Abstract
Purpose
Unstructured data are an untapped source for surgical prediction. Modern image analysis and machine learning (ML) can harness unstructured data in medical imaging. Incisional hernia (IH) is a pervasive surgical disease, well-suited for prediction using image analysis. Our objective was to identify optimal biomarkers (OBMs) from preoperative abdominopelvic computed tomography (CT) imaging which are most predictive of IH development.
Methods
Two hundred and twelve rigorously matched colorectal surgery patients at our institution were included. Preoperative abdominopelvic CT scans were segmented to derive linear, volumetric, intensity-based, and textural features. These features were analyzed to find a small subset of OBMs, which are maximally predictive of IH. Three ML classifiers (Ensemble Boosting, Random Forest, SVM) trained on these OBMs were used for prediction of IH.
Results
Altogether, 279 features were extracted from each CT scan. The most predictive OBMs found were: (1) abdominopelvic visceral adipose tissue (VAT) volume, normalized for height; (2) abdominopelvic skeletal muscle tissue volume, normalized for height; and (3) pelvic VAT volume to pelvic outer aspect of body wall skeletal musculature (OAM) volume ratio. Among ML prediction models, Ensemble Boosting produced the best performance with an AUC of 0.85, accuracy of 0.83, sensitivity of 0.86, and specificity of 0.81.
Conclusion
These OBMs suggest increased intra-abdominopelvic volume/pressure as the salient pathophysiologic driver and likely mechanism for IH formation. ML models using these OBMs are highly predictive for IH development. The next generation of surgical prediction will maximize the utility of unstructured data using advanced image analysis and ML.
Keywords: Unstructured data, Image analysis, Machine learning, Computed tomography, Incisional hernia
Introduction
Surgical complications are a major burden on patients and the health care system [1]. Generally speaking, current surgical prediction techniques are reliant on organized and easily interpretable information stored within Electronic Health Records (EHRs) [2–4], which we will refer to as structured data. However, these data are often limited in scope and may not be appropriate surrogates for underlying drivers of surgical outcomes. There are potentially revealing factors in unstructured data (e.g., medical images and clinical notes). In fact, it is estimated that 80% of healthcare data are of this latter form [5]. With the power of modern analysis techniques, we can now harness information from unstructured data. In particular, advanced image analysis and machine learning (ML) techniques allow us to systematically decipher medical images to improve understanding of surgical disease with potential implications for clinical practice [6–9]. Doing so can elucidate key proximate drivers for surgical outcomes related to a patient’s body habitus and improve surgical prediction.
Incisional hernia (IH) is a quintessential surgical complication amenable to exploration of unstructured data, as it is a common, long-term complication of abdominopelvic surgery that is incompletely understood [10–12]. IH is hallmarked by failed fascial healing resulting in protrusion of the abdominopelvic contents, leading to chronic pain, diminished physical function, poor mental health, and unsuitable appearance [13–17]. IH is a prevalent public health concern because it is estimated 1-in-8 abdominal surgery patients develop it [10, 12, 18–20]. Furthermore, nearly one-third of IH repairs fail, leading to a vicious cycle of disability and costs [15, 21, 22]. For surgeons to employ risk mitigation strategies, prediction models, such as the Penn Hernia Risk Calculator, have been developed using hundreds of structured data elements, yet have modest prediction ability [4, 23–25]. Features from preoperative medical imaging may elucidate proximate drivers of IH, enable deeper understand of its pathophysiology, and, thus, better prediction and prevention efforts.
We have developed methods to systematically derive image-based features from preoperative abdominopelvic computed tomography (CT) imaging of abdominal surgery patients [6, 7, 26]. Our recent pilot study identified some features which have discriminative capacity for IH [26]. The primary objective of this study is to adapt the optimal biomarker (OBM) method [8, 9] to identify key image-based features from preoperative abdominopelvic CT imaging with the best risk prediction abilities for IH. The second objective was to employ various ML classifiers to predict IH formation using these OBMs and compare performance.
Methods
Patient selection and image analysis
This study was approved by the Institutional Review Board (IRB) at the University of Pennsylvania (Protocol # 843,902). Informed consent was waived by the IRB due to the retrospective and deidentified nature of the study. Two hundred and twelve adult patients undergoing elective colorectal surgery at our institution between 2005 and 2016 were matched 1:1 for the outcome of developing IH. Patients were exactly matched on 10 preoperative characteristics important to IH risk: age (± 4 years), sex, race, ethnicity, smoking status, history of diabetes mellitus, pre-index ventral incisional hernia repair, pre-index abdominal surgery, index laparoscopic surgery, and index open surgery. Their preoperative CT scans were retrospectively obtained and evaluated for 107 linear and morphometric features as in our previous work [26]. The same CT images were now assessed additionally for textural and intensity-based features. These tissual properties were evaluated as mean, median, standard deviation, first quartile, third quartile, maximum, and minimum for various tissues in the abdomen, pelvis, and abdomen-pelvis. In aggregate, there were 84 texture-based features and 42 intensity-based features.
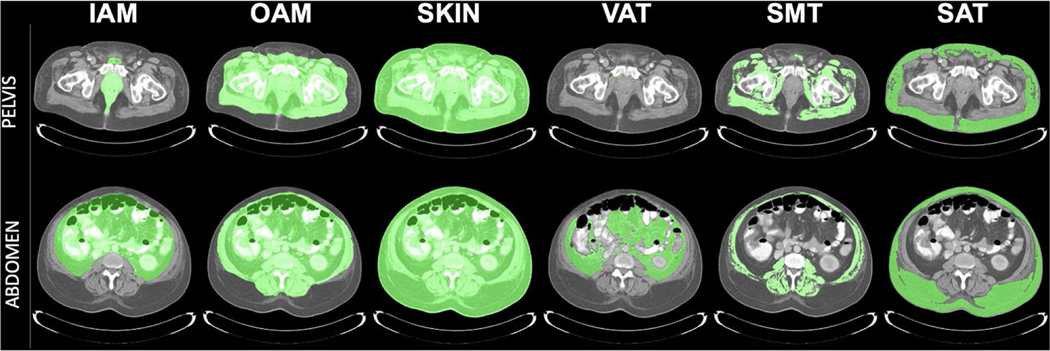
Proportional measurements between morphometric features were also considered as additional derived features, such as visceral adipose tissue (VAT) volume as a fraction of inner aspect of body wall skeletal musculature (IAM) volume within the abdomen, pelvis, and abdomen-pelvis. Examples of delineated regions on slices for which volumetric measurements are computed are depicted in Fig. 1 (adapted with permission from McAuliffe, et al.) [26]. In total, there were 44 ratio features considered. All previous and new features were included in the analysis which resulted in a total of 279 features.
Fig. 1.
CT-derived volumetric measurements of patient abdominopelvic body habitus. Delineations overlaid on CT slice displays show tissue regions from which volumetric measurement are derived. IAM inner aspect of the body wall skeletal musculature, OAM outer aspect of the body wall skeletal musculature, Skin skin and interior contents, VAT visceral adipose tissue, SMT skeletal muscle tissue, SAT subcutaneous adipose tissue. Adapted with permission from McAuliffe, et al. [26]
Optimal biomarker (OBM) methodology
A general Optimal Biomarker (OBM) methodology was previously developed by our team and validated in different applications to identify an optimal set of predictive features [8, 9, 27]. The OBM methodology finds a small subset from a large set of features that are both discriminative for the outcome of interest and independent from other features. In the current study, this meant finding features which (i) had a low magnitude of correlation (r ≤ 0.5) with most of the other features in the given set; (ii) were maximally discriminative in separating patients who developed IH from those who did not, as determined by p value; and (iii) which provided the best prediction accuracy when used with all other features of the subset in a ML prediction model. While there were 279 features identified per CT, there are no known practical methods to find the best subset among 2279− 1 possible subsets. Therefore, we instead identified the subset of the most potent six features as an approximate solution [8]. In addition, in this work, we modified the original OBM method to handle data given as matched pairs. The details of the implementation are as follows.
Our matched cohort of 212 patients (106 pairs) was randomly partitioned into a 128:42:42 training:validating:testing split wherein pairing was maintained, with the ratio for pairs being 64:21:21. The training set was used to determine the top six most potent among 279 features following the aforementioned OBM methodology such that the features are (i) least correlated and (ii) most discriminative.
Once the top six most potent features were identified, an ensemble boosting ML classifier was then trained on the training data set separately for each subset among the 26− 1 = 63 possible subsets of the six-feature set. The resulting 63 models were tested on the validation data set for predicting IH formation. The subset of features with the highest prediction accuracy on the validation data set was considered to represent the OBMs.
Finally, three ML classifiers—support vector machine (SVM), random forest, and ensemble boosting—were trained on the training + validation data sets (170 scans or 85 pairs) for the OBMs found for final predictive performance evaluation on the remaining 42 scans (21 pairs) set aside solely for testing [28–30].
To handle paired data effectively, the feature values were transformed from the measured values to the difference between the measured value and the mean of its pair. In mathematical terms, for each feature f among the 279 features, let its paired values be (a, b), where a and b denote the values of f for the Hernia and no-Hernia groups, respectively. (a, b) is modified to the pair (a’, b’) where a’ = a − (a + b)/2 and b’ = b − (a + b)/2. In other words, a’ and b’ denote the signed distance of a and b, respectively, from the mean. These transformed values were used for training, validation, and testing experiments.
Results
The OBM method revealed that the most potent CT features for identifying IH are all morphometric in nature: abdominopelvic VAT volume normalized for abdominopelvic height, abdominopelvic IAM volume normalized for abdominopelvic height, pelvic VAT volume normalized for pelvic height, abdominopelvic IAM/OAM volume ratio, pelvic VAT/OAM volume ratio, and pelvic IAM volume normalized for pelvic height. Table 1 lists these features and their summary statistics for the two groups.
Table 1.
Top six most potent features and their mean and standard deviation for the two classes for prediction of incisional hernia
| Top six potent feature | No hernia [mean (SD)] | Hernia [mean (SD)] | p-value |
|---|---|---|---|
| AbdPlvs VAT vol normalized (m3) | 0.06 (0.04) | 0.08 (0.04) | 0.02 |
| AbdPlvs IAM vol normalized (m3) | 0.18 (0.05) | 0.19 (0.05) | 0.03 |
| Plvs VAT vol normalized (m3) | 0.11 (0.06) | 0.13 (0.07) | 0.01 |
| AbdPlvs IAM/OAM | 0.46 (0.05) | 0.48 (0.06) | 0.02 |
| Plvs VAT/OAM | 0.12 (0.06) | 0.14 (0.06) | 0.02 |
| Plvs IAM vol normalized (m3) | 0.29 (0.08) | 0.32 (0.08) | 0.01 |
Abd Abdomen, Plvs pelvis, AbdPlvs abdomen-pelvis, VAT visceral adipose tissue, IAM inner aspect of body wall skeletal musculature, OAM outer aspect of body wall skeletal musculature, Vol volume, Normalized normalized to body region height, SD standard deviation
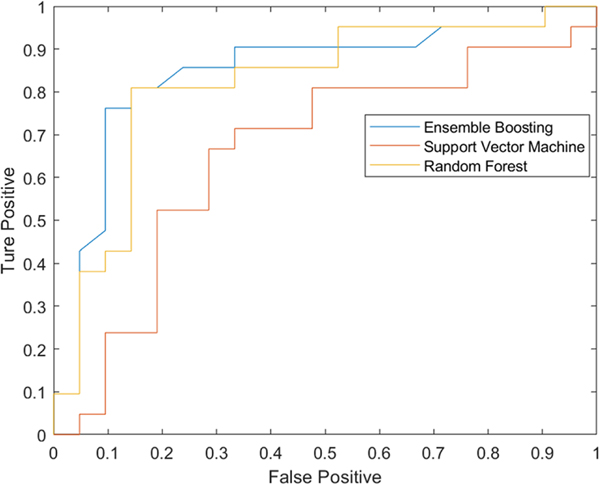
The six most potent feature biomarkers are all related to three fundamental volumes of the lower abdomen and pelvis: VAT, IAM, and OAM volumes. Figure 2 shows three-dimensional depictions of the surfaces of these volumes from CT images in two matched-pair of subjects, one of whom developed IH and the other who did not. Videos 1 and 2 are complete views of these three-dimensional renditions of key body volumes. The validation stage for the best ensemble boosting classifier is depicted in Table 2, where the best subset found with 1, 2, …, 6 features is shown along with the corresponding accuracy, sensitivity, specificity, and AUC metrics. The best subset included three features (i.e., OBMs): abdominopelvic VAT volume normalized for abdominopelvic height, abdominopelvic IAM volume normalized for abdominopelvic height, and pelvic VAT/OAM volume ratio. These OBMs from the training and validation sets were used to train the three ML classifiers, with ROC curves for prediction on the testing set shown in Fig. 3. The accuracy, sensitivity, specificity, and AUC of the classifiers are listed in Table 3. The ensemble boosting classifier yielded the best performance across all prediction metrics for the paired approach.
Fig. 2.
Optimal volumetric features for prediction of incisional hernia (IH), depicted from a sample of matched patients as 3D rendered images of the surfaces of the volumetric objects. VAT visceral adipose tissue, OAM outer aspect of the body wall skeletal musculature, IAM inner aspect of the body wall skeletal musculature
Table 2.
Performance of best combinations of top features using an ensemble classifier on the validation cohort for prediction of incisional hernia
| The number of features in best subsets | Index of features in best subsets | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| 1 | 19 | 0.69 | 0.62 | 0.76 | 0.79 |
| 2 | 19, 107 | 0.81 | 0.81 | 0.81 | 0.85 |
| 3 | 109, 19, 120 | 0.83 | 0.86 | 0.81 | 0.85 |
| 4 | 109, 19, 107, 120 | 0.83 | 0.86 | 0.81 | 0.84 |
| 5 | 109, 19, 107, 112, 120 | 0.81 | 0.81 | 0.81 | 0.82 |
| 6 | 109, 19, 107, 112, 120, 1 3 | 0.67 | 0.71 | 0.62 | 0.73 |
The optimal biomarkers are those in the best performing subset of three, as this subset has the best accuracy, sensitivity, specificity, and AUC (bolded)
Feature 19–AbdPelvis IAM volume norm, Feature 107–Pelvis VAT volume norm, Feature 109–AbdPelvis VAT volume norm, Feature 120–Pelvis VAT/OAM volume ratio, Feature 112–AbdPelvis IAM/OAM volume ratio, Feature 13–Pelvis IAM volume norm
Abd Abdomen, Plvs pelvis, AbdPlvs abdomen-pelvis, VAT visceral adipose tissue, IAM inner aspect of body wall skeletal musculature, OAM outer aspect of body wall skeletal musculature, Vol volume, Norm normalized to body region height, AUC area under the curve
Fig. 3.
Receiver operating characteristic (ROC) curves of three machine learning models trained using the best combination of optimal biomarkers for prediction of incisional hernia. SVM support vector machine, AUC area under the curve
Table 3.
Prediction of incisional hernia using combined three optimal biomarkers, using three machine learning classifiers: ensemble boosting, random forest, SVM
| Classifier | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| Ensemble boosting | 0.83 | 0.86 | 0.81 | 0.85 |
| Random forest | 0.79 | 0.76 | 0.81 | 0.83 |
| SVM | 0.67 | 0.67 | 0.67 | 0.68 |
The ensemble boosting classifier model had the best performance in terms of accuracy, sensitivity, specificity, and AUC (bolded)
SVM Support vector machine, AUC area under the curve
Discussion
This study demonstrates that morphometric image-based features may denote key drivers of surgical risk and IH formation. Moreover, these optimal features characterize a singular physiologic domain, increased intra-abdominopelvic volume, which likely represents a more proximate pathophysiologic driver of IH. While results are preliminary, the study finds that ML modeling of IH using these optimal image-based features is not only possible, but also can predict IH formation with excellent accuracy, albeit in a limited cohort. The results further suggest that image-based prediction pairing OBMs with ML modeling are crucial in augmenting our understanding and management of surgical outcomes. Such features must be validated in a larger cohort and can eventually be integrated to build a comprehensive risk model using healthcare datasets of both structured and imaging data elements.
Our previous work determined that biometric features discriminative for IH formation fell into three pathophysiologic domains: widening of the rectus complex and linea alba, increased visceral volume, and atrophy of the abdominopelvic wall skeletal musculature [26]. The current study significantly expands upon this pilot work by finding the six most optimal biomarkers from CT image analysis. All six features characterize a single physiologic domain: increased intra-abdominopelvic volume. This suggests that increased intra-abdominopelvic volume is a critical pathophysiologic mechanism for IH formation. Previous literature agrees with this notion as it has been suggested that visceral obesity is a mechanistic driver of IH formation [31–33].
Further investigation must be done to mechanistically connect increased visceral volume with IH. We propose two potential explanations. The first is that increased visceral adiposity may contribute to IH directly through molecular mechanisms of wound healing. Abdominal adipocyte hypertrophy typical of obesity is coupled with inadequate vascularization [34]. The resulting hypoxia can delay wound healing and lead to a pro-fibrotic environment. This dovetails well with existing literature establishing impaired wound healing as mechanistically significant in IH pathogenesis. Altered collagen subtypes and fibroblast phenotypes, resulting in altered expression of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs), lead to disorganization of the wound extracellular matrix [35–37]. This results in impaired fascial healing and decreased integrity of the abdominal wall. Another possible explanation is that the optimal biomarkers indicating increased intra-abdominopelvic volume may actually be a proxy for increased pressure, possibly an even more proximate driver of IH. Such a mechanical stimulus may affect fibroblast homeostasis during the fibroproliferative phase of wound healing [35]. In an analogous finding, Besancenot et al. recently identified chronic obstructive pulmonary disease (COPD) as a risk factor for IH, again possibly due to intrathoracic air trapping referring increased pressure in the abdomen [38]. This increased intra-abdominopelvic pressure may also cause early micro-gapping and failed fascial healing. More exploration is needed to better elucidate the mechanistic pathways, to identify whether it is intra-abdominopelvic volume or pressure that is the more proximate driving force for IH, and whether there are ways to mitigate this biomechanical tendency peri-operatively to prevent IH.
The current study found ensemble boosting to be the most accurate ML classifier for predicting IH using image-based features. Ensemble methods are a machine learning technique which iterate multiple ML algorithms over datasets, evaluate each base model, and combine the base models into a larger ensemble model to perform more effective classification [39–42]. Indeed, the results find that ensemble boosting outperforms other ML methods, such as random forests and SVMs. The best risk model derived in this study using just three image-based features, with an AUC of 0.85, compares favorably with the Penn Hernia Calculator, a model using on hundreds of clinical data points derived from thousands of patients, with an AUC of 0.83 [4]. While the comparison is not entirely fungible given the stringent criteria and preliminary nature of the current study, it demonstrates the potential of harnessing image-based data in surgical risk prediction. This study provides the groundwork for future image analysis to employ ensemble boosting methods with other image-based features.
This study creates high-performing predictive models using exclusively image-based features. However, the results are still very preliminary for ultimate clinical translation. This study uses a small, matched cohort of patients from a single academic institution, which may confound signals of potentially important image-based features. Future work must be done using a larger cohort of surgical patients with preoperative imaging to better establish the OBMs. With greater signal, it is possible that other features related to morphometry, intensity, and texture from CT imaging may become more important in predicting IH. After establishing in a larger cohort, the OBMs must be externally validated across surgical specialties and centers. In this future work, other types of machine learning models, which take advantage of ensemble methods, should be developed using these OBMs to compare their performance in risk prediction [43]. For clinical translation, this line of work must improve IH risk prediction and mitigation ability. To that end, OBMs will need to be routinely analyzed from preoperative imaging. Other sources of unstructured data, such as clinical documentation or genetic profile, should also be analyzed to reveal even more potential features. The ultimate goal is to build an integrated, multi-modal risk model using multidimensional healthcare data sets constructed of structured and unstructured clinical data from the EHR. Eventually, such a model will facilitate development of a point-of-care tool for surgeons to preoperatively assess patient-specific risk of IH formation and guide care recommendations.
Conclusion
Imaging data are a powerful, untapped source for studying surgical outcomes. This study applied advanced image analysis on preoperative CT images to identify optimal biomarkers (OBMs) predictive of incisional hernia (IH) formation. These OBMs suggest increased abdominopelvic volume is a salient pathophysiologic mechanism for IH. Machine learning models trained on these OBMs can provide high-performing risk prediction for IH. The next generation of surgical prediction will maximize the information stored in EHRs in a framework that incorporates both structured and medical imaging healthcare data.
Supplementary Material
Acknowledgements
JPF has full access to all the data in the study and takes responsibility for the integrity and accuracy of the data analysis.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (Award Number UL1TR001878); the Institute for Translational Medicine and Therapeutics’ (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics Grant; and by The Plastic Surgery Foundation. This funding support was not associated with any role in the study design, writing, and submission of this manuscript.
Abbreviations
- CT
C omputed tomography
- EHR
Electronic health record
- IAM
Inner aspect of body wall skeletal musculature
- IH
I ncisional hernia
- OAM
Outer aspect of body wall skeletal musculature
- OBM
O ptimal biomarker
- MIPG
Medical image processing group
- ML
Machine learning
- SVM
Support vector machine
- VAT
Visceral adipose tissue
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10029-023-02835-7.
Declarations
Conflict of interest JKU and DAT are co-founders of Quantitative Radiology Solutions, LLC. JPF has received funding from 3 M, Becton Dickinson, Integra, Gore, and Allergan for speaking and teaching, honoraria, and consulting fees. The other authors have no other conflicts of interest.
Ethical approval and Informed consent This study was approved by the Institutional Review Board (IRB) at the University of Pennsylvania (Protocol # 843,902). Informed consent was waived by the IRB due to the retrospective and deidentified nature of the study.
Human and animal rights statement The ethics committee and review board at our institution approved the study protocol.
Data availability
Not applicable.
References
- 1.Stokes SM, Scaife CL, Brooke BS, Glasgow RE, Mulvihill SJ, Finlayson SRG, Varghese TK (2022) Hospital costs following surgical complications: a value-driven outcomes analysis of cost savings due to complication prevention. Ann Surg 275:e375–e381. 10.1097/SLA.0000000000004243 [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 217(833–842):e1–3. 10.1016/j.jamcollsurg.2013.07.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, Lipori G, Hogan WR, Efron PA, Moore F, Moldawer LL, Wang DZ, Hobson CE, Rashidi P, Li X, Momcilovic P (2019) MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg 269:652–662. 10.1097/SLA.0000000000002706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basta MN, Kozak GM, Broach RB, Messa CA, Rhemtulla I, Dematteo RP, Serletti JM, Fischer JP (2019) Can we predict incisional hernia?: Development of a surgery-specific decision-support interface. Ann Surg. 10.1097/SLA.0000000000003472 [DOI] [PubMed] [Google Scholar]
- 5.Kong H-J (2019) Managing unstructured big data in healthcare system. Healthc Inform Res 25:1–2. 10.4258/hir.2019.25.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grevera G, Udupa J, Odhner D, Zhuge Y, Souza A, Iwanaga T, Mishra S (2007) CAVASS: a computer-assisted visualization and analysis software system. J Digit Imaging 20:101. 10.1007/s10278-007-9060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Pan J, Torigian DA, Xu P, Miao Q, Tong Y, Udupa JK (2020) ABCNet: a new efficient 3D dense-structure network for segmentation and analysis of body tissue composition on body-torso-wide CT images. Med Phys 47:2986–2999. 10.1002/mp.14141 [DOI] [PubMed] [Google Scholar]
- 8.Tong Y, Udupa JK, Sin S, Liu Z, Wileyto EP, Torigian DA, Arens R (2016) MR image analytics to characterize the upper airway structure in obese children with obstructive sleep apnea syndrome. PLoS ONE 11:e0159327. 10.1371/journal.pone.0159327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong Y, Udupa JK, Wang C, Chen J, Venigalla S, Guzzo TJ, Mamtani R, Baumann BC, Christodouleas JP, Torigian DA (2018) Radiomics-guided therapy for bladder cancer: using an optimal biomarker approach to determine extent of bladder cancer invasion from t2-weighted magnetic resonance images. Adv Radiat Oncol 3:331–338. 10.1016/j.adro.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 10.1007/s10029-011-0879-9 [DOI] [PubMed] [Google Scholar]
- 11.Le Huu NR, Mege D, Ouaïssi M, Sielezneff I, Sastre B (2012) Incidence and prevention of ventral incisional hernia. J Visc Surg 149:e3–14. 10.1016/j.jviscsurg.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Carney MJ, Weissler JM, Fox JP, Tecce MG, Hsu JY, Fischer JP (2017) Trends in open abdominal surgery in the United States—observations from 9,950,759 discharges using the 2009–2013 National Inpatient Sample (NIS) datasets. Am J Surg. 10.1016/j.amjsurg.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Jensen KK, Munim K, Kjaer M, Jorgensen LN (2017) Abdominal wall reconstruction for incisional hernia optimizes truncal function and quality of life: a prospective controlled study. Ann Surg 265:1235–1240. 10.1097/sla.0000000000001827 [DOI] [PubMed] [Google Scholar]
- 14.Van Ramshorst GH, Eker HH, Hop WCJ, Jeekel J, Lange JF (2012) Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg. 10.1016/j.amjsurg.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 15.Holihan JL, Alawadi Z, Martindale RG, Roth JS, Wray CJ, Ko TC, Kao LS, Liang MK (2015) Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg 221:478–485. 10.1016/j.jamcollsurg.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 16.Mauch JT, Enriquez FA, Shea JA, Barg FK, Rhemtulla IA, Broach RB, Thrippleton SL, Fischer JP (2020) The abdominal hernia-Q: development, psychometric evaluation, and prospective testing. Ann Surg. 10.1097/SLA.0000000000003144 [DOI] [PubMed] [Google Scholar]
- 17.Patel V, Cunning JR, Rios-Diaz AJ, Mauch JT, Nathan SL, Messa CA, Whitely CB, Kozak GM, Broach RB, Fischer JP (2020) Prospective assessment of the abdominal hernia-Q (AHQ)-patient burden, reliability, and longitudinal assessment of quality of life in hernia repair. Ann Surg. 10.1097/SLA.0000000000004713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosanquet DC, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A, Davies L, Glasbey JCD, Frewer KA, Frewer NC, Russell D, Russell I, Torkington J (2015) Systematic review and meta-regression of factors affecting midline Incisional hernia rates: analysis of 14,618 Patients. PLoS ONE. 10.1371/journal.pone.0138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brosi P, Glauser PM, Speich B, Käser SA, Maurer CA (2018) Prophylactic intraperitoneal onlay mesh reinforcement reduces the risk of incisional hernia, two-year results of a randomized clinical trial. World J Surg 42:1687–1694. 10.1007/s00268-017-4363-2 [DOI] [PubMed] [Google Scholar]
- 20.Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM (2010) Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 10.1097/SLA.0b013e3181d973e4 [DOI] [PubMed] [Google Scholar]
- 21.Shubinets V, Carney MJ, Colen DL, Mirzabeigi MN, Weissler JM, Lanni MA, Braslow BM, Fischer JP, Kovach SJ (2018) Management of infected mesh after abdominal hernia repair: systematic review and single-institution experience. Ann Plast Surg 80:145–153. 10.1097/SAP.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman RD, Danos DM, Lau FH (2021) National health disparities in incisional hernia repair outcomes: an analysis of the healthcare cost and utilization project national inpatient sample (HCUP-NIS) 2012–2014. Surgery 169:1393–1399. 10.1016/j.surg.2020.11.028 [DOI] [PubMed] [Google Scholar]
- 23.Fischer JP, Basta MN, Mirzabeigi MN, Bauder AR, Fox JP, Drebin JA, Serletti JM, Kovach SJ (2016) A risk model and cost analysis of incisional hernia after elective abdominal surgery based on 12,373 cases. The case for targeted prophylactic intervention. Ann Surg. 10.1097/SLA.0000000000001394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissler JM, Lanni MA, Hsu JY, Tecce MG, Carney MJ, Kelz RR, Fox JP, Fischer JP (2017) Development of a clinically actionable incisional hernia risk model after colectomy using the healthcare cost and utilization project. J Am Coll Surg 225:274–284.e1. 10.1016/j.jamcollsurg.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Tecce MG, Basta MN, Shubinets V, Lanni MA, Mirzabeigi MN, Cooney L, Senapati S, Haggerty AF, Weissler JM, Hernandez JA, Fischer JP (2017) A risk model and cost analysis of postoperative incisional hernia following 2,145 open hysterectomies-defining indications and opportunities for risk reduction. Am J Surg 213:1083–1090. 10.1016/j.amjsurg.2016.09.047 [DOI] [PubMed] [Google Scholar]
- 26.McAuliffe PB, Desai AA, Talwar AA, Broach RB, Hsu JY, Serletti JM, Liu T, Tong Y, Udupa JK, Torigian DA, Fischer JP (2022) Preoperative computed tomography morphological features indicative of incisional hernia formation after abdominal surgery. Ann Surg. 10.1097/SLA.0000000000005583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson MR, Easthausen I, Gallagher G, Udupa J, Tong Y, Torigian D, Diamond JM, Porteous MK, Palmer SM, Snyder LD, Benvenuto L, Aversa M, Arcasoy S, Greenland JR, Hays SR, Kukreja J, Cantu E, Kim JS, Gallagher D, Baldwin MR, Barr RG, Lederer DJ, Christie JD, Singer JP (2020) Skeletal muscle adiposity and outcomes in candidates for lung transplantation: a lung transplant body composition cohort study. Thorax 75:801–804. 10.1136/thoraxjnl-2019-214461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cristianini N, Shawe-Taylor J (2000) An introduction to support vector machines and other kernel-based learning methods. Cambridge University Press, Cambridge [Google Scholar]
- 29.Breiman L (2001) Random forests. Mach Learn 45:5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 30.Freund Y, Schapire RE (1997) A decision-theoretic generalization of on-line learning and an application to boosting. J Comput Syst Sci 55:119–139. 10.1006/jcss.1997.1504 [DOI] [Google Scholar]
- 31.Yamamoto M, Takakura Y, Ikeda S, Itamoto T, Urushihara T, Egi H (2018) Visceral obesity is a significant risk factor for incisional hernia after laparoscopic colorectal surgery: a single-center review. Asian J Endosc Surg. 10.1111/ases.12466 [DOI] [PubMed] [Google Scholar]
- 32.Aquina CT, Rickles AS, Probst CP, Kelly KN, Deeb A-P, Monson JRT, Fleming FJ, Muscle and Adiposity Research Consortium (MARC) (2015) Visceral obesity, not elevated BMI, is strongly associated with incisional hernia after colorectal surgery. Dis Colon Rectum 58:220–227. 10.1097/DCR.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 33.Baastrup NN, Jensen KK, Christensen JK, Jorgensen LN (2022) Visceral obesity is a predictor of surgical site occurrence and hernia recurrence after open abdominal wall reconstruction. Hernia 26:149–155. 10.1007/s10029-021-02522-5 [DOI] [PubMed] [Google Scholar]
- 34.Corvera S, Solivan-Rivera J, Yang Loureiro Z (2022) Angiogenesis in adipose tissue and obesity. Angiogenesis 25:439–453. 10.1007/s10456-022-09848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thankam FG, Palanikumar G, Fitzgibbons RJ, Agrawal DK (2019) Molecular mechanisms and potential therapeutic targets in incisional hernia. J Surg Res 236:134–143. 10.1016/j.jss.2018.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reilly MJ, Larsen NK, Agrawal S, Thankam FG, Agrawal DK, Fitzgibbons RJ (2021) Selected conditions associated with an increased incidence of incisional hernia: a review of molecular biology. Am J Surg 221:942–949. 10.1016/j.amjsurg.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 37.Durukan U, Agcaoglu O, Ozoran E, Karahan SN, Ozata I, Duzkoylu Y, Pasaoglu E, Aren A (2022) The role of tissue inhibitor of metalloproteinases in the aetiology of inguinal and incisional hernias. Int Wound J 19:1502–1508. 10.1111/iwj.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besancenot A, du Mont LS, Lejay A, Heranney J, Delay C, Chakfé N, Rinckenbach S, Thaveau F (2022) Risk factors of long-term incisional hernia after open surgery for abdominal aortic aneurysm: a bicentric study. Ann Vasc Surg 83:62–69. 10.1016/j.avsg.2021.10.074 [DOI] [PubMed] [Google Scholar]
- 39.Maclin R, Opitz D (1999) Popular ensemble methods: an empirical study. Jair 11:169–198. 10.1613/jair.614 [DOI] [Google Scholar]
- 40.Zhou Z-H (2012) Ensemble methods: foundations and algorithms. CRC Press, Boca Raton [Google Scholar]
- 41.Hosni M, Abnane I, Idri A, Carrillo de Gea JM, Fernández Alemán JL (2019) Reviewing ensemble classification methods in breast cancer. Comput Methods Programs Biomed 177:89–112. 10.1016/j.cmpb.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 42.Chandra A, Yao X (2006) Ensemble learning using multi-objective evolutionary algorithms. J Math Model Algor 5:417–445. 10.1007/s10852-005-9020-3 [DOI] [Google Scholar]
- 43.Chatzimparmpas A, Martins RM, Kucher K, Kerren A (2021) StackGenVis: alignment of data, algorithms, and models for stacking ensemble learning using performance metrics. IEEE Trans Visual Comput Graphics 27:1547–1557. 10.1109/TVCG.2020.3030352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.