Abstract
BACKGROUND
Subarachnoid hemorrhage is a severe neurological condition that requires prompt and appropriate treatment to prevent complications. Aneurysms are the most common cause of spontaneous subarachnoid hemorrhage. Conversely, basilar artery perforator aneurysms (BAPAs) are a rare etiology. There is no consensus on the optimal management of ruptured BAPAs in the acute setting.
CASE SUMMARY
We present a case series of 3 patients with ruptured BAPAs who were treated at our institution. Two patients had a modified Fisher grade of I, and one had a grade of IV on initial presentation. The aneurysms were detected by computed tomography angiography in two cases and conventional angiography in one case. The 3 patients underwent endovascular treatment with Guglielmi detachable coils. Post-treatment, the patients had good clinical outcomes, and follow-up brain computed tomography scans showed reduced subarachnoid hemorrhage without any new hemorrhage. However, one patient experienced a cerebral infarction 2 months later and eventually succumbed to the condition. The other 2 patients showed progressive recovery, and no aneurysm recurrence was observed at the 2-year follow-up.
CONCLUSION
Endovascular treatment may be a preferable approach for managing ruptured BAPAs compared with surgical intervention or conservative management. Early detection and prompt treatment is important to achieve favorable patient outcomes.
Keywords: Basilar artery, Intracranial aneurysm, Endovascular treatment, Subarachnoid hemorrhage, Case report
Core Tip: Basilar artery perforator aneurysms are a rare cause of subarachnoid hemorrhage. This case series of 3 patients treated with endovascular coiling suggested that this approach may be preferable for managing ruptured basilar artery perforator aneurysms compared with surgical intervention or conservative management. Early detection and prompt treatment are critical. Treatment plans should be individualized based on the patient’s specific circumstances. Further research is needed to establish evidence-based guidelines for the optimal management of this rare condition.
INTRODUCTION
Basilar artery perforator aneurysms (BAPAs) are an uncommon etiology of subarachnoid hemorrhage[1]. Their diagnosis necessitates a high degree of suspicion because they are often small, partially thrombosed, and supplied by diminutive vessels. Because of slow blood flow, BAPAs may exhibit delayed filling, making them challenging to detect on initial angiography[2]. In this case series, we presented 3 patients with ruptured BAPAs who underwent successful endovascular coiling. Two patients demonstrated good clinical outcomes; one patient succumbed to cerebral infarction 2 months post-treatment.
The optimal management of BAPAs remains debatable, with surgical, endovascular, and conservative approaches all being viable options depending on individual patient circumstances. Larger studies are essential to determine the natural history of these lesions and refine treatment strategies. This case series underscored the importance of meticulous angiographic techniques and heightened awareness for accurate diagnosis and timely intervention to achieve favorable patient outcomes.
CASE PRESENTATION
Chief complaints
Case 1: A 58-year-old female presented with sudden onset of dizziness and nausea.
Case 2: A 79-year-old female presented with a 1-h history of headache and vomiting.
Case 3: A 31-year-old female presented with a 30-min history of headache, nausea, and vomiting.
History of present illness
Case 1: The patient initially had a Glasgow Coma Scale (GCS) score of 15 but rapidly deteriorated becoming poorly responsive with bilateral gaze deviation and a GCS score of 3.
Case 2: The patient presented with a 1-h history of headache and vomiting.
Case 3: The patient suddenly developed poor responsiveness, coma (GCS 3), and desaturation (SpO2: 78%).
History of past illness
Case 1: The patient had a history of hypertension.
Case 2: The patient had a history of hypertension.
Case 3: This patient had a disease-free medical history.
Personal and family history
Cases 1-3: None of the patients had a family history of neurovascular disease.
Physical examination
Case 1: Blood pressure: 154/89 mmHg; Level of consciousness: Comatose (GCS 3); Pupillary response: Sluggish bilaterally; Extraocular movements: Bilateral gaze deviation; Corneal reflex: Absent bilaterally; Motor response: Flaccid posturing; and Babinski sign: Positive bilaterally.
Case 2: Blood pressure: 198/79 mmHg; Level of consciousness: Drowsy (GCS 10); Pupillary response: Sluggish; Extraocular movements: Nystagmus; Motor response: Bilateral weakness; Meningeal signs: Neck stiffness, Kernig’s sign, and Brudzinski’s sign were present.
Case 3: Blood pressure: 146/76 mmHg; Oxygen saturation: Decreased (SpO2 78%); Level of consciousness: Comatose (GCS 3); Pupillary response: Non-reactive, fixed pupils bilaterally; Extraocular movements: Absent or minimal response to oculocephalic reflex (Doll’s eye); Corneal reflex: Absent bilaterally; Gag reflex: Absent; Motor response: Flaccid or decerebrate posturing; Babinski sign: Positive bilaterally.
Laboratory examinations
Cases 1-3: All 3 patients exhibited normal findings on laboratory examinations including: Hemoglobin concentration with an average of 12.3 g/dL; Renal function: Serum urea with an average of 3.5 mmol/L; Serum creatine with an average of 78 μmol/L; Liver function: serum aspartate aminotransferase with an average of 23 U/L and serum alanine aminotransferase with an average of 34 U/L; Sodium with an average of 140 mmol/L; potassium with an average of 3.9 mmol/L; and Chloride with an average of 103 mmol/L.
Imaging examinations
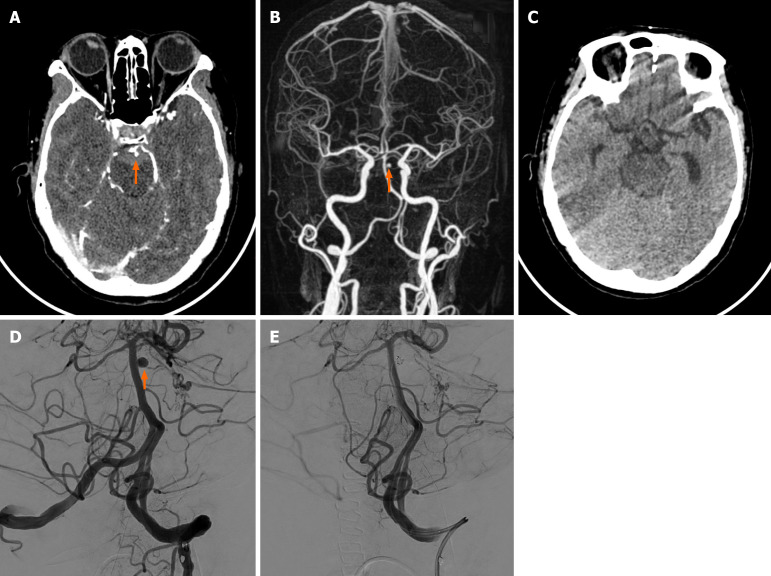
Case 1: Urgent brain CT and CT angiography revealed a ruptured left superior cerebellar artery aneurysm with pontine and right cerebellar hematomas (Figure 1). Angiography showed the aneurysm noted in the left side of the basilar artery (Figure 1).
Figure 1.
Case 1. A: Axial enhanced brain computed tomography of a 58-year-old female showed a tiny aneurysm found in the left superior cerebellar artery. Subarachnoid hemorrhage was noted; B: Three-dimensional reconstruction showed a 4.2 mm × 3.0 mm × 2.6 mm aneurysm found in the left superior cerebellar artery; C: Follow-up brain computed tomography (24 months post-operation) showed no new subarachnoid hemorrhage; D: Angiography showed the aneurysm noted on the left side of the basilar artery was clearly visualized by the frontal and lateral views; E: A 3.0 mm × 80.0 mm metallic coil was impacted into the aneurysm sac. Follow-up angiogram confirmed complete embolization and parent artery was intact.
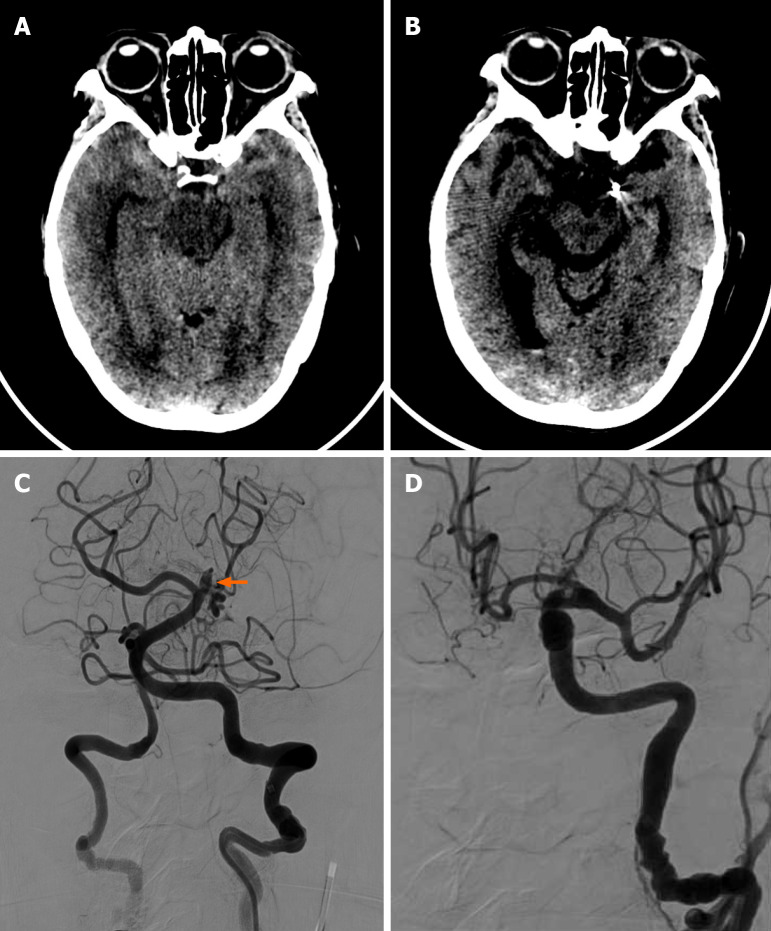
Case 2: Head CT showed bilateral temporal lobe subarachnoid hemorrhage and fourth ventricle hematoma (Figure 2). Cerebral angiography revealed a small (2.0 mm × 2.0 mm) wide-necked aneurysm in the P2 segment of the left posterior cerebral artery (Figure 2).
Figure 2.
Case 2. A: Axial brain computed tomography of a 79-year-old female showed bilateral subarachnoid hemorrhage in the bilateral temporal region; B: Axial brain computed tomography post-treatment showed the previous subarachnoid hemorrhage in the bilateral temporal region had subsided. No intracranial hemorrhage was found; C: Angiography showed a bulging aneurysm in the basilar perforated branches; D: Final angiography showed complete embolization of the aneurysm.
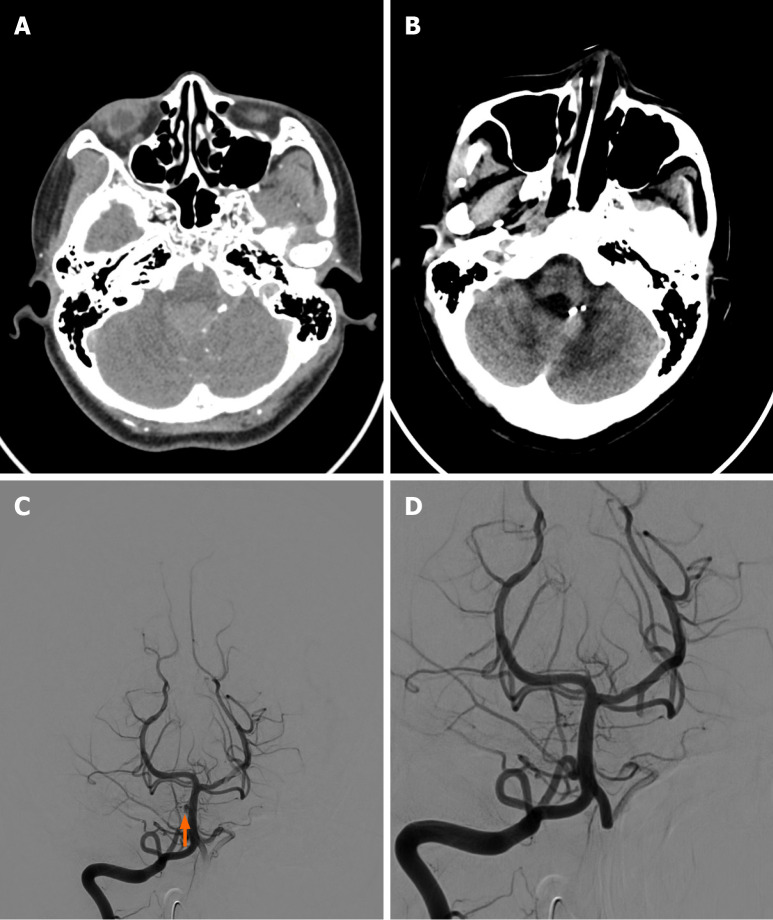
Case 3: Urgent brain CT revealed a ruptured aneurysm of the meningeal branch of the left vertebral artery with cerebellar vermis hematoma and intraventricular hemorrhage (Figure 3). Angiography showed a double berry-shaped aneurysm supplied by the left posterior inferior cerebellar artery branch and an unruptured right-sided basilar artery perforator aneurysm (Figure 3).
Figure 3.
Case 3. A: Axial brain computed tomography with contrast of a 31-year-old female showed an irregular saccular structure found in one of the left distal vertebral artery meningeal branches; B: Follow-up (24 months) axial brain non-contrast computed tomography of the same patient showed the previous subarachnoid hemorrhage regressed, and no new subarachnoid hemorrhage was noted; C: Angiography showed a tiny semi-circle saccular lesion noted on the right side of the basilar artery, and it was considered an unruptured basilar artery perforator aneurysm; D: Metallic coil embolization of these aneurysms was performed.
FINAL DIAGNOSIS
Case 1
Ruptured left superior cerebellar artery aneurysm with pontine and right cerebellar hematomas.
Diagnostic reasoning: The patient presented with sudden onset headache, nausea, and vomiting, consistent with subarachnoid hemorrhage. CT and digital subtraction angiography imaging confirmed the presence of a ruptured aneurysm arising from the left superior cerebellar artery, with associated pontine and contralateral cerebellar hematomas. Other considerations included arteriovenous malformation, dural arteriovenous fistula, and hemorrhage from a tumor, but the imaging findings were most consistent with a ruptured saccular aneurysm. The location arising from a basilar perforating artery is unusual.
Prognostic characteristics: The presence of brainstem and bilateral cerebellar hematomas suggested a more severe initial presentation, which may be associated with a higher risk of neurological deficits and complications. However, timely endovascular treatment and management of hydrocephalus with an external ventricular drain can improve outcomes.
Case 2
Ruptured small wide-necked aneurysm in the P2 segment of the left posterior cerebral artery.
Diagnostic reasoning: The patient presented with headache and meningism, raising suspicion for subarachnoid hemorrhage, which was confirmed on CT. Cerebral angiography revealed a small (2.5 mm) aneurysm with a relatively wide neck arising from the P2 segment of the left posterior cerebral artery. Given the location and morphology, alternate diagnoses such as dissection or vasculitis were considered less likely. Perforator aneurysms in this location are uncommon.
Prognostic characteristics: The small size of the aneurysm and the absence of significant intraparenchymal hemorrhage were favorable prognostic factors. However, the wide neck of the aneurysm may make endovascular treatment more challenging, potentially impacting the risk of complications and long-term outcome.
Case 3
Ruptured aneurysm of the meningeal branch of the left vertebral artery with cerebellar vermis hematoma and intraventricular hemorrhage and an unruptured right-sided basilar artery perforator aneurysm.
Diagnostic reasoning: This patient presented with thunderclap headache and depressed consciousness. Imaging confirmed subarachnoid, intraventricular, and intracerebellar hemorrhage. The unusual location of the ruptured aneurysm arising from a meningeal branch of the left vertebral artery made it challenging to initially identify and required repeat angiography. The additional finding of a small unruptured aneurysm arising from a right basilar perforator was also noted. Other diagnostic considerations included vascular malformations or hemorrhage from a tumor.
Prognostic characteristics: The presence of intraventricular hemorrhage and cerebellar hematoma suggested a more severe initial presentation, which may be associated with a higher risk of complications such as hydrocephalus and neurological deficits. The unruptured basilar artery perforator aneurysm added complexity to the management and required careful follow-up. Early endovascular treatment and management of hydrocephalus can improve outcomes, but the overall prognosis may be guarded given the extent of the initial hemorrhage.
TREATMENT
The management of posterior fossa aneurysms requires a tailored approach based on the specific location, morphology, and presentation of each aneurysm. In the presented cases, a combination of surgical and endovascular interventions was used to address the immediate life-threatening situations and prevent further hemorrhage.
Case 1
The patient underwent an emergency posterior fossa craniectomy to decompress the brainstem and cerebellum, followed by endovascular coiling of the ruptured left superior cerebellar artery aneurysm. This two-pronged approach was intended to alleviate the mass effect caused by the pontine and right cerebellar hematomas while securing the aneurysm to promote healing.
Case 2
Metallic coils were used for the successful endovascular embolization of a small, wide-necked aneurysm in the P2 segment of the left posterior cerebral artery. Because of the challenging location and morphology of the aneurysm, the parent artery was sacrificed to achieve complete occlusion and minimize the risk of future rupture. Although this approach effectively secured the aneurysm, it also had the potential risk of ischemic complications in the territory supplied by the sacrificed artery.
Case 3
The patient presented with a unique combination of a ruptured aneurysm of the meningeal branch of the left vertebral artery and an unruptured right-sided BAPA. Both aneurysms were successfully treated in a single session using endovascular coil embolization, highlighting the benefit of this minimally invasive approach in managing multiple aneurysms while reducing the patient’s overall procedural risk.
OUTCOME AND FOLLOW-UP
Case 1
The patient’s GCS score improved significantly from 3 to 10 following the emergency posterior fossa craniectomy and endovascular coiling of the aneurysm. Follow-up brain CT scans at 6 months and 24 months post-treatment demonstrated no aneurysm recurrence, indicating a favorable long-term outcome.
Case 2
Although the post-treatment CT showed resolution of the subarachnoid hemorrhage, the patient unfortunately experienced a left cerebellar infarction 2 months later, likely due to the sacrifice of the parent artery during the embolization procedure. This ischemic complication ultimately led to the patient’s demise, underscoring the delicate balance between aneurysm occlusion and preservation of the arterial supply in the posterior fossa.
Case 3
The patient’s condition improved following the successful endovascular coil embolization of both the ruptured aneurysm of the meningeal branch of the left vertebral artery and the unruptured right-sided BAPA. Follow-up CT scans at 6 months and 24 months post-treatment revealed no aneurysm recurrence, suggesting a favorable long-term outcome and the effectiveness of the endovascular approach in this case.
Literature review
We analyzed 80 BAPA cases including the three cases reported here (Tables 1 and 2[1,3-29]). The mean age of the patients was 56.7 years (range: 2-82 years), and the mean aneurysm diameter was 2.1 mm (range: 0.2-7.0 mm). Forty-three percent of the aneurysms were located in the distal third of the basilar artery, whereas 57% were in the middle third. Ninety-eight percent of BAPAs presented with diffuse subarachnoid hemorrhage or focal perimesencephalic/prepontine subarachnoid hemorrhage on CT. Notably, up to 60% of the lesions were not visible on initial catheter angiography.
Table 1.
Demographic and treatment data for each patient
|
Patient
|
Age in yr
|
Sex
|
Modified Fisher grade
|
Detection on initial angiography
|
Size in mm
|
Location of perforating artery of BA
|
Treatment
|
Material for embolization
|
Infarction post-treatment
|
Rebleed after treatment
|
Clinical outcome
|
Length of follow-up
|
| 1 | 58 | F | 1 | Yes | 4.2 × 2.6 × 3.0 | Mid 1/3 | Endovascular | Coils | No | No | Stable | 18 months |
| 2 | 79 | F | 1 | Yes | 2.0 × 2.0 × 2.5 | Distal 1/3 | Endovascular | Coils | Yes | No | Dead | 2 months |
| 3 | 31 | F | 4 | Yes | 3.0 × 2.5 × 3.0 | Mid 1/3 | Endovascular | Coils | No | No | Stable | 24 months |
BA: Basilar artery; F: Female.
Table 2.
Review of previously published basilar artery perforated aneurysm cases
|
Author
|
Yr of publication
|
Age in yr
|
Location of perforating artery of BA
|
Treatment
|
Pontine infarction
|
Rebleed subarachnoid hemorrhage
|
Clinical outcome
|
Length of follow-up
|
| Ghogawala et al[1] | 1996 | 56 | Distal | Surgery | No | No | Stable | 6 months |
| Hamel et al[11] | 2005 | 44 | Mid | Surgery | No | No | Residual ataxia | 7 months |
| Fiorella et al[12] | 2006 | 13 | Distal 1/3 | Endovascular | No | No | Stable | 6 months |
| Sanchez-Meija et al[7] | 2007 | 27 | Distal 1/3 | Surgery | No | No | Stable | Unknown |
| 68 | Mid 1/3 | Surgery | No | No | Stable | Unknown | ||
| 2 | Mid 1/3 | Surgery | No | No | Stable | Unknown | ||
| Park et al[13] | 2009 | 54 | Distal 1/3 | Conservative | No | No | Stable | 16 months |
| 67 | Distal 1/3 | Conservative | No | No | Stable | 16 months | ||
| 53 | Distal 1/3 | Conservative | No | No | Stable | 1 month | ||
| Mathieson et al[12] | 2010 | 51 | Distal 1/3 | Surgery | No | No | Mild amnesia | Unknown |
| Chen et al[8] | 2012 | 66 | Mid 1/3 | Endovascular | No | No | Stable | 24 months |
| 28 | Mid 1/3 | Endovascular | No | No | Stable | 18 months | ||
| Nyberg et al[15] | 2013 | 50 | Mid 1/3 | Endovascular | No | No | Stable | 2 months |
| 60 | Mid 1/3 | Endovascular | No | No | Stable | 4 months | ||
| Ding et al[9] | 2013 | 58 | Distal 1/3 | Conservative | No | No | Stable | 6 wk |
| 55 | Distal 1/3 | Conservative | Yes | No | Stable | 19 months | ||
| 62 | Distal 1/3 | Endovascular | Yes | No | Hemiparesis | 22 months | ||
| Chalouhi et al[16] | 2014 | 65 | Mid 1/3 | Endovascular | No | No | Stable | 6 months |
| Chavent et al[4] | 2014 | 55 | Distal 1/3 | Conservative | No | No | Stable | 6 months |
| 39 | Distal 1/3 | Conservative | No | No | Stable | 12 months | ||
| 59 | Distal 1/3 | Conservative | No | No | Stable | 6 months | ||
| Forbrig et al[5] | 2015 | 71 | Mid 1/3 | Conservative | Yes | Yes | Hemiparesis | 11 months |
| 65 | Mid 1/3 | Conservative | Yes | No | Hemiparesis | 15 months | ||
| 82 | Mid 1/3 | Conservative | Yes | No | Hemiparesis | 6 months | ||
| 60 | Distal 1/3 | Conservative | Yes | No | Stable | 78 months | ||
| 53 | Distal 1/3 | Conservative | No | No | Stable | 6 months | ||
| 72 | Distal 1/3 | Conservative | No | No | Mild cognitive impairment | 5 months | ||
| 59 | Distal 1/3 | Endovascular | No | Yes | Hemiparesis | 23 months | ||
| 71 | Distal 1/3 | Endovascular | No | No | Hemiparesis | 60 months | ||
| Aboukais et al[17] | 2016 | 67 | Distal | Conservative | No | No | Stable | 6 wk |
| Daruwalla et al[3] | 2016 | 76 | Mid | Conservative | No | No | Dead | 16 d |
| Finitsis et al[10] | 2017 | 59 | Distal | Conservative | No | No | Stable | 6 wk |
| 78 | Distal | Conservative | Yes | No | Quadriparesis | 12 months | ||
| 53 | Mid | Conservative | No | No | Stable | 6 wk | ||
| 62 | Mid | Endovascular | No | No | Stable | 3 months | ||
| Chau et al[19] | 2017 | 53 | Distal | Endovascular | No | No | Stable | 6 months |
| Satti et al[6] | 2017 | 52 | Mid | Endovascular | No | No | Stable | 7 months |
| Buell et al[18] | 2018 | 62 | Mid 1/3 | Endovascular | No | No | Stable | 49 months |
| 63 | Mid | Endovascular | No | No | Stable | 50 months | ||
| Bhogal et al[20] | 2019 | 65 | Mid | Endovascular | No | No | Stable | 3 months |
| 55 | Distal | Endovascular | No | No | Stable | 9 months | ||
| 66 | Mid | Endovascular | No | No | Stable | 12 months | ||
| 41 | Mid | Endovascular | Yes | No | Stable | 5 months | ||
| 52 | Mid | Endovascular | No | No | Stable | 4 months | ||
| 39 | Mid | Endovascular | No | No | Stable | 3 months | ||
| 59 | Mid | Conservative | No | No | Stable | 3 months | ||
| 57 | Distal | Conservative | No | No | Stable | 1 month | ||
| 62 | Distal | Conservative | No | Yes | Hemiparesis | 2 months | ||
| Enomato et al[22] | 2020 | 60 | Distal 1/3 | Conservative | Yes | No | Stable | 2 months |
| Nathan et al[24] | 2020 | 62 | Distal 1/3 | Conservative | No | No | Stable | 30 months |
| 48 | Distal 1/3 | Conservative | No | No | Stable | 12 months | ||
| Mizuno et al[21] | 2020 | 69 | Mid 1/3 | Endovascular | No | No | Stable | 1 month |
| Sattur et al[26] | 2020 | 62 | Mid 1/3 | Endovascular | No | No | Stable | 3 months |
| Inoue et al[28] | 2020 | 72 | Mid 1/3 | Endovascular | Yes | No | Stable | 3 months |
| Ma et al[23] | 2021 | 52 | Distal 1/3 | Endovascular | No | No | Stable | 6 months |
| 2021 | 48 | Mid 1/3 | Endovascular | No | No | Stable | 18 months | |
| 2021 | 65 | Mid 1/3 | Endovascular | No | No | Dead | N/A | |
| Kuhn et al[25] | 2021 | Unknown | Mid 1/3 | Endovascular | Yes | No | Stable | 12 d |
| Mutlu et al[27] | 2022 | 64 | Mid 1/3 | Endovascular | No | No | Stable | 2 wk |
| Elsheikh et al[29] | 2022 | 57 (40-78 ± 10.7) | N/A | Endovascular | Yes (1 case) | Yes (2 cases) | Stable (16 cases); dead (2 cases) | Median: 180 d |
BA: Basilar artery; N/A: Not available.
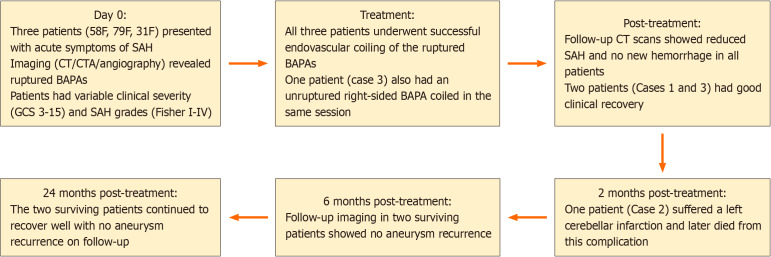
Twenty-five patients received conservative treatment with a mean follow-up of 10.2 months (range: 1-78 months). Seven patients (11.1%) developed brainstem infarction, and two patients experienced rebleeding after the initial subarachnoid hemorrhage. Among the 52 surgically treated cases, 6 underwent open surgery, and 46 received endovascular therapy using various embolic materials, including coils (6 cases), stents (6 cases), Onyx (2 cases), flow diverters (28 cases), electrothrombosis (3 cases), and a flow redirection endoluminal device (1 case). The treatment and follow-up timeline of our BAPA patients is shown in Figure 4.
Figure 4.
Treatment and follow-up timeline of patients with basilar artery perforator aneurysm. BAPA: Basilar artery perforator aneurysm; CT: Computed tomography; CTA: Computed tomography angiography; F: Female; GCS: Glasgow Coma Score; SAH: Subarachnoid hemorrhage.
DISCUSSION
Subarachnoid hemorrhage is a common neurological disease; its causes can be divided into traumatic and nontraumatic. Nontraumatic subarachnoid hemorrhage is usually due to aneurysm rupture. BAPA is a rare disease whose diagnosis requires a high degree of suspicion. The first case was reported by Ghogawala et al[1] in 1996. Even with high suspicion, BAPA is easy to ignore because the aneurysm is small, usually partly thrombosed, and supplied by small-caliber blood vessels[2,3]. They usually show delayed filling secondary to slow blood flow, which may be difficult to see[4,5]. Sixty-one percent of the lesions were not found in the first angiography, and the lesions became obvious on repeated angiography. Therefore, in the process of angiography, good patient fixation and sufficient injection are the guarantee of diagnosis. Two of our cases were identified by CT angiography, and aneurysms could also be found in the subsequent angiography.
The etiology of BAPA is unknown. It is considered to originate from separation, and some thrombosis may occur at the time of diagnosis. They are considered to be more benign than other ruptured aneurysms. Among the patients treated conservatively, 2 patients bled again 15 d after the attack[5,20]. However, 7 patients with conservative treatment also had spontaneous perforator stroke, and the clinical consequences ranged from mild to severe[5,9,10,22].
From such a small series of case reports, we cannot draw a decisive conclusion about the optimal treatment algorithm. Surgical, endovascular, or conservative treatment should be carefully discussed according to the actual situation of each patient.
Endovascular techniques include coiling, overlapping stents, liquid embolization, and shunt placement. Direct coiling of BAPAs is rarely possible because it requires catheterization of very small diameter perforators, which are usually at right angles to the main basilar artery. In addition, these aneurysms are too small to safely place coils, and the preservation of perforator patency is another problem. In the published cases, the aneurysms were in the middle of the basilar artery, most of which were treated with coils, and there was no rebleeding or cerebral embolism[8,18,21]. Among the reported cases of BAPA treated with overlapping stents, 6 patients had no complications of embolization. Stenting is technically easier than coil embolization because it does not require a microcatheter to pass through the perforator[6,15,19,26,28].
Flow diverters are sometimes deployed for BAPA treatment; this off-label application is considered to reduce flow enough to cause the occlusion of these thrombosis-prone aneurysms while maintaining the patency of the parent vessel to meet ongoing physiological needs. Elsheikh et al[29] described the use of a flow diverter in the treatment of BAPA. Eighteen patients were treated with flow diversion for BAPA, and one case of puncture embolism and two cases of rebleeding occurred. Kuhn et al[25] published a case report on the use of a flow redirection endoluminal device (FRED) to treat BAPA. The FRED is a double-layered, self-expanding braided nickel titanium alloy mesh fluid diverter. The double-layered shunt part of the device can be accurately positioned above the aneurysm, and the flared end can fix the device in the parent vessel without affecting the flow into the branch vessel/perforator. This is particularly important along the basilar artery because the device reduces the coverage of uninvolved perforators. Other flow diverters do not have this special design and will cover more orifices over the entire length of the unit. There were no postoperative complications. However, the number of cases was small, and more cases are needed to prove the efficacy of FRED[25].
In addition to the embolization methods mentioned above, Ma et al[23] reported a case of treatment of BAPA with electrothrombosis. There are two potential advantages of intravascular electrothrombosis. First, immediate hemostasis is effective. This concentrated current reduces treatment time compared with coil embolization. In addition, intravascular electrothrombosis may alleviate bleeding earlier than flow diverter implantation. Second, the overall risk of bleeding was reduced. Compared with catheterization and coiling, electrothrombosis improves the efficiency of microsurgery, and the wire head can be inserted more safely into the aneurysm[23]. Efficiency of microsurgery, and the wire head can be inserted more safely into the aneurysm[23].
All of our cases were treated with coiling because the neck of the aneurysms were quite wide, and we successfully embolized the aneurysms using a microcatheter. Patients with endovascular treatment had better clinical outcomes. Another patient was treated with conservative treatment, and the clinical condition gradually improved after treatment. All patients underwent follow-up brain CT after 1 month, which showed that the previous subarachnoid hemorrhage had been absorbed and no new subarachnoid hemorrhage had been noted. Two patients recovered well and were in good condition at the 24 months follow-up; the other patient experienced cerebral infarction and died.
CONCLUSION
Rupture of BAPA is a rare cause of subarachnoid hemorrhage. Careful angiographic techniques and repeated diagnostic cerebral angiography may be necessary for correct diagnosis. BAPA can spontaneously form thrombosis, but it may also rebleed or be related to basilar artery perforator infarction. In selected cases, these lesions can initially be treated with the most appropriate treatment. Larger studies are needed to fully understand the natural history and improve the treatment strategy for these lesions.
Footnotes
Informed consent statement: Informed consent of the patient for publication, including personal data and pictures, was given.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Kaneko J, Japan S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Zheng XM
Contributor Information
Ieong-Chon Man, Department of Radiology, Hospital Conde S. Januário, Macao SAR 999078, China.
Tam-Man Pan, Department of Radiology, Hospital Conde S. Januário, Macao SAR 999078, China.
Kuok-Cheong U, Department of Radiology, Hospital Conde S. Januário, Macao SAR 999078, China. kuokcu1@gmail.com.
References
- 1.Ghogawala Z, Shumacher JM, Ogilvy CS. Distal basilar perforator artery aneurysm: case report. Neurosurgery. 1996;39:393–396. doi: 10.1097/00006123-199608000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Abraham MK, Chang WW. Subarachnoid Hemorrhage. Emerg Med Clin North Am. 2016;34:901–916. doi: 10.1016/j.emc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Daruwalla VJ, Syed FH, Elmokadem AH, Hurley MC, Shaibani A, Ansari SA. Large basilar perforator pseudoaneurysm: A case report. Interv Neuroradiol. 2016;22:662–665. doi: 10.1177/1591019916659261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavent A, Lefevre PH, Thouant P, Cao C, Kazemi A, Mourier K, Ricolfi F. Spontaneous resolution of perforator aneurysms of the posterior circulation. J Neurosurg. 2014;121:1107–1111. doi: 10.3171/2014.7.JNS132411. [DOI] [PubMed] [Google Scholar]
- 5.Forbrig R, Eckert B, Ertl L, Patzig M, Brem C, Vollmar C, Röther J, Thon N, Brückmann H, Fesl G. Ruptured basilar artery perforator aneurysms--treatment regimen and long-term follow-up in eight cases. Neuroradiology. 2016;58:285–291. doi: 10.1007/s00234-015-1634-1. [DOI] [PubMed] [Google Scholar]
- 6.Satti SR, Vance AZ, Fowler D, Farmah AV, Sivapatham T. Basilar artery perforator aneurysms (BAPAs): review of the literature and classification. J Neurointerv Surg. 2017;9:669–673. doi: 10.1136/neurintsurg-2016-012407. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Mejia RO, Lawton MT. Distal aneurysms of basilar perforating and circumferential arteries. Report of three cases. J Neurosurg. 2007;107:654–659. doi: 10.3171/JNS-07/09/0654. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Chen E, Chotai S, Tian X. An endovascular approach to ruptured aneurysms of the circumferential branch of the basilar artery. J Clin Neurosci. 2012;19:527–531. doi: 10.1016/j.jocn.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 9.Ding D, Starke RM, Jensen ME, Evans AJ, Kassell NF, Liu KC. Perforator aneurysms of the posterior circulation: case series and review of the literature. J Neurointerv Surg. 2013;5:546–551. doi: 10.1136/neurintsurg-2012-010557. [DOI] [PubMed] [Google Scholar]
- 10.Finitsis S, Derelle AL, Tonnelet R, Anxionnat R, Bracard S. Basilar Perforator Aneurysms: Presentation of 4 Cases and Review of the Literature. World Neurosurg. 2017;97:366–373. doi: 10.1016/j.wneu.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Hamel W, Grzyska U, Westphal M, Kehler U. Surgical treatment of a basilar perforator aneurysm not accessible to endovascular treatment. Acta Neurochir (Wien) 2005;147:1283–1286. doi: 10.1007/s00701-005-0615-2. [DOI] [PubMed] [Google Scholar]
- 12.Fiorella D, Kelly ME, Albuquerque FC, Nelson PK. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212–7; discussion 217. doi: 10.1227/01.NEU.0000337576.98984.E4. [DOI] [PubMed] [Google Scholar]
- 13.Park SQ, Kwon OK, Kim SH, Oh CW, Han MH. Pre-mesencephalic subarachnoid hemorrhage: rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien) 2009;151:1639–1646. doi: 10.1007/s00701-009-0416-0. [DOI] [PubMed] [Google Scholar]
- 14.Mathieson CS, Barlow P, Jenkins S, Hanzely Z. An unusual case of spontaneous subarachnoid haemorrhage - a ruptured aneurysm of a basilar perforator artery. Br J Neurosurg. 2010;24:291–293. doi: 10.3109/02688690903572095. [DOI] [PubMed] [Google Scholar]
- 15.Nyberg EM, Chaudry MI, Turk AS, Spiotta AM, Fiorella D, Turner RD. Report of two cases of a rare cause of subarachnoid hemorrhage including unusual presentation and an emerging and effective treatment option. J Neurointerv Surg. 2013;5:e30. doi: 10.1136/neurintsurg-2012-010387. [DOI] [PubMed] [Google Scholar]
- 16.Chalouhi N, Jabbour P, Starke RM, Zanaty M, Tjoumakaris S, Rosenwasser RH, Gonzalez LF. Treatment of a basilar trunk perforator aneurysm with the pipeline embolization device: case report. Neurosurgery. 2014;74:E697–701; discussion 701. doi: 10.1227/NEU.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 17.Aboukais R, Zairi F, Estrade L, Quidet M, Leclerc X, Lejeune JP. A dissecting aneurysm of a basilar perforating artery. Neurochirurgie. 2016;62:263–265. doi: 10.1016/j.neuchi.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Buell TJ, Ding D, Raper DMS, Chen CJ, Hixson HR, Crowley RW, Evans AJ, Jensen ME, Liu KC. Posterior circulation perforator aneurysms: a proposed management algorithm. J Neurointerv Surg. 2018;10:55–59. doi: 10.1136/neurintsurg-2016-012891. [DOI] [PubMed] [Google Scholar]
- 19.Chau Y, Sachet M, Sédat J. Super-selective coil embolization of a basilar perforator artery aneurysm previously treated by the stent-in-stent technique, using an extremely soft bare coil delivered through a one-marker microcatheter. Interv Neuroradiol. 2017;23:492–496. doi: 10.1177/1591019917720807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhogal P, AlMatter M, Hellstern V, Pérez MA, Lehmberg J, Ganslandt O, Bäzner H, Henkes H. Basilar artery perforator aneurysms: Report of 9 cases and review of the literature. J Clin Neurosci. 2019;63:122–129. doi: 10.1016/j.jocn.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno H, Wakabayashi K, Shimizu T, Tomita Y, Koga H, Yoshimoto Y. Deconstructive Endovascular Treatment of Ruptured Serpentine Basilar Artery Aneurysm by Mid-Basilar Occlusion. World Neurosurg. 2021;146:40–44. doi: 10.1016/j.wneu.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto N, Shinno K, Tamura T, Shikata E, Shono K, Takase K. Ruptured Basilar Artery Perforator Aneurysm: A Case Report and Review of the Literature. NMC Case Rep J. 2020;7:93–100. doi: 10.2176/nmccrj.cr.2019-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma H, Zhao R, Fang Y, Li Q, Yang P, Huang Q, Xu Y, Hong B, Liu JM. Endovascular electrothrombosis: A promising alternative for basilar artery perforator aneurysm treatment. Interv Neuroradiol. 2021;27:511–515. doi: 10.1177/1591019920987913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlobin NA, Cantrell DR, Ansari SA, Hurley MC, Shaibani A, Jahromi BS, Potts MB. Conservative Management and Natural History of Ruptured Basilar Perforator Artery Aneurysms: Two Cases and Literature Review. World Neurosurg. 2020;138:218–222. doi: 10.1016/j.wneu.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn AL, Puri AS, Massari F, Singh J. Intravascular Wrap for Treatment of Basilar Artery Perforator Aneurysm. Cureus. 2021;13:e18021. doi: 10.7759/cureus.18021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattur MG, Gunasekaran A, Spiotta AM, Lena JR. Basilar Artery Perforator Aneurysms and their Contemporary Management. Neurol India. 2020;68:1301–1306. doi: 10.4103/0028-3886.304111. [DOI] [PubMed] [Google Scholar]
- 27.Mutlu U, Kortman H, Boukrab I. A giant basilar artery perforator aneurysm. Radiol Case Rep. 2022;17:911–913. doi: 10.1016/j.radcr.2021.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Y, Kusaka N, Ikushima K, Edaki H, Shinji Y, Itami H, Otsuka S, Nishiura T, Ogihara K. A case of simple stenting for ruptured basilar perforator aneurysm. J Stroke Cerebrovasc Dis. 2020;29:104855. doi: 10.1016/j.jstrokecerebrovasdis.2020.104855. [DOI] [PubMed] [Google Scholar]
- 29.Elsheikh S, Möhlenbruch M, Seker F, Berlis A, Maurer C, Kocer N, Jamous A, Behme D, Taschner C, Urbach H, Meckel S. Flow Diverter Treatment of Ruptured Basilar Artery Perforator Aneurysms : A Multicenter Experience. Clin Neuroradiol. 2022;32:783–789. doi: 10.1007/s00062-021-01133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]