Abstract
BACKGROUND
Castleman's disease (CD) is a rare lymphoproliferative, emulating both benign and malignant diseases. The diagnosis of CD is formulated upon the combination of clinical and laboratory criteria and ultimately confirmed by histopathological assessment. Due to its rarity, CD presents a challenge in treatment selection, with available options encompassing surgery, chemotherapy, and autologous stem cell transplantation. However, studies suggest that surgical resection of the lesion is the most effective treatment modality, especially for unicentric CD (UCD).
CASE SUMMARY
Here, we describe the case of a 25-year-old woman who presented with painless left thigh swelling for 10 wk. She had been following a low-fat diet to lose weight and had normal laboratory results. Magnetic resonance imaging revealed a well-circumscribed, demarcated cystic lesion located in the left inguinal region with eccentrically positioned signal void vascular structures, measuring 4.3 cm × 3 cm × 3.2 cm, likely of lymphoid origin. The patient underwent surgical resection, and the final histopathology showed a vascular proliferation and hyalinization of the vessel walls, along with atretic germinal centers traversed by penetrating vessels, consistent with CD. The patient was discharged home one day after the procedure in good condition, with a follow-up appointment scheduled in our outpatient clinic.
CONCLUSION
Although surgical resection is the mainstay for UCD, a multidisciplinary approach is needed due the lack of specific diagnostic features and treatments.
Keywords: Castleman’s disease, Lymph nodes, Surgical resection, Lymphoproliferative disorder, Case report
Core Tip: Castleman's disease (CD) is a rare disorder primarily affecting lymph nodes and associated tissues. Clinical characteristics and survival vary significantly among the three histological subtypes of CD. Diagnosis of CD primarily relies on histopathological examination, supported by imaging modalities such as computed tomography scan, magnetic resonance imaging, and ultrasound. Histopathological examination is crucial for diagnosing both unicentric-CD (UCD) and multicentric-CD, especially after ruling out other disorders, including infections, malignancies, and autoimmune conditions. Despite its rarity, CD presents a range of treatment options. However, studies consistently highlight surgical resection as the optimal treatment modality, particularly for UCD.
INTRODUCTION
Castleman's disease (CD), alternatively referred to as giant lymph node hyperplasia or angiofollicular lymph node hyperplasia, encompasses a spectrum of rare diseases within the family of lymphoproliferative disorders. This non-malignant proliferation manifests in two distinct forms: Unicentric CD (UCD) and multicentric CD (MCD)[1].
MCD constitutes 15% of the total CD cases and has an incidence of approximately 3.4 per million per year in the United States[2,3]. MCD type can be further classified into two subtypes including human herpesvirus 8 virus (HHV)-8-positive and HHV-8-negative (or idiopathic) MCD. Different from UCD, MCD is a systemic, progressive, and frequently fatal disorder affecting multiple nodes and accompanied by generalized signs and symptoms. These include lymphadenopathy, hepatosplenomegaly, fever, fatigue, and weight loss, with more severe cases complicated by thromboembolic disorders, anemia, and hypoalbuminemia. These events are triggered by the increased production of proinflammatory markers such as interleukin 6 (IL-6) and fibrinogen. MCD treatment often requires a combination of corticosteroids, immunomodulatory agents, and chemotherapy[4].
UCD is the most common type of CD, with an approximate incidence of 16-19 per million per year in the United States[2,3]. UCD typically manifests in the third to fourth decade of life, with no apparent gender preference[3,5], although a mild preponderance among females was reported[6]. To date, the cause of the disease remains elusive, though several hypotheses have been postulated[1]. UCD typically affects one or multiple lymph nodes in a single location, more frequently the mediastinum, abdominal cavity, retroperitoneum, pelvis, neck, and less commonly, in the axillary or inguinal regions[5,7]. As UCD usually involves the slow growth of lymph nodes at a single anatomical site, clinical symptoms predominantly result from the local mass effect[4].
Histopathologically, CD can be distinguished in three subtypes: Hyaline vascular (HV), plasma cell (PC), and mixed. The majority of UCD cases exhibit a HV-type histology (74%–91%), although cases with PC-type histology or mixed features have also been documented (9%–26%)[8]. Considering its nonmalignant nature and isolated location, surgical resection is often curative and thus the preferred approach[5].
Here we present a case study concerning UCD located in the inguinal region in a young 25-year-old female.
CASE PRESENTATION
Chief complaints
A 25-year-old woman presented to our clinic with a painless left thigh swelling persisting for 10 wk following a low-fat diet for weight loss.
History of present illness
10 wk earlier, the patient noticed painless swelling in her left thigh after starting a low-fat diet for weight loss. She visited our hospital to seek further evaluation and assessment.
History of past illness
The patient denied any history of sweating, fatigue, anorexia, or jaundice. Additionally, she had no chronic conditions such as vascular diseases, connective tissue disorders, infections, malignancies, autoimmune diseases, viral illnesses like human immunodeficiency virus (HIV) or HHV-8, diabetes, trauma, interventions, or concurrent medication use.
Personal and family history
The patient married at an appropriate age, had two daughters, a healthy spouse and parents, and no family history of connective tissue disorders, vascular diseases, infections, malignancies, or autoimmune diseases. She also denied a history of smoking or alcohol consumption.
Physical examination
On examination, a mobile, non-tender subcutaneous mass measuring approximately 5 cm × 3 cm was noted in the left inguinal region. Vascular and neurological examinations of the lower limb were unremarkable. Chest and abdominal examinations revealed no tenderness or palpable masses. A comprehensive lymph node examination was within normal limits.
Laboratory examinations
Preoperative complete blood count and coagulation profile showed: Haemoglobin: 13.1 g/dL; leukocyte count: 8.70 × 109/L; hematocrit: 35.5%; platelet count: 359 × 109/L; prothrombin time 11.5 s; partial thromboplastin time: 27.9 s; international normalized ratio: 0.98. Liver and renal function tests, erythrocyte sedimentation rate (ESR), and C-reactive protein were all within the normal range. Systemic levels of IgG (0.8 g/L), IgM (0.6 g/L), and IL-6 (10 pg/mL) were also normal. Viral screening for HHV-8 and HIV was negative.
Imaging examinations
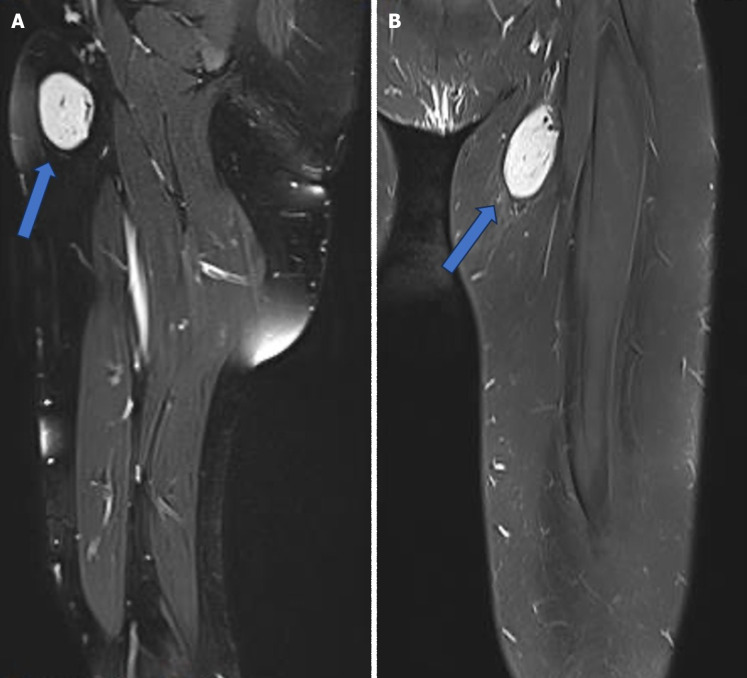
The initial workup included an ultrasound of the left thigh, which revealed an ovoid, intensely hypervascular mass lesion located at the left inner thigh, approximately 1 cm beneath the skin surface, measuring 3.5 cm × 1.6 cm × 5.2 cm. Magnetic resonance imaging (MRI) demonstrated a well-circumscribed, oval-shaped cystic lesion with markedly high-signal intensity on short tau inversion recovery sequences and low signal intensity on T1-weighted images, projected in the left inguinal region. Eccentrically positioned signal void vascular structures were observed, indicative of vascular involvement. The lesion measured 4.3 cm × 3 cm × 3.2 cm, likely of lymphoid origin (Figure 1).
Figure 1.
Magnetic resonance imaging showed a well-circumscribed and demarcated cystic lesion projected in the left inguinal region with eccentrically positioned signal void vascular, likely lymphoid in nature (arrow). A: Sagittal view. B: Coronal view.
Histological findings
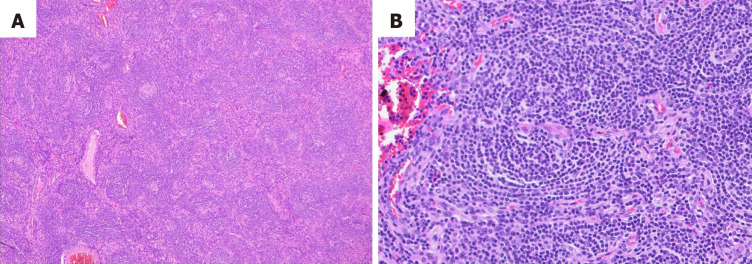
The final histopathological examination revealed vascular proliferation, hyalinization of vessel walls, and atretic germinal centers traversed by penetrating vessels. The mantle zones exhibited thickening with lymphocytes arranged in layers (Figure 2).
Figure 2.
Hematoxylin and eosin staining showing. A: Regressed follicles with increased endothelial venules; B: Higher magnification of one of the regressed follicles showing a prominent mantle zone (onion skin appearance) and penetrated by vessel.
FINAL DIAGNOSIS
UCD.
TREATMENT
The patient underwent surgical resection of the mass.
OUTCOME AND FOLLOW-UP
The patient was discharged home one day after the procedure in good condition, and a follow-up appointment was scheduled in our outpatient clinic. At 1 year, a computed tomography (CT) scan of the chest, abdomen, and pelvis did not reveal any involvement of other lymph nodes. Viral screening for HHV-8 and HIV remained negative.
DISCUSSION
CD represents a rare lymphoproliferative disorder encompassing a spectrum of diverse conditions, including UCD and MCD, with or without association with HHV8[1,4,5]. This case highlights a unique presentation of UCD in the inguinal region, contributing to the limited literature on this uncommon manifestation. Various aspects of this clinical case offer avenues for discussion. The risk factors for UCD are not clear, and its etiology remains largely unknown, mirroring the complexity of CD as a whole.
In MCD, the relationship with HHV-8 is not consistently identified, while other MCD cases are associated with the so-called polyneuropathy, organomegaly, endocrinopathy, monoclonal PC disorder, skin changes syndrome, also known as Crow-Fukase syndrome[9]. This syndrome is considered a variant of plasma cell dyscrasia, typically involving the overproduction of abnormal PC in the bone marrow[10].
Regarding UCD, while various hypotheses have been proposed, including the involvement of infectious agents and autoimmune processes, no definitive cause has been identified. This underscores the complexity of the disease and emphasizes the need for further research to elucidate its underlying mechanisms. Evidence suggests that UCD is likely a clonal neoplastic condition, with the stromal cells, particularly follicular dendritic cells (FDCs), being the most probable cell of origin[11]. Given this hypothesis, an investigation employing next-generation sequencing of UCD lymph node tissue revealed somatic mutations in the platelet-derived growth factor receptor β gene in nearly 20% of cases. These mutations were predominantly found in CD45− cells, likely representing stromal cells[12]. Notably, the authors performed in vitro studies and further confirmed that this mutation leads to a gain of function, providing proliferative and survival advantages[13]. It was also noted that there were no documented monoclonal immunoglobulin and T-cell receptor gene rearrangements[14]. Nonetheless, the presence of dysplastic FDCs may explain the increased propensity of these individuals to develop FDC sarcoma[4,15].
From a clinical perspective, UCD frequently presents as a painless mass confined to a solitary anatomical site, consistent with the presentation observed in our patient. Compression on contiguous anatomical structures can lead to various symptoms; for example, nodules in the chest can cause dyspnea and coughing[4]. In the PC subtype of UCD, patients often present with symptoms such as fever and fatigue, significantly impacting their quality of life. These symptoms may persist over time and vary in intensity. Additionally, laboratory abnormalities are commonly observed in this subtype, including elevated IL-6 levels[4]. Polyneuropathy, Hodgkin disease, non-Hodgkin lymphoma, and FDC sarcoma can be found in up to 18% of UCD cases[16].
Although occurrences in regions such as the inguinal area are less frequent compared to sites like the mediastinum or neck, UCD can indeed affect these less common areas. Therefore, conducting a thorough differential diagnosis to distinguish UCD from other lymph node masses becomes crucial in clinical practice. In this context, imaging modalities, including ultrasound, CT, and MRI (Figure 1), play crucial roles in the diagnosis and characterization of UCD lesions, aiding in treatment planning. Given the specific anatomical site of the lesion in the inguinal region, we opted for MRI over CT to provide better visualization of the surrounding soft tissues and vascular structures. MRI offers superior soft tissue contrast resolution, making it particularly useful for delineating the anatomy of the inguinal region and identifying any associated vascular abnormalities. Additionally, MRI is capable of demonstrating flow void structures, which are indicative of vascular channels and can help identify feeding vessels supplying the lesion. This capability is especially relevant in cases where vascular involvement or tumor vascularity needs to be assessed[15].
In numerous cases, the presence of an enlarged lymph node may prompt suspicion of lymphomas. Consequently, histopathological examination remains the gold standard for diagnosing UCD. The histopathological aspects vary, with subtypes including HV, PC (found especially in HHV8+ and idiopathic MCD cases), or mixed[17]. However, in unicentric forms, the HV subtype predominates. In our case, the investigations facilitated a straightforward diagnosis, showing characteristic findings such as vascular proliferation, hyalinization of vessel walls, and atretic germinal centers (Figure 2)[8]. Although histopathology was conclusive for a typical HV UCD form, given the concern for additional unrecognized lesions, we conducted viral investigations. Our patient's negative viral screening results for HHV-8 and HIV suggested that UCD in this case was not associated with these viral infections, which are commonly implicated in the multicentric forms of the disease. This underscores the importance of comprehensive diagnostic evaluation, including viral screening, to differentiate between different subtypes of CD and guide appropriate management strategies[18].
Generally, the treatment of CD depends on the disease subtype, with curative surgery being the gold standard for UCD and monoclonal antibody-based immunotherapy being the standard of care for MCD[4].
Surgical excision of the affected lymph node in UCD provides both diagnostic confirmation and therapeutic benefit[5,19], with an overall survival > 90% at 5 years and prompt resolution of local and systemic symptoms (if present) following resection[4]. The role of radiotherapy is controversial, although this approach can be considered in non-resectable cases[20]. According to a recent international evidence-based consensus, unresectable asymptomatic UCD may be monitored, whereas symptomatic unresectable UCD usually requires treatment with rituximab combined or not with steroids, or anti-IL6 antibody therapy, followed by surgical excision[4]. Strategies for embolization have been also proposed[21].
On the other hand, the treatment of MCD is primarily based on immunomodulatory agents. Due to the central role of IL-6 in MCD pathophysiology, the use of anti-IL-6 therapies (e.g., rituximab, tocilizumab, siltuximab) is recommended as the first-line treatment in all patients with idiopathic MCD, with the addition of corticosteroids in case of severe symptoms. In case of inadequate response, cytotoxic chemotherapy as per lymphoma or myeloma regimens can also be considered[4].
In our patient's case, excisional biopsy successfully removed the lesion, resulting in the resolution of symptoms. However, follow-up is essential to monitor for any (rare) recurrence or development of complications[22]. The main characteristics of UCD are outlined in Table 1.
Table 1.
Main features of unicentric Castleman disease
|
|
Features
|
Ref.
|
| Epidemiology | Incidence (United States): 16–19 cases per million person-years. No gender predominance | [2,3] |
| Cause | Probable clonal neoplastic condition: Benign neoplasm of FDCs | [4,10-14] |
| Risk factors | Not known | [10] |
| Clinical presentation | Single lymph node mass (non-tender lymphadenopathy): Asymptomatic or manifesting as pain, sensation of heaviness, compression of organs, and anatomic structures (e.g., chest lesions). In the plasma cell subtype: Fever, fatigue, and laboratory abnormalities (IL-6 secretion: Elevated ESR, elevated CRP, anemia) | [1,4-7,9,15,16,20,21] |
| Disorders associated | Polyneuropathy, Hodgkin disease, non-Hodgkin lymphoma, and follicular dendritic cell sarcoma | [16,20,21] |
| Site | Mediastinum, neck, abdominal cavity, retroperitoneum, and pelvis. Less frequently in axillary or inguinal regions | [5,7] |
| Imaging | Useful for differential diagnosis: Ultrasonography, CT, MRI | [15,20] |
| Differential diagnosis | Lymphomas; cancer (e.g., lymph node metastasis); benign proliferative lesions (e.g., thymoma); inflammatory processes (e.g., sarcoidosis); infections (e.g., tuberculosis) | [17,18] |
| Histopathology | Confirm the diagnosis: Hyaline vascular or plasma cell-type aspects | [8,20] |
| Virology | HHV-8 negative | [18] |
| Therapy | Surgical excision is preferred. Radiotherapy, embolization, or systemic therapy for non-resectable symptomatic lesions | [5,19-21] |
| Outcome | Long-term follow-up to monitor for potential recurrences | [22] |
FDCs: Follicular dendritic cells; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; CT: Computed tomography; MRI: Magnetic resonance imagining; HHV-8: Herpesvirus/human herpesvirus-8.
CONCLUSION
CD is a lymphoproliferative disorder causing an overgrowth of cells in the lymph nodes throughout the body. The diagnosis is primarily made through CT scan, MRI, ultrasound, and histopathologic examination. Treatment varies depending on the subtype. However, since CD involves the enlargement of lymph nodes, complete surgical resection of the tumors is the standard approach across all subtypes. CD necessitates a multidisciplinary approach in evaluating and treating patients with this rare pathology, mainly due to the condition's lack of specific features and limited treatment modalities.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for the publication of this report and any accompanying images. A copy of the written consent form is available for review by the editor-in-chief of this journal upon request.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Saudi Arabia
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Li JJ, China S-Editor: Liu H L-Editor: A P-Editor: Guo X
Contributor Information
Sultan AlSheikh, Division of Vascular Surgery, Department of Surgery, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia.
Abdulmajeed Altoijry, Division of Vascular Surgery, Department of Surgery, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia.
Husain Al-Mubarak, Division of Vascular Surgery, Department of Surgery, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia.
Ofays Dakkam Alsallum, Division of Vascular Surgery, Department of Surgery, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia; Division of Vascular Surgery, Department of Surgery, King Khalid Hospital Najran, Najran 11321, Saudi Arabia.
Fadi Alakeel, Department of Pathology, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia.
Tariq Alanezi, Division of Vascular Surgery, Department of Surgery, College of Medicine, King Saud University, Riyadh 11322, Saudi Arabia. alanezitariq@gmail.com.
References
- 1.Hoffmann C, Hentrich M, Tiemann M, Rosenwald A, Weber F, Willenbacher W, Hübel K. Recent Advances in Castleman Disease. Oncol Res Treat. 2022;45:693–704. doi: 10.1159/000526640. [DOI] [PubMed] [Google Scholar]
- 2.Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56:1252–1260. doi: 10.3109/10428194.2014.953145. [DOI] [PubMed] [Google Scholar]
- 3.Masaki Y, Kawabata H, Fujimoto S, Kawano M, Iwaki N, Kotani T, Nakashima A, Kurose N, Takai K, Suzuki R, Aoki S. Epidemiological analysis of multicentric and unicentric Castleman disease and TAFRO syndrome in Japan. J Clin Exp Hematop. 2019;59:175–178. doi: 10.3960/jslrt.19021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone A, Borok M, Damania B, Gloghini A, Polizzotto MN, Jayanthan RK, Fajgenbaum DC, Bower M. Castleman disease. Nat Rev Dis Primers. 2021;7:84. doi: 10.1038/s41572-021-00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molacek J, Treska V, Skalicky T, Vodicka J, Ferda J, Ferdova E, Baxa J, Mach C, Jungova A, Michal M. Unicentric form of Castleman´s disease, pitfalls of diagnosis and surgical treatment. Front Oncol. 2023;13:1057683. doi: 10.3389/fonc.2023.1057683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talat N, Schulte KM. Castleman's disease: systematic analysis of 416 patients from the literature. Oncologist. 2011;16:1316–1324. doi: 10.1634/theoncologist.2011-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RSM. Unicentric Castleman Disease. Hematol Oncol Clin North Am. 2018;32:65–73. doi: 10.1016/j.hoc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16:236–246. doi: 10.1097/PAP.0b013e3181a9d4d3. [DOI] [PubMed] [Google Scholar]
- 9.Oksenhendler E, Boutboul D, Fajgenbaum D, Mirouse A, Fieschi C, Malphettes M, Vercellino L, Meignin V, Gérard L, Galicier L. The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol. 2018;180:206–216. doi: 10.1111/bjh.15019. [DOI] [PubMed] [Google Scholar]
- 10.Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135:1353–1364. doi: 10.1182/blood.2019000931. [DOI] [PubMed] [Google Scholar]
- 11.Chang KC, Wang YC, Hung LY, Huang WT, Tsou JH, M Jones D, Song HL, Yeh YM, Kao LY, Medeiros LJ. Monoclonality and cytogenetic abnormalities in hyaline vascular Castleman disease. Mod Pathol. 2014;27:823–831. doi: 10.1038/modpathol.2013.202. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Lan X, Li C, Zhang Y, Wang Y, Xue W, Lu L, Jin M, Zhou Z, Wang X, Li L, Zhang L, Li X, Fu X, Sun Z, Wu J, Zhang X, Yu H, Nan F, Chang Y, Yan J, Wu X, Wang G, Zhang D, Zhang Y, Young KH, Zhang M. Recurrent PDGFRB mutations in unicentric Castleman disease. Leukemia. 2019;33:1035–1038. doi: 10.1038/s41375-018-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson JS. Diagnosis and Management of Castleman Disease. J Natl Compr Canc Netw. 2019;17:1417–1419. doi: 10.6004/jnccn.2019.5037. [DOI] [PubMed] [Google Scholar]
- 14.Bonekamp D, Horton KM, Hruban RH, Fishman EK. Castleman disease: the great mimic. Radiographics. 2011;31:1793–1807. doi: 10.1148/rg.316115502. [DOI] [PubMed] [Google Scholar]
- 15.Boutboul D, Fadlallah J, Chawki S, Fieschi C, Malphettes M, Dossier A, Gérard L, Mordant P, Meignin V, Oksenhendler E, Galicier L. Treatment and outcome of Unicentric Castleman Disease: a retrospective analysis of 71 cases. Br J Haematol. 2019;186:269–273. doi: 10.1111/bjh.15921. [DOI] [PubMed] [Google Scholar]
- 16.Meignin V, Parrens M. La maladie de Castleman: aspects anatomopathologiques. Rev Med Interne. 2022;43:10S10–10S16. doi: 10.1016/S0248-8663(23)00020-6. [DOI] [PubMed] [Google Scholar]
- 17.Nabel CS, Sameroff S, Shilling D, Alapat D, Ruth JR, Kawano M, Sato Y, Stone K, Spetalen S, Valdivieso F, Feldman MD, Chadburn A, Fosså A, van Rhee F, Lipkin WI, Fajgenbaum DC. Virome capture sequencing does not identify active viral infection in unicentric and idiopathic multicentric Castleman disease. PLoS One. 2019;14:e0218660. doi: 10.1371/journal.pone.0218660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677–684. doi: 10.1097/SLA.0b013e318249dcdc. [DOI] [PubMed] [Google Scholar]
- 19.Mitsos S, Stamatopoulos A, Patrini D, George RS, Lawrence DR, Panagiotopoulos N. The role of surgical resection in Unicentric Castleman's disease: a systematic review. Adv Respir Med. 2018;86:36–43. doi: 10.5603/ARM.2018.0008. [DOI] [PubMed] [Google Scholar]
- 20.van Rhee F, Oksenhendler E, Srkalovic G, Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S, Streetly M, Wong R, Wu D, Maillard I, Brandstadter J, Munshi N, Bowne W, Elenitoba-Johnson KS, Fössa A, Lechowicz MJ, Chandrakasan S, Pierson SK, Greenway A, Nasta S, Yoshizaki K, Kurzrock R, Uldrick TS, Casper C, Chadburn A, Fajgenbaum DC. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. 2020;4:6039–6050. doi: 10.1182/bloodadvances.2020003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam Z, Řehák Z, Adamová Z, Koukalová R, Pour L, Krejčí M, Boichuk I, Krejčí M, Štork M, Ševčíková S, Král Z. Unicentric Castlemans disease. Symptoms, diagnostics and therapy. Vnitr Lek. 2021;67:465–473. [PubMed] [Google Scholar]
- 22.Wang W, Medeiros LJ. Castleman Disease. Surg Pathol Clin. 2019;12:849–863. doi: 10.1016/j.path.2019.03.003. [DOI] [PubMed] [Google Scholar]