meewan M., Mohan M., Rock F. L., Sexton H., Zhang S., Zhou Y., Wan B., Wang Y., Franzblau S. G., Woolhiser L., Gruppo V., Lenaerts A. J., O’Malley T., Parish T., Cooper C. B., Waters M. G., Ma Z., Ioerger T. R., Sacchettini J. C., Rullas J., Angulo-Barturen I., Pérez-Herrán E., Mendoza A., Barros D., Cusack S., Plattner J. J., Alley M. R. K., Discovery of novel oral protein synthesis inhibitors of Mycobacterium tuberculosis that target leucyl-tRNA synthetase. Antimicrob. Agents Chemother. 60, 6271–6280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Q.-H., Liu R.-J., Fang Z.-P., Zhang J., Ding Y.-Y., Tan M., Wang M., Pan W., Zhou H.-C., Wang E.-D., Discovery of a potent benzoxaborole-based anti-pneumococcal agent targeting leucyl-tRNA synthetase. Sci. Rep. 3, 2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibba M., Soll D., Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Lincecum T. L., Tukalo M., Yaremchuk A., Mursinna R. S., Williams A. M., Sproat B. S., Van Den Eynde W., Link A., Van Calenbergh S., Grøtli M., Martinis S. A., Cusack S., Structural and mechanistic basis of pre- and posttransfer editing by leucyl-tRNA synthetase. Mol. Cell 11, 951–963 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Cvetesic N., Palencia A., Halasz I., Cusack S., Gruic-Sovulj I., The physiological target for LeuRS translational quality control is norvaline. EMBO J. 33, 1639–1653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann G., Le Gorrec M., Mestdach E., Cusack S., Salmon L., Jensen M. R., Palencia A., Adenosine-dependent activation mechanism of prodrugs targeting an aminoacyl-tRNA synthetase. J. Am. Chem. Soc. 145, 800–810 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor M. J., von Geldern T. W., Ford L., Hübner M. P., Marsh K., Johnston K. L., Sjoberg H. T., Specht S., Pionnier N., Tyrer H. E., Clare R. H., Cook D. A. N., Murphy E., Steven A., Archer J., Bloemker D., Lenz F., Koschel M., Ehrens A., Metuge H. M., Chunda V. C., Ndongmo Chounna P. W., Njouendou A. J., Fombad F. F., Carr R., Morton H. E., Aljayyoussi G., Hoerauf A., Wanji S., Kempf D. J., Turner J. D., Ward S. A., Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci. Transl. Med. 11, eaau2086 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Bakowski M. A., Shiroodi R. K., Liu R., Olejniczak J., Yang B., Gagaring K., Guo H., White P. M., Chappell L., Debec A., Landmann F., Dubben B., Lenz F., Struever D., Ehrens A., Frohberger S. J., Sjoberg H., Pionnier N., Murphy E., Archer J., Steven A., Chunda V. C., Fombad F. F., Chounna P. W., Njouendou A. J., Metuge H. M., Ndzeshang B. L., Gandjui N. V., Akumtoh D. N., Kwenti T. D. B., Woods A. K., Joseph S. B., Hull M. V., Xiong W., Kuhen K. L., Taylor M. J., Wanji S., Turner J. D., Hübner M. P., Hoerauf A., Chatterjee A. K., Roland J., Tremblay M. S., Schultz P. G., Sullivan W., Chu X.-J., Petrassi H. M., McNamara C. W., Discovery of short-course antiwolbachial quinazolines for elimination of filarial worm infections. Sci. Transl. Med. 11, eaav3523 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Hong W. D., Benayoud F., Nixon G. L., Ford L., Johnston K. L., Clare R. H., Cassidy A., Cook D. A. N., Siu A., Shiotani M., Webborn P. J. H., Kavanagh S., Aljayyoussi G., Murphy E., Steven A., Archer J., Struever D., Frohberger S. J., Ehrens A., Hübner M. P., Hoerauf A., Roberts A. P., Hubbard A. T. M., Tate E. W., Serwa R. A., Leung S. C., Qie L., Berry N. G., Gusovsky F., Hemingway J., Turner J. D., Taylor M. J., Ward S. A., O’Neill P. M., AWZ1066S, a highly specific anti-Wolbachia drug candidate for a short-course treatment of filariasis. Proc. Natl. Acad. Sci. U.S.A. 116, 1414–1419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrens A., Lunde C. S., Jacobs R. T., Struever D., Koschel M., Frohberger S. J., Lenz F., Fendler M., Turner J. D., Ward S. A., Taylor M. J., Freund Y. R., Stefanakis R., Easom E., Li X., Plattner J. J., Hoerauf A., Hübner M. P., In vivo efficacy of the boron-pleuromutilin AN11251 against Wolbachia of the rodent filarial nematode Litomosoides sigmodontis. PLOS Negl. Trop. Dis. 14, e0007957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs R. T., Lunde C. S., Freund Y. R., Hernandez V., Li X., Xia Y., Carter D. S., Berry P. W., Halladay J., Rock F., Stefanakis R., Easom E., Plattner J. J., Ford L., Johnston K. L., Cook D. A. N., Clare R., Cassidy A., Myhill L., Tyrer H., Gamble J., Guimaraes A. F., Steven A., Lenz F., Ehrens A., Frohberger S. J., Koschel M., Hoerauf A., Hübner M. P., McNamara C. W., Bakowski M. A., Turner J. D., Taylor M. J., Ward S. A., Boron-pleuromutilins as anti-Wolbachia agents with potential for treatment of onchocerciasis and lymphatic filariasis. J. Med. Chem. 62, 2521–2540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Collaborative Computational Project , The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Williams C. J., Headd J. J., Moriarty N. W., Prisant M. G., Videau L. L., Deis L. N., Verma V., Keedy D. A., Hintze B. J., Chen V. B., Jain S., Lewis S. M., Arendall W. B., Snoeyink J., Adams P. D., Lovell S. C., Richardson J. S., Richardson D. C., MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettersen E. F., Goddard T. D., Huang C. C., Meng E. C., Couch G. S., Croll T. I., Morris J. H., Ferrin T. E., UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laskowski R. A., Swindells M. B., LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Morin A., Eisenbraun B., Key J., Sanschagrin P. C., Timony M. A., Ottaviano M., Sliz P., Collaboration gets the most out of software. eLife 2, e01456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S11
Tables S1 to S3