Abstract
MxA is a large, interferon-induced GTPase with antiviral activity against RNA viruses. It forms large oligomers, but whether oligomerization and GTPase activity are important for antiviral function is not known. The mutant protein MxA(L612K) carries a lysine-for-leucine substitution at position 612 and fails to form oligomers. Here we show that monomeric MxA(L612K) lacks detectable GTPase activity but is capable of inhibiting Thogoto virus in transiently transfected Vero cells or in a Thogoto virus minireplicon system. Likewise, MxA(L612K) inhibited vesicular stomatitis virus multiplication. These findings indicate that MxA monomers are antivirally active and suggest that GTP hydrolysis may not be required for antiviral activity. MxA(L612K) is rapidly degraded in cells, whereas wild-type MxA is stable. We propose that high-molecular-weight MxA oligomers represent a stable intracellular pool from which active MxA monomers are recruited.
The interferon-induced human MxA protein belongs to the group of large GTP-binding proteins that includes dynamin and the human guanylate-binding protein 1 (GBP1) (17, 26, 27). A common feature of these proteins is their ability to form high-molecular-weight oligomers (3, 14, 25). Large GTPases serve diverse cellular functions, such as endocytosis (28) and intracellular protein trafficking (10, 21), but the functional significance of oligomerization is not clear in most cases. MxA is unique in having broad antiviral activity against several RNA viruses (5, 7, 15), including Thogoto virus (THOV), a tick-transmitted orthomyxovirus that transcribes and replicates its genome in the cell nucleus (6). Upon infection of MxA-expressing cells with THOV, MxA recognizes the incoming viral ribonucleoprotein complexes (vRNPs) and retains them in the cytoplasm, thereby preventing the translocation of the viral genome into the nucleus (12). Likewise, MxA has recently been shown to be inhibitory in a THOV minireplicon system (29) in which recombinant vRNPs are reconstituted from cloned cDNAs (30). Using this in vivo reconstitution system, we demonstrated that MxA recognizes assembled vRNPs rather than single viral components (29). However, the mechanistic details of viral target recognition and the roles of oligomerization and GTP hydrolysis in antiviral function remain unclear.
Several domains have been proposed to be responsible for the formation of MxA oligomers. Two leucine zipper motifs in the murine Mx1 protein, LZ1 and LZ2, originally described by Melén and coworkers for (14), are of particular importance for oligomerization (3, 22). Schumacher and Staeheli (22) have previously shown that the carboxyl-terminal region of MxA folds back onto an internal region within a central interaction domain (CID) (4). It has been proposed that this backfolding is required for GTPase activity (23) and assembly into MxA homooligomers (3). Alternatively, the LZ1 domain of one molecule could fold back onto the CID of a neighboring molecule (22). When many molecules interact in this way, large multimeric structures are formed.
Here we asked whether oligomerization and GTP hydrolysis are required for antiviral activity or whether MxA monomers are also antivirally active. Assuming that an interaction of the LZ1 domain with the CID is critical for oligomerization and GTPase activity, mutations in LZ1 should abolish backfolding and oligomerization and presumably reduce the enzymatic activity of the GTPase. A mutation was introduced into LZ1 by site-directed mutagenesis leading to the replacement of the leucine residue at position 612 with a lysine residue. This exchange destroys the amphipathic character of helix LZ1 without disturbing the overall alpha-helical structure of the domain (3). The resulting MxA(L612K) mutant was unable to form large multimeric complexes as demonstrated both in yeast and mammalian cells (3, 22). Therefore, we used this particular mutant to assess the importance of oligomerization and GTP hydrolysis for antiviral function of MxA.
MxA(L612K) lacks detectable GTPase activity.
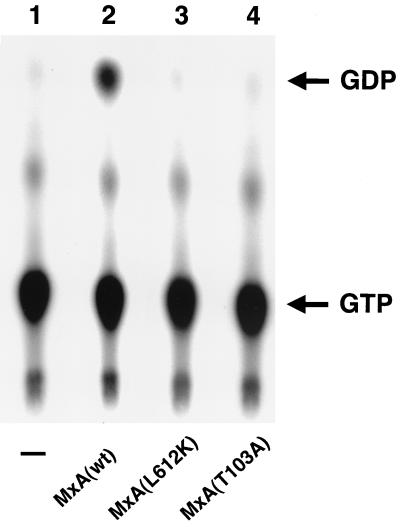
Histidine-tagged MxA(L612K) protein was purified from Escherichia coli by nickel agarose affinity chromatography followed by gel filtration chromatography (Hi Load16/6 Superdex 200; Amersham-Pharmacia) as previously described (13, 19). The monomeric mutant protein eluted from the S200 gel filtration column in a single fraction expected to contain proteins of approximately 80 kDa (data not shown). Histidine-tagged wild-type MxA protein and the dominant-negative mutant MxA(T103A) (16) were purified in the same way and eluted mostly with the void volume of the column, indicating that they formed large oligomers, as expected (19, 23). MxA(T103A) has a threonine-to-alanine exchange in the GTP binding domain at position 103 that abolishes GTP binding and antiviral activity (16) and was used as a negative control in these assays. The purified proteins (0.2 μg) were incubated at 37°C for 60 min in a standard GTPase assay as previously described (13, 19). The reaction products were resolved by thin-layer chromatography and detected by autoradiography. Figure 1 shows that MxA(L612K) lacked detectable GTPase activity, as did MxA(T103A). This is in agreement with a previous report (2) and demonstrates that efficient GTPase activity depends on proper protein folding and oligomerization. Most likely, interactions of the C terminus with the N-terminal catalytic domain are necessary for efficient catalytic activity of Mx proteins (4), as discussed for dynamin elsewhere (25). Since the GTP binding domain of MxA(L612K) is left intact, GTP binding probably still occurs, but this remains to be formally demonstrated.
FIG. 1.
MxA(L612K) lacks detectable GTPase activity. GTP hydrolysis of mutant MxA(L612K) protein was compared with that of wild-type MxA [MxA(wt)] or inactive mutant MxA(T103A) in a standard GTPase assay. Lane 1 is a control without protein in the reaction mixture.
Antiviral activity of MxA(L612K) in transfected cells.
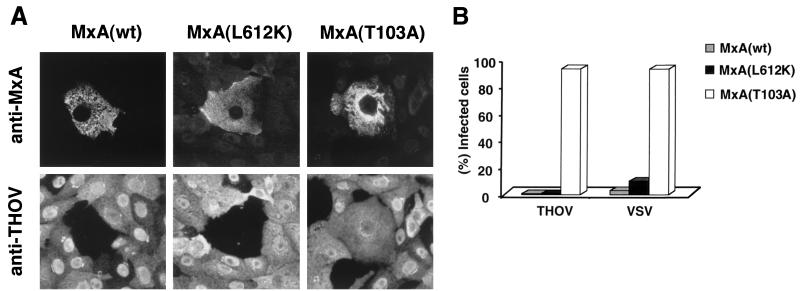
Vero cells were transiently transfected with expression constructs encoding either monomeric MxA(L612K), wild-type MxA, or mutant MxA(T103A) and subsequently assayed for sensitivity to THOV infection by double-immunofluorescence analysis. Transfection was performed with 2 μg of plasmids pHMG-MxA(L612K), pHMG-MxA(wt), or pHMG-MxA(T103A) using Lipofectamine (GIBCO-BRL, Wiesbaden, Germany), according to the manufacturer's instructions. Twenty-four hours later, the cells were infected with 50 PFU of THOV strain SiAr126 per cell (1). Accumulation of viral antigens in infected cells was examined 9 h after infection with a hyperimmune guinea pig antiserum (kindly provided by P. A. Nuttall, NERC Institute, Oxford, United Kingdom) (9). Simultaneously, MxA proteins were detected with the mouse monoclonal antibody M143 that is directed against a conserved epitope in the N-terminal half of the MxA molecule (4). Cells were then stained with fluorescent secondary antibodies and observed using a microscope equipped with epifluorescence as described previously (16). Figure 2A shows that no viral proteins were produced in cells expressing MxA(L612K) or wild-type MxA, indicating that the cells were protected from infection. In contrast, nontransfected cells were fully permissive, as were Vero cells expressing antivirally inactive MxA(T103A). A quantitative analysis of this experiment is shown in Fig. 2B. Approximately 95% of MxA(T103A)-expressing cells were infected with THOV and accumulated viral antigens, but none of the MxA(L612K)-expressing cells (total of 173 cells analyzed) or wild-type-MxA-expressing cells (total of 220 cells analyzed) were infected. These results indicate that the monomeric form of MxA is as effective against THOV as the wild-type molecule. Similar results were obtained when vesicular stomatitis virus (VSV) was used as the challenge virus (Fig. 2B). Only 14 of 141 cells expressing MxA(L612K) (10%) and 6 of 180 cells expressing wild-type MxA (6%) were positive for viral antigens. In contrast, 220 of 235 cells expressing MxA(T103A) (93%) were infected. To investigate the antiviral effect of MxA(L612K) in more detail, we turned to a THOV minireplicon system that allows a quantitative analysis of MxA action (29).
FIG. 2.
MxA(L612K) is antivirally active. (A) Vero cells transfected with cDNAs encoding either wild-type MxA [MxA(wt)] or mutant protein MxA(L612K) or MxA(T103A) were infected with 50 PFU of THOV per cell. Cells were fixed 9 h later, and MxA proteins or viral proteins were detected by double immunofluorescence using a monoclonal antibody directed against MxA and a polyclonal antiserum directed against THOV antigens. (B) Quantitative analysis of infection experiments with THOV and VSV. The percentage of infected cells expressing either wild-type MxA [MxA(wt)], MxA(L612K), or MxA(T103A) is shown. Cells were infected with 10 PFU of VSV serotype Indiana per cell (15).
MxA(L612K) blocks reporter gene expression in a THOV minireplicon system.
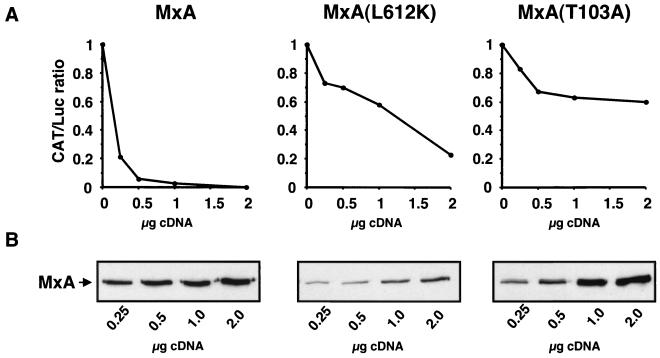
We have recently established a minireplicon system in which recombinant vRNPs of THOV are reconstituted from cloned cDNAs (30). In this system, a model minigenome RNA containing the chloramphenicol acetyltransferase (CAT) gene in the negative-sense orientation, flanked by THOV regulatory genomic sequences, is coexpressed together with the three polymerase subunits (PA, PB1, and PB2) and the viral nucleoprotein (NP). Coexpression leads to the formation of transcriptionally active vRNPs that, in turn, direct the synthesis of CAT mRNA. The amount of CAT synthesized in this system faithfully reflects the transcriptional activity of the reconstituted vRNPs. We have previously shown that wild-type MxA, but not mutant MxA(T103A), inhibits the transcriptional activity of these reconstituted transcription units and that the degree of inhibition is directly proportional to the amount of MxA protein present (29). To assess the antiviral activity of MxA(L612K), COS-1 cells were transfected with expression plasmids coding for either wild-type MxA, MxA(L612K), or MxA(T103A) in the presence of the components of the THOV minireplicon system, and CAT synthesis was measured as described previously (29). In agreement with previous findings, coexpression of wild-type MxA led to a dose-dependent inhibition of CAT synthesis, whereas mutant MxA(T103A) had only a small effect that did not correlate with the amount of plasmid transfected and was, therefore, considered to be nonspecific (Fig. 3A). Like wild-type MxA, monomeric MxA(L612K) also led to significant inhibition of CAT synthesis in a dose-dependent manner. However, to achieve an effect comparable to that obtained with wild-type MxA, a 10-fold-higher concentration of expression plasmid was required. Since protein expression levels, rather than the plasmid concentrations used, are critical in this assay, we determined the amounts of wild-type and mutant MxA proteins in cell lysates by Western blot analysis (Fig. 3B). Approximately 10-fold-higher plasmid concentrations were required for MxA(L612K) than for wild-type MxA to reach similar protein levels. Thus, MxA(L612K) appears to be as active as wild-type MxA when protein expression levels are taken into account.
FIG. 3.
MxA(L612K) inhibits reporter gene expression in a THOV minireplicon system. COS-1 cells were transfected with T7 promoter constructs coding for the components of a THOV minireplicon system as previously described (29). In this system, synthesis of CAT protein reflects the activity of the reconstituted vRNPs (30). Wild-type or mutant MxA was expressed under the control of the T7 promoter using increasing amounts of expression plasmid pBS-T7/MxA, pBS-T7/MxA(L612K), or pBS-T7/MxA(T103A). (A) CAT protein concentration as determined by a colorimetric immunoassay (Boehringer Mannheim). The amounts of CAT and luciferase activity were determined in the cell lysates. Luciferase activity was used to normalize CAT expression, and the ratio of CAT protein concentration to luciferase activity (CAT/Luc ratio) was calculated as described previously (29). The CAT/luciferase ratio of experiments without MxA were set at 1. (B) Expression of MxA proteins. Aliquots of the cell lysates (15 μg of protein per lane) were analyzed by Western blotting using a polyclonal antiserum directed against MxA.
Intracellular stability of MxA(L612K).
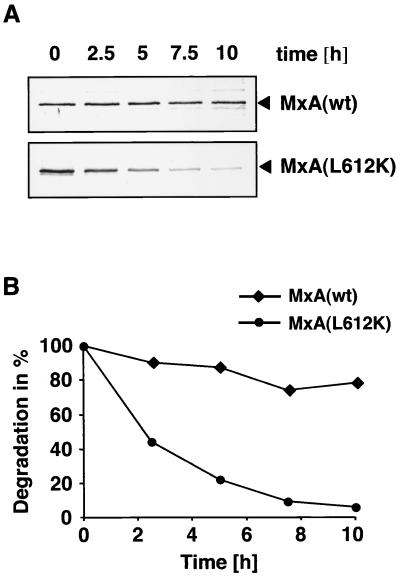
Next, we asked whether the relatively poor accumulation of MxA(L612K) observed in transfected COS-1 cells could be due to the higher turnover rates for MxA(L612K) than for wild-type MxA. To resolve this question, pulse-chase experiments were performed after expression of both types of MxA proteins in COS-1 cells. Newly synthesized proteins were metabolically labeled with [35S]methionine for 2 h as described previously (11) and then chased for 2, 5, 7.5, and 10 h. Wild-type MxA and MxA(L612K) were immunoprecipitated from cell lysates with monoclonal antibody M143 and analyzed by polyacrylamide gel electrophoresis and autoradiography (11). Figure 4A shows that the wild-type protein was extremely stable, as previously reported (8, 20). In contrast, MxA(L612K) was rapidly degraded. A quantitative PhosphoImager analysis of the immunoprecipitated proteins revealed that MxA(L612K) had a half-life of approximately 2 h, whereas 80% of wild-type MxA protein was still detectable after a chase of 10 h (Fig. 4B). It is conceivable that the high turnover rate of MxA(L612K) is due to its monomeric form and that oligomerization has a stabilizing effect on wild-type MxA. However, we cannot exclude the possibility that the L612K amino acid exchange contributes to rapid degradation of MxA(L612K) by influencing the conformation of the protein or its interactions with putative cellular partner molecules.
FIG. 4.
MxA(L612K) is rapidly degraded. COS-1 cells were transfected with expression plasmids coding for either MxA or MxA(L612K). Cells were then labeled for 2 h with 35 μCi of [35S]methionine per ml and chased for 2.5, 5, 7.5, and 10 h. (A) Wild-type and mutant MxA proteins were immunoprecipitated from cell lysates using a monoclonal antibody directed against MxA and analyzed by polyacrylamide gel electrophoresis and autoradiography. (B) PhosphoImager analysis of immunoprecipitated proteins. MxA(wt), wild-type MxA.
Conclusions.
The present results clearly demonstrate that MxA monomers can inhibit virus replication in infected cells. Most remarkably, GTP hydrolysis seems not to be required for antiviral activity in vivo. This is in line with evidence from previous in vitro studies. We have shown before that GTP binding is necessary and sufficient for viral target recognition by MxA (11). We have postulated that binding of GTP leads to a conformational change of the molecule, allowing tight binding to target structures, such as viral nucleocapsids. This interaction can be stabilized by GTPγS, a nonhydrolyzable analogue of GTP (11, 13). Likewise, studies with an in vitro VSV transcription system suggested that GTP binding is sufficient for inhibition by MxA, with no need for GTP hydrolysis (24).
Previous studies have shown that wild-type MxA forms large oligomers when purified to homogeneity (19). Gel filtration studies indicated that these oligomers were approximately 2,000 kDa (19). In MxA-expressing cells, immunofluorescence analysis reveals punctate granula (5, 15) (Fig. 2A) that most likely correspond to high-molecular-weight forms of MxA in the cytoplasm, although other interpretations are also possible. Thus, all available data indicate that MxA forms large aggregates in vitro and in vivo. What could be the role of these aggregates? MxA proteins need to be present in cells for a considerable time to combat invading viruses but are produced only for a short time in interferon-stimulated cells. Oligomerization may be instrumental in providing an intracellular pool of MxA that is relatively stable and from which antivirally active monomers can be recruited over prolonged periods of time. We therefore propose the following scenario. Intramolecular backfolding of LZ1 allows for a conformational change and the formation of a large intermolecular complex (3). The self-assembled aggregates represent the stable storage form of MxA. Monomers are released from such oligomeric structures by conformational changes that may involve GTP hydrolysis. It is also possible that virus infection somehow triggers recruitment and activation of functional MxA monomers, as previously discussed by Di Paolo (2). In fact, virus-induced activation of antiviral proteins has been shown to occur in other instances, for example with the double-stranded RNA-dependent protein kinase PKR and the 2′-5′ oligoadenylate synthetases (18, 31). It is conceivable that monomers represent an activated form of MxA and are able to recognize viral target structures. In the case of THOV, these targets are the functionally active vRNPs (11). Upon binding to their targets, MxA monomers may acquire an antivirally active conformation and block specific viral functions. In the absence of a viral target structure, MxA monomers may join again the pool of resting oligomers or are rapidly degraded.
(This research was conducted by C. Janzen in partial fulfillment of the requirements for a Ph.D. degree from the Faculty of Biology of the University of Freiburg, Freiburg, Germany, 2000.)
Acknowledgments
We thank Jovan Pavlovic for kindly providing plasmid pHMG-MxA(L612K) and for helpful suggestions and Friedemann Weber and Michael Frese for advice.
This work was supported in part by grant Ko 1579/1-2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Albanese M, Bruno-Smiraglia C, Di Di Cuonza G, Lavagnino A, Srihongse S. Isolation of Thogoto virus from Rhipicephalus bursa ticks in western Sicily. Acta Virol. 1972;16:267. [PubMed] [Google Scholar]
- 2.Di Paolo C. Role of the carboxy-terminal domain of MxA protein in antiviral activity and protein-protein interactions. Ph.D. thesis. Zurich, Switzerland: University of Zurich; 1998. [Google Scholar]
- 3.Di Paolo C, Hefti H P, Meli M, Landis H, Pavlovic J. Intramolecular backfolding of the carboxyl-terminal end of MxA protein is a prerequisite for its oligomerization. J Biol Chem. 1999;274:32071–32078. doi: 10.1074/jbc.274.45.32071. [DOI] [PubMed] [Google Scholar]
- 4.Flohr F, Schneider-Schaulies S, Haller O, Kochs G. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 1999;463:24–28. doi: 10.1016/s0014-5793(99)01598-7. [DOI] [PubMed] [Google Scholar]
- 5.Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70:915–923. doi: 10.1128/jvi.70.2.915-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus. J Virol. 1995;69:3904–3909. doi: 10.1128/jvi.69.6.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hefti H P, Frese M, Landis H, Di Paolo C, Aguzzi A, Haller O, Pavlovic J. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J Virol. 1999;73:6984–6991. doi: 10.1128/jvi.73.8.6984-6991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakschies D, Hochkeppel H, Horisberger M, Deicher H, Von Wussow P. Emergence and decay of the human Mx homolog in cancer patients during and after interferon-alpha therapy. J Biol Response Modif. 1990;9:305–312. [PubMed] [Google Scholar]
- 9.Jones J D, Nuttall P A. The effect of virus-immune hosts on Thogoto virus infection of the tick, Rhipicephalus appendiculatus. Virus Res. 1989;14:129–140. doi: 10.1016/0168-1702(89)90034-8. [DOI] [PubMed] [Google Scholar]
- 10.Jones S M, Howell K E, Henley J R, Cao H, McNiven M A. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 11.Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae) J Biol Chem. 1999;274:4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- 12.Kochs G, Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochs G, Trost M, Janzen C, Haller O. MxA GTPase: oligomerization and GTP-dependent interaction with viral RNP target structures. Methods Companion Methods Enzymol. 1998;15:255–263. doi: 10.1006/meth.1998.0629. [DOI] [PubMed] [Google Scholar]
- 14.Melén K, Ronni T, Broni B, Krug R M, von Bonsdorff C-H, Julkunen L. Interferon-induced Mx proteins form oligomers and contain a putative leucine zipper. J Biol Chem. 1992;267:25898–25907. [PubMed] [Google Scholar]
- 15.Pavlovic J, Zürcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponten A, Sick C, Weeber M, Haller O, Kochs G. Dominant-negative mutants of human MxA protein: domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J Virol. 1997;71:2591–2599. doi: 10.1128/jvi.71.4.2591-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakash B, Praefcke G J K, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403:567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 18.Rebouillat D, Hovanessian A G. The human 2′,5′-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties. J Interferon Cytokine Res. 1999;19:295–308. doi: 10.1089/107999099313992. [DOI] [PubMed] [Google Scholar]
- 19.Richter M F, Schwemmle M, Herrmann C, Wittinghofer A, Staeheli P. Interferon-induced MxA protein: GTP binding and GTP hydrolysis properties. J Biol Chem. 1995;270:13512–13517. [PubMed] [Google Scholar]
- 20.Ronni T, Melen K, Malygin A, Julkunen I. Control of IFN-inducible MxA gene expression in human cells. J Immunol. 1993;150:1715–1726. [PubMed] [Google Scholar]
- 21.Rothman J H, Raymond C K, Gilbert T, O'Hara P J, Stevens T H. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher B, Staeheli P. Domains mediating intramolecular folding and oligomerization of MxA GTPase. J Biol Chem. 1998;273:28365–28370. doi: 10.1074/jbc.273.43.28365. [DOI] [PubMed] [Google Scholar]
- 23.Schwemmle M, Richter M F, Herrmann C, Nassar N, Staeheli P. Unexpected structural requirements for GTPase activity of the interferon-induced MxA protein. J Biol Chem. 1995;270:13518–13523. [PubMed] [Google Scholar]
- 24.Schwemmle M, Weining K C, Richter M F, Schumacher B, Staeheli P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 25.Sever S, Muhlberg A, Schmid S. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- 26.Staeheli P, Pitossi F, Pavlovic J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 1993;3:268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 27.van der Bliek A M. Functional diversity in the dynamin family. Trends Cell Biol. 1999;9:96–102. doi: 10.1016/s0962-8924(98)01490-1. [DOI] [PubMed] [Google Scholar]
- 28.Warnock D E, Schmid S L. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 29.Weber F, Haller O, Kochs G. MxA GTPase blocks reporter gene expression of reconstituted Thogoto virus ribonucleoprotein complexes. J Virol. 2000;74:560–563. doi: 10.1128/jvi.74.1.560-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber F, Jambrina E, Gonzalez S, Dessens J T, Leahy M, Kochs G, Portela A, Nuttall P A, Haller O, Ortin J, Zurcher T. In vivo reconstitution of active Thogoto virus polymerase: assays for the compatibility with other orthomyxovirus core proteins and template RNAs. Virus Res. 1998;58:13–20. doi: 10.1016/s0168-1702(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 31.Williams B R. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]