Abstract
Background
Despite the WHO's report of 24 available SARS-CoV-2 vaccines, limited data exist regarding vaccination policies for liver transplant (LT) patients. To address this, we conducted a global multi-society survey (EASL-ESOT-ELITA-ILTS) in LT centers.
Methods
A digital questionnaire assessing vaccine policies, safety, efficacy, and center data was administered online to LT centers.
Results
Out of 168 responding centers, 46.4%, 28%, 13.1%, 10.7%, and 1.8% were from European, American, Western Pacific, Southeast Asian, and Eastern Mediterranean Regions. Most LT centers prioritized COVID-19 vaccine access for LT patients (76%) and healthcare workers (86%), while other categories had lower priority (30%). One-third of responders recommended mRNA vaccine exclusively, while booster doses were widely recommended (81%). One-third conducted post-vaccine liver function tests post COVID-19 vaccine. Only 16% of centers modified immunosuppression, and mycophenolate discontinuation or modification was the main approach. Side effects were seen in 1 in 1,000 vaccinated patients, with thromboembolism, acute rejection, and allergic reaction being the most severe. mRNA showed fewer side effects (−3.1, p = 0.002).
Conclusion
COVID-19 vaccines and booster doses were widely used among LT recipients and healthcare workers, without a specific vaccine preference. Preventative immunosuppression adjustment post-vaccination was uncommon. mRNA vaccines demonstrated a favorable safety profile in this population.
Keywords: vaccine, SARS-CoV-2, liver, transplant, survey, policy, side effect, worldwide activity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus that was first described in Wuhan, China, in December 2019 (1). Since then, SARS-CoV-2 has spread globally, causing the coronavirus disease 2019 (COVID-19) pandemic, resulting in >611 million infections, with a death toll exceeding 6.5 million as of September 20th, 2022 according to the World Health Organization COVID-19 dashboard (2).
-
1.
The health emergency caused by the coronavirus pandemic dramatically changed clinical practice during the pandemic and beyond. The first wave impacted liver transplantation differently across the world, with particularly detrimental effects on the countries that sustained a severe hit by the virus (3). In 2020, The European Association for the Study of Liver disease (EASL), the European Liver and Intestine Transplant Association (ELITA) of the European Society of Organ Transplantation (ESOT), and the International Liver Transplantation Society (ILTS) task force demonstrated that the resilience of the entire transplant network enabled continued organ donation and transplantation, ultimately improving the lives of patients with end-stage liver disease (https://www.covid-19vaccinetracker.org/).
With dedicated financial support and worldwide commitment from the scientific community, on August 18th 2022, the WHO reported 276 vaccines in development, 109 in clinical trials, and 24 in use (4 with four prevalent types in the United States and Europe).
Currently, the data surrounding vaccination policies in liver transplant centers across the world are lacking. Consequently, EASL-ESOT-ELITA-ILTS task force convened and formulated a plan to investigate the variable approaches of liver transplant centers across the world in utilizing vaccines against SARS CoV-2 by means of an online survey. Herein, we report the results of this global survey, which may guide LT centers as they continue to operate and encounter the sequelae of the current pandemic and potentially optimize the future approach to vaccination.
Materials/patients and methods
This is a cross-sectional survey aimed at exploring the approach to COVID-19 vaccines across adult liver transplant centers worldwide. A digital questionnaire composed of four sections assessing (i) vaccine policies, (ii) COVID-19 safety assessment, (iii) COVID-19 efficacy assessment, and (iv) center data was designed using the Google Surveys platform (https://surveys.google.com), including multiple choice and open question tools. Each section was composed of 6, 6, 2, and 3 questions, respectively (see Supplementary Table 7). The questionnaire was proposed by the authors and pretested among LT centers of the EASL-ESOT-ELITA-ILTS task force during a period of one month, correcting and clarifying the content. The final version of the digital survey was published online in October 2021 and remained available until February 2022. The global target population was obtained estimating the number of adult LT centers registered worldwide at the Global Database on Donation and Transplantation (GODT, http://www.transplant-observatory.org), and the corresponding physician staff were reached throughout the main international liver transplant societies (EASL, ESOT, ELITA, and ILTS) during the annual meeting and through their respective webpages. Furthermore, a periodic reminder promoting the survey was sent through the mailing list of the societies and was posted on Twitter and Facebook.
Results from the survey were integrated with the data from the Global Database on Donation and Transplantation to conceptualize information of COVID-19 vaccination in relation to global transplant activity.
A convenience sampling was applied, while sample size was not calculated given the exploratory purpose of the study. In order to prevent multiple participation, the e-mail and the name of the associated institution were required. Thus, double entries and unclear or incomplete data were resolved by contacting the participants. Responders that reported side effects of COVID-19 vaccination were re-contacted to clarify the type of side effect and the number of patients who suffered them.
The study was approved by the Medical Ethics Committee of Erasmus MC (MEC-2016-277). The study follows the CROSS guideline for survey studies (4).
Statistical analysis
A survey analysis was performed, considering the hierarchical structure formed by three primary sampling units: (i) World Health Organization Regions, (ii) countries and (iii) LT centers, adjusted by the corresponding sampling weights. The sampling weights were obtained by considering 6 WHO Regions (https://www.who.int/countries), 86 countries with active LT programs, and 810 LT adult centers around the world (http://www.transplant-observatory.org). Stratification and finite population corrections were not used for the study. Data were presented by percentages and frequencies with the corresponding 95% confidence intervals. Distribution of continuous variables was assessed by the Shapiro–Wilk test. Comparisons were performed using multinomial and logistic regression according to their distribution. P values were expressed after Bonferroni correction in subgroup analyses.
A sensitivity analysis was performed by multiple imputation analysis of missing data that was considered missing completely at random. A multivariate chained model using predictive mean matching imputation was used to fit 100 imputations for each missing value. A p-value lower than 0.05 was considered statistically significant. Statistical analysis was performed using Stata 15 Software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
WHO regions responders
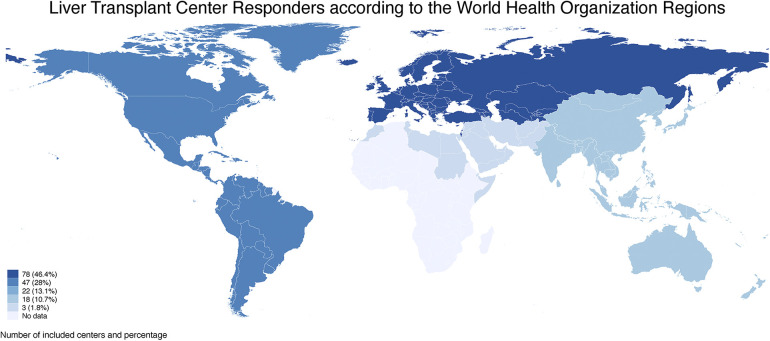
According to the World Health Organization (WHO) Classification, the Regions that participated in the survey were European (46.4%, n = 78 Countries), American (28%, n = 47 Countries), Western Pacific (13.1%, n = 22 Countries), South East Asian (10.7%, n = 18 Countries), and Eastern Mediterranean (1.8%, n = 3 Countries) with a total of 168 corresponding centers, with no responders from African Regions (Figure 1).
Figure 1.
The number of liver transplant centers included in the survey are graphically represented with a blue scale according to the world health organization regions. European and American Regions had the highest number of included centers (78 and 47, respectively), followed by Western Pacific, South East Asian, and Eastern Mediterranean Regions with 22 (13.1%), 18 (10.7%) and 3 (1.8%) centers, respectively. No centers were included from African Regions.
Country responders
Overall, 54.7% of the Countries in the World responded to this survey (47 out of 86 countries that have an LT program).
Of the 168 centers, United States of America (USA), Spain, India, and Italy were the most frequent centers that responded to the survey (17.9%, n = 31 centers, 13.3%, n = 23; 11%, n = 16; 9.25%, n = 16 centers, respectively) followed by Australia with 5 centers (2.9%); China, Germany, Japan, Russia, and Turkey with 4 centers (2.3%); Austria, Australia, Canada, Croatia, Mexico, and the United Kingdom with 3 centers (1.7%); Argentina, Brazil, Czech Republic, Egypt, France, Netherlands, Sweden, Taiwan, and Vietnam with 2 centers (1.2%); and Belgium, Chile, Colombia, Costa Rica, Finland, Georgia, Greece, Ireland, Malaysia, Nepal, New Zealand, Norway, Oman, Panama, Peru, Philippines, Romania, Thailand, and Trinidad and Tobago with 1 center (0.6%).
We estimated a total number of 810 adult active liver transplant (LT) centers across the included countries, excluding the Philippines and Taiwan where this information was lacking. Countries with 100% LT center participation were Australia, Austria, Czech Republic, Finland, Georgia, Greece, Ireland, New Zealand, Norway, Oman, Panama, Romania, Trinidad and Tobago, and Sweden, followed by Spain and Italy with 85.2% and 76.2%, respectively (Table 1).
Table 1.
Liver transplant activity was summarized according to the World Health Organization Regions and Countries. Percentages of included centers per country were also described. The global activity per Regions and Countries were similar. However, the included centers represented approximately 21% of this population.
| WHO Regions | LT in 2020/2021 | Countries | LT in 2020/2021 | Center included n (%) |
Center per Country n/N (%) |
|---|---|---|---|---|---|
| AR | 12,329/12,765 | Argentina | 316/438 | 2 (1.19%) | 2/35 (5.71%) |
| Brazil | 2,075/1,944 | 2 (1.19%) | 2/77 (2.6%) | ||
| Canada | 565/589 | 3 (1.79%) | 3/7 (42.9%) | ||
| Chile | 127/125 | 1 (0.60%) | 1/9 (11.11%) | ||
| Colombia | 199/229 | 1 (0.60%) | 1/8 (12.5%) | ||
| Costa Rica | 15/20 | 1 (0.60%) | 1/2 (50%) | ||
| Mexico | 72/135 | 3 (1.79%) | 3/20 (15%) | ||
| Panama | 3/0 | 1 (0.60%) | 1/1 (100%) | ||
| Peru | 17/20 | 1 (0.60%) | 1/3 (33.3%) | ||
| Trinidad & Tobago | 0/0 | 1 (0.60%) | 1/1 (100%) | ||
| United State | 8,901/9,236 | 31 (18.45%) | 31/142 (21.8%) | ||
| ER | 9,219/9,333 | Austria | 158/159 | 3 (1.79%) | 3/3 (100%) |
| Azerbaijan | 45/NA | 1 (0.60%) | 1/3 (33.33%) | ||
| Belgium | 235/268 | 1 (0.60%) | 1/6 (16.7%) | ||
| Croatia | 95/104 | 2 (1.19%) | 2/3 (66.7%) | ||
| Czech Republic | 172/186 | 2 (1.19%) | 2/2 (100%) | ||
| Finland | 75/75 | 1 (0.60%) | 1/1 (100%) | ||
| France | 1,128/1,225 | 2 (1.19%) | 2/26 (7.7%) | ||
| Georgia | 10/15 | 1 (0.60%) | 1/1 (100%) | ||
| Germany | 826/834 | 4 (2.38%) | 4/27 (14.8%) | ||
| Greece | 32/24 | 1 (0.60%) | 1/1 (100%) | ||
| Ireland | 37/35 | 1 (0.60%) | 1/1 (100%) | ||
| Italy | 1,202/1,396 | 16 (9.52%) | 16/21 (76.2%) | ||
| Netherlands | 186/181 | 2 (1.19%) | 2/3 (66.7%) | ||
| Norway | 88/98 | 1 (0.60%) | 1/1 (100%) | ||
| Portugal | 193/202 | 2 (1.19%) | 2/3 (66.7%) | ||
| Romania | 62/53 | 1 (0.60%) | 1/1 (100%) | ||
| Russia | 559/524 | 4 (2.38%) | 4/27 (14.8%) | ||
| Spain | 1,034/1,078 | 22 (13.10%) | 23/27 (85.2%) | ||
| Sweden | 172/170 | 2 (1.19%) | 2/2 (100%) | ||
| Switzerland | 135/151 | 2 (1.19%) | 2/4 (50%) | ||
| Turkey | 1,320/1,528 | 4 (2.38%) | 4/11 (36.36%) | ||
| United Kingdom | 823/821 | 3 (1.79%) | 3/7 (42.9%) | ||
| WPR | 8,160/7,778 | Australia | 277/254 | 5 (2.89%) | 5/5 (100%) |
| China | 5,828/5,822 | 4 (2.38%) | 4/114 (3.5%) | ||
| Japan | 380/421 | 4 (2.38%) | 4/25 (16%) | ||
| Malaysia | 28/15 | 1 (0.60%) | 1/2 (50%) | ||
| New Zealand | 55/53 | 1 (0.60%) | 1/1 (100%) | ||
| Philippines | 0/0 | 1 (0.60%) | N/A | ||
| South Korea | 1,542/1,531 | 2 (1.19%) | 2/40 (5%) | ||
| Taiwan | 543/508 | 2 (1.19%) | N/A | ||
| Vietnam | NA/NA | 2 (1.19%) | 2/9 (22.2%) | ||
| SEAR | 1,914/2,936 | India | 1,780/2,847 | 16 (9.52%) | 16/95 (16.8%) |
| Nepal | 0/8 | 1 (0.60%) | 1/3 (33.3%) | ||
| Thailand | 125/89 | 1 (0.60%) | 1/9 (11.1%) | ||
| EMR | 930/1,450 | Egypt | 395/515 | 2 (1.19%) | 2/20 (10%) |
| Oman | 0/2 | 1 (0.60%) | 1/1 (100%) | ||
| Total | 32,552/34,262 | 31,830/33,928 | 168 (100%) | 168/810a (20.74%) |
LT, liver transplant; WHO, World Health Organization; AR, American Regions; ER, European Regions; WPE, Western Pacific Regions; SEAR, South East Asia Regions; EMR, Easter Mediterranean Regions.
Estimated total given the lacking information on Taiwan and Philippines number of active centers and the possible discrepancy on real active Liver transplant Centers.
Liver transplant activity in 2020 and 2021 across the world
The number of LT performed across the WHO Regions in 2020 and 2021 were 12,329 and 12,765 in American R. (AR), 930 and 1,450 in Eastern Mediterranean R. (EMR), 9,219 and 9,333 in European R. (ER), 1,914 and 2,936 in South East Asian R. (SEAR) and 8,160 and 7,778 in Western Pacific R. (WPR), respectively.
Overall, the median number of LT performed in 2020 and 2021 per Country was 172 (Q1-Q3, 45–565) and 181 (53–589), respectively, with an increase in 2021 (p = 0.043). Out of 47 Countries, 53.2% (n = 25) had a significantly increased number of LT, 36.2% (n = 17) a significantly lower transplant activity, and 6.4% (n = 3) similar activity (2 missing data, Table 1).
Survey results
Vaccine policies
Most LT centers prioritized access to COVID-19 vaccines for LT patients (76%, 95%CI 41%–94.1%) and health care workers (86.2%; 67.3%–95%), while cohabitants, life partners of LT patients, and other categories were rarely included in these prioritization policies (26.2% and 13.5%, respectively). WPR, SEAR, AR, and ER were more likely to prioritize vaccination of LT patients than other regions.
Most LT centers had no specific recommendation about the type of COVID-19 vaccine administration (69.8%; 95%CI 43.6%–87.4%), while one-third restricted it just to mRNA vaccines (30.2%; 95%CI 12.6%–56.4%).
Booster (third) dose for mRNA Pfizer® vaccine was widely recommended in LT patients (80.5%; 95%CI 35.2%–96.9%). ER were more likely recommended mRNA vaccines for LT patients, while booster doses were largely implemented in American Regions compared with the other regions.
Approximately one-third of centers reported the presence of barriers for COVID-19 vaccine administration (31.8%, 95%CI 14.3%–56.4%), mainly because of patient concerns (89.4%; 48.8%–98.7%) and regional/national public health policies (35.7%; 95%CI 26%–46.7%). Regions with a higher presence of barriers were mainly EMR and WPR.
The main administering providers of COVID-19 vaccines were health-care and governmental authorities (61.9%; 95%CI 31.5%–85.1%) followed by transplant providers (25.3%; 95%CI 9.2%–52.9%) and primary care physicians (12.9%; 95%CI 3%–41.1%). WPR had a higher involvement of health care authorities in ordering COVID-19 vaccines.
Most centers routinely recommended other types of vaccines (e.g., flu) (89.4%; 95%CI 63.7%–97.6%) that were provided by transplant centers (56.8%; 95%CI 31.9%–78.6%) or by primary care clinics (43.2%; 95%CI 21.4%–68.1%). ER and AR were more likely to recommend other routine vaccinations, see Table 2 and Supplementary Table S1.
Table 2.
Policies for COVID-19 vaccination showed that transplant patients and health care workers were prioritized in the vast majority of centers without a specific restriction for the type of vaccine and promoting a booster dose and other vaccines (e.g flu).
| Proportion | [95% Conf. interval] | |||
|---|---|---|---|---|
| Categories of prioritized access | Transplant patients | 0.768 | 0.410 | 0.941 |
| Health care workers | 0.862 | 0.673 | 0.950 | |
| Co-habitants/life | 0.262 | 0.129 | 0.460 | |
| Other categories | 0.135 | 0.090 | 0.198 | |
| Type of vaccine recommended | No specific restrictions | 0.698 | 0.436 | 0.874 |
| Only mRNA vaccines | 0.302 | 0.126 | 0.564 | |
| Booster (third) dose | 0.805 | 0.352 | 0.969 | |
| Barriers for vaccination administration | Presence of any of the follow | 0.318 | 0.143 | 0.564 |
| Patient's fear | 0.894 | 0.488 | 0.987 | |
| Regional or National Public health policy | 0.357 | 0.260 | 0.467 | |
| Age | 0.146 | 0.035 | 0.444 | |
| Logistics/Organization | 0.106 | 0.019 | 0.421 | |
| Funding | 0.048 | 0.003 | 0.423 | |
| Others | 0.265 | 0.126 | 0.476 | |
| Facility ordering the vaccination | Health-care authorities | 0.619 | 0.315 | 0.851 |
| Primary care physician | 0.129 | 0.030 | 0.411 | |
| Transplant provider | 0.253 | 0.092 | 0.529 | |
| Other vaccines (flu, etc.) routinely recommended | Routinely recommended | 0.894 | 0.637 | 0.976 |
| Provided by Primary Care Clinic | 0.432 | 0.214 | 0.681 | |
| Provided by Transplant Center | 0.568 | 0.319 | 0.786 | |
COVID-19 safety assessment
Approximately one-third of the responders implemented routine liver-related biochemical testing post COVID19 vaccine (29.6%; 95%CI 22.1%–38.4%), mainly at 2 and 4 weeks after its administration (38.2%; 95%CI 10.9%–75.8% and 37.6%; 95%CI 15.5–66.5%, respectively). Monitoring was reported mainly by outpatient clinics (62.7%; 95%CI 30.6%–86.5%) and via telemedicine (26.7%; 95%CI 8.7%–58.3%). Liver biochemical tests were mainly assessed by EMR and ER regions, while telemedicine was likely preferred by AR and SEAR.
A small proportion of centers modified the immunosuppression before COVID-19 vaccination (16.1%; 95%CI 5.9%–37.3%), with MF discontinuation or reduction being the most common approach (41.4%; 95%CI 10.4%–81.1% and 37.8%; 95%CI 7.6%–81.9%, respectively), followed by steroid reduction (29.4%; 95%CI 3.6%–82.1%). Preventive dose or type modification of IS were more frequent in SEAR and ER, as shown in Table 3 and Supplementary Table S2.
Table 3.
Safety assessment after COVID-19 vaccination was scarcely implemented as well as preemptive immunosuppression modification, where mycophenolate adjustment was the most common approach.
| Proportion | [95%_Conf. interval] | |||
|---|---|---|---|---|
| Liver function test (LFT) monitoring | 0.296 | 0.221 | 0.384 | |
| Time of LFT monitoring | 2 weeks | 0.376 | 0.155 | 0.665 |
| 1 month | 0.382 | 0.109 | 0.758 | |
| 3 months | 0.145 | 0.022 | 0.559 | |
| Other | 0.097 | 0.010 | 0.545 | |
| Via report of vaccine monitorization | Telemedicine | 0.267 | 0.087 | 0.583 |
| Outpatient clinic | 0.627 | 0.306 | 0.865 | |
| Patient self-report | 0.070 | 0.015 | 0.264 | |
| Other | 0.036 | 0.004 | 0.266 | |
| Type or dose of immunosuppression modification pre-vaccine | 0.161 | 0.059 | 0.373 | |
| CNI reduction | 0.093 | 0.014 | 0.420 | |
| MF reduction | 0.378 | 0.076 | 0.819 | |
| MF interruption | 0.414 | 0.104 | 0.811 | |
| Steroids reduction | 0.294 | 0.036 | 0.821 | |
| Steroids interruption | 0.166 | 0.016 | 0.715 | |
| Others | 0.073 | 0.008 | 0.444 | |
LFT, liver function test; CNI, calcineurin inhibitor; MF, mycophenolate.
Overall 35.7% (95%CI 20.9%–53.9%) of the centers reported any of the following significant adverse drug events (ADEs) after COVID-19 vaccine: 13.8% of centers (95%CI 6.6%–26.5%) reported ≥one occurrence of thrombosis or thromboembolic event, 13% (95%CI 2.8%–43.6%) acute rejection, 9.5% (95%CI 1.6%–40.7%) other significant liver related alterations different from isolated biochemical alteration, 4.3% (95%CI 0.9%–18.8%) allergic reaction and 14.6% (95%CI 8.2%–24.7%) other side effects. Moreover, 29.5% (95%CI 15.8%–47.7%) of centers reported LFT alterations. Out of 38 centers that reported side effects, only 14 specified the number of patients involved. Excluding liver-related biochemical alteration, the estimated rate of side effects per patient was about 1 case (95%CI 0%–3%) per 1,000 vaccinated patients. Significant side effects post-vaccination were more frequently reported in EMR, AR, and ER, see Table 4 and Supplementary Table S3.
Table 4.
Side effects were reported in 36% of the centers, with thrombosis and thromboembolic events being the most relevant. The estimated rate of side effects excluding liver enzymes alteration was 1 case per 1000 of vaccinated patients. The vaccination efficacy was rarely evaluated, and 12% of the center reported ≥1 severe infection despite the vaccination.
| Side effects post vaccination (questions 9–11) | Proportion | [95%_Conf. interval] | ||
|---|---|---|---|---|
| Liver enzymes elevation | 0.295 | 0.158 | 0.477 | |
| Thrombosis or thromboembolic event | 0.138 | 0.066 | 0.265 | |
| Acute graft rejection | 0.130 | 0.028 | 0.436 | |
| Liver related | 0.095 | 0.016 | 0.407 | |
| Allergy related | 0.043 | 0.009 | 0.188 | |
| Others significant adverse eventa | 0.146 | 0.082 | 0.247 | |
| Total side effects (except liver enzymes elevation) | 0.357 | 0.209 | 0.539 | |
| Rate of significant side effects per patientb | 0.001 | 0.000 | 0.003 | |
| Efficacy assessment (questions 12–13) | ||||
| Centers testing antibodies | 0.252 | 0.065 | 0.621 | |
| Timing of test | 2 weeks | 0.062 | 0.010 | 0.310 |
| 1 month | 0.391 | 0.181 | 0.651 | |
| 3 months | 0.275 | 0.141 | 0.468 | |
| 6 months | 0.021 | 0.004 | 0.108 | |
| Other | 0.008 | 0.001 | 0.042 | |
| Infection post vaccine | Yes | 0.221 | 0.072 | 0.508 |
| Severe | 0.553 | 0.275 | 0.802 | |
Myocardial infarction (n = 2); post-transplant lymphoproliferative disease (n = 2); cholangitis (n = 1); Guillain Barré (n = 1); leukopenia (n = 1); thrombocytopenia (n = 1) lymphadenitis (n = 1); retinal detachment (n = 1); menorrhagia/dysmenorrhea (n = 1); unknow (n = 1).
Estimation of significant side effects rate per patients based on 14 centers who specified the number of patients with side effects over 38 that reported any of them.
After adjusting for the WHO Regions, viral vector-based vaccines were significantly associated with a higher risk of thrombotic event (contrast 20.3; 95% CI 3.152, 37.48; p = 0.029). Combined mRNA plus viral vector formulation or other formulations were associated with lower risk of allergic side effects (−1.935, p = 0.072 and −21.082; p = 0.002, respectively). Furthermore, other complications different from liver biochemical alteration, thrombosis, acute rejection, and allergy were significantly higher in viral vector-based vaccines (23.553; p = 0.034), while combined formulation had a tendency for lower risk of these complications (−5.931, p = 0.059), see Table 5.
Table 5.
Viral vector vaccines were significantly associated with a greater risk of thrombosis (p = 0.029) and other complications (p = 0.034), while acute rejection, allergy and liver related side effects were not associated with a specific type of vaccine.
| Side effects | Contrast | [95% Conf. interval] | P > t | Sig | ||
|---|---|---|---|---|---|---|
| mRNA (yes vs. mean) | All side effects | −1.685 | −13.787 | 10.417 | 1.000 | |
| Viral vector | 8.183 | −2.797 | 19.162 | 0.119 | ||
| Mix | −0.332 | −4.518 | 3.855 | 1.000 | ||
| Other vaccine | −6.348 | −20.824 | 8.128 | 0.400 | ||
| mRNA (yes vs. mean) | Thrombosis | 4.319 | −15.060 | 23.699 | 0.959 | |
| Viral vector | 20.316 | 3.151 | 37.482 | 0.029 | ** | |
| Mix | −2.195 | −12.939 | 8.549 | 1.000 | ||
| Other vaccine | −22.441 | −44.024 | −0.858 | 0.044 | ** | |
| mRNA (yes vs. mean) | Acute rejection | −1.074 | −36.103 | 33.956 | 1.000 | |
| Viral vector | −9.876 | −32.656 | 12.904 | 0.409 | ||
| Mix | 6.237 | −4.179 | 16.653 | 0.209 | ||
| Other vaccine | 4.432 | −27.971 | 36.834 | 1.000 | ||
| mRNA (yes vs. mean) | Allergy | 14.868 | −21.188 | 50.923 | 0.446 | |
| Viral vector | 8.149 | −33.844 | 50.142 | 1.000 | ||
| Mix | −1.935 | −4.113 | 0.244 | 0.072 | * | |
| Other vaccine | −21.082 | −29.198 | −12.966 | 0.002 | *** | |
| mRNA (yes vs. mean) | Liver related | −0.322 | −11.443 | 10.798 | 1.000 | |
| Viral vector | −8.301 | −23.078 | 6.476 | 0.242 | ||
| Mix | 12.511 | 2.693 | 22.329 | 0.022 | ||
| Other vaccine | −4.185 | −19.902 | 11.532 | 0.809 | ||
| mRNA (yes vs. mean) | Others | −8.618 | −42.805 | 25.569 | 0.856 | |
| Viral vector | 23.553 | 2.633 | 44.472 | 0.034 | ** | |
| Mix | −5.931 | −12.170 | 0.308 | 0.059 | * | |
| Other vaccine | −9.195 | −38.357 | 19.967 | 0.665 | ||
p < 0.1.
p < 0.05.
p < 0.01.
Efficacy assessment
Approximately one-fourth of the centers tested for COVID-19 antibodies (25.2%; 95% CI 6.5%–62.1%), which was commonly carried out 1 and 3 months after its administration (39.1%; 95% CI 14.1%–46.8% and 27.5%; 95% CI 14.1%–46.8%, respectively). ER together with WPR were more likely to test for antibodies after COVID-19 vaccination.
Approximately half of the centers reported COVID-19 infections in LT patients despite vaccination (56.1%, 95% CI 25.7%–82.6%); among these, one-fourth had a severe infection (25.8%, 95% CI 7.3%–60.3%). COVID-19 infection post-vaccine was more frequent in EMR, followed by ER and AR, yet more severe infection was more likely reported in SEAR and AR regardless of the type of vaccine administered (see Table 4 and Supplementary Table S4).
Center data
The mean number of alive LT patients per center was 698.5 (504.3–892.6), yet the data were available in only 57.7% (n = 97) of responders.
Patients were mostly vaccinated 12 months after LT (73.8%; 95% CI 66.1%–81.4%). Only one-fourth of LT patients were vaccinated within 3 months post-transplant (25.1%; 95% CI 9.8%–40.4%).
COVID-19 vaccine formulation was mRNA-based (Pfizer®/Moderna®) in 49.6% (95% CI 17.5%–81.8%), viral vector (Astra-Zeneca®/Janssen®) in 30.8% (95% CI 1.5%–60.1%), and a mix of them in only 4.7% (95% CI 0.5%–8.9%) of cases. About one-sixth of the patients received a different formulation (15%; 95% CI −4.7% to 34.6%), mainly in SEA and ER (7/17 and 6/17 centers). Globally, AR, WPR, and ER were the regions with higher percentages of vaccinated patients; mRNA vaccine was largely preferred in ER and AR, while viral vector was more frequently used in EMR and SEAR (Table 6 and Supplementary Table S5).
Table 6.
Data at the center level is summarized. The mean number of alive patients at the time of the survey was approximately 700. The percentage of patients fully vaccinated was around 87%. The most common type of vaccine was mRNA based in 50% of centers, followed by viral vector type in 31% of the centers.
| Proportion (n) | [95%_Conf. interval] | |||
|---|---|---|---|---|
| LT patients alive (n) | 698.459 | 504.334 | 892.584 | |
| Timing of vaccination administration | <3 months (%) | 25.116 | 9.809 | 40.423 |
| 3–6 months (%) | 50.869 | 21.674 | 80.063 | |
| 6–12 months (%) | 58.748 | 45.334 | 72.162 | |
| >12 months (%) | 72.682 | 66.977 | 78.388 | |
| Globally vaccinated (%) | 86.570 | 81.550 | 91.590 | |
| Patients vaccinated (n)a | 667.120 | 332.030 | 1,002.210 | |
| Type of vaccine administered (%) | mRNA | 49.646 | 17.523 | 81.770 |
| Viral vector | 30.811 | 1.515 | 60.107 | |
| Viral vector and mRNA | 4.695 | 0.502 | 8.888 | |
| Others | 14.968 | −4.681 | 34.618 | |
This estimation is based on the number of alive patients (available in 58.9%, n = 99) and the rate of fully vaccinated patients per center (available in 52.5%, n = 88).
Sensitivity analysis
Missing data for LT patients alive, vaccinated for COVID-19, and suffering significant ADEs per each center were obtained by multiple imputation considering hierarchical LT activity (globally, WHO regions and centers) in 2020 and 2021, country, and the number of centers per country. A total of 144 centers had complete data (see Supplementary Table S6): the mean rate of LT alive patients per center was 697.08 (95%CI, 360.56–1,033), the mean rate of COVID-19 vaccinated patients was 87.1%(95% CI 71.75%–100%), the estimated number of patients vaccinated per center at the time of the survey was 620.48 (273.3–967.66), and the mean rate of patients with ADEs was 0.78 (95% CI 0–0.233) per 1,000 patients.
Given the estimated rate of ADEs, mRNA vaccines showed a higher safety profile compared with the remainder vaccines, with −3.998 (−6.466, −1.631; p = 0.013) cases per 1,000 vaccinated patients, yet vial vector vaccines showed a significant increase rate of ADEs once compared with the remainder (3.544, 95% CI 0.762; 6.326; p = 0.028).
Discussion
Vaccination against COVID-19 is a major tool in the fight against the pandemic and has profoundly impacted its progression, with an estimated 14.4 million deaths worldwide prevented by vaccines during the first year of the vaccination program (from December 2020 to December 2021) (5). Liver transplant recipients are a high-risk group for COVID-19 infection, mainly due to their medical comorbidities, rather than to their immunosuppressed status. Scientific societies have recommended vaccination in LT recipients (6). However, access to vaccines and the related policies around the world have not been uniform.
This multi-society global survey is the largest study exploring current practices and policies regarding COVID-19 vaccination in LT centers across the world. A previous study (7) reported significant heterogeneity in COVID-19 vaccination mandate policies among centers in the US. In our study, most of the centers prioritized LT recipients and healthcare workers for COVID-19 vaccination and recommended a booster dose. This recommendation is in line with EASL and AASLD current recommendations (6, 8). However, cohabitants and life partners of LT recipients were rarely prioritized. A specific type of vaccine was not recommended in more than two thirds of programs (69.8%), and the main type of vaccine administered was an mRNA-based vaccine in approximately half of the studied centers. Importantly, concurrent routine vaccinations such as flu vaccination were recommended in most centers (89%). Of note, previous vaccination with flu has been described as a predictive factor for acceptance to receive vaccination against COVID-19 (9).
We also explored the different barriers to vaccination. The main reported barrier to vaccination was the patient's fear in 89% of cases. Vaccination hesitancy is another factor that is frequently encountered in the general population and has also been described in the transplant population (10). Counseling the patient about vaccine safety is crucial in order to increase the vaccination rate in transplant recipients. In more than one third of survey responses, public health policies were reported as a barrier to vaccination.
Interestingly, vaccination policies were slightly different in some regions; EM and WP Regions reported a higher presence of barriers and EMR had more difficulties in prioritizing COVID-19 vaccination in LT patients. Moreover, mRNA vaccines were more likely to be recommended in ER and a booster dose was more commonly implemented in AR. Finally, AR and ER were more prone to recommend other routine vaccinations.
Timing of COVID vaccination in LT recipients is not clearly defined, but it has been suggested that it should be performed after 3 months post-LT, when immunosuppression is lower. In our study, vaccination was mostly performed >12 months post-LT (73%). Only 25% of centers reported vaccination of patients in the first 3 months post-LT.
Efficacy assessment by COVID-19 antibodies post-vaccination at 1 and 3 months was limited, probably due to the paucity of data regarding the immune correlates of protection (CoP) after vaccination, the lack of a clear cut-off of neutralizing antibody titer against COVID-19, and the concern about the rapidly emerging variants with capacity to escape the specific antibody protection, especially in immunocompromised patients (11–13).
Preventive adjustment of immunosuppression regimen or dose before COVID-19 vaccination was uncommon across the assessed centers, occurring in just 16% of them. Among these, the most common approach was MF discontinuation or reduction of dose. This approach was probably driven by the known cytotoxic effect of MF on activated lymphocytes and the arising data showing absent or suboptimal antibody responses to COVID-19 vaccination in patients on MF (14, 15).
The most relevant side effects following COVID-19 vaccination were thrombosis or thromboembolic events, acute rejection, and significant liver dysfunction in 13.8%, 13%, and 9.5% of assessed centers. It is important to highlight that these rates are reflective of the number of centers that reported occurrence of ≥1 of these adverse events divided by the number of all participating centers in this survey and that these rates do not reflect the possibility of occurrence per patient. Interestingly, viral vector vaccines were associated with an increased risk of thrombotic events by 20.3 points (95% CI 3.152, 37.48; p = 0.029). Several reports showed an increased risk of splenic, porto-mesenteric, and hepatic thrombosis, cerebral arterial thromboembolism, and thrombotic microangiopathy (16–18) for adenoviral vector vaccines, contrary to observations for mRNA-based vaccines (19). Despite being unable to assess the incidence of thrombotic events, which based on the literature should be considered as a rare event (6), it provides evidence that the type of vaccine is highly relevant.
In order to better clarify the incidence and the type of side effects at the patient level, all centers that reported any of the aforementioned side effects were re-contacted. Unfortunately, the responders that specified the number of patients affected by side effects remained low (36.8%), precluding a clear estimation of its incidence. Thus, a sensitivity analysis using a complex imputation model replacing the missing data allowed for an estimate of a global ADEs incidence of 0.781 (95%CI 0; 0.233) per 1,000 vaccinated patients. This incidence is challenging to compare with what has been reported in literature as it reflects a pooled estimation of specific and unspecific side effects such as thrombosis, acute rejection, allergy, liver related, and others. Despite the fact that this model was unable to assess the specific incidence for each side effect, it globally confirmed that mRNA vaccine had a better safety profile compared with the other vaccine types (3 cases of ADEs less for every thousand vaccinated patients, p = 0.002).
The main limitations of this study are related to its nature, a cross sectional survey study. Indeed, we cannot rule out a selection bias with a relatively small proportion of centers worldwide that answered the questionnaire (20.7%). This challenges the extrapolation of the results, especially for WHO regions with low or no respondents (e.g., Eastern Mediterranean and African Regions). However, we estimated that the centers we interviewed account for approximately 45.6% of all liver transplant procedures performed in the included WHO regions during 2020 and 2021. Although the number of reported ADEs was double-checked directly with the corresponding center for case-by-case verification, the absence of ADE reports from the remainder centers relied on responder recall. The limited availability of patient level data is another limitation. Moreover, some of the questions had a low rate of response, perhaps because of the complexity in addressing the query. The study recognizes the challenges posed by the fast-changing landscape of vaccines and evolving guidelines compared to the 2021–2022 period. This study did not fully cover the latest COVID-19 vaccination recommendations for liver transplant patients, which now favor mRNA vaccines starting 3 months post-transplant (20). While viral vector vaccines are still advised in combination with mRNA vaccines, the focus has shifted towards mRNA vaccines. Nonetheless, the comprehensive nature of the analyses performed, including the sensitivity analysis addressing the missing data for the most relevant outcomes, provides valuable insights despite the acknowledged limitations.
In conclusion, this is the first multi-society survey assessing COVID-19 vaccination policies worldwide and it sheds light on the practices of LT centers in the new era of pandemic. Despite the obvious heterogeneity across WHO regions, this study was able to show that most centers prioritize COVID-19 vaccination in LT recipients and healthcare workers, recommending a booster dose and generally preferring mRNA-based vaccines, mainly administered 1 year after LT. There was no universal strategy to assess the efficacy of the vaccines or to adjust immunosuppression before COVID-19 vaccine administration, even though MF reduction or discontinuation was the most common approach. Finally, this study seems to confirm that adenoviral vaccine has an increased risk of thrombotic events, though it should be considered as a rare event.
Acknowledgments
We sincerely and deeply acknowledge all respondents for their precious participation in this survey (see Supplementary Table 7). We would like to acknowledge Luca Segantini from the European Society for Organ Transplantation for the preparation of the survey, Rossana Mirabella, Joel Walicki, and Ben Hainsworth from The European Association for the Study of the Liver and Jwana Ribeiro da Silva from the International Liver Transplant Society for their continuous technical support throughout the survey period.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
TD received support from the Juan Rodes Research Grant (JR18/00008) and the Mobility Grant Research (MV21/00066), which were funded by the Instituto de Salud Carlos III. Additionally, CIBERehd is partially funded by Instituto de Salud Carlos III.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Erasmus MC (MEC-2021-0818). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because the study did not involve direct contact with patients and relied on retrospective data collection.
Author contributions
TD: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. CV: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MI: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. FP: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. VK: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. AR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. LB: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. WP: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. TB: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. MB: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2023.1332616/full#supplementary-material
References
- 1.Russo FP, Burra P, Zanetto A. COVID-19 and liver disease: where are we now? Nat Rev Gastroenterol Hepatol. (2022) 19(5):277–8. 10.1038/s41575-022-00607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) dashboard > cases [dashboard]. (2023). Available at: https://data.who.int/dashboards/covid19/cases (accessed September 20, 2022).
- 3.Russo FP, Izzy M, Rammohan A, Kirchner VA, Di Maira T, Belli LS, et al. Global impact of the first wave of COVID-19 on liver transplant centers: a multi-society survey (EASL-ESOT/ELITA-ILTS). J Hepatol. (2022) 76(2):364–70. 10.1016/j.jhep.2021.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. (2021) 36(10):3179–87. 10.1007/s11606-021-06737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. (2022) 22(9):1293–1302. 10.1016/S1473-3099(22)00320-6. Erratum in: Lancet Infect Dis. (2023) 23(10):e400. PMID: 35753318; PMCID: PMC9225255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marjot T, Eberhardt CS, Boettler T, Belli LS, Berenguer M, Buti M, et al. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: an updated EASL position paper. J Hepatol. (2022) 77(4):1161–97. 10.1016/j.jhep.2022.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hippen BE, Axelrod DA, Maher K, Li R, Kumar D, Caliskan Y, et al. Survey of current transplant center practices regarding COVID-19 vaccine mandates in the United States. Am J Transplant. (2022) 22(6):1705–13. 10.1111/ajt.16995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AASLD. AASLD COVID-19 guidance document. (2022). Available at: https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf (accessed October 6, 2022).
- 9.Reuken PA, Albers S, Rauchfuss F, Strnad P, Settmacher U, Trautwein C, et al. Acceptance of SARS-CoV-2 vaccines by liver transplant recipients and candidates. Z Gastroenterol. (2021) 59(12):1288–96. 10.1055/a-1649-8568 [DOI] [PubMed] [Google Scholar]
- 10.Kates OS, Stohs EJ, Pergam SA, Rakita RM, Michaels MG, Wolfe CR, et al. The limits of refusal: an ethical review of solid organ transplantation and vaccine hesitancy. Am J Transplant. (2021) 21(8):2637–45. 10.1111/ajt.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadj Hassine I. COVID-19 vaccines and variants of concern: a review. Rev Med Virol. (2022) 32(4):e2313. 10.1002/rmv.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galmiche S, Luong Nguyen LB, Tartour E, de Lamballerie X, Wittkop L, Loubet P, et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect. (2022) 28(2):163–77. 10.1016/j.cmi.2021.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuh AJ, Satheshkumar PS, Dietz S, Bull-Otterson L, Charles M, Edens C, et al. SARS-CoV-2 convalescent Sera binding and neutralizing antibody concentrations compared with COVID-19 vaccine efficacy estimates against symptomatic infection. Microbiol Spectr. (2022) 10(4):e0124722. 10.1128/spectrum.01247-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines (Basel). (2021) 9:1422. 10.3390/vaccines9121422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toniutto P, Falleti E, Cmet S, Cussigh A, Veneto L, Bitetto D, et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol. (2022) 77(1):152–62. 10.1016/j.jhep.2022.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. (2021) 384(23):2254–6. 10.1056/NEJMe2106315 Erratum in: N Engl J Med. (2021) 384(23):e92. PMID: 33861524; PMCID: PMC8063912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. (2021) 138:2256–68. 10.1182/blood.2021013231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Öcal O, Stecher S-S, Wildgruber M. Portal vein thrombosis associated with ChAdOx1 nCov-19 vaccination. Lancet Gastroenterol Hepatol. (2021) 6:676. 10.1016/S2468-1253(21)00197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). (2021) 2(8):965–978.e5. 10.1016/j.medj.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AASLD Expert panel consensus statement: COVID-19 clinical best practice advice for hepatology and liver transplant providers. Released: October 6, 2022. Available at: https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf (Accessed December 8, 2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.