Abstract
There is a knowledge gap regarding the effect of extracorporeal shockwave treatment (ESWT) on the stress response and immunomodulatory and anti-inflammatory properties of equine umbilical cord blood mesenchymal stromal cells (CB-MSCs). The objective of this study was to investigate the presence of cellular oxidative stress, inflammatory response, and production of growth factors in CB-MSCs after treatment with ESWT. We hypothesized that CB-MSCs treated with ESWT will experience higher levels of cellular stress and increased production of anti-inflammatory cytokines and growth factors compared to untreated CB-MSCs.
Résumé
Il existe un manque de connaissances concernant l’effet du traitement extracorporel par ondes de choc (ESWT) sur la réponse au stress et les propriétés immunomodulatrices et anti-inflammatoires des cellules stromales mésenchymateuses du sang de cordon ombilical équin (CB-MSCs). L’objectif de cette étude était d’étudier la présence de stress oxydatif cellulaire, de réponse inflammatoire et de production de facteurs de croissance dans les CB-MSCs après un traitement par ESWT. Nous avons émis l’hypothèse que les CB-MSCs traitées par ESWT connaîtront des niveaux plus élevés de stress cellulaire et une production accrue de cytokines anti-inflammatoires et de facteurs de croissance par rapport aux CB-MSCs non traitées.
(Traduit par Docteur Serge Messier)
Introduction
Horses engaged in athletic pursuits are consistently exposed to the potential for musculoskeletal injuries, with about 76% of the observed injuries occurring in their limbs on racetracks (1). Nearly 46% of these are directly linked to tendon-related issues (1). Tendinopathies have a high economic impact on the equine industry due to their career- or life-threatening potential. Consequently, new therapeutic approaches are needed that repair the lesion and allow the horse to return to its previous athletic function. Mesenchymal stromal cells (MSCs) have emerged as a promising therapeutic option for treating tendinopathies in horses (2), with intralesional injection improving ultrasonographic appearance, histological healing, and biomechanical properties compared to controls such as saline (3–10). Horses treated with MSCs also exhibit lower reinjury rates than with other treatment methods such as controlled exercise or hyaluronan (11,12). Extracorporeal shockwave therapy (ESWT) is another commonly used therapy (13), which enhances neovascularization and histomorphological appearance, reduces inflammation, and increases expression of transforming growth factor beta (TGF-β1) (14–16).
Mesenchymal stromal cells (MSCs) and ESWT are combined in equine treatment despite limited scientific evidence of their effectiveness, although studies in humans and animals suggest that tissue heals better with this combination (17–24). The reasons for these findings are unclear, but some studies have shown that stimulating MSCs with ESWT increases proliferation (25) and differentiation (26). Moreover, MSCs derived from equine umbilical cord blood (CB) that are treated with ESWT maintained their differentiation capacity and showed higher potential for differentiation towards the adipogenic and osteogenic lineages (27). Stimulation with ESWT seems to enhance the proliferation and differentiation of MSCs, potentially through activation of the cellular stress response (28,29) or by modulation of cytokines and growth factors, leading to improved tissue repair and a balanced inflammatory response (30,31).There is a knowledge gap regarding the effect of ESWT on the stress response and the immunomodulatory and anti-inflammatory properties of equine CB-MSCs. The objective of this study was to investigate the presence of cellular oxidative stress, inflammatory response, and production of growth factors in CB-MSCs after treatment with ESWT. We hypothesized that CB-MSCs treated with ESWT will experience higher levels of cellular stress and increased production of anti-inflammatory cytokines and growth factors compared to untreated CB-MSCs.
Materials and methods
Culture of equine CB — MSCs Cord blood was collected immediately after foaling and before the umbilical cord broke spontaneously or was broken according to farm management protocol. Venipuncture of the umbilical vein was carried out with a 16-G hypodermic needle attached to a 450-mL blood transfusion collection bag containing citrate phosphate dextrose adenine as the anticoagulant solution. The blood was then immediately transported to the laboratory at ambient temperature.
The equine MSCs were isolated as described in a previous study (32). Briefly, the mononuclear cell fraction (MNCF) was cultured and non-adherent cells were removed through successive complete medium changes; the isolation medium consisted of low-glucose Dulbecco’s modified Eagle medium (DMEM-LG; Lonza, Walkersville, Maryland, USA), supplemented with 30% fetal bovine serum (FBS; Invitrogen, Burlington, Ontario), low dexamethasone (10−7 M) (Sigma-Aldrich, Oakville, Ontario), 100 U penicillin-streptomycin (Invitrogen), and 2 mM L-glutamine (Sigma-Aldrich). When cell numbers allowed, the cells were passaged and expanded until they were cryopreserved in hypothermic biopreservation medium (CryoStor CS10; BioLife Solutions, Bothell, Washington, USA) in 1-mL aliquots at a concentration of 1 × 106 cells/mL for later use.
Mesenchymal stromal cells (MSCs) isolated from 3 different donors were used in this study [1801 (Filly, Thoroughbred), 1803 (Filly, Warmblood), and 1810 (Colt, Warmblood)]. To increase the number of technical replicates, 3 cryovials of each donor were used in the experiment for a total of 9 cryovials. The cryovials had been stored in liquid nitrogen for 2 y prior to the study and all cells were contained in passage 3. The cells were thawed, seeded at a cell density of 5000 cells/cm2 of culture surface area, and expanded until 80 to 90% confluence to obtain sufficient cell numbers for all the experiments and assays of this study.
The culture medium consisted of low-glucose DMEM (DMEM-LG; Lonza), 10% FBS (Invitrogen), 2 mM L-glutamine (Sigma-Aldrich), and 100 U penicillin-streptomycin (Invitrogen), and passaging was done by enzymatic digestion with 0.25% trypsin-EDTA.
In-vitro extracorporeal shockwave treatment
The in-vitro ESWT treatment was carried out as described in previous studies (27,33) using a specially designed water bath with pre-warmed water (37°C) and an electrohydraulic shockwave generator (Versatron; Pulse Veterinary Technologies, Alpharetta, Georgia, USA). Equine MSCs were treated at 80 to 90% confluence in T-25 cell culture flasks. The flasks were placed in front of the applicator, 5 cm from the shockwave probe (R80 Trode, focal depth 50 mm to 110 mm) to the cell layer inside. The focused shock waves were applied with an energy flux density of 0.1 mJ/mm2, a frequency of 180 pulses per min, and 300 impulses (27). The control group was maintained in the same culture conditions, consisting of the expansion medium without exposure to shock waves.
After the ESWT, the cell cultures were returned to the incubator for 3 h before oxidative stress and cytokines were determined. The 3-hour time frame was chosen to evaluate the initial effects of ESWT based on prior studies examining its early impact on oxidative stress (34,35). The experiment was conducted in technical triplicates, wherein each week, 1 cryovial of each of the 3 cell lines was cultured and subjected to the shockwave and determination of oxidative stress and cytokine levels. The experiment was repeated with new cryovials at a 1-week interval.
Production of conditioned medium of CB-MSC co-cultured with peripheral blood mononuclear cells
Before carrying out the cytokine enzyme-linked immunosorbent assays (ELISAs), a conditioned medium of equine MSCs co-cultured with peripheral blood mononuclear cells (PBMCs) was obtained as a positive control for cytokine production. For this, PBMCs from 5 unrelated equine donors were isolated and frozen as described by Lepage et al (36).
Frozen PBMCs from all 5 donors were thawed, pooled in equal ratios, then incubated overnight in complete Roswell Park Memorial Institute medium (RPMI 1640; Thermo Fisher Scientific, Burlington, Ontario), 2 mM L-glutamine (Sigma-Aldrich), 100 U penicillin-streptomycin (Invitrogen), and 10% horse serum.
Equine MSCs from 3 different donors (1801, 1803, and 1810) were seeded at 10 000 cells/well in a 48-well plate in MSC expansion medium and incubated overnight. The next day, PBMCs were activated with Concanavalin A mitogen (Sigma), final concentration: 5 μg/mL. MSCs were washed 1× with PBS before adding activated PBMCs in a complete RPMI medium at a ratio of 10 PBMC:1 MSC. After 5 d of PBMC:MSC co-culture, the conditioned media was collected, centrifuged at 1000 × g for 15 min to remove the cell debris, and was then aliquoted and stored at −80°C until carrying out the ELISAs to determine PGE2, TGF-β1, IL-1ra, and IL-10 profiles.
Measurement of mitochondrial membrane potential and reactive oxygen species
The culture medium was removed 3 h after shockwave treatment and 4 mL of a 25-nM solution of MitoTracker Red CM-H2XRos (Invitrogen) was added to the T-25 flask and incubated for 15 min to measure mitochondrial membrane potential (MMP). The cells were then rinsed twice with pre-warmed 1× PBS to remove excess stain. After that, 4 mL of a 5-μM solution of 2′,7′-dich lorodihydrofluorescein diacetate H2DCFDA (Invitrogen) for measuring reactive oxygen species (ROS) was added to the T-25 flask and incubated for 30 min. The cells were rinsed 3 times with pre-warmed 1× PBS to remove excess stain.
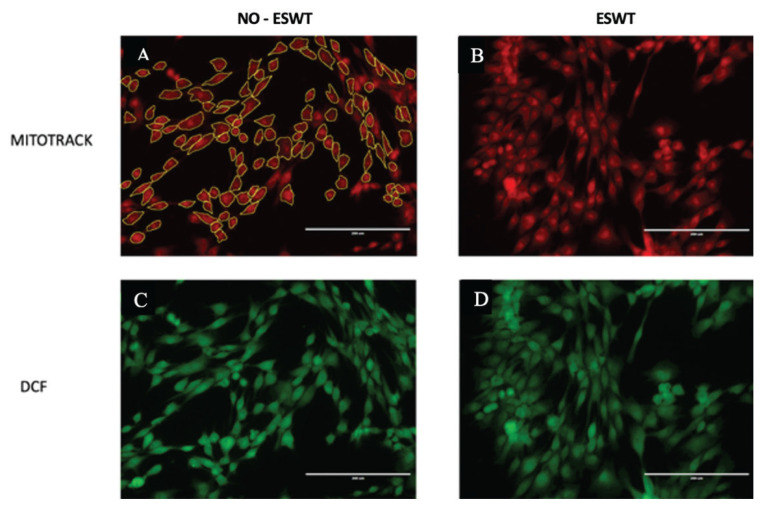
The stained T-25 flasks were then visualized under the fluorescent microscope at 40× and 10 field pictures of each flask were randomly obtained in areas of high cellular density. Using the Image J software (Rasband W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA), the cells in the pictures were manually highlighted; overlapping cells or those with cut margins were not included. The highlighted cells were added to the region of interest (ROI) manager and the fluorescence intensity [integrated density (intden)] was measured (Figure 1).
Figure 1.
Equine CB-MSCs stained with Mitotracker (red) and H2DCFDA (green). A — Control non-ESWT-treated CB-MSCs stained with Mitotracker and highlighted with Image J/Fiji for measurement of the integrated density. B — ESWT-treated CB-MSCs stained with Mitotracker. C — Control non-ESWT-treated CB-MSCs stained with H2DCFDA. D — ESWT-treated CB-MSCs stained with H2DCFDA.
Determination of PGE2, TGF-β1, IL-1ra, and IL-10 profiles
The culture medium was collected 3 h after ESWT treatment and centrifuged at 400 × g for 5 min. Supernatants were then collected and stored at −80°C until further analysis. Supernatants were collected from control MSC cultures in a similar fashion. Concentrations of prostaglandin E2 (PGE2) and transforming growth factor-β1 (TGF-β1) were determined using human PGE2 and TGF-β1 ELISA assays, previously validated for horses, according to the manufacturer’s instructions (Parameter Assay Kit and Quantikine Human TGF-β1 ELISA; R&D Systems, Minneapolis, Minnesota, USA). Interleukin-10 (IL-10) and interleukin-1 receptor antagonist (IL-1ra) were determined using equine IL-10 and IL-1ra ELISA assays, according to the manufacturer’s instructions (IL-10 Equine ELISA Kit; Invitrogen and Equine IL-1ra/IL-1F3 DuoSet ELISA; R&D Systems).
Statistical analysis
Data normality was assessed using normal probability Q-Q plots and the Kolmogorov-Smirnov test. Descriptive statistics, such as mean and standard deviation (SD), were employed. The impact of ESWT on mitochondrial membrane potential (MMP), reactive oxygen species (ROS) (MitoTracker and H2DCFDA), and PGE2 concentration was analyzed using a mixed model analysis of variance (ANOVA) test to account for random effects.
The model for stain intensity (MitoTracker and H2DCFDA) included the fixed effect of treatment (ESWT) and random effects of the cell line (1801, 1803, and 1810), image, and subsample within the image. Random effects for the PGE2 model included the date. The normality of residuals was assessed using the Kolmogorov-Smirnov test. Statistical significance was defined at P < 0.05.
Results
Effect of ESWT on mitochondrial membrane potential and reactive oxygen species
The different values of Integrated Density [intden (Arbitrary Units)], reflecting the fluorescence intensity, for the various treatments and cell lines of ESWT-treated and non-treated CB-MSCs are presented in Table I. No significant difference was observed in the levels of oxidative stress, as indicated by changes in mitochondrial membrane potential (MMP) or reactive oxygen species (ROS), between ESWT-treated CB-MSC cells (MMP = intden 729 AU ± 64 and ROS = intden 560 AU ± 69) and non-treated cells (MMP = intden 995 AU ± 397 and ROS = intden 903 AU ± 483) (MMP: P = 0.36; ROS: P = 0.29).
Table I.
Values of integrated density [intden (A.U.)], indicative of fluorescence intensity, of the different cell times and treatments of ESWT-treated and control non-ESWT-treated equine CB-MSCs.
| Cell line/Treatment | MitoTrack control | MitoTrack ESWT | H2DCFDA control | H2DCFDA ESWT |
|---|---|---|---|---|
| 1801/TR1 | 622.92 ± 194.63 | 743.47 ± 193.65 | 336.45 ± 107.57 | 385.73 ± 132.44 |
| 1803/TR1 | 722.03 ± 211.21 | 788.54 ± 243.57 | 226.34 ± 95.75 | 228.330 ± 88.13 |
| 1810/TR1 | 635.77 ± 189.14 | 687.04 ± 169.34 | 264.33 ± 90.14 | 243.70 ± 79.41 |
| 1801/TR2 | 3108.47 ± 5928.58 | 812.65 ± 232.21 | 3373.75 ± 6224.95 | 895.98 ± 288.19 |
| 1803/TR2 | 976.83 ± 297.77 | 827.39 ± 274.91 | 987.15 ± 351.06 | 725.08 ± 315.52 |
| 1810/TR2 | 798.26 ± 234.20 | 723.98 ± 193.49 | 790.29 ± 266.24 | 702.48 ± 225.52 |
| 1801/TR3 | 644.32 ± 167.12 | 606.20 ± 151.43 | 737.90 ± 241.83 | 608.37 ± 201.69 |
| 1803/TR3 | 922.55 ± 329.22 | 783.48 ± 235.36 | 930.95 ± 407.30 | 658.88 ± 300 |
| 1810/TR3 | 593.77 ± 182.30 | 605.06 ± 170.78 | 549.93 ± 199.35 | 547.77 ± 217.84 |
Data presented as mean ± SD.
MitoTrack — Stain for mitochondrial membrane potential (MMP); H2DCFDA — Stain for reactive oxygen species (ROS); ESWT — Extracorporeal shock wave therapy; AU — Arbitrary units.
Effect of ESWT on PGE2, TGF-β1, IL-1ra, and IL-10 concentrations
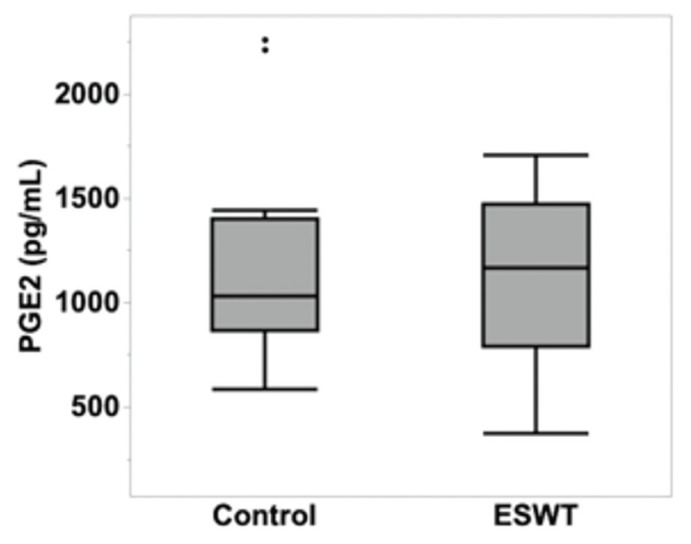
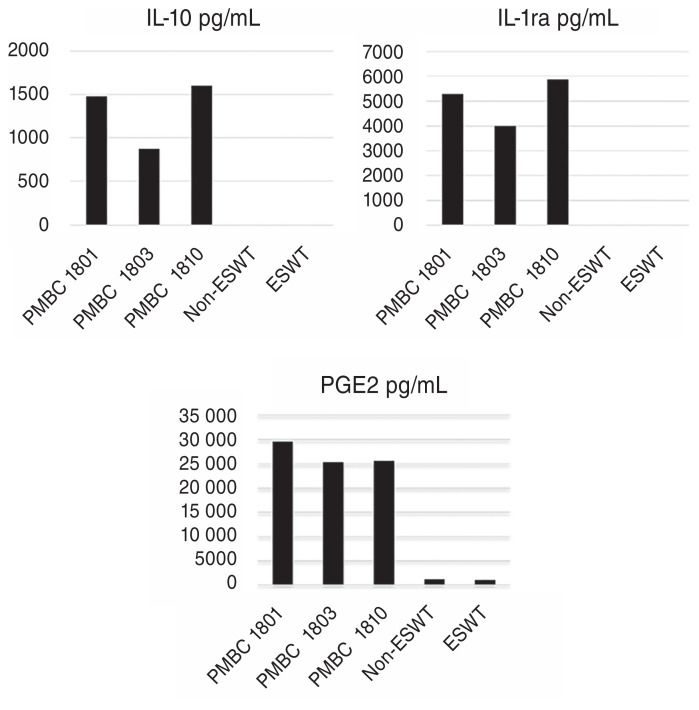
The multivariable mixed model analysis revealed that there were no statistically significant differences in the concentration of PGE2 between the non-treated (1203.95 pg/mL ± 199.43) and ESWT-treated (1095.29 pg/mL ± 199.43) CB-MSCs (P = 0.5416) (Figure 2). In addition, no TGF-β1, IL-1ra, or IL-10 production was observed in non-treated or ESWT-treated CB-MSCs. The CB-MSCs preconditioned with peripheral blood mononuclear cells (PBMCs), which served as a positive control for the ELISA tests, exhibited the production of IL-1ra or IL-10 and showed an increase in the amount of secreted PGE2 (Figure 3).
Figure 2.
Concentrations of PGE2 in control non-ESWT treated CB-MSCs and ESWT-treated CB-MSCs. There were no differences in the concentration of PGE2 of both groups of MSCs (P = 0.5416).
Figure 3.
Concentrations of IL-10, IL-1ra, and PGE2 in control non-ESWT treated CB-MSCs, ESWT-treated CB-MSCs and the conditioned medium of CB-MSCs co-cultured with PMBCs.
Discussion
This study examined the effects of extracorporeal shockwave therapy (ESWT) on equine cord blood mesenchymal stromal cells (CB-MSCs) in-vitro. Results indicate that ESWT did not induce the anticipated increase in cellular oxidative stress or the production of PGE2, TGF-β1, IL-1ra, and IL-10, nor did it elevate mitochondrial membrane potential or ROS production, thus rejecting our initial hypothesis.
In previous studies, treating adipose-derived MSCs with ESWT has increased cellular proliferation and differentiation (25,26). These observed effects in equine MSCs may be associated with mechano-transduction driven by ESWT, as applying shockwave to human MSCs triggers the release of ATP-activating Erk 1/2 and MAPK signalling leading to proliferation and differentiation (28,29).
It has also been reported that treating human mesenchymal progenitor cells with ESWT results in increased cellular oxidative stress, which has been linked with dose-dependent increased osteogenic potential of these cells (31). This is consistent with other literature describing the association between oxidative stress and proliferation and differentiation of human MSCs (37–39), which provided a rationale to examine the role of ROS in equine MSCs treated with ESWT.
Our previous study showed increased proliferation and differentiation of equine CB-MSCs after ESWT treatment (27). However, the results from our current study suggest that applying shockwave with an energy flux density of 0.1 mJ/mm2, frequency of 180 p/m, and 300 impulses did not appear to affect oxidative stress in equine MSCs 3 h after the exposure. This contradicts the results from a similar study in human cord blood-derived mesenchymal progenitors described above that demonstrated an increase in ROS within 60 min of exposure to ESWT (31).
Interestingly, whole cord blood was initially exposed to shockwave in the study by Wang et al (31), whereas in the present study, only isolated CB-MSCs were exposed to ESWT. Although isolated CB-MSCs are more relevant clinically, as they represent the cell product being transplanted, the oxidative stress observed in their study could have resulted from stimulation of cellular populations other than MSCs present in the cord blood. Further studies are required to determine the origin of oxidative stress.
Secondly, ROS concentrations were determined using different methods. In our current study, H2DCFDA was used as an indicator of intracellular ROS, whereas in the study by Wang et al (31), Lucigenin chemiluminescence was used, which only detects extracellular ROS. More specifically, the H2DCFDA assay is more sensitive for measuring concentrations of hydrogen peroxide (H2O2) and peroxynitrite (ONOO−), whereas Lucigenin chemiluminescence is more sensitive to extracellular superoxide (40). As we would expect intracellular ROS to be associated with a response to external mechanical stimuli such as ESWT, this is what we probed for. However, it is unknown if extracellular ROS levels increased with ESWT-treated CB-MSCs.
In our current study, cultured CB-MSCs produced detectable concentrations of PGE2 in both ESWT-treated and non-ESWT-treated cells. This finding aligns with previous research documenting the production of PGE2 by bone marrow-derived MSCs without any external stimulation (41). It is unknown how this could explain the effect of MSCs on tissue repair. However, PGE2 has been associated with the activation of endogenous mesenchymal cells, immunomodulation, and stimulation of neovascularization by activating prostanoid receptors (42).
Our results also indicate that stimulation of CB-MSCs with ESWT did not alter the production of PGE2, TGF-β1, IL-1ra, or IL-10, which suggests that ESWT might not influence the production of these cytokines. This finding agrees with a previous study showing that ESWT does not increase VEGF, TGF-β1, and PGE2 production (41). In contrast to the present study, stimulation of human tendon cells and mesenchymal progenitors with ESWT has increased the production of IL-10 and TGF-β1, respectively. These changes in the concentration of IL-10 and TGF-β1 are observed from 15 to 24 h after exposure to ESWT (30,31).
In the present study, PGE2, TGF-β1, IL-1ra, and IL-10 concentrations were measured 3 h after treatment with ESWT to assess the early response of CB-MSCs to ESWT exposure. In addition, this timepoint reduced the risk of other sources of cellular stress, such as trypsinization and over-confluency, which have been found to increase cytokine expression and apoptosis, respectively (43,44).
It is possible that any ESWT-associated production of PGE2, TGF-β1, IL-1ra, and IL-10 required more than 3 h to be observed. Further studies with different time points are necessary to estimate when these mechanisms to produce PGE2, TGF-β1, IL-1ra, and IL-10 start unveiling.
As a part of our study, we used CB-MSCs preconditioned with PBMCs as a positive control when carrying out the ELISA to determine the concentrations of PGE2, TGF-β1, IL-1ra, and IL-10. The production of different cytokines, such as VEGF, HGF, IGF-1, TGF-β1, PGE-2, IDO, iNOS, and interleukins including IL-1, IL-1ra, IL-3, IL-6 IL-10, upon preconditioning of MSCs has been demonstrated (45–48).
Similarly, we discovered that equine CB-MSCs preconditioned with PBMCs produced IL-10 and IL-1ra and increased production of PGE2 compared to non-preconditioned CB-MSCs. To the authors’ knowledge, this is the first study reporting the secretion of PGE2 and interleukins by equine CB-MSCs preconditioned with PBMCs. Further studies are required to categorize the cytokine and interleukin profiles of equine CB-MSC.
We acknowledge that our study was limited by a small sample size, which may have hindered our ability to detect smaller differences between the groups. A post-hoc power analysis revealed that, due to the small differences in fluorescence intensity between the groups, our study had a power of only 40%. Although our study’s sample size may have influenced our results, it is important to consider the cumulative evidence from similar studies that have achieved notable findings despite similar limitations (27,33).
Additional limitations warrant mentioning. Firstly, our study only assessed a limited number of cytokines. Moreover, these cytokines were measured at a single point after the MSCs were exposed to ESWT. This approach might have hindered our ability to detect cytokines with early expression patterns or those essential to the mechanism of action of ESWT.
Secondly, we acknowledge the existence of numerous ESWT protocols varying in energy flux densities, frequencies, and shock numbers for both in-vitro and in-vivo applications. The findings from our study are confined to the experimental settings of ESWT chosen here and, importantly, should not be extrapolated to clinical scenarios.
In conclusion, we did not observe an increase in MMP, production of ROS, or the production of PGE2, TGF-β1, IL-1ra, and IL-10 by CB-MSCs 3 h after treatment with ESWT. Further in-vitro and in-vivo studies are warranted to elucidate the previously reported mechanism that has influenced the changes in metabolic activity and differentiation of MSCs treated with ESWT. These studies should include calculating a proper sample size based on our findings, incorporating different sampling times after treatment with ESWT, encompassing a larger spectrum of cytokines, and including multiple ESWT protocols.
References
- 1.Williams RB, Harkins LS, Hammond CJ, Wood JLN. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet J. 2001;33:478–486. doi: 10.2746/042516401776254808. [DOI] [PubMed] [Google Scholar]
- 2.Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35:99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 3.Crovace A, Lacitignola L, De Siena R, Rossi G, Francioso E. Cell therapy for tendon repair in horses: An experimental study. Vet Res Commun. 2007;31:281–283. doi: 10.1007/s11259-007-0047-y. [DOI] [PubMed] [Google Scholar]
- 4.Crovace A, Lacitignola L, Rossi G, Francioso E. Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet Med Int. 2010;2010:250978. doi: 10.4061/2010/250978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 2008;69:928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho AM, Badial PR, Álvarez LE, et al. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: A randomized controlled trial. Stem Cell Res Ther. 2013;4:85. doi: 10.1186/scrt236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RKW, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;25(8):e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Loon VJ, Scheffer CJ, Genn HJ, Hoogendoorn AC, Greve JW. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet Q. 2014;34:92–97. doi: 10.1080/01652176.2014.949390. [DOI] [PubMed] [Google Scholar]
- 10.Salz RO, Elliott CRB, Zuffa T, Bennet ED, Ahern BJ. Treatment of racehorse superficial digital flexor tendonitis: A comparison of stem cell treatments to controlled exercise rehabilitation in 213 cases. Equine Vet J. 2023;55:979–987. doi: 10.1111/evj.13922. [DOI] [PubMed] [Google Scholar]
- 11.Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RKW. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012 Jan;44(1):25–32. doi: 10.1111/j.2042-3306.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 12.Dyson SJ. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000) Equine Vet J. 2004;36:415–419. doi: 10.2746/0425164044868422. [DOI] [PubMed] [Google Scholar]
- 13.O’Meara B, Bladon B, Parkin TDH, Fraser B, Lischer CJ. An investigation of the relationship between race performance and superficial digital flexor tendonitis in the Thoroughbred racehorse. Equine Vet J. 2010;42:322–326. doi: 10.1111/j.2042-3306.2009.00021.x. Available from: https://beva.onlinelibrary.wiley.com/doi/10.1111/j.2042-3306.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- 14.McClure SR. Shock wave therapy. In: Ross MW, Dyson SJ, editors. Diagnosis and Management of Lameness in the Horse. Elsevier; 2011. pp. 914–919. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9781416060697000961. [Google Scholar]
- 15.Wang CJ, Wang FS, Yang KD, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ, Wang CJ, Yang KD, et al. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-β1 and IGF-I expression. J Orthop Res. 2004;22:854–861. doi: 10.1016/j.orthres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Kersh KD, Mcclure SR, Van Sickle D, Evans RB. The evaluation of extracorporeal shock wave therapy on collagenase induced superficial digital flexor tendonitis. Vet Comp Orth Traumatol. 2006;19:99–105. [PubMed] [Google Scholar]
- 18.Yin TC, Wu RW, Sheu JJ, et al. Combined therapy with extracorporeal shock wave and adipose-derived mesenchymal stem cells remarkably improved acute ischemia-reperfusion injury of quadriceps muscle. Oxid Med Cell Longev. 2018;2018:6012636. doi: 10.1155/2018/6012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen KH, Hsiao HY, Wallace CG, et al. Combined adipose-derived mesenchymal stem cells and low-energy extracorporeal shock wave therapy protect the brain from brain death-induced injury in rat. J Neuropathol Exp Neurol. 2019;78:65–77. doi: 10.1093/jnen/nly108. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CC, Cheng JH, Wang CJ, Ko JY, Hsu SL, Hsu TC. Shockwave therapy combined with autologous adipose‐derived mesenchymal stem cells is better than with human umbilical cord Wharton’s jelly‐derived mesenchymal stem cells on knee osteoarthritis. Int J Mol Sci. 2020;21:1217. doi: 10.3390/ijms21041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JH, Yen KT, Chou WY, et al. Autologous adipose-derived mesenchymal stem cells combined with shockwave therapy synergistically ameliorates the osteoarthritic pathological factors in knee joint. Pharmaceuticals (Basel) 2021;14:318. doi: 10.3390/ph14040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu JJ, Lee FY, Yuen CM, et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69–83. doi: 10.1016/j.ijcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 23.Chen XJ, Zhang X, Jiang K, et al. Adjunctive mesenchymal stem/stromal cells augment microvascular function in poststenotic kidneys treated with low-energy shockwave therapy. J Cell Physiol. 2020;235:9806–9818. doi: 10.1002/jcp.29794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai L, Ma XL, Jiang C, Zhang B, Liu ST, Xing GY. Human autologous mesenchymal stem cells with extracorporeal shock wave therapy for nonunion of long bones. Indian J Orthop. 2016;50:543–550. doi: 10.4103/0019-5413.189602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raabe O, Shell K, Goessl A, et al. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. [Last accessed May 23, 2024];Am J Stem Cells. 2013 2:62–73. Available from: www.AJSC.us. [PMC free article] [PubMed] [Google Scholar]
- 26.Schuh CMAP, Heher P, Weihs AM, et al. In vitro extracorporeal shock wave treatment enhances stemness and preserves multipotency of rat and human adipose-derived stem cells. Cytotherapy. 2014;16:1666–1678. doi: 10.1016/j.jcyt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Salcedo-Jiménez R, Koenig JB, Lee OJ, Gibson TWG, Madan P, Koch TG. Extracorporeal shock wave therapy enhances the in vitro metabolic activity and differentiation of equine umbilical cord blood mesenchymal stromal cells. Front Vet Sci. 2020;7:554306. doi: 10.3389/fvets.2020.554306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Liao H, Ma Z, et al. Focal adhesion kinase signaling mediated the enhancement of osteogenesis of human mesenchymal stem cells induced by extracorporeal shockwave. Sci Rep. 2016;6:20875. doi: 10.1038/srep20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weihs AM, Fuchs C, Teuschl AH, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289:27090–27104. doi: 10.1074/jbc.M114.580936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Girolamo L, Stanco D, Galliera E, et al. Soft-focused extracorporeal shock waves increase the expression of tendon-specific markers and the release of anti-inflammatory cytokines in an adherent culture model of primary human tendon cells. Ultrasound Med Biol. 2014;40:1204–1215. doi: 10.1016/j.ultrasmedbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang FS, Yang KD, Wang CJ, et al. Shockwave stimulates oxygen radical-mediated osteogenesis of the mesenchymal cells from human umbilical cord blood. J Bone Miner Res. 2004;19:973–982. doi: 10.1359/JBMR.040121. [DOI] [PubMed] [Google Scholar]
- 32.Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:1–9. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holfeld J, Tepeköylü C, Kozaryn R, Mathes W, Grimm M, Paulus P. Shock wave application to cell cultures. J Vis Exp. 2014;86:51076. doi: 10.3791/51076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark DL, Connors BA, Evan AP, Handa RK, Gao S. Effect of shock wave number on renal oxidative stress and inflammation. BJU Int. 2011;107:318–322. doi: 10.1111/j.1464-410X.2010.09311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng B, Dong Z, Wang Y, et al. Li-ESWT treatment reduces inflammation, oxidative stress, and pain via the PI3K/AKT/FOXO1 pathway in autoimmune prostatitis rat models. Andrology. 2021;9:1593–1602. doi: 10.1111/andr.13027. [DOI] [PubMed] [Google Scholar]
- 36.Lepage SIM, Lee OJ, Koch TG. Equine cord blood mesenchymal stromal cells have greater differentiation and similar immunosuppressive potential to cord tissue mesenchymal stromal cells. Stem Cells Dev. 2019;28:227–237. doi: 10.1089/scd.2018.0135. [DOI] [PubMed] [Google Scholar]
- 37.Denu RA, Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid Med Cell Long. 2016 doi: 10.1155/2016/2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi M, Dusting GJ, Peshavariya H, et al. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–888. doi: 10.1089/scd.2012.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. In: Parker GC, editor. Stem Cells and Development. Vol. 24. New Rochelle, New York: Mary Ann Liebert Inc; 2015. pp. 1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 41.Colbath AC, Kisiday JD, Phillips JN, Goodrich LR. Can extracorporeal shockwave promote osteogenesis of equine bone marrow-derived mesenchymal stem cells in vitro? Stem Cells Dev. 2020;29:110–118. doi: 10.1089/scd.2019.0202. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H, Huang H, Guo Z, Chang Y, Li Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics. 2021;11:8836–8854. doi: 10.7150/thno.63396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 44.Song IH, Caplan AI, Dennis JE. Dexamethasone inhibition of confluence-induced apoptosis in human mesenchymal stem cells. J Ortho Res. 2009;27:216–221. doi: 10.1002/jor.20726. [DOI] [PubMed] [Google Scholar]
- 45.Baberg F, Geyh S, Waldera-Lupa D, et al. Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim Biophys Acta Proteins Proteom. 2019;1867:434–441. doi: 10.1016/j.bbapap.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Trzyna A, Banaś-Zabczyk A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy”. Biomolecules. 2021;11:878. doi: 10.3390/biom11060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romanov YA, Volgina NE, Vtorushina VV, et al. Comparative analysis of secretome of human umbilical cord- and bone marrow-derived multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2019;166:535–540. doi: 10.1007/s10517-019-04388-1. [DOI] [PubMed] [Google Scholar]
- 48.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]