OVERVIEW
Renal cell carcinoma (RCC) is one of the 10 most commonly diagnosed solid tumors. Most RCCs are histologically defined as clear cell, comprising approximately 75% of diagnoses. However, the remaining RCC cases are composed of a heterogeneous combination of diverse histopathologic subtypes, each with unique pathogeneses and clinical features. Although the therapeutic approach to both localized and metastatic RCCs has dramatically changed, first with the advent of antiangiogenic targeted therapies and more recently with the approval of immune checkpoint inhibitor (ICI)–based combinations, these advances have primarily benefited the clear cell RCC patient population. As such, there remains critical gaps in the optimization of treatment regimens for patients with non–clear cell, or variant, RCC histologies. Herein, we detail recent advances in understanding the biology of RCC with variant histology and how such findings have guided novel clinical studies investigating precision oncology approaches for these rare subtypes. Among the most common variant histology RCCs are papillary RCC, comprising approximately 15%-20% of all diagnoses. Although a histopathologically diverse subset of tumors, papillary RCC is canonically associated with amplification of the MET protooncogene; recently completed and ongoing trials have investigated MET-directed therapies for this patient population. Finally, we discuss the unique biology of RCC with sarcomatoid dedifferentiation and the recent clinical findings detailing its paradoxical sensitivity to ICIs.
INTRODUCTION
Renal cell carcinoma (RCC) is among the 10 most common cancer diagnoses in the United States, with over 80,000 new cases expected to be diagnosed in 2024 alone.1 RCCs are subclassified on the basis of histopathologic features: clear cell RCC (ccRCC) represents the most common subtype, accounting for approximately 75% of diagnoses. ccRCC is typically defined by nests of PAX8+, CAIX+ dysplastic cells with clear lipid-laden cytoplasm, and canonically arises secondary to loss of chromosome 3p containing the VHL tumor suppressor gene.2,3 However, while ccRCC is the major RCC subtype, various histologically and biologically unique subtypes, termed variant histologies, exist (Fig 1). These variant histologies include papillary RCC (approximately 15% of diagnoses), chromophobe RCC (approximately 5% of cases), and multiple other subtypes such as collecting duct carcinoma, translocation RCC, renal medullary carcinoma, and mucinous tubular and spindle cell carcinoma of the kidney. Furthermore, sarcomatoid and rhabdoid features present an additional class of histologic features that can arise from any of the above-stated subtypes and which carry their own unique biology and clinical challenges.4
FIG 1.
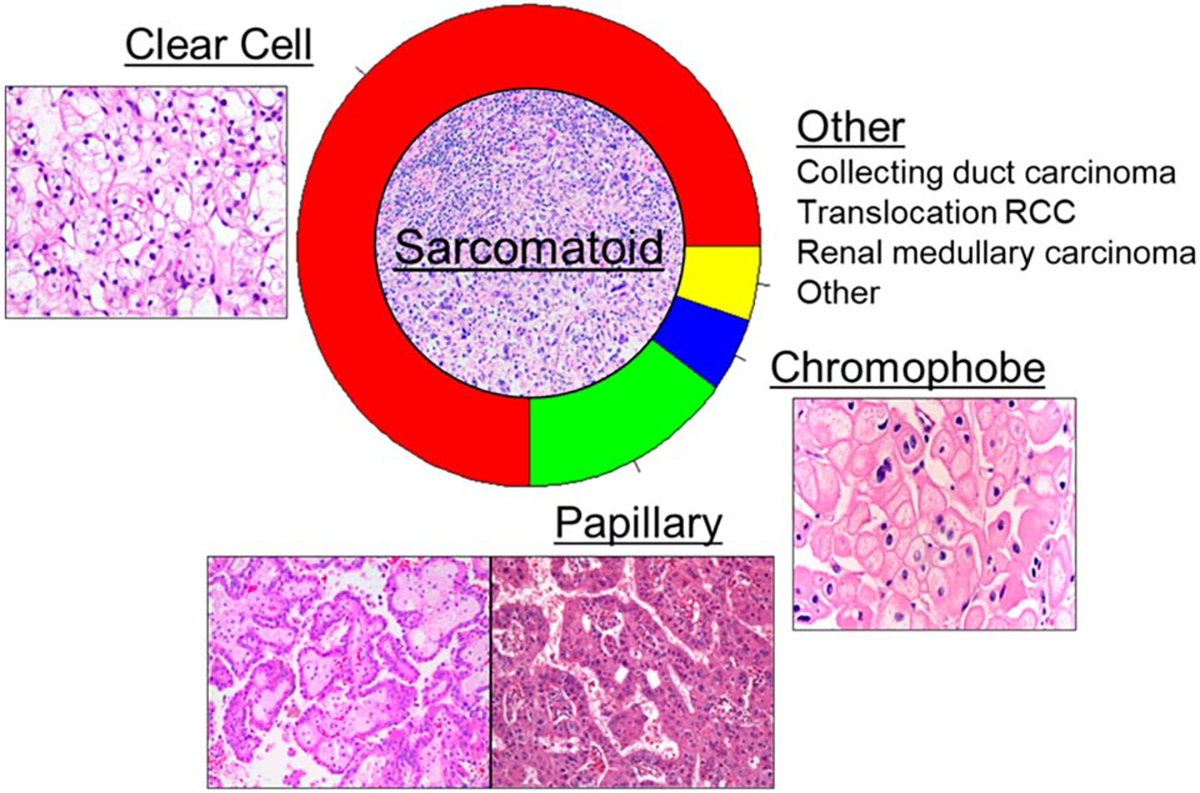
Overview and relative proportion of histologic subtypes comprising RCC diagnoses. Pathology images retrieved from pathologyoutlines.com. RCC, renal cell carcinoma.
The treatment paradigm for metastatic RCC is rapidly evolving, having undergone three distinctive eras within the past 25 years. RCC was among the few solid tumor types to exhibit responsiveness to cytokine therapies, and resultingly, systemic interleukin-2 and interferon-α were approved for use in metastatic RCC.5–7 However, these agents were limited by a poor efficacy-to-toxicity ratio, with durable responses noted in <10% of patients and median overall survival (OS) plateauing at approximately 1 year. Beginning with the approval of sorafenib in 2005, agents targeting vascular endothelial growth factor (VEGF) and the VEGF receptor (VEGFR) became the front-line therapies of choice for metastatic RCC. These agents include bevacizumab, sunitinib, axitinib, pazopanib, lenvatinib, cabozantinib, and tivozanib.8–13 Additional small molecule inhibitors of the mTOR signaling cascade—everolimus and temsirolimus—were also approved for metastatic RCC during this period.14,15 More recently, RCC has benefitted from the advent of immune checkpoint inhibitors (ICIs). Initial approvals for ICI therapy in RCC came after the successful CheckMate-025 trial comparing nivolumab, an ICI agent targeting the PD-1 checkpoint molecule on T cells, to everolimus in patients with metastatic RCC refractory to previous systemic therapy.16 This accelerated study of ICIs in the frontline treatment setting and has resulted in the approval of multiple ICI-based combinations for metastatic RCC, including nivolumab with ipilimumab (an ICI targeting the cytotoxic T-cell lymphocyte-4 checkpoint molecule), axitinib with avelumab (an inhibitor of the PD-L1 checkpoint), axitinib with pembrolizumab (anti–PD-1), lenvatinib with pembrolizumab, and cabozantinib with nivolumab.17–21
Intriguingly, these approved agents have been developed and received approval on the basis of their activity in ccRCC, yet have historically been used in a histology-agnostic fashion for the treatment of non-ccRCC as well, with limited success. Therefore, developing novel strategies for the treatment of variant histology RCCs has been a paramount need. Herein, we detail the ongoing efforts using precision oncology approaches to address the unique biology of papillary and sarcomatoid RCC.
PAPILLARY RCC
Histology and Biology
Papillary RCC (pRCC) is the most common non-ccRCC, accounting for approximately 15% of all RCC diagnoses. Historically, pRCC has been divided into two subtypes on the basis of cytoarchitecture: type 1 pRCC and type 2 pRCC. Type 1 pRCC is identified by papillary cores comprising small cuboidal cells with or without psammoma bodies, and often stains positive for CK7 and MUC1.22 Genomic alterations in the MET oncogene have been identified in at least one third of patients with type 1 pRCC.23 Additional work also highlighted MET copy-number variations in over 80% of patients with type 1 pRCC, yielding a potentially actionable target for precision oncology strategies in the clinical setting.24 Additional alterations described in type 1 pRCC include TERT-promoter variants and CDKN2A/2B mutations.
Type 2 pRCC is a more heterogeneous classification, and recent genomic interrogations have revealed two distinct subtypes within type 2 pRCC distinguished by differences in copy-number alterations.25 Histologically, type 2 pRCC presents with large, pseudostratified, eosinophilic cells, and typically bears higher nuclear grades. Like type 1 pRCC, TERT-promoter and CDKN2A/B alterations are common; however, type 2 pRCC does not harbor MET alterations to the same degree as type 1 pRCC.23
In the most recent WHO classification, the designation of type 1 and type 2 disease has been abandoned in favor of two main subcategories of papillary renal tumors—renal papillary adenoma and papillary RCC.26 Prominent in these new guidelines are molecularly characterized subsets of RCC including TFE3-rearranged RCC, TFEB-altered RCC, FH-deficient RCC, ALK-rearranged RCC, and so on. It is of note that several of these molecular subtypes may coalesce with papillary RCC—for instance, our group has defined a series of patients with pRCC bearing ALK mutation.27 The new designation schema underscores the importance of molecular classification of patients with rare histologies of RCC.
VEGF-/VEGFR- and mTOR-Directed Targeted Therapies
Given the numerous approvals of VEGF- and mTOR-directed therapies in RCC, multiple targeted therapy agents were trialed in pRCC patient cohorts. Among the initial endeavors was the single-arm RAPTOR trial (ClinicalTrials.gov identifier: NCT00688753) of everolimus as first-line therapy in patients with pRCC. 28 RAPTOR enrolled 88 patients with a median progression-free survival (PFS) of 4.1 months and median OS of 21.4 months reported. The SUPAP trial (ClinicalTrials.gov identifier: NCT00541008) was similarly structured, investigating sunitinib in treatment-naïve patients with metastatic pRCC in a nonrandomized fashion.29 Median PFS with sunitinib was 6.6 months for patients with type 1 pRCC and 5.5 months for those with type 2 pRCC. OS outcomes for type 1 and type 2 pRCC were 17.8 and 12.4 months, respectively.
The ESPN (ClinicalTrials.gov identifier: NCT01185366) and ASPEN (ClinicalTrials.gov identifier: NCT01108445) trials randomly assigned patients with metastatic non-ccRCC to receive sunitinib or everolimus in the frontline setting.30,31 ESPN was structured to allow for crossover at the time of disease progression. Notably, ESPN and ASPEN both allowed for various non-ccRCC histologies, including pRCC, chromophobe RCC, and unclassified RCC. In total, 27 (25%) and 70 patients (65%) with pRCC were enrolled in ESPN and ASPEN, respectively. There was no significant difference in median PFS in the ESPN intention-to-treat population (6.1 months with sunitinib; 4.1 months with everolimus; P = .60). Similarly, no significant difference in median PFS between sunitinib and everolimus was observed in ASPEN (8.3 months with sunitinib; 5.6 months with everolimus; P = .16). Within pRCC subgroups of both ESPN and ASPEN, PFS trends were in line with the full study cohorts, with median PFS of 5.7 months and 8.1 months with frontline sunitinib and median PFS of 4.1 months and 5.5 months with frontline everolimus in ESPN and ASPEN, respectively.
Further studies investigated targeted therapies beyond sunitinib and everolimus for pRCC. The AXIPAP trial (ClinicalTrials.gov identifier: NCT02489695) of axitinib as frontline therapy in 44 patients with pRCC yielded a median PFS of 6.6 months, while a trial of lenvatinib with everolimus in 31 patients with non-ccRCC (including 20 pRCC) reported an objective response rate of 15%, a median PFS of 9.2 months, and a median OS of 11.7 months.10,32
MET-Directed Targeted Therapies
The adoption of next-generation sequencing has allowed for the profiling of patient tumors to identify potentially actionable targets at both the individual level and disease type level. As detailed above, these protocols have revealed the common presence of alterations to the MET protooncogene, resulting in an increase in MET expression and signaling in pRCC. As such, recent trials have attempted to leverage this biology to improve patient outcomes in pRCC by directly targeting MET.
Among the first agents to be trialed was foretinib, a multikinase inhibitor targeting both MET and VEGFR, among other receptors. Foretinib was investigated in a phase II trial (ClinicalTrials.gov identifier: NCT00726323) for patients with pRCC who were either naïve to systemic treatment or received up to one previous line of therapy. In total, 74 patients were enrolled onto this nonrandomized trial, with a median PFS of 9.3 months reached. Germline sequencing was performed on the study population, and of note, five of 10 patients with germline mutations in MET had a clinical response to foretinib. This preceded the activation of SAVOIR (ClinicalTrials.gov identifier: NCT03091192), a phase III randomized trial of savolitinib (a MET tyrosine kinase inhibitor) compared against sunitinib.33 SAVOIR was designed as a biomarker-driven trial; inclusion criteria required MET amplification, MET tyrosine kinase domain mutation, hepatocyte growth factor (the cognate ligand for MET) alteration, or chromosome 7 gain (genetic loci of the MET gene) for enrollment. Unfortunately, SAVOIR was halted before completion because of a lack of efficacy signal, with only 60 patients enrolled at closure. In the enrolled population, savolitinib yielded a 27% objective response rate compared with only 7% for sunitinib.
Although SAVOIR did not result in meaningful PFS improvement with savolitinib, an additional randomized phase II study of MET inhibitors in pRCC was recently reported out. The PAPMET trial (ClinicalTrials.gov identifier: NCT02761057) initially randomly assigned patients to receive either (1) sunitinib, (2) cabozantinib, (3) crizotinib, or (4) savolitinib.34 Importantly, unlike the SAVOIR trial, PAPMET enrollment was agnostic to MET-biomarker status. The crizotinib and savolitinib arms were closed to randomization early after futility analysis at a prespecified interim analysis, whereas the sunitinib and cabozantinib arms remained open to accrual through the duration of the study. In total, 29 patients received crizotinib, 28 received savolitinib, 44 received cabozantinib, and 46 received sunitinib. Although both crizotinib and savolitinib demonstrated inferior PFS compared with sunitinib, cabozantinib yielded superior PFS (9.0 months v 5.6 months, P = .019). Cabozantinib also elicited an improved objective response rate relative to sunitinib (23% v 4%, P = .010). To date, PAPMET represents the only randomized trial showing a significant survival benefit for MET inhibition in pRCC relative to VEGFR inhibitors and thus promotes cabozantinib as the small molecule inhibitor of choice for patients with metastatic pRCC.
ICIs
Further efforts have emerged to explore the efficacy of ICI regimens in pRCC and other non–clear cell histologies. The CheckMate-374 trial (ClinicalTrials.gov identifier: NCT02596035) investigated the anti–PD-1 agent, nivolumab, as monotherapy for patients with metastatic non-ccRCC.35 Patients were allowed up to three previous lines of systemic therapy in CheckMate-374. Patients with pRCC represented 24 of 44 enrollees (55%) and accounted for two of the five reported partial responses on trial. Across the entire non-ccRCC cohort, median PFS was 2.2 months and median OS was 16.3 months. Cohort B of the phase II KEYNOTE-427 (ClinicalTrials.gov identifier: NCT02853344) study investigated pembrolizumab monotherapy in patients with non-ccRCC who, unlike in CheckMate-374, had not experienced previous systemic therapy for metastatic disease.36 Of the 165 total patients enrolled, 118 (72%) had pRCC. In subgroup analysis, patients with pRCC experienced an objective response rate of 28.8% and a disease control rate of 47.5%. The median PFS for the entire cohort was 4.2 months and the median OS was 28.9 months. The phase IIIb/IV CheckMate-920 (ClinicalTrials.gov identifier: NCT02982954) used a similar patient population, interrogating nivolumab plus ipilimumab in treatment-naïve metastatic pRCC.37 In total, 52 patients were enrolled, of whom 22 (42.3%) had pRCC. One patient with pRCC experienced a complete response and seven received a partial response. The median PFS across the full cohort was 3.7 months and the median OS was 21.2 months.
Combination ICIs and Primarily VEGF-Directed Therapies
The clinical activity of combinations with ICI and VEGF-directed therapies in ccRCC prompted exploration of these combinations in variant histology RCCs. Initial investigations in this space included testing bevacizumab in combination with atezolizumab (ClinicalTrials.gov identifier: NCT02724878) (anti–PD-L1 agent).38 In total, 12 (20%) patients with pRCC were enrolled, of whom three (25%) had an objective response. The median PFS across the full cohort was 8.3 months, while the median OS was not reached. A more recent single-arm trial with first-line pembrolizumab plus lenvatinib across non-ccRCC histologies, KEYNOTE-B61 (ClinicalTrials.gov identifier: NCT04704219), showed promising clinical outcomes.39 The majority of patients (59%) had pRCC, of whom 54% of patients were noted to have a confirmed objective response, including 8 (9%) patients with a complete response with lenvatinib/pembrolizumab. Notably, 33% of patients had a best response of stable disease. Although these results compare favorably with the other prospective trials in pRCC, interpreting activity of combinations with ICI and primarily VEGF-targeted agents in comparison with MET-directed approaches remains limited because of the single-arm nonrandomized fashion of these investigations.
Combining ICIs With MET-Directed Therapies
Leveraging the improved outcomes with MET-directed therapies and the promising results of ICIs in the pRCC population, combinations of ICI with MET-directed therapies have been an active area of investigation with a number of studies recently reported out and many underway (Table 1). Nivolumab was combined with cabozantinib (ClinicalTrials.gov identifier: NCT03635892) for patients with variant histology RCC who had received zero or one previous systemic agent and had not previously received an ICI.40 Cohort 1 of this study comprised patients with papillary, unclassified, or translocation RCC, resulting in 40 patients enrolled. The median PFS in this cohort was 12.5 months, with a median OS of 28 months.
TABLE 1.
Recently Completed and Ongoing Trials Combining MET Inhibitors With Immune Checkpoint Inhibitors
| Trial | Arms | No. of Patients With Papillary RCC | ORR | PFS | OS |
|---|---|---|---|---|---|
| NCT03635892 | Nivolumab + cabozantinib (ClinicalTrials.gov identifier: NCT03635892) | 32 | 47% | 12.5 monthsa | 28.0 monthsa |
| COSMIC-021 (ClinicalTrials.gov identifier: NCT03170960) | Atezolizumab + cabozantinib | 15 | 47% | 9.5 monthsa | Not reported |
| CALYPSO (ClinicalTrials.gov identifier: NCT02819596) | Durvalumab + savolitinib | 41 | 27% | 4.9 months | 14.1 months |
| PAPMET2 (ClinicalTrials.gov identifier: NCT05411081) | Atezolizumab + cabozantinib v cabozantinib | 200 (expected) | – | – | – |
| SAMETA (ClinicalTrials.gov identifier: NCT05043090) | Durvalumab + savolitinib v durvalumab v sunitinib | 220 (expected) | – | – | – |
| STELLAR-304 (ClinicalTrials.gov identifier: NCT05678673) | Nivolumab + zanzalintinib v sunitinib | 291a | – | – | – |
Abbreviations: ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma.
Includes full non–clear cell RCC cohort.
The phase Ib dose-escalation/cohort expansion study, COSMIC-021 (ClinicalTrials.gov identifier: NCT03170960), enrolled patients with non-ccRCC to receive cabozantinib with atezolizumab.41 Within the non-ccRCC cohort, 15 patients (47%) had pRCC. No complete responses were reported; however, partial responses were observed in 47% of patients with pRCC. Furthermore, the disease control rate across all variant histology patients was a remarkable 94%. The completion of COSMIC-021, demonstrating favorable toxicity profiles and survival outcomes with the combination of a MET-inhibitor and an ICI agent, preceded the development of PAPMET2 (ClinicalTrials.gov identifier: NCT05411081). PAPMET2is an ongoing phase II randomized trial set toenroll 200 participants that compares cabozantinib alone to the combination of cabozantinib with atezolizumab in patients with metastatic pRCC who have not previously received ICI therapy (Fig 2A).42 The primary end point of PAPMET2 is PFS, with secondary end points including OS, objective response rate, and adverse event reporting.
FIG 2.
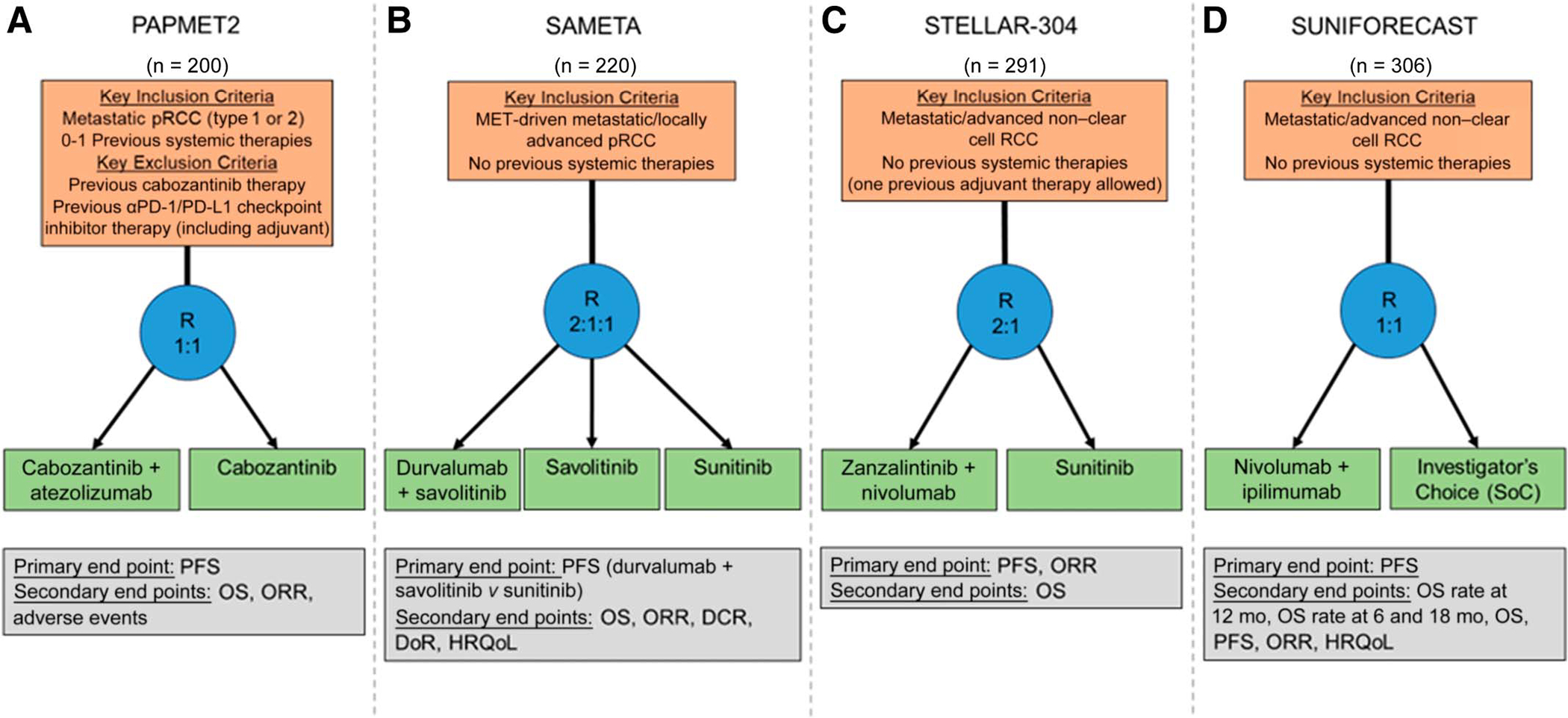
Schematics of ongoing randomized trials for patients with pRCC: (A) PAPMET2 (ClinicalTrials.gov identifier: NCT05411081), (B) SAMETA (ClinicalTrials.gov identifier: NCT05043090), (C) STELLAR-304 (ClinicalTrials.gov identifier: NCT05678673), and (D) SUNIFORECAST (ClinicalTrials.gov identifier: NCT03075423). DCR, disease control rate; DoR, duration of response; HRQoL, health-related quality of life; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; pRCC, papillary renal cell carcinoma; R, randomize; RCC, renal cell carcinoma; SoC, standard of care.
Furthermore, the CALYPSO trial (ClinicalTrials.gov identifier: NCT02819596) also combined MET inhibition with ICI, pairing savolitinib with durvalumab (anti–PD-L1).43 This single-arm trial allowed for patients with treatment-refractory pRCC, enrolling 41 patients. Objective response was observed in 29% of the overall cohort and, among the 27 patients with MET-driven tumors, the objective response rate was 53%. The phase III SAMETA trial (ClinicalTrials.gov identifier: NCT05043090) builds from the findings of CALYPSO, randomly assigning patients with MET-driven pRCC with no previous lines of systemic therapy to savolitinib plus durvalumab, durvalumab alone, or sunitinib in a 2:1:1 fashion.44 SAMETA is expected to enroll 220 patients with PFS as the primary end point (Fig 2B). The STELLAR-304 trial (ClinicalTrials.gov identifier: NCT05678673) is similar in design to both PAPMET2 and SAMETA. STELLAR-304 is comparing the combination of nivolumab with zanzalintinib, a next-generation multikinase inhibitor targeting MET, VEGR, and additional receptor tyrosine kinases, with sunitinib.45,46 Patients will be randomly assigned 2:1 to the experimental combination and control arms, respectively (Fig 2C). Unlike PAPMET2 and SAMETA, however, STELLAR-304 will allow for inclusion of any non–clear cell variant RCC histology. An additional randomized phase II trial, SUNIFORECAST (ClinicalTrials.gov identifier: NCT03075423), is randomly assigning patients with variant histology RCC, including pRCC, to receive the combination of nivolumab plus ipilimumab versus a physician’s choice regimen on the basis of current standards of care (Fig 2D).47 PAPMET2, SAMETA, STELLAR-304, and SUNIFORECAST represent a new push toward exploring therapeutic activity through randomized trials in pRCC and other non-ccRCC subtypes.
Investigations into the optimal therapeutic strategies for pRCC remain ongoing. In recent years, we have seen advancement from PFS <5 months with VEGF- and mTOR-directed therapies to PFS durations surpassing 1 year with newer precision approaches such as MET-directed agents and ICIs, alone or in combination. Ongoing work exploring these strategies in randomized studies will provide more clarity into the appropriate frontline treatments of choice for patients with metastatic pRCC. On the basis of the recent success of KEYNOTE-564 in the adjuvant space, future efforts are needed to assess the utility of adjuvant therapy for patients with localized pRCC after nephrectomy.48
RCC WITH SARCOMATOID DEDIFFERENTIATION
Histology and Biology
RCC with sarcomatoid dedifferentiation (sRCC) is defined by the presence of pathognomonic spindled cells. These features of dedifferentiation demonstrate loss of epithelial components with high cell density and nuclear atypia.49 sRCC presents a unique challenge compared with other variant histology RCCs, as it is not truly a distinct histotype. Indeed, sRCC typically exists as discrete dedifferentiated features embedded within another RCC histology, most commonly ccRCC or chromophobe RCC.4 Thus, sRCCs are fundamentally heterogeneous in their histopathologic presentation and biology.
Although the precise etiology of sarcomatoid feature development is still unclear, the process is commonly associated with epithelial-to-mesenchymal transition (EMT).50–52 EMT is a cellular plasticity process that underlies invasion, metastasis, and aggressiveness across tumor types. As such, sRCC is historically among the most aggressive RCC subtypes. Although only accounting for approximately 5% of all RCCs, sRCCs are enriched for in metastatic RCC, comprising approximately 20% of metastatic cases.53,54 Furthermore, median OS for patients with sRCC has infrequently surpassed 1 year and the presence of sarcomatoid features portends worse survival outcomes compared with nonsarcomatoid-containing RCCs irrespective of disease stage.55 Importantly, sRCC has also shown minimal responsiveness to canonical anticancer therapies, including cytokines, VEGF(R) inhibitors, and chemotherapeutics, as detailed below. Notably, however, recent subgroup analyses from the trials of ICIs in RCC have revealed a preferential responsiveness for ICI among sRCC cases, resulting in a welcome advancement in the clinical management of this aggressive disease. Studies of sRCC patient specimens have elucidated correlative features that may underlay this responsiveness, including increased PD-L1 expression, increased inflammatory gene programs, and increased TP53 mutations in sRCC relative to ccRCC and other nonsarcomatoid RCC subtypes.4,52,56,57
Chemotherapeutic Approaches
Among the first experimental strategies for sRCC was doxorubicin, on the basis of previous evidence of doxorubicin activity in sarcoma. A single-center analysis of 44 patients with metastatic sRCC included those treated with doxorubicin, interferon, or hormonal therapy.58 Intriguingly, two patients treated with doxorubicin had complete response and interferon portended the longest OS durations. Subsequent investigations into doxorubicin-based chemotherapy strategies commenced. A phase II study enrolling 25 patients (including 23 evaluable patients) with sRCC investigated doxorubicin with ifosfamide showed minimal benefit for this regimen: median PFS was 2.2 months and OS did not surpass 4 months.59 An additional study of doxorubicin, this time with gemcitabine, was performed in 18 patients.60 Seven patients achieved an objective response (two complete responders), and the median duration of response was 5 months. ECOG 8802 (ClinicalTrials.gov identifier: NCT01164228) offered a further interrogation on the basis of the previous data, evaluating doxorubicin with gemcitabine in a prospective phase II trial.61 In total, 39 patients were enrolled onto ECOG 8802; objective responses were seen in six (16%) patients, including one complete response. The median PFS for the study cohort was 3.5 months, while the median OS was 8.8 months.
Targeted Therapies Alone or in Combination
As targeted therapies were approved throughout the 2000s, prospective studies commenced to investigate the impact of these agents on sRCC outcomes. Sorafenib was trialed in nine patients previously treated with gemcitabine plus doxorubicin. The time to treatment progression with sorafenib was 10.9 months and a partial remission was reported in one patient.62 Additional work also interrogated the addition of targeted agents to cytotoxic chemotherapies. Capecitabine, gemcitabine, and bevacizumab were combined in a phase II trial enrolling 34 patients with sRCC.63 Objective responses were observed in 20% of patients, including one complete responder. The median PFS in this cohort reached 5.5 months, with a median OS of 12 months. The ECOG 1808 trial investigated targeted therapy with or without addition of chemotherapy for sRCC.64 Patients in ECOG 1808 were randomly assigned to receive sunitinib monotherapy or sunitinib with gemcitabine. In total, 47 patients were randomly assigned to the sunitinib plus gemcitabine arm and 40 to the sunitinib monotherapy arm. Response rates were 20% and 11% for the combination and monotherapy arms, respectively. The median PFS with the combination strategy was 5.3 months and median OS was 9.4 months, compared with a median PFS of 3.0 months and median OS of 7.6 months with sunitinib alone. To date, ECOG 1808 remains the only randomized trial to be performed exclusively in a sRCC patient population.
ICIs
The adoption of ICIs in RCC has perhaps had no greater impact than on the sRCC patient population. As detailed above, previous therapeutic approaches for sRCC yielded minimal survival improvements. However, subgroup analyses of multiple phase III trials have shown dramatic survival benefits for ICI-based combinations relative to VEGF-directed targeted therapies (Table 2). Within the CheckMate-214 trial (ClinicalTrials.gov identifier: NCT02231749) comparing nivolumab plus ipilimumab to sunitinib as frontline therapy for metastatic RCC, 139 patients with sRCC were enrolled.65 Comparisons of PFS demonstrated clear benefit with nivolumab plus ipilimumab (26.5 months v 5.1 months; P = .009). This survival benefit was reaffirmed in the analysis of objective response rate (61% v 23%) and complete response rate (19% v 3%). Long-term follow-up of this population demonstrated maintenance of these PFS and response rate trends, and further revealed a difference in median OS of 48.6 months versus 14.2 months between patients receiving nivolumab plus ipilimumab and sunitinib, respectively.66 The findings from CheckMate-214 represent a seismic shift in survival outcomes for patients with sRCC, improving OS from <1 year to beyond 4 years with ICI.
TABLE 2.
Subgroup Analyses of Sarcomatoid RCC Patient Populations Within Randomized Phase III Trials of Immune Checkpoint Inhibitor Combinations
| Trial | Arms | No. of Patients with Sarcomatoid RCC | ORR | CR Rate | PFS | OS |
|---|---|---|---|---|---|---|
| CheckMate-214 (ClinicalTrials.gov identifier: NCT02231749) | Nivolumab + ipilimumab v sunitinib | 139a | 60.8% v 23.1% | 23.0% v 6.2% | 26.5 months v 5.5 months (HR = 0.50) | 48.6 months v 14.2 months (HR = 0.46) |
| JAVELIN 101 (ClinicalTrials.gov identifier: NCT02684006) | Avelumab + axitinib v sunitinib | 108 | 46.8% v 21.3% | 4.3% v 0% | 7.0 months v 4.0 months (HR = 0.57) | 12-month OS rate: 83.0% v 67.0% |
| IMmotion151 (ClinicalTrials.gov identifier: NCT02420821) | Atezolizumab + bevacizumab v sunitinib | 142 | 49% v 14% | 10% v 3% | 8.3 months v 5.3 months (HR = 0.52) | 21.7 months v 15.4 months (HR = 0.64) |
| KEYNOTE-426 (ClinicalTrials.gov identifier: NCT02853331) | Pembrolizumab + axitinib v sunitinib | 105 | 58.8% v 31.5% | 11.8% v 0% | NR v 8.4 months (HR = 0.54) | 12-month OS rate: 83.4% v 79.5% (HR = 0.58) |
| CLEAR (ClinicalTrials.gov identifier: NCT02811861) | Pembrolizumab + lenvatinib v sunitinib | 49 | 60.7% v 23.8% | 0.8% v 0% | 11.1 months v 5.5 months (HR = 0.39) | NR v NR (HR = 0.91) |
| CheckMate-9ER (ClinicalTrials.gov identifier: NCT03141177) | Nivolumab + cabozantinib v sunitinib | 75 | 58.8% v 22.0% | Not reported | 13.8 months v 4.2 months (HR = 0.34) | 37.6 months v 22.1 months (HR = 0.38) |
Abbreviations: CR, complete response; HR, hazard ratio; NR, not reached; ORR, objective response rate; OS, overall survival, PFS, progression-free survival; RCC, renal cell carcinoma.
International Metastatic Renal Cell Carcinoma Database Consortium intermediate/poor risk only.
The positive findings from CheckMate-214 have been recapitulated in subsequent trials of ICIs for metastatic RCC. In JAVELIN 101 (ClinicalTrials.gov identifier: NCT02684006), 108 patients had sRCC (47 receiving axitinib plus avelumab and 61 receiving sunitinib).67 Again, patients with sRCC receiving axitinib plus avelumab experienced improved PFS (7.0 v 4.0 months) and objective response rate (47% v 21%). IMmotion151 (ClinicalTrials.gov identifier: NCT02420821), comparing bevacizumab plus atezolizumab to sunitinib, reported similar survival improvements with ICI in sRCC.68 In total, 142 patients with sRCC were enrolled onto IMmotion151 and median PFS favored the bevacizumab with atezolizumab arm (8.3 months v 5.3 months). Similar to previous trials, treatment with bevacizumab plus atezolizumab resulted in higher objective response rates (49% v 14%) and complete response rates (10% v 3%) relative to sunitinib. The afore-mentioned trial of bevacizumab with atezolizumab in variant RCC histologies also allowed for patients with >20% sarcomatoid feature presence irrespective of background histology.38 In total, 26 patients with sarcomatoid features were enrolled, including 18 with ccRCC and 8 with variant histologies. Among patients harboring ccRCC with sarcomatoid features, the objective response rate reached 50%, whereas the response rate for patients with variant histology and sarcomatoid features was 38%.
The more recently reported CLEAR (ClinicalTrials.gov identifier: NCT02811861) study of lenvatinib plus pembrolizumab compared with sunitinib also reported similar outcomes with ICI combination relative to targeted therapy alone for patients with sRCC.69 Forty-nine patients with sRCC were enrolled in CLEAR; among patients with sRCC, the median PFS reached 11.1 months with lenvatinib and pembrolizumab compared with 5.5 months with sunitinib. CheckMate-9ER (ClinicalTrials.gov identifier: NCT03141177), also among the more recent phase III trials in metastatic RCC comparing nivolumab plus cabozantinib to sunitinib, has also reported both PFS (13.8 months v 4.2 months) and OS (37.6 months v 22.1 months) benefits with ICI combination relative to sunitinib for patients with sRCC.70
Taken together, these subgroup analyses of ICI efficacy provide a remarkable advancement in survival outcomes for patients with sRCC. Efforts are ongoing to better understand the biology underlying these responses, with hopes that such discoveries can further improve outcomes in this aggressive disease type. Furthermore, for those patients with sRCC who experience disease progression on an ICI, it remains unclear what the best treatment option is. Although ICI rechallenge did not provide benefit for patients with mRCC regardless of primary histology who previously had progression on an ICI therapy in the CONTACT-03 trial (ClinicalTrials.gov identifier: NCT043382569), 10% of the trial population had sRCC.71 It will be interesting to note if any differences in ICI rechallenge response are noted in the sRCC population compared with the intention-to-treat population in future subgroup analyses.
CONCLUSIONS
Variant histology RCCs account for 25% of all RCC diagnoses, yet therapeutic approaches specific to the biology of each subtype has only recently expanded. The most common variant histology RCC, pRCC, is canonically associated with MET hyperactivity, and as such, novel efforts have shown improved clinical outcomes with MET inhibitors in patients with pRCC. Ongoing efforts in this domain are investigating the addition of ICI regimens to MET inhibition for patients with pRCC, with the goal of further improving upon the advances in survival made over the past 5 years. Furthermore, the discovery that sRCC tumors are highly responsive to ICI represents among the most meaningful clinical revelations in the ICI-therapy era. For a patient population with historical survivals of <1 year, ICI combinations can now improve OS beyond the 4-year time point. More work is needed to better understand the biology underlying these preferential responses, and in doing so, we may develop novel strategies to augment ICI and further improve patient responses moving forward.
Although not highlighted within this article, there have been numerous advancements in our understanding of both the biology and clinical management for additional variant RCC histologies, including chromophobe RCC, translocation RCC, and renal medullary carcinoma.72–81 As progress continues for the more prevalent non-ccRCC subtypes detailed within, clinical translation of novel findings for these rare histologies will provide further development and refinement of clinical strategies to improve the lives of patients with variant histology RCC.
The previous 5 years have seen a rapid expansion of pre-clinical and clinical investigation into understanding and treating variant histology RCCs. These efforts have coalesced into meaningful improvements in clinical outcomes for patients diagnosed with non-ccRCCs. Ongoing and future work, including trials detailed herein and beyond, promise to further improve the outcomes and well-being of patients diagnosed with variant histology RCC.
PRACTICAL APPLICATIONS.
Renal cell carcinoma (RCC) can be classified on the basis of histopathology; clear cell RCC is the most common, while non–clear cell variant histologies account for 25% of cases and comprise multiple unique subtypes.
Papillary RCC is the second most common RCC histology, comprising 15% of cases, and is frequently associated with amplifications and alterations of the MET protooncogene.
Targeting of the MET signaling pathway with cabozantinib has proven efficacious in papillary RCC; ongoing trials are investigating MET inhibitors in combination with immune checkpoint inhibitors (ICIs).
Sarcomatoid dedifferentiation is a unique entity based on the presence of pathognomonic spindle-cell morphology, which can arise within any RCC histologic background.
RCC with sarcomatoid dedifferentiation has historically been a highly aggressive tumor type, but recent evidence has demonstrated preferential responsiveness of these tumors to ICIs.
ACKNOWLEDGMENT
N.J.S. is supported by the NCI training grant T32CA085183.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated.
Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc.
Sumanta K. Pal
Travel, Accommodations, Expenses: Exelixis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/259259
Nazli Dizman
Stock and Other Ownership Interests: Several pharmaceutical companies (I)
Consulting or Advisory Role: Vivreon Biosciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Giaquinto AN, Jemal A: Cancer statistics, 2024. CA Cancer J Clin 74:12–49, 2024 [DOI] [PubMed] [Google Scholar]
- 2.Reuter VE, Argani P, Zhou M, et al. : Best practices recommendations in the application of immunohistochemistry in the kidney tumors: Report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol 38:e35–e49, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WGJ: The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol 14:2703–2711, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Blum KA, Gupta S, Tickoo SK, et al. : Sarcomatoid renal cell carcinoma: Biology, natural history and management. Nat Rev Urol 17:659–678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atzpodien J, Kirchner H, Jonas U, et al. : Interleukin-2- and interferon alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: A prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). J Clin Oncol 22:1188–1194, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Klapper JA, Downey SG, Smith FO, et al. : High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 113:293–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fosså SD: Interferon in metastatic renal cell carcinoma. Semin Oncol 27:187–193, 2000. ° [PubMed] [Google Scholar]
- 8.Rini BI: Vascular endothelial growth factor–targeted therapy in renal cell carcinoma: Current status and future directions. Clin Cancer Res 13:1098–1106, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814–1823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Glen H, et al. : Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16:1473–1482, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Gore M: Axitinib for the management of metastatic renal cell carcinoma. Drugs R D 11:113–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Pal SK, Escudier BJ, et al. : Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): A phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol 21:95–104, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Buti S, Leonetti A, Dallatomasina A, et al. : Everolimus in the management of metastatic renal cell carcinoma: An evidence-based review of its place in therapy. Core Evid 11:23–36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, et al. : Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Penkov K, Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103–1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116–1127, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289–1300, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829–841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alshenawy HA: Immunohistochemical panel for differentiating renal cell carcinoma with clear and papillary features. J Microsc Ultrastruct 3:68–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal SK, Ali SM, Yakirevich E, et al. : Characterization of clinical cases of advanced papillary renal cell carcinoma via comprehensive genomic profiling. Eur Urol 73:71–78, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Albiges L, Guegan J, Le Formal A, et al. : MET is a potential target across all papillary renal cell carcinomas: Result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res 20:3411–3421, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network; Linehan WM, Spellman PT, et al. : Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374:135–145, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo J, Ohashi R, Amin MB, et al. : WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology 81:426–438, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Pal SK, Bergerot P, Dizman N, et al. : Responses to alectinib in ALK-rearranged papillary renal cell carcinoma. Eur Urol 74:124–128, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Escudier B, Molinie V, Bracarda S, et al. : Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer 69:226–235, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Ravaud A, Oudard S, De Fromont M, et al. : First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase II study (SUPAP) by the French Genitourinary Group (GETUG)†. Ann Oncol 26:1123–1128, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Tannir NM, Jonasch E, Albiges L, et al. : Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol 69:866–874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong AJ, Halabi S, Eisen T, et al. : Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol 17:378–388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negrier S, Rioux-Leclercq N, Ferlay C, et al. : Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: Results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer 129:107–116, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Choueiri TK, Heng DYC, Lee JL, et al. : Efficacy of savolitinib vs sunitinib in patients with MET-driven papillary renal cell carcinoma: The SAVOIR phase 3 randomized clinical trial. JAMA Oncol 6:1247–1255, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal SK, Tangen C, Thompson IM Jr, et al. : A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 397:695–703, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelzang NJ, Olsen MR, McFarlane JJ, et al. : Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase IIIb/IV CheckMate 374 study. Clin Genitourin Cancer 18:461–468.e3, 2020 [DOI] [PubMed] [Google Scholar]
- 36.McDermott DF, Lee JL, Ziobro M, et al. : Open-Label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol 39:1029–1039, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tykodi SS, Gordan LN, Alter RS, et al. : Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer 10:e003844, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGregor BA, McKay RR, Braun DA, et al. : Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol 38:63–70, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albiges L, Gurney H, Atduev V, et al. : Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): A single-arm, multicentre, phase 2 trial. Lancet Oncol 24:881–891, 2023 [DOI] [PubMed] [Google Scholar]
- 40.Lee CH, Voss MH, Carlo MI, et al. : Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol 40:2333–2341, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal SK, McGregor B, Suárez C, et al. : Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: Results from the COSMIC-021 study. J Clin Oncol 39:3725–3736, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maughan BL, Plets M, Pal SK, et al. : SWOG S2200 (PAPMET2): A phase II randomized trial of cabozantinib with or without atezolizumab in patients with advanced papillary renal cell carcinoma (PRCC). J Clin Oncol 42, 2024. (suppl 4; abstr TPS493) [Google Scholar]
- 43.Suáez C, Larkin JMG, Patel P, et al. : Phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol 41:2493–2502, 2023 [DOI] [PubMed] [Google Scholar]
- 44.Chouieri T: SAMETA: A phase III study of savolitinib + durvalumab vs sunitinib and durvalumab monotherapy in patients with MET-driven, unresectable, locally advanced/metastatic papillary renal cell carcinoma. Oncologist 28:S11–S12, 2023. (suppl 1) [Google Scholar]
- 45.Pal SK, Powles T, Kanesvaran R, et al. : 1912TiP STELLAR-304: A randomized phase III study of zanzalintinib (XL092) and nivolumab in non-clear cell renal cell carcinoma (nccRCC). Ann Oncol 34: S1028–S1029, 2023 [Google Scholar]
- 46.Motzer RJ, Choueiri TK, Garmezy B, et al. : Zanzalintinib in combination with immune checkpoint inhibitors: Design of the renal cell carcinoma expansion stage cohorts in STELLAR-002. Oncologist 28:S10–S11, 2023. (suppl 1) [Google Scholar]
- 47.Ahrens M, Escudier B, Boleti E, et al. : A randomized phase II study of nivolumab plus ipilimumab versus standard of care in previously untreated and advanced non-clear cell renal cell carcinoma (SUNIFORECAST). J Clin Oncol 38, 2020. (suppl 15; abstr TPS5103) [Google Scholar]
- 48.Choueiri TK, Tomczak P, Park SH, et al. : Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385:683–694, 2021 [DOI] [PubMed] [Google Scholar]
- 49.Delahunt B, Cheville JC, Martignoni G, et al. : The International Society of Urological Pathology (ISUP) Grading System for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37:1490–1504, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Delahunt B: Sarcomatoid renal carcinoma: The final common dedifferentiation pathway of renal epithelial malignancies. Pathology 31:185–190, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Conant JL, Peng Z, Evans MF, et al. : Sarcomatoid renal cell carcinoma is an example of epithelial–mesenchymal transition. J Clin Pathol 64:1088–1092, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Bakouny Z, Braun DA, Shukla SA, et al. : Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun 12:808, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alevizakos M, Gaitanidis A, Nasioudis D, et al. : Sarcomatoid renal cell carcinoma: Population-based study of 879 patients. Clin Genitourin Cancer 17:e447–e453, 2019 [DOI] [PubMed] [Google Scholar]
- 54.Shuch B, Said J, La Rochelle JC, et al. : Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology--is up-front resection indicated and, if not, is it avoidable? J Urol 182:2164–2171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trudeau V, Larcher A, Sun M, et al. : Comparison of oncologic outcomes between sarcomatoid and clear cell renal cell carcinoma. World J Urol 34:1429–1436, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Motzer RJ, Banchereau R, Hamidi H, et al. : Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 38:803–817.e4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malouf GG, Ali SM, Wang K, et al. : Genomic characterization of renal cell carcinoma with sarcomatoid dedifferentiation pinpoints recurrent genomic alterations. Eur Urol 70:348–357, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Sella A, Logothetis CJ, Ro JY, et al. : Sarcomatoid renal cell carcinoma. A treatable entity. Cancer 60:1313–1318, 1987 [DOI] [PubMed] [Google Scholar]
- 59.Escudier B, Droz JP, Rolland F, et al. : Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: A phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol 168:959–961, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Nanus DM, Garino A, Milowsky MI, et al. : Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer 101:1545–1551, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Haas NB, Lin X, Manola J, et al. : A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol 29:761–767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staehler M, Haseke N, Roosen A, et al. : Sorafenib after combination therapy with gemcitabine plus doxorubicine in patients with sarcomatoid renal cell carcinoma: A prospective evaluation. Eur J Med Res 15:287–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maiti A, Nemati-Shafaee M, Msaouel P, et al. : Phase 2 trial of capecitabine, gemcitabine, and bevacizumab in sarcomatoid renal-cell carcinoma. Clin Genitourin Cancer 16:E47–E57, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas NB, Puligandla M, McDermott DF, et al. : ECOG 1808: Randomized phase II trial of sunitinib with or without gemcitabine in advanced kidney cancer with sarcomatoid features. J Clin Oncol 34, 2016. (suppl 15; abstr 4511) [Google Scholar]
- 65.Tannir NM, Signoretti S, Choueiri TK, et al. : Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res 27:78–86, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rini BI, Signoretti S, Choueiri TK, et al. : Long-term outcomes with nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. J Immunother Cancer 10:e005445, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choueiri TK, Larkin J, Pal S, et al. : Efficacy and correlative analyses of avelumab plus axitinib versus sunitinib in sarcomatoid renal cell carcinoma: Post hoc analysis of a randomized clinical trial. ESMO Open 6:100101, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rini BI, Motzer RJ, Powles T, et al. : Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: A prespecified subgroup analysis of the IMmotion151 clinical trial. Eur Urol 79:659–662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grünwald V, Powles T, Eto M, et al. : Phase 3 CLEAR study in patients with advanced renal cell carcinoma: Outcomes in subgroups for the lenvatinib-plus-pembrolizumab and sunitinib arms. Front Oncol 13:1223282, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motzer RJ, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol 23:888–898, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pal SK, Albiges L, Tomczak P, et al. : Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicentre, randomised, open-label, phase 3 trial. Lancet 402:185–195, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henske EP, Cheng L, Hakimi AA, et al. : Chromophobe renal cell carcinoma. Cancer Cell 41:1383–1388, 2023 [DOI] [PubMed] [Google Scholar]
- 73.Msaouel P, Walker CL, Genovese G, et al. : Molecular hallmarks of renal medullary carcinoma: More to c-MYC than meets the eye. Mol Cell Oncol 7:1777060, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nze C, Msaouel P, Derbala MH, et al. : A phase II clinical trial of pembrolizumab efficacy and safety in advanced renal medullary carcinoma. Cancers 15:3806, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soeung M, Perelli L, Chen Z, et al. : SMARCB1 regulates the hypoxic stress response in sickle cell trait. Proc Natl Acad Sci USA 120:e2209639120, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thibault C, Fléchon A, Albiges L, et al. : Gemcitabine plus platinum-based chemotherapy in combination with bevacizumab for kidney metastatic collecting duct and medullary carcinomas: Results of a prospective phase II trial (BEVABEL-GETUG/AFU24). Eur J Cancer 186:83–90, 2023 [DOI] [PubMed] [Google Scholar]
- 77.Xiao Y, Clima R, Busch J, et al. : Decreased mitochondrial DNA content drives OXPHOS dysregulation in chromophobe renal cell carcinoma. Cancer Res 80:3830–3840, 2020 [DOI] [PubMed] [Google Scholar]
- 78.Priolo C, Khabibullin D, Reznik E, et al. : Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc Natl Acad Sci USA 115:E6274–e6282, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prakasam G, Mishra A, Christie A, et al. : Comparative genomics incorporating translocation renal cell carcinoma mouse model reveals molecular mechanisms of tumorigenesis. J Clin Invest 134:e170559, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Damayanti NP, Cordova RA, Rupert C, et al. : TFE3-splicing factor fusions represent functional drivers and druggable targets in translocation renal cell carcinoma. Cancer Res 84:1286–1302, 2024 [DOI] [PubMed] [Google Scholar]
- 81.Achom M, Sadagopan A, Bao C, et al. : A genetic basis for cancer sex differences revealed in Xp11 translocation renal cell carcinoma. bioRxiv 2023.08.04.552029 [Google Scholar]


