Abstract
Abnormal uterine bleeding (AUB) is an acute/chronic variation in the normal menstrual cycle that affects adolescents, women of reproductive age and perimenopausal women. AUB affects approximately 3–30% of reproductive-aged women worldwide, and reduces their quality of life and productivity whilst increasing the overall healthcare burden. Its management requires thorough medical evaluation and individualized treatment. Depending on the severity and cause of AUB, its treatment ranges from lifestyle modifications and hormonal therapies to more invasive procedures or surgery. Although hormonal therapy is the preferred first-line measure in AUB, the available pharmacological options have various adverse effects. There exists a need for safer and more efficient treatment regimens with high patient compliance to effectively treat AUB. Norethisterone, also known as norethindrone, is a widely used synthetic analogue of progestogen. Controlled release formulations of norethisterone/ norethisterone acetate help maintain constant drug levels in the blood and exert minimal side-effects; therefore, they are promising therapeutic agents for effective AUB management. The present review summarizes the epidemiology and diagnosis of AUB, with a focus on the safety, efficacy and tolerability of norethisterone/ norethisterone acetate in AUB management. We also report a case of AUB in a 40-year-old woman, who was treated with NETA tablets. The treatment resulted in favourable outcomes, and patient satisfaction.
Keywords: dysfunctional uterine bleeding, leiomyoma, metrorrhagia, norethisterone, norethisterone acetate, progestogen
Introduction
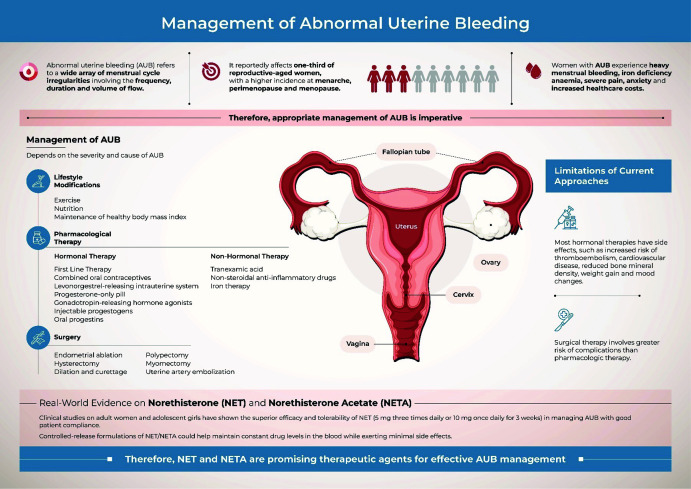
Abnormal uterine bleeding (AUB) refers to a wide array of menstrual irregularities involving the frequency, duration and volume of flow. It is a common debilitating condition that is typically life-altering and rarely life-threatening. The prevalence of AUB varies with age and geographical region. AUB reportedly affects one-third of reproductive-aged women, with a higher incidence at menarche, perimenopause and postmenopause.1–3
The most common symptom of AUB is heavy menstrual bleeding (HMB).4 Women with AUB also experience severe pain, mood fluctuations, anxiety, abdominal bloating, diarrhoea, breast tenderness and increased urinary frequency. These symptoms create feelings of self-consciousness, embarrassment, fear of leaking or staining and worry regarding going out.5 In adolescence, AUB is associated with pain, fatigue, limited physical and social activity, school absenteeism, concerns regarding breakthrough spotting and increased healthcare costs.6–8 Thus, AUB significantly impacts women’s daily lives, including their family, job and lifestyle, and creates an economic burden.5
Although these effects are an important motivation to seek medical care, several women first self-treat and wait to see if their condition improves.5 In developing countries, where menstruation is often considered highly personal and secretive, fewer women seek medical attention for AUB.9 However, the management of AUB requires thorough medical evaluation and individualized treatment. The present narrative review therefore aims to summarize the epidemiology and diagnosis of AUB, with a focus on the safety, efficacy and tolerability of the available first-line interventions for effective AUB management (Figure 1).
Figure 1.
Management of abnormal uterine bleeding. AUB, abnormal uterine bleeding.
Methods
A PubMed search was conducted using keywords “abnormal uterine bleeding”, “norethisterone”, “uterine bleeding management”, “role of norethisterone in bleeding disorders” and “abnormal uterine bleeding and management”. In addition, MeSH terms “uterine bleeding”, “dysfunctional uterine bleeding”, “norethisterone” and “norethisterone acetate” were used during the search. This review included clinical trials (open trials, non-randomized controlled trials and randomized controlled trials), observational studies (retrospective and prospective, survey-based questionnaires, case reports and case series) and reviews (narrative reviews, clinical guidelines and meta-analyses) published between 2008 and 2023. The keywords and MeSH terms were also used to search a second database, Google Scholar, and results from the first five pages were screened for inclusion. In addition, we performed snowballing of relevant articles retrieved from both databases. Only articles published in English were included. The SANRA (Scale for the Assessment of Narrative Review Articles) criteria were followed for this narrative and non-systematic review.10 Information retrieved through the above-mentioned searches was used to compile the present article.
Review
Global prevalence of AUB
The global prevalence of AUB amongst reproductive-aged women ranges between 3% and 30%, with a higher incidence at menarche and perimenopause.3 Kazemijaliseh et al.11 observed an AUB prevalence of 35.8% in 1393 reproductive-aged women from Iran. Similarly, Gerema et al.12 observed an AUB prevalence of 34.1% among 660 women from Jimma town, Southwest Ethiopia; of the different types of AUB, the incidence of metrorrhagia was highest. A multicentre cross-sectional study from Brazil indicated an AUB prevalence of 31.4% in 1761 reproductive-aged women.13 In Beijing, a questionnaire survey of 2356 women aged 18–50 years showed that 18.2% experienced HMB.14 The prevalence of AUB in the Apple Women’s Health Study cohort from the USA was 16.4%, with a higher prevalence amongst black women and women with obesity.15
Prevalence of AUB in India
The prevalence of AUB ranges from 10% to 30% in India and varies across regions.16–18 A doctor-centric questionnaire survey by Faruqui19 showed that 32.72% of women visiting 141 gynaecologists across the country presented with AUB symptoms. Further, women of reproductive age accounted for most menstrual irregularity complaints, and HMB was the most commonly reported condition. An observational study by Choudhury and Nath showed that the prevalence of AUB in Silchar, Assam, was 20.48%; AUB was more frequent in women aged 41–45 years, and leiomyoma was the most common type of AUB.20 Vaidya et al.18 retrospectively identified an AUB prevalence of 18.3% in Kozhikode, Kerala. Similarly, Sharma and Dogra observed an AUB prevalence of 17.09% in Shimla, Himachal Pradesh; HMB was the most frequent type of AUB observed.16
Aetiology and patterns of AUB
AUB is chiefly caused by a hormonal imbalance (relative oestrogen excess over progesterone).21 Its other causes include structural abnormalities of the uterus (polyps, fibroids and adenomyosis), polycystic ovarian syndrome (PCOS), intrauterine devices, infection of the uterus, cervix or endometrium, thickening of the uterine wall, cancer or precancer of the uterus, cervix, endometrium or ovaries, stress, lifestyle factors, thyroid disorders, coagulation disorders and reproductive disorders, such as ectopic pregnancy and placental trauma or infection.22–24
The clinical patterns of AUB were previously reported using descriptive terms, such as polymenorrhagia, menorrhagia, metrorrhagia, menometrorrhagia, hypomenorrhea and oligomenorrhea.25 However, inconsistent nomenclature and the lack of a standardized classification for potential aetiologies hindered AUB diagnosis and management.26 In 2011, the International Federation of Gynecology and Obstetrics (FIGO) introduced the acronym PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic and not yet classified) to describe and classify the underlying aetiology of AUB.26 The first section of the acronym, PALM, refers to structural entities, whereas the second part, COEIN, refers to non-structural entities.26 In 2018, FIGO updated the nomenclature and definitions of AUB symptoms to include intermenstrual bleeding.2 Indian experts have found the PALM-COEIN system to be clinically useful and a crucial step forward in AUB management.27 However, an overlap between the features of the conditions described by the PALM-COEIN system may make diagnosis and management of AUB complex.28,29
AUB has also been classified as acute and chronic. Acute AUB refers to sudden and excessive blood flow requiring immediate medical attention to control bleeding and address the underlying cause. On the other hand, chronic AUB refers to ongoing or recurring bleeding, which is abnormal in volume, regularity or timing, from the uterine corpus for the majority of the past 6 months. Although chronic AUB is not as critical as acute AUB, its root cause must be diagnosed and managed.26 In addition, ovulatory AUB must be distinguished from anovulatory AUB.30
Risk factors of AUB
AUB has been associated with several risk factors, including hypertension, diabetes and thyroid disorders.18 Uterine fibroids, obesity, PCOS, adenomyosis and endometrial intraepithelial neoplasia are other risk factors associated with AUB.22,31 Premenopausal women aged >40 years with thickened endometrium (endometrial thickness >13 mm), body mass index (BMI) >25 kg/m2 and hypothyroidism, are at a higher risk of developing endometrial hyperplasia and endometrial cancer.32 The initiation of AUB at menarche in the absence of structural or hormonal abnormality may indicate haemostatic disorders such as von Willebrand disease.33 AUB may be associated with other coagulation factor deficits, haemophilia and platelet function abnormalities.34 The endometrial disorder (AUB-E) and iatrogenic (AUB-I) types of AUB are most likely linked to abnormal endometrial angiogenesis and poor vascular maturation.35 Mood and anxiety disorders, depression, generalized anxiety disorder and obsessive-compulsive disorder have also been reported as risk factors for AUB.36
Impact of AUB
Women with AUB have a high risk of developing iron deficiency and iron deficiency anaemia (IDA).37 Iron deficiency and IDA impair oxygen delivery and enzyme reactions, affecting all metabolic pathways of the body and causing headaches, fatigue, impaired cognition and decreased exercise capacity.38,39 AUB impacts women’s social, mental, physical and psychological behaviour. Women with AUB often experience mental health disorders, including anxiety and depression.36,40 AUB also leads to social isolation due to fear of unexpected bleeding episodes41 and can cause discomfort and pain, which affects the quality of life and overall well-being of women.22 Structural causes of AUB, such as fibroids and polyps, can lead to pregnancy-related complications, minimize the possibility of natural conception and lower the chances of success in assisted reproduction.42 Frequent doctor’s visits and missed workdays due to AUB can impact a woman’s job prospects and income.43 Moreover, AUB leads to increased healthcare costs for diagnosis and treatment.5,44 The 2018 UK NICE guidelines recommend hysteroscopy for AUB, which may further increase treatment costs.45
Diagnosis of AUB
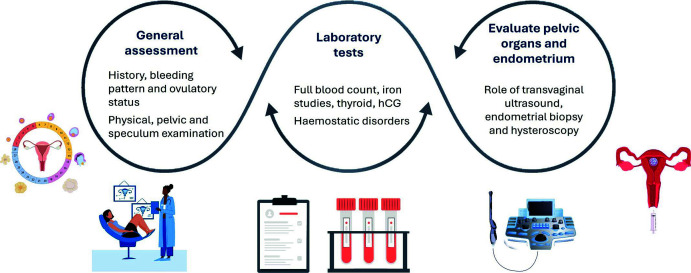
The menstrual history of the patient must be carefully examined, including the age at menarche, last menstrual period, frequency, duration, regularity and volume of flow, clot size and frequency of sanitary product changes.3 The patient’s medical and surgical history (PCOS, thyroid disorders, bleeding or clotting disorders, kidney disease, hepatic disease, celiac disease or malignancy), past or current use of medications (non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulants, vitamins/supplements, hormonal contraception), family history (menstrual history, bleeding or clotting disorders) and social history (diet, exercise, sexual history) should be examined (Figure 2).46
Figure 2.
Assessment of abnormal uterine bleeding.
hCG, human chorionic gonadotropin.
Physical examination must involve evaluation of general appearance, vital signs, signs of endocrine or haematologic disorders and external genitalia.46 Bimanual examination of the pelvis helps detect genital tract infections (Figure 2).47
Cervical and vaginal swabs help exclude infection. A Pap smear test with HPV DNA may be performed to detect cervical cancer.48 Laboratory investigations include tests for pregnancy, complete blood count, blood glucose, thyroid stimulating hormone, gonorrhoea or chlamydia (in patients at high risk), serum iron/ferritin and blood hormone levels and targeted screening for bleeding disorders (Figure 2).49
Pelvic imaging through transvaginal ultrasonography and MRI may be performed to diagnose structural uterine abnormalities (uterine fibroids, polyps, endometriosis, adenomyosis or ovarian anomalies).3,49 Although MRI provides comprehensive images, it is not the preferred first-line imaging modality due to its higher cost. Other modalities for diagnosing uterine anatomic changes/peritoneal endometriotic lesions include hysteroscopy and sonohysterography (Figure 2).49
Endometrial biopsy should be considered for patients with AUB at high risk for hyperplasia or malignancy, especially those aged >45 years.3 It should also be considered for women aged <45 years with unopposed oestrogen exposure, obesity, PCOS, failure of therapy or persistent bleeding.3
Management of AUB
The management of AUB depends on several factors, such as the acute or chronic nature of AUB, its aetiology, patient desire to maintain fertility, clinical stability and other health-related comorbidities.3 AUB treatment is categorized as non-pharmacological, hormonal, non-hormonal and surgical (Figure 1).
Non-pharmacological therapy
A higher BMI (≥25 kg/m2) often increases the risk of ovulatory dysfunction, PCOS and heavy or irregular menstrual periods.50,51 Therefore, lifestyle factors, including regular exercise and maintenance of a healthy BMI, are often recommended for women with HMB.52,53 Exercise and a nutritious diet also help reduce IDA, increase energy levels and improve quality of life.52
Hormonal therapy
Pharmacological hormonal therapy is the first-line treatment to restore regularity and flow volume of menses.41 It involves the use of combined oral contraceptives (OCs), levonorgestrel-releasing intrauterine system (LNG-IUS), progesterone-only pill, gonadotropin-releasing hormone agonists, injectable progestogens and oral progestins.52
Combined OCs help reduce blood loss and regulate menstrual bleeding. They may be prescribed for 3 weeks followed by a pill-free week to facilitate withdrawal bleeding.52 Their side-effects include mood changes, increased risk of thromboembolism, stroke, cardiovascular disease or breast cancer.52 However, they are the treatment of choice in women with known von Willebrand disease who desire contraception.54
LNG-IUS is another effective treatment option for patients requiring contraception. A randomized meta-analysis by Endrikat et al.55 showed that LNG-IUS is a reliable, long-term treatment option for women with idiopathic HMB. The side-effects of LNG-IUS include intermenstrual bleeding infection, mood changes and breast tenderness.52
Progesterone-only pills are recommended to patients if no other options are safe. However, in contrast to combined OCs, the progesterone-only pill is associated with irregular and unpredictable blood loss. Therefore, it is not usually recommended for HMB.52 Gonadotropin-releasing hormone agonists are used to treat HMB caused by a leiomyoma-associated hormonal imbalance.56 However, these compounds are expensive and have significant side-effects, such as hot flashes, vaginal dryness, headaches, decreased libido, depression and trabecular bone loss, which limit their long-term use.52,56 Therefore, they must be prescribed for a short term and add-back therapy may be considered to mitigate the side-effects.57 Further, recent reports suggest that the combination of relugolix/oestradiol/norethisterone acetate (NETA) is well tolerated and effective in treating HMB associated with uterine fibroids.58,59
Injectable progestogens are an alternative to oral tablets and intrauterine devices in AUB treatment. Injectable progestogens like depot medroxyprogesterone acetate (MPA) have been shown to provide effective contraception and induce amenorrhea in 50% of women with AUB.55 Their side-effects include loss of bone mineral density, weight gain, greasy skin and hair, acne and bloating.51
Oral progestogens/progestins, such as MPA, norethisterone (NET) and megestrol acetate, are synthetic analogues of progesterone and the most commonly used hormonal therapy to manage acute and heavy irregular bleeding.56 Progestogens can be administered continuously or cyclically. Cyclic progestin medication once a month for 10–14 days enables synchronized withdrawal bleeding. Continuous progestin medication in higher doses is administered to patients with endometrial hyperplasia. However, its long-term use can cause endometrial atrophy.60 Of the oral progestogens, NET is the most commonly used to treat AUB. It is administered to approximately 38% of women with AUB due to its fewer side-effects and cost-effectiveness.61 A dosage of 5 mg thrice daily from day 5 to day 26 of the menstrual cycle can reduce blood loss by approximately >80%.52
Role of NET and NETA in the management of AUB
NET, also known as norethindrone, is a widely used synthetic analogue of progestogen. NETA is the acetic acid ester of NET. Following oral administration, NETA is rapidly absorbed from the gastrointestinal tract and transformed into the active form, NET, upon removal of the acetate group. NET binds mainly to albumin (61%) and partly to sex hormone-binding globulins (36%), and the non-protein bound form of NET (3–4%) remains in circulation.62 Both NETA and NET exhibit strong progestogenic, tissue-specific androgenic and oestrogenic characteristics as well as antigonadotropic and antioestrogenic effects due to their interactions with different steroid receptors in the body.62,63
Besides oral contraception, NETA is used to treat AUB, endometriosis and endometrial cancer, in hormone replacement therapy and to delay menstruation.62,64 NET is more effective than combined OC pills in delaying menstruation, and preventing breakthrough bleeding.65 Controlled-release NETA helps maintain drug levels in the blood, reduces dosing frequency and helps avoid night-time dosing. It also improves patient compliance, and reduces gastrointestinal irritation and dose-related side-effects.66 The dose for controlled-release NETA varies from 10 mg to 15 mg per day according to the indications for therapeutic use. The clinical studies on the therapeutic use, dose regimen, efficacy and side-effects of NET and NETA have been summarized in Table 1.61,67–75
Table 1.
Clinical trials on the safety and efficacy of norethisterone acetate alone or in comparison with placebo.
| Reference | Study design | Location | Population | Age group | Intervention | Comparator | Efficacy | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Boruah et al. (2021)61 | Retrospective analysis of women with ovulatory AUB | 40 centres in India | 308 women with ovulatory AUB | 18–45 years | 10 mg/day CR-NET for 10 days | None | Most women (63%) experienced styptic action of 10 mg CR-NET within 4 hours of administration 61% did not report any incidence of breakthrough bleeding 70% reported withdrawal bleeding within 24–72 hours of taking the last dose |
No adverse events |
| Bonassi Machado et al. (2023)67 | Multicentre, double-blind, placebo-controlled, randomized clinical trial | Brazil | 118 postmenopausal women with moderate to severe vasomotor symptoms | 45–60 years | Combination of 17β-E2 and NETA: 0.5 mg 17β-E2/0.1 mg NETA |
Placebo | The frequency of vasomotor symptoms reduced by 77.1% in the treatment group versus 49.9% in the placebo group (p=0.0001) | Headache, breast tenderness, increased vaginal bleeding, hypercholesterolaemia |
| Ferrero et al. (2010)68 | Prospective pilot study | Italy | 40 women with colorectal endometriosis, who had pain and gastrointestinal symptoms | 33.7±4.4 years | 2.5 mg/day NETA In case of breakthrough bleeding after 2 months of treatment, the NETA dose was increased by 2.5 mg/day (maximum dose of 5 mg/day) |
None | NETA intake significantly improved the intensity of chronic pelvic pain, deep dyspareunia and dyschezia | Uterine bleeding/spotting (scanty bleeding not requiring usual sanitary protection); breakthrough bleeding (light or moderate bleeding requiring sanitary protection); and metrorrhagia (more than normal menstruation) |
| Morotti et al. (2017)69 | Retrospective cohort study | Italy | 103 women with pain symptoms caused by rectovaginal endometriosis | 30.5±3.5 years | 2.5 mg/day, up to 5 mg/day, NETA | None | 68.8% of the 61 women completing the study were satisfied or very satisfied with long-term NETA treatment | Weight gain, breakthrough bleeding, migraine attacks, decreased libido, lipid alteration, depression |
| Taniguchi et al. (2017)70 | Preliminary study | Japan | 6 women with unilateral ovarian endometriomas | 39–48 years | 5 mg/day NET | None | The size of ovarian endometrioma decreased after treatment; all patients were relieved from dysmenorrhea | No serious adverse effects of NET use |
| Santos et al. (2014)71 | Retrospective cohort study to assess the effectiveness of NET taper in the management of acute heavy menstrual bleeding in adolescents | USA | 176 adolescent females | 14.8±2.3 years | 0.35 mg NET | None | 20 patients required NET taper for heavy bleeding; of this group, 78.9% experienced complete cessation of bleeding within 7 days | Irregular bleeding and systemic side-effects (nausea/vomiting, mood swings, hot flashes) were associated with discontinuation; no serious adverse events (venous thromboembolism, cardiovascular events) were reported |
| Muneyyirci-Delale et al. (2012)72 | Retrospective chart review to study the effectiveness of NETA in the management of adenomyosis | USA | 28 premenopausal women with moderate to severe pelvic pain and bleeding | 27–49 years | 5 mg NETA daily at beginning of menstrual cycle as “3 weeks on” and “1 week off” regimen | None | Pain and bleeding decreased from pretreatment levels (p<0.001); dysmenorrhea scores before and after treatment were 62.5±9.1 versus 11.3±3.1, respectively (p<0.001); bleeding scores before and after treatment were 28.1±2.4 and 8.1±8.5, respectively (p<0.001); none of the patients reported breakthrough bleeding after 2 months | Fewer and milder side-effects |
| Papapanagiotou et al. (2019)73 | Prospective audit focused on the effect of high NET doses in adolescents with AUB | Greece | 29 females | 11–17 years | Girls <55 kg were administered 5 mg NET two to three times a day, whereas for heavier adolescents, the starting dose was higher at 5–10 mg three times a day | None | Vaginal bleeding stopped at a mean of 46.1 (range, 8–120) hours after initiating NET | No serious adverse events |
| Taneja et al. (2019)74 | Prospective observational study on women with AUB post-abortion | India | 30 women with AUB post-abortion | 31–34 years | 10 mg oral NET twice daily × 3 weeks, for a maximum of three cycles | None | Most patients had complete resolution of symptoms after a single 3-week course, whilst some remained symptomatic and required a second course | NET was well tolerated, and none of the patients suffered any major adverse effects |
| Boruah et al. (2021)61 | Retrospective analysis of women with ovulatory AUB | 40 centres in India | 308 women with ovulatory AUB | 18–45 years | 10 mg/day CR-NET for 10 days | None | Most women (63%) experienced styptic action of 10 mg CR-NET within 4 hours of administration; 61% did not report any incidence of breakthrough bleeding; 70% reported withdrawal bleeding within 24–72 hours of taking the last dose | No adverse events |
| Ayalon et al. (2022)75 | Prospective questionnaire-based study to examine the effectiveness of NETA on vaginal bleeding caused by POP | Israel | 120 women experiencing vaginal bleeding due to POP | 20–44 years | 5 mg/day NETA after a 5-day pause after taking POP | None | Women who added 5 mg NETA to POP contraception reported a significant reduction in the frequency of bleeding after 2, 4 and 6 weeks of treatment | No adverse effects |
17β-E2, 17β-oestradiol; AUB, abnormal uterine bleeding; CR, controlled release; NET, norethisterone; NETA, norethisterone acetate; POP, progesterone-only pills.
Muneyyirci-Delale et al.72 studied the effect of 5 mg/day NETA in managing adenomyosis in women with moderate or severe pelvic pain and bleeding. They observed that NETA significantly improved both dysmenorrhea and bleeding amongst the participants. The authors had adopted the ‘3 weeks on, 1 week off’ regimen to minimize the common side effect of breakthrough bleeding, which results in incomplete suppression of the hypothalamic– pituitary–ovarian axis and a reduced hypo-oestrogenic effect. Further, a 5–10 mg dose of NETA every 6 hours can help manage acute bleeding in adolescents and girls.76 Papapanagiotou et al.73 prospectively assessed the effectiveness of NET in managing AUB in adolescents at a tertiary care centre. They found that a daily intake of NET (5–10 mg two or three times a day) for 21 days was an effective and reliable treatment option for adolescents requiring AUB control in an acute setting. Further, a retrospective study of adolescent women aged <21 years showed that 5–10 mg/day NET suppressed menstrual bleeding in 83% of women with a bleeding disorder.77 A prospective, observational study by Taneja et al.74 showed that a 3-week course of twice-daily 10 mg NET helped completely resolve AUB symptoms in 56.7% of women. In India, a retrospective analysis of reproductive-aged Indian women with ovulatory AUB showed that once-daily administration of 10 mg controlled-release NET for a shorter duration (10 days) was effective, safe and associated with a high compliance rate.61 A prospective, observational study of women (18–35 years) with AUB treated with NET (5 mg × 25 days) and MPA (10 mg × 21 days) over 3 months suggested that NET was more effective than MPA in managing AUB.78 Clinical studies comparing the safety and efficacy of NET with that of other hormonal treatments for different types of AUB have been summarized in Table 2.65,77–87
Table 2.
Clinical trials on the safety and efficacy of norethisterone acetate in comparison with other methods of treatment.
| Reference | Study design | Location | Population | Age group | Intervention | Comparator | Efficacy | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Dean et al. (2019)65 | Case–control study and a pilot randomized controlled study | USA | 4 women who presented late in their cycle and desired avoiding vaginal bleeding within 10 days before wedding | 18–26 years | 5 mg NET | Age-matched controls that received OC pills | NET, begun on or before cycle day 12, is superior for women who desire to avoid breakthrough bleeding and maintain fertility when compared to OC pills; patient satisfaction was significantly higher in the NET group, with 80% willing to choose this method again | Spotting, weight gain, bloating, nausea, headache |
| Alaqzam et al. (2018)77 | Retrospective chart review to identify the most effective option for management of HMB in adolescents | USA | 73 adolescents with HMB | <21 years | 52 mg LNG-IUS (Mirena) | 5–10 mg/day NETA, combined OC, transdermal contraceptive patch, DMPA and tranexamic acid | LNG-IUS group (89%) had the highest rate of menstrual suppression followed by the NETA (83%) and transdermal patch (80%) groups | No adverse side-effects were observed |
| Kader et al. (2023)78 | Prospective observational study | India | 71 patients with AUB | 18–35 years | Group 1: NET (5 mg × 25 days) | Group 2: MPA (10 mg × 21 days) | NET was more effective than MPA | The drugs had no serious adverse effects |
| Vercellini et al. (2005)74 | Open-label, parallel-group, randomized, controlled trial | Italy | 90 women experiencing recurrent moderate or severe pelvic pain following conservative surgery for rectovaginal endometriosis | 18–35 years | 0.01 mg oral ethinyl oestradiol plus 3 mg/day cyproterone acetate | 2.5 mg/day NETA | Proportion of satisfied women was moderately, though not significantly, higher in the NETA group (73%) than in the intervention group (62%) | Weight gain, headache, nausea, depression, decreased libido, acne, bloating or swelling, breast tenderness, hypertriglyceridaemia, erythematous cutaneous reaction |
| Vercellini et al. (2012)90 | Patient preference, parallel cohort study | Italy | 154 women with persistent/recurrent severe deep dyspareunia after first-line surgery | Surgery: 35.0±4.7 years NETA: 34.3±5.0 years |
Conservative surgery at laparoscopy | Low dose of NETA (2.5 mg/day) | 43% women in the surgery group and 59% in the progestin group were satisfied (p=0.015) Surgery group: marked and rapid short-term dyspareunia score reduction, followed by partial recurrence of pain NETA group: pain relief effect was more gradual but progressive throughout the study period |
Weight gain, breakthrough bleeding, decreased libido, vaginal dryness, spotting, breast tenderness, bloating/swelling, headache, depression, nausea |
| Ferrero et al. (2014)81 | Prospective, non-randomized, open-label trial | Italy | 40 women with unilateral single endometrioma | 34.2±3.6 years | Oral NETA (2.5 mg/day) or a combination of oral letrozole (2.5 mg/day) | NETA (2.5 mg/day) | Volume of the endometriomas significantly decreased compared to baseline in both groups; it was smaller in the intervention group (p=0.026) | Breakthrough bleeding, depression, weight gain, insomnia, decreased libido, vaginal dryness, hot flashes, weight gain |
| Scala et al. (2018)82 | Patient preference prospective study | Italy | Women with endometriosis | 32.5±5.3 years | Group A: continuous oral treatment with 2.5 mg/day NETA | Group B: 91-day extended-cycle oral contraception (LNG/EE 150/30 μg for 84 days and EE 10 μg for 7 days) | No statistically significant difference in the rate of satisfied patients at 12 months between Group A (82.2%) and Group B (68.4%) (p=0.143) | Unscheduled bleeding/spotting |
| Lee et al. (2016)83 | Not mentioned | South Korea | 64 women who underwent laparoscopic surgery for endometriosis | GnRHa: 30.6±6.1 years Dienogest: 29.0±5.9 years |
GnRH agonist plus 17β-E2 and NETA (1.0 mg/day of 17β-E2 and 0.5 mg/day of NETA) | Dienogest (2 mg/day) | Visual analogue scale pain scores decreased significantly in both group with no significant inter-group differences | Menstruation-like bleeding and spotting, hot flashes, genital dryness, depression, sleep disorder, acne, headache, weight gain, decreased libido |
| Genazzani et al. (2013)84 | Double-blind, randomized, controlled study | 48 centres in Austria, Argentina, Denmark, Italy, Mexico, Russia and the USA | 662 postmenopausal women with spontaneous amenorrhea or spontaneous amenorrhea with serum follicle stimulating hormone levels 40 mIU/ml or had a bilateral oophorectomy without hysterectomy | 40–65 years | 0.25 mg drospirenone/0.5 mg 17β-E2 combination | 0.5 mg NETA/1.0 mg 17β-E2 | None of the participants groups had an endometrial biopsy result of ‘hyperplasia or worse’ | Breast pain, postmenopausal haemorrhage, cervical dysplasia, headache, endometrial hypertrophy, uterine leiomyoma, hot flash, weight gain, nausea, vulvovaginal mycotic infection, abdominal pain |
| Malik et al. (2020)85 | Comparative study on treatment of idiopathic HMB | Pakistan | 76 patients with regular bleeding and HMB and decreased haemoglobin levels | 35–45 years | Group A: LNG-IUS (Mirena) inserted into the uterus | Group B: 5 mg NET tablet prescribed thrice daily | Mean number of treatment days: Group A: 2.00±0.81 Group B: 3.63±0.75 LNG-IUS was more effective and associated with higher satisfaction rate |
No adverse side-effects |
| Rezk et al. (2016)86 | Randomized clinical trial | Egypt | 150 perimenopausal women with endometrial hyperplasia without atypia | >40 years | Group 1: LNG-IUS | Group 2: 15 mg MPA Group 3: 15 mg NETA |
Resolution of endometrial hyperplasia did not differ significantly between the three groups (p<0.05); LNG-IUS had the highest cost and acceptability (p<0.001) | Group 1: vaginal discharge Group 2: acne Group 3: nausea |
| Patel et al. (2012)87 | Randomized clinical study | India | 60 young girls | Age of menarche to 19 years with HMB | 5 mg NETA | Combined OC | Total mean improvement in the Menorrhagia Impact Questionnaire scores was higher in the NETA group than in the Combined OC group after three treatment cycles (21 versus 17); treatment failure was less in the NETA group than in the combined OC group | Breast tenderness, withdrawal bleeding, nausea, vomiting and water retention |
17β-E2, 17β-oestradiol; AUB, abnormal uterine bleeding; DMPA, depo-medroxyprogesterone acetate; GnRHa, gonadotropin-releasing hormone agonist; HMB, heavy menstrual bleeding; LNG-EE, extended-cycle oral contraception; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; NET, norethisterone; NETA, norethisterone acetate; OC oral contraceptive.
Non-hormonal therapy
Non-hormonal treatment of AUB is suitable for women who wish to achieve pregnancy or avoid hormonal side-effects. Two common non-hormonal treatment options for AUB include NSAIDs and antifibrinolytics. Tranexamic acid is an antifibrinolytic with a short half-life. According to the FDA, 1.3 g of tranexamic acid taken thrice daily for up to 5 days can reduce HMB.88 It is well tolerated, safe and has few gastrointestinal side-effects.89 Mefenamic acid is the most common NSAID used to treat HMB. A mefenamic acid dose of 500 mg thrice daily for 4–5 days from the onset of menstruation reduces blood loss by 25–50%. Its side-effects include gastrointestinal symptoms, and this medication should be avoided in women with a history of coagulation disorders or gastric ulcer.87 Iron therapy is recommended in AUB to replenish iron stores. Intravenous iron-like iron sucrose is administered at 200 mg in 200 mL of normal saline over 30 min, three times a week. Its side-effects are chiefly gastrointestinal.90
Surgical therapy
Surgical therapy is invasive and involves greater risk than pharmacological therapy. It is adopted for clinically unstable patients who have a structural aetiology of AUB or have failed to respond to medical management. The selection of surgical therapy depends on the underlying structural cause of AUB, patient age, preference, contraceptive needs, effects on the quality of life and desire for future pregnancy.91
Surgical modalities for AUB management include endometrial ablation, hysterectomy, dilation and curettage (D&C), hysteroscopy with D&C, polypectomy, myomectomy and uterine artery embolization.92 Endometrial ablation may be considered for patients without uterine abnormality who desire reduced menstrual blood loss and dysmenorrhea. It involves the use of an energy source to destroy the endometrial lining, and should therefore be avoided in women of childbearing age.91 Hysterectomy is a definitive surgical treatment and should only be considered for women past their childbearing years.93 Hysteroscopy with D&C, polypectomy or myomectomy may be required if a structural aetiology of AUB (uterine fibroids, polyps) is suspected.34 Uterine artery embolization and myomectomy are performed for AUB secondary to fibroids and adenomyosis.94
Management of an acute AUB episode with controlled-release NETA: a case report
A 40-year-old woman presented to the Apollo International Hospital, Guwahati, with an acute episode of HMB. Prior to this severe episode, her periods were normal with flow lasting 4–5 days and an average of 3–4 pads used per day. She had a medical history of sub-fertility and had undergone laparoscopic salpingectomy for a disturbed ectopic pregnancy, 2 years ago. She had a normal appetite, weight and sleeping patterns. She reported no substantial changes in bowel and bladder habits or recent initiation of a new medication. Her father had diabetes mellitus and hypertension.
The patient was alert and co-operative but lethargic. Her blood pressure level was normal with a pulse rate of 104 bpm. She had an afebrile respiratory rate of 16 breaths per minute. She was pale without koilonychia, and no cyanosis, jaundice or mouth ulcers were observed. Dependent oedema was absent. Gynaecological examination revealed a bulky irregular mobile uterus without adnexal pathology. Basic laboratory tests demonstrated a haemoglobin level of 8.9 gm%, euglycaemia, euthyroidism and a normal coagulation profile and liver function. The peripheral smear and complete blood count tests revealed moderate iron deficiency. Transvaginal ultrasound examination revealed an irregular, enlarged uterus with an endometrial thickness of 8–9 mm without obvious pathology. Urine hCG findings were negative for pregnancy.
Endometrial biopsy was planned to obtain an accurate diagnosis but the patient denied undergoing an invasive procedure. Following extensive counselling, the patient received 10 mg/day controlled-release NETA tablets (GYNASET CR 10, Mankind Pharma) from day 5 of the cycle/flow for 20 days. Follow-up revealed the satisfactory response of the patient to this treatment; therefore, 10 mg/day controlled-release NETA was continued cyclically from day 16 to day 25 for the next three cycles. Re-evaluation at the subsequent follow-up visit indicated that the patient was highly satisfied with the treatment and its outcomes.
Unmet clinical needs in AUB management and future research
The prevalence of AUB has been studied in a few regions of India. Therefore, future survey studies are required to determine AUB prevalence in different parts of India, and elucidate the impact of geographical location and dietary patterns on the incidence of AUB in the Indian sub-continent.
Further, the underlying cause of AUB can sometimes present a diagnostic challenge. Whilst aetiologies such as hormonal imbalance, uterine fibroids or polyps are commonly identified, the cause may remain unclear in some cases despite extensive evaluation. Advanced tools, such as non-invasive imaging and use of molecular biomarkers, could enhance diagnostic accuracy. In a recent review, Chodankar et al.95 highlighted potential biomarkers of AUB, including metalloproteinases, epithelial-to-mesenchymal transition and chromosomal rearrangements on HMG2A, amongst several others. However, studies on biomarkers have been performed on a small number of samples, and further validation is necessary. Deep phenotyping of women with AUB may help to provide individualized care.95
AUB often requires long-term management. Whilst short-term interventions, such as hormonal therapies or surgical procedures, may provide temporary relief, there is a need for a sustainable, long-term management strategy that can minimize recurrence and optimize a patient’s quality of life. Moreover, the side-effects of conventional treatment options often limit compliance and efficacy. AUB can significantly impact fertility, particularly when endometriosis or adenomyosis are present.96 Preserving fertility whilst effectively managing AUB is a crucial consideration for many women of reproductive age. There is a clear unmet need for innovative and effective approaches that can ensure bleeding control, fertility preservation and patient compliance.52
Most clinical studies have reported the efficacy and tolerability of NET (5 mg three times daily or 10 mg once daily for 3 weeks) in AUB management.73,74 However, a shorter treatment duration may improve patient compliance and minimize adverse side-effects. Based on the severity of AUB, a thrice-daily 5 mg dose may be replaced by a single daily dose of 10 mg or 15 mg NET/NETA from day 5 to day 26 of the menstrual cycle to decrease the dosing frequency, and thereby improve patient compliance. Further, a daily dose of 10 mg/15 mg NET instead of a thrice-daily 5 mg dose for approximately 2 weeks could help effectively manage acute AUB. Therefore, future clinical trials are necessary to determine the efficacy of 15 mg NET taken once daily for 3 weeks in AUB management.
Interventions for adolescents and young women with HMB and dysmenorrhea should be developed and implemented based on the needs of the patients to improve their quality of life. Education programmes for parents and teachers of adolescents with HMB and dysmenorrhea, health professionals (including general practitioners and specialists) and the general public, are likely to be useful in addressing these issues and managing expectations.7 Therefore, future research should also focus on quantifying these needs in a large sample.
Conclusion
AUB is indeed a significant health concern that profoundly impacts women’s lives, that of their families and society as a whole. Raising awareness regarding AUB, and ensuring that women have access to appropriate healthcare services is essential to mitigate its impact on individuals and society. Depending on the severity and cause of AUB, its management ranges from lifestyle modifications and hormonal therapies to more invasive procedures or surgery. Unfortunately, most hormonal therapies have side-effects that limit their efficacy. However, controlled-release formulations of NET/NETA could help maintain constant drug levels in the blood and exert minimal side-effects. Therefore, NET and NETA are promising therapeutic agents for effective AUB management.
Acknowledgements
The authors acknowledge Mankind Pharma and medical editors Dr Ritika Bishnoi and Dr Sonal Nafade from Neovation Consultancy Services Pte. Ltd for editorial assistance.
Footnotes
Contributions: All authors contributed extensively to the work presented in this paper. AMB and AG contributed to conception, design, drafting and reviewing of the manuscript. DB, FB, SM, RS and SS have drafted and reviewed the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Boruah AM declared receiving Gynaset – CR 10 from Mankind Pharma Ltd. All other authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2024/05/dic.2024-4-1-COI.pdf
Funding declaration: Funding was provided by Mankind Pharma for editorial assistance facilitated by Neovation Consultancy Services Pte. Ltd.
Correct attribution: Copyright © 2024 Boruah AM, Banerjee D, Bhardwaj F, Mallya S, Singal R, Sharma S, Gautam A. https://doi.org/10.7573/dic.2024-4-1. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Munro M, Critchley H, Fraser I. Research and clinical management for women with abnormal uterine bleeding in the reproductive years: More than PALM COEIN. BJOG. 2017;124(2):185–189. doi: 10.1111/1471-0528.14431. [DOI] [PubMed] [Google Scholar]
- 2.Munro MG, Critchley HOD, Fraser IS. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;143(3):393–408. doi: 10.1002/ijgo.12666. [DOI] [PubMed] [Google Scholar]
- 3.Davis E, Sparzak P. StatPearls. StatPearls Publishing; [Accessed March 20, 2024]. Abnormal Uterine Bleeding. Updated September 4, 2023. https://www.ncbi.nlm.nih.gov/books/NBK532913/ [PubMed] [Google Scholar]
- 4.Hernandez A, Dietrich JE. Abnormal uterine bleeding in the adolescent. Obstet Gynecol. 2020;135(3):615–621. doi: 10.1097/AOG.0000000000003693. [DOI] [PubMed] [Google Scholar]
- 5.Fraser IS, Langham S, Uhl-Hochgraeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Rev Obstet Gynecol. 2009;4(2):179–189. doi: 10.1586/17474108.4.2.179. [DOI] [Google Scholar]
- 6.Pawar A, Krishnan R, Davis K, Bosma K, Kulkarni R. Perceptions about quality of life in a school-based population of adolescents with menorrhagia: implications for adolescents with bleeding disorders. Haemophilia. 2008;14(3):579–583. doi: 10.1111/j.1365-2516.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellis EK, Li AD, Jayasinghe YL, et al. Exploring the unmet needs of parents of adolescent girls with heavy menstrual bleeding and dysmenorrhea: a qualitative study. J Pediatr Adolesc Gynecol. 2020;33(3):271–277. doi: 10.1016/j.jpag.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Pike M, Chopek A, Young NL, et al. Quality of life in adolescents with heavy menstrual bleeding: validation of the Adolescent Menstrual Bleeding Questionnaire (aMBQ) Res Pract Thromb Haemost. 2021;5(7):e12615. doi: 10.1002/rth2.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walraven G, Ekpo G, Coleman R, Scherf C, Morison L, Harlow SD. Menstrual disorders in rural Gambia. Stud Fam Plann. 2002;33(3):261–268. doi: 10.1111/j.1728-4465.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- 10.Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazemijaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Khalili D, Hosseinpanah F, Azizi F. A population-based study of the prevalence of abnormal uterine bleeding and its related factors among Iranian reproductive-age women: an updated data. Arch Iran Med. 2017;20(9):558–563. [PubMed] [Google Scholar]
- 12.Gerema U, Kene K, Abera D, et al. Abnormal uterine bleeding and associated factors among reproductive age women in Jimma town, Oromia Region, Southwest Ethiopia. Women’s Health. 2022;18:174550572210775. doi: 10.1177/17455057221077577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezende GP, Yela Gomes DA, Benetti-Pinto CL. Prevalence of abnormal uterine bleeding in Brazilian women: association between self-perception and objective parameters. PLoS One. 2023;18(3):e0282605. doi: 10.1371/journal.pone.0282605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding C, Wang J, Cao Y, et al. Heavy menstrual bleeding among women aged 18–50 years living in Beijing, China: prevalence, risk factors, and impact on daily life. BMC Womens Health. 2019;19(1):27. doi: 10.1186/s12905-019-0726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CY, Li H, Zhang S, et al. Abnormal uterine bleeding patterns determined through menstrual tracking among participants in the Apple Women’s Health Study. Am J Obstet Gynecol. 2023;228(2):213e1–213.e22. doi: 10.1016/j.ajog.2022.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Dogra Y. Trends of AUB in tertiary centre of Shimla hills. J Midlife Health. 2013;4(1):67. doi: 10.4103/0976-7800.109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotagasti T. Prevalence of different menstrual irregularities in women with abnormal uterine bleeding (AUB)-an observational study. Int J Cur Res Rev. 2015;7(10):66–70. [Google Scholar]
- 18.Vaidya R, Vinayachandran S, Devi S, et al. Prevalence of abnormal uterine bleeding and its associated risk factors in women of perimenopausal age group-a retrospective study. J Clin Diagn Res. 2022;16(12):9–13. doi: 10.7860/JCDR/2022/59994.17252. [DOI] [Google Scholar]
- 19.Faruqui AA. Abnormal uterine bleeding: a doctor centric survey on prevalence, management and limitations in Indian Context. Obstet Gynecol Res. 2019;2:59–66. doi: 10.26502/ogr022. [DOI] [Google Scholar]
- 20.Choudhury SA, Nath P. Abnormal uterine bleeding; its prevalence, causes and management in a tertiary care hospital. New Indian J OBGYN. 2020;7(1):52–57. doi: 10.21276/obgyn.2020.7.11. [DOI] [Google Scholar]
- 21.Bhardwaj TT, Hiwale KM, Vagha S. Correlation of morphological findings of endometrium with concerned hormone levels in patients with abnormal uterine bleeding: a narrative review. Cureus. 2022;14(10):e30063. doi: 10.7759/cureus.30063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker L, Critchley HOD. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016;34:54–65. doi: 10.1016/j.bpobgyn.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khrouf M, Terras K. Diagnosis and management of formerly called “dysfunctional uterine bleeding” according to PALM-COEIN FIGO classification and the new guidelines. J Obstet Gynaecol India. 2014;64(6):388–393. doi: 10.1007/s13224-014-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams PL, Laifer-Narin SL, Ragavendra N. US of abnormal uterine bleeding. RadioGraphics. 2003;23(3):703–718. doi: 10.1148/rg.233025150. [DOI] [PubMed] [Google Scholar]
- 25.Fraser IS, Critchley HOD, Munro MG, Broder M. A process designed to lead to international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding. Fertil Steril. 2007;87(3):466–476. doi: 10.1016/j.fertnstert.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Munro MG, Critchley HOD, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113(1):3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Bandi ND, Arumugam CP, Venkata MRN, Nannam L. Utility of the PALM-COEIN classification of abnormal uterine bleeding for Indian gynecologists. Int J Gynaecol Obstet. 2016;133(2):196–198. doi: 10.1016/j.ijgo.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Mishra D, Sultan S. FIGO’s PALM-COEIN classification of abnormal uterine bleeding: a clinico-histopathological correlation in Indian setting. J Obstet Gynaecol India. 2017;67(2):119–125. doi: 10.1007/s13224-016-0925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain V, Munro MG, Critchley HOD. Contemporary evaluation of women and girls with abnormal uterine bleeding: FIGO Systems 1 and 2. Int J Gynaecol Obstet. 2023;162(Suppl 2):29–42. doi: 10.1002/ijgo.14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iram S, Musonda P, Ewies AAA. Premenopausal bleeding: when should the endometrium be investigated?—a retrospective non-comparative study of 3006 women. Eur J Obstet Gynecol Reprod Biol. 2010;148(1):86–89. doi: 10.1016/j.ejogrb.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Zeng LH, Rana S, Hussain L, et al. Polycystic ovary syndrome: a disorder of reproductive age, its pathogenesis, and a discussion on the emerging role of herbal remedies. Front Pharmacol. 2022;13:874914. doi: 10.3389/fphar.2022.874914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha S, Singh A, Sinha HH, Bhadani P, Anant M, Agarwal M. Rate of premalignant and malignant endometrial lesion in “low-risk” premenopausal women with abnormal uterine bleeding undergoing endometrial biopsy. Obstet Gynecol Sci. 2021;64(6):517–523. doi: 10.5468/ogs.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain S, Agrawal NR, Tilak V, Piplani KS. Prevalence of von Willebrand disease in patients with heavy menstrual bleeding: an Indian perspective. J South Asian Fed Obstet Gynecol. 2022;13(6):369–373. doi: 10.5005/jp-journals-10006-1968. [DOI] [Google Scholar]
- 34.Committee on Gynecologic Practice, The American College of Obstetricians and Gynecologists. Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121(4):891–896. doi: 10.1097/01.AOG.0000428646.67925.9a. [DOI] [PubMed] [Google Scholar]
- 35.Middelkoop MA, Don EE, Hehenkamp WJK, Polman NJ, Griffioen AW, Huirne JAF. Angiogenesis in abnormal uterine bleeding: a narrative review. Hum Reprod Update. 2023;29(4):457–485. doi: 10.1093/humupd/dmad004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayhan F, Alptekin H, Kayhan A. Mood and anxiety disorders in patients with abnormal uterine bleeding. Eur J Obstet Gynecol Reprod Biol. 2016;199:192–197. doi: 10.1016/j.ejogrb.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 37.Nazaryan H, Watson M, Ellingham D, et al. Impact of iron supplementation on patient outcomes for women with abnormal uterine bleeding: a protocol for a systematic review and meta-analysis. Syst Rev. 2023;12(1):121. doi: 10.1186/s13643-023-02222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55–67. doi: 10.1016/j.bpobgyn.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Munro MG. Abnormal uterine bleeding: a well-travelled path to iron deficiency and anemia. Int J Gynaecol Obstet. 2020;150(3):275–277. doi: 10.1002/ijgo.13180. [DOI] [PubMed] [Google Scholar]
- 40.Lee HN, Ju HR, Seo JM, Um GS, Kim MJ. Clinical factors associated with anxiety and depression in Korean women with abnormal uterine bleeding. Clin Exp Obstet Gynecol. 2021;48(2):323–330. doi: 10.31083/j.ceog.2021.02.2329. [DOI] [Google Scholar]
- 41.Farrukh JB, Towriss K, McKee N. Abnormal uterine bleeding: taking the stress out of controlling the flow. Can Fam Physician. 2015;61(8):693–697. [PMC free article] [PubMed] [Google Scholar]
- 42.Jain V, Chodankar RR, Maybin JA, Critchley HOD. Uterine bleeding: how understanding endometrial physiology underpins menstrual health. Nat Rev Endocrinol. 2022;18(5):290–308. doi: 10.1038/s41574-021-00629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry C, Jefferies R, Ekeroma A, Filoche S. Beyond the numbers—understanding women’s experiences of accessing care for abnormal uterine bleeding (AUB): a qualitative study. BMJ Open. 2020;10(11):e041853. doi: 10.1136/bmjopen-2020-041853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10(3):183–194. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 45.National Institute for Health and Care Excellence. Heavy Menstrual Bleeding: Assessment and Management. National Institute for Health and Care Excellence; 2018. [Accessed March 20, 2024]. Updated May 24, 2021. https://www.nice.org.uk/guidance/ng88 . [Google Scholar]
- 46.Miller K, Konal J, Brown K, Cabral MD. Abnormal uterine bleeding: a narrative review. Pediatric Medicine. 2019;2:27–27. doi: 10.21037/pm.2019.06.11. [DOI] [Google Scholar]
- 47.Telner DE, Jakubovicz D. Approach to diagnosis and management of abnormal uterine bleeding. Can Fam Physician. 2007;53(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- 48.Marnach ML, Laughlin-Tommaso SK. Evaluation and management of abnormal uterine bleeding. Mayo Clin Proc. 2019;94(2):326–335. doi: 10.1016/j.mayocp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Achanna KS, Nanda J. Evaluation and management of abnormal uterine bleeding. Med J Malaysia. 2022;77(3):374–383. [PubMed] [Google Scholar]
- 50.Tang Y, Chen Y, Feng H, Zhu C, Tong M, Chen Q. Is body mass index associated with irregular menstruation: a questionnaire study. BMC Womens Health. 2020;20(1):226. doi: 10.1186/s12905-020-01085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itriyeva K. The effects of obesity on the menstrual cycle. Curr Probl Pediatr Adolesc Health Care. 2022;52(8):101241. doi: 10.1016/j.cppeds.2022.101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maybin JA, Critchley HO. Medical management of heavy menstrual bleeding. Women’s Health. 2016;12(1):27–34. doi: 10.2217/whe.15.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mena GP, Mielke GI, Brown WJ. Prospective associations between physical activity and BMI with irregular periods and heavy menstrual bleeding in a large cohort of Australian women. Hum Reprod. 2021;36(6):1481–1491. doi: 10.1093/humrep/deab055. [DOI] [PubMed] [Google Scholar]
- 54.James AH, Manco-Johnson MJ, Yawn BP, Dietrich JE, Nichols WL. Von Willebrand disease. Obstet Gynecol. 2009;114(3):674–678. doi: 10.1097/AOG.0b013e3181b191ea. [DOI] [PubMed] [Google Scholar]
- 55.Endrikat J, Vilos G, Muysers C, Fortier M, Solomayer E, Lukkari-Lax E. The levonorgestrel-releasing intrauterine system provides a reliable, long-term treatment option for women with idiopathic menorrhagia. Arch Gynecol Obstet. 2012;285(1):117–121. doi: 10.1007/s00404-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 56.Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol. 2016;214(1):31–44. doi: 10.1016/j.ajog.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 57.Moroni RM, Martins WP, Ferriani RA, et al. Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst Rev. 2015;2015(3):CD010854. doi: 10.1002/14651858.CD010854.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali M, Chen HY, Chiang YF, Badary OA, Hsia SM, Al-Hendy A. An evaluation of relugolix/estradiol/norethindrone acetate for the treatment of heavy menstrual bleeding associated with uterine fibroids in premenopausal women. Expert Opin Pharmacother. 2022;23(4):421–429. doi: 10.1080/14656566.2022.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart EA, Archer DF, Owens CD, et al. Reduction of heavy menstrual bleeding in women not designated as responders to elagolix plus add back therapy for uterine fibroids. J Womens Health. 2022;31(5):698–705. doi: 10.1089/jwh.2021.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews ML. Abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol Clin North Am. 2015;42(1):103–115. doi: 10.1016/j.ogc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Boruah AM, Jaiswal P, Chinda M, Jaiswal A. Real-world safety and effectiveness analysis of norethisterone in the management of abnormal uterine bleeding. J South Asian Fed Obstet Gynecol. 2022;14(3):313–316. doi: 10.5005/jp-journals-10006-2048. [DOI] [Google Scholar]
- 62.Huvinen E, Holopainen E, Heikinheimo O. Norethisterone and its acetate–what’s so special about them? BMJ Sex Reprod Health. 2021;47(2):102–109. doi: 10.1136/bmjsrh-2020-200619. [DOI] [PubMed] [Google Scholar]
- 63.He J, Wang Q, Ma X, et al. Probing the binding of two 19-nortestosterone derivatives to human serum albumin: insights into the interactions of steroid hormone drugs with functional biomacromolecule. J Mol Recognit. 2016;29(9):415–425. doi: 10.1002/jmr.2540. [DOI] [PubMed] [Google Scholar]
- 64.Read WL, Trivedi S, Williams F. Norethindrone substituted for megestrol in the treatment of metastatic endometrial carcinoma: three cases. Gynecol Oncol Rep. 2017;22:75–77. doi: 10.1016/j.gore.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dean J, Kramer KJ, Akbary F, et al. Norethindrone is superior to combined oral contraceptive pills in short-term delay of menses and onset of breakthrough bleeding: a randomized trial. BMC Womens Health. 2019;19(1):70. doi: 10.1186/s12905-019-0766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nadkarni P. Conservative modalities for management of AUB: role of norethisterone. [Accessed November 11, 2023];Fertility Blaze. 2016 3(2) https://wbhf.walterbushnell.com/publications/fertility-blaze/item/360-conservative-modalities-for-management-of-aub-role-of-norethisterone . [Google Scholar]
- 67.Bonassi Machado R, de Melo Pompei L, Nahas EAP, et al. Efficacy and safety of ultra-low-dose estradiol and norethisterone in postmenopausal Brazilian women. Climacteric. 2023;26(4):401–407. doi: 10.1080/13697137.2023.2190507. [DOI] [PubMed] [Google Scholar]
- 68.Ferrero S, Camerini G, Ragni N, Venturini PL, Biscaldi E, Remorgida V. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25(1):94–100. doi: 10.1093/humrep/dep361. [DOI] [PubMed] [Google Scholar]
- 69.Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;213:4–10. doi: 10.1016/j.ejogrb.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 70.Taniguchi F, Enatsu A, Ikebuchi A, et al. Efficacy of norethisterone in patients with ovarian endometrioma. Yonago Acta Med. 2017;60(3):182–185. [PMC free article] [PubMed] [Google Scholar]
- 71.Santos M, Hendry D, Sangi-Haghpeykar H, Dietrich JE. Retrospective review of norethindrone use in adolescents. J Pediatr Adolesc Gynecol. 2014;27(1):41–44. doi: 10.1016/j.jpag.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Muneyyirci-Delale O, Chandrareddy A, Mankame S, Osei-Tutu N, von Gizycki H. Norethindrone acetate in the medical management of adenomyosis. Pharmaceuticals. 2012;5(10):1120–1127. doi: 10.3390/ph5101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papapanagiotou IK, Charamanta M, Roidi S, Al-Achmar NS, Soldatou A, Michala L. The use of norethisterone for the treatment of severe uterine bleeding in adolescents: an audit of our experience. J Pediatr Adolesc Gynecol. 2019;32(6):596–599. doi: 10.1016/j.jpag.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Taneja A, Chopra I, Kaur H, et al. Successful management of abnormal uterine bleeding from uterine arteriovenous malformations with progesterone in postabortal patients. J Obstet Gynaecol Res. 2019;45(6):1114–1117. doi: 10.1111/jog.13939. [DOI] [PubMed] [Google Scholar]
- 75.Vilk Ayalon N, Segev L, Samson AO, et al. Norethisterone reduces vaginal bleeding caused by progesterone-only birth control pills. J Clin Med. 2022;11(12):3389. doi: 10.3390/jcm11123389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yaşa C, Güngör Uğurlucan F. Approach to abnormal uterine bleeding in adolescents. J Clin Res Pediatr Endocrinol. 2020;12(1):1–6. doi: 10.4274/jcrpe.galenos.2019.2019.S0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alaqzam TS, Stanley AC, Simpson PM, Flood VH, Menon S. Treatment modalities in adolescents who present with heavy menstrual bleeding. J Pediatr Adolesc Gynecol. 2018;31(5):451–458. doi: 10.1016/j.jpag.2018.02.130. [DOI] [PubMed] [Google Scholar]
- 78.Kader MIA, Karthikeyan V, Sabitha J. A comparative study on efficacy of norethisterone and medroxyprogestrone in the management of dysfunctional uterine bleeding: a prospective observational study. Biosci Biotechnol Res Asia. 2023;20(2):617–625. doi: 10.13005/bbra/3115. [DOI] [Google Scholar]
- 79.Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen–progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375–1387. doi: 10.1016/j.fertnstert.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 80.Vercellini P, Somigliana E, Consonni D, Frattaruolo MP, De Giorgi O, Fedele L. Surgical versus medical treatment for endometriosis-associated severe deep dyspareunia: I. Effect on pain during intercourse and patient satisfaction. Hum Reprod. 2012;27(12):3450–3459. doi: 10.1093/humrep/des313. [DOI] [PubMed] [Google Scholar]
- 81.Ferrero S, Remorgida V, Venturini PL, Leone Roberti Maggiore U. Norethisterone acetate versus norethisterone acetate combined with letrozole for the treatment of ovarian endometriotic cysts: a patient preference study. Eur J Obstet Gynecol Reprod Biol. 2014;174:117–122. doi: 10.1016/j.ejogrb.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 82.Scala C, Leone Roberti Maggiore U, Barra F, Venturini PL, Ferrero S. Norethindrone acetate versus extended-cycle oral contraceptive (Seasonique®) in the treatment of endometriosis symptoms: a prospective open-label comparative study. Eur J Obstet Gynecol Reprod Biol. 2018;222:89–94. doi: 10.1016/j.ejogrb.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 83.Lee DY, Lee JY, Seo JW, Yoon BK, Choi D. Gonadotropin-releasing hormone agonist with add–back treatment is as effective and tolerable as dienogest in preventing pain recurrence after laparoscopic surgery for endometriosis. Arch Gynecol Obstet. 2016;294(6):1257–1263. doi: 10.1007/s00404-016-4184-9. [DOI] [PubMed] [Google Scholar]
- 84.Genazzani AR, Schmelter T, Schaefers M, Gerlinger C, Gude K. One-year randomized study of the endometrial safety and bleeding pattern of 0.25 mg drospirenone/0.5 mg 17β-estradiol in postmenopausal women. Climacteric. 2013;16(4):490–498. doi: 10.3109/13697137.2013.783797. [DOI] [PubMed] [Google Scholar]
- 85.Malik FO, Sara SA, Kasi RU. Comparative trial of levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. Pak J Med Health Sci. 2020;14:1184–1186. [Google Scholar]
- 86.Rezk M, Kandil M, Salih S, Shaheen A. Comparison of levonorgestrel-releasing intrauterine system, medroxyprogesterone and norethisterone for treatment of endometrial hyperplasia without atypia: a randomized clinical trial. Obstet Gynecol Int J. 2016;5(4):00163. doi: 10.15406/ogij.2016.05.00163. [DOI] [Google Scholar]
- 87.Patel N, Patel S, Damor R, Pandya M. Comparison of the efficacy and safety of norethisterone vs combined oral contraceptive pills for the management of puberty menorrhagia. Int J Basic Clin Pharmacol. 2012;1(3):191–195. doi: 10.5455/2319-2003.ijbcp003512. [DOI] [Google Scholar]
- 88.Benetti-Pinto CL, de Rosa-e-Silva ACJ, Yela DA, Soares JM., Júnior Abnormal uterine bleeding. Revista Brasileira de Ginecologia e Obstetrícia / RBGO Gynecol Obstetr. 2017;39(7):358–368. doi: 10.1055/s-0037-1603807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leminen H, Hurskainen R. Tranexamic acid for the treatment of heavy menstrual bleeding: efficacy and safety. Int J Womens Health. 2012;4:413–421. doi: 10.2147/IJWH.S13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheong Y, Cameron IT, Critchley HOD. Abnormal uterine bleeding. Br Med Bull. 2017;123(1):103–114. doi: 10.1093/bmb/ldx027. [DOI] [PubMed] [Google Scholar]
- 91.Davidson BR, DiPiero CM, Govoni KD, Littleton SS, Neal JL. Abnormal uterine bleeding during the reproductive years. J Midwifery Womens Health. 2012;57(3):248–254. doi: 10.1111/j.1542-2011.2012.00178.x. [DOI] [PubMed] [Google Scholar]
- 92.MacGregor B, Munro MG, Lumsden MA. Therapeutic options for the management of abnormal uterine bleeding. Int J Gynaecol Obstet. 2023;162(S2):43–57. doi: 10.1002/ijgo.14947. [DOI] [PubMed] [Google Scholar]
- 93.Goldstein SR, Lumsden MA. Abnormal uterine bleeding in perimenopause. Climacteric. 2017;20(5):414–420. doi: 10.1080/13697137.2017.1358921. [DOI] [PubMed] [Google Scholar]
- 94.Oberman E, Rodriguez-Triana V. Abnormal uterine bleeding: treatment options. Clin Obstet Gynecol. 2018;61(1):72–75. doi: 10.1097/GRF.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 95.Chodankar R, Critchley HOD. Biomarkers in abnormal uterine bleeding. Biol Reprod. 2019;101(6):1155–1166. doi: 10.1093/biolre/ioy231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. doi: 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]