Abstract
Background and purpose
Rituximab (RTX) is frequently used off‐label in multiple sclerosis. However, studies on the risk–benefit profile of RTX in pediatric‐onset multiple sclerosis are scarce.
Methods
In this multicenter retrospective cohort study, patients with pediatric‐onset multiple sclerosis from Sweden, Austria and Germany, who received RTX treatment were identified by chart review. Annualized relapse rates, Expanded Disability Status Scale scores and magnetic resonance imaging parameters (new T2 lesions and contrast‐enhancing lesions) were assessed before and during RTX treatment. The proportion of patients who remained free from clinical and disease activity (NEDA‐3) during RTX treatment was calculated. Side effects such as infusion‐related reactions, infections and laboratory abnormalities were assessed.
Results
Sixty‐one patients received RTX during a median (interquartile range) follow‐up period of 20.9 (35.6) months. The annualized relapse rate decreased from 0.6 (95% confidence interval [CI] 0.38–0.92) to 0.03 (95% CI 0.02–0.14). The annual rate of new T2 lesions decreased from 1.25 (95% CI 0.70–2.48) to 0.08 (95% CI 0.03–0.25) and annual rates of new contrast‐enhancing lesions decreased from 0.86 (95% CI 0.30–3.96) to 0. Overall, 70% of patients displayed no evidence of disease activity (NEDA‐3). Adverse events were observed in 67% of patients. Six patients discontinued treatment due to ongoing disease activity or adverse events.
Conclusion
Our study provides class IV evidence that RTX reduces clinical and radiological activity in pediatric‐onset multiple sclerosis.
Keywords: central nervous system, cohort study, disease‐modifying therapy, pediatric‐onset multiple sclerosis, rituximab
INTRODUCTION
Approximately 2%−5% of all multiple sclerosis (MS) patients present before the age of 18 years [1]. Patients with pediatric‐onset MS (POMS) show a two to three times higher relapse rate [2] and acquire physical and cognitive deficits at a younger age compared to their adult counterparts [3, 4]. Yet at disease onset brain volumes are smaller compared to age‐matched controls and brain growth is occurring at slower rates [5]. Healthcare resource utilization and costs are high in this population and patients report a reduction in quality of life and significant fatigue compared to age‐matched controls [6].
Early initiation of high‐efficacy treatment leads to better long‐term outcomes in both adult‐onset MS and POMS [7, 8]. However, only few disease‐modifying therapies (DMTs) approved for adults have been investigated in randomized controlled trials in the pediatric MS population [9, 10, 11] and access to treatment is highly variable around the globe.
In MS, treatment strategies targeting anti‐CD20 show excellent efficacy in reducing clinical and magnetic resonance activity [12, 13] and slowing disease progression [14]. Both ocrelizumab and ofatumumab are currently tested in phase III pediatric trials but, still, access in the pediatric MS population is limited, depending on local healthcare systems.
Rituximab (RTX) is currently used off‐label in many neuroimmunological disorders both in children and adults due to its excellent risk–benefit profile [15, 16, 17, 18, 19, 20]. In Sweden, RTX has become the most prescribed DMT for MS [21]. With respect to the pediatric MS population, increasing data from the United States [22, 23] as well as from Sweden [24] report high effectiveness and few side effects in their cohorts.
The aim of the present observational study is to report the safety and effectiveness of RTX in a cohort of 61 pediatric MS patients from Sweden, Austria and Germany.
PATIENTS AND METHODS
Study population and design
For this retrospective multicenter observational cohort study, patients with a diagnosis of pediatric MS (disease onset before the age of 18) were identified according to the current diagnostic criteria [25, 26] between 1 February 2009 and 1 February 2022 from local databases in Sweden, Austria and Germany. Patients who started RTX treatment before the age of 19 were eligible for the study.
Demographic data included age at first MS symptoms, age at MS diagnosis, sex, age at onset of first DMT, age at onset of RTX, time from disease onset to RTX onset, and the Expanded Disability Status Scale (EDSS) at first presentation. The use of DMTs before the initiation of RTX therapy as well as RTX infusion schedules were recorded. CD19+ counts were followed, if available.
Magnetic resonance imaging (MRI) scans were reviewed by local experienced radiologists (J. Sv., Sweden, for patients from Sweden; RI. M., Austria, for patients from Austria and Germany). The imaging variables were the number of T2 and contrast‐enhancing lesions (CELs) at baseline and at the predefined time points “before RTX” and “during RTX”.
Outcome measures
With respect to effectiveness, the annualized relapse rates (ARRs), the number of new T2 lesions and the number of CELs from disease onset to the onset of RTX as well as from RTX onset to the last available MRI during RTX therapy were calculated. EDSS scores at the time of RTX onset and the last available EDSS were also recorded. No evidence of disease activity (NEDA‐3), as defined by the absence of relapses, the absence of MR activity as well as the absence of EDSS progression, was assessed.
Adverse events
Adverse events (AEs) were grouped into infusion‐related AEs, infections and laboratory abnormalities such as hypogammaglobulinemia and lymphopenia.
Statistical analyses
Patient characteristics were assessed using descriptive statistics. The distribution of numerical data was assessed visually with histograms and quantile−quantile plots. According to distribution and sample size, continuous and discrete numerical variables are reported as median and interquartile range (IQR) and/or range where appropriate. Categorical variables are reported as frequency and percentage. Denominators of given frequency rates represent patients with available data.
Annualized relapse rates (ARRs), rates of new T2 lesions and new CELs were assessed using unadjusted negative binomial regression with log‐link. The data were separated into two groups, before and during RTX therapy, based on the start of RTX therapy. ARRs were estimated by modelling the number of relapses from disease onset to the start of RTX therapy and from RTX therapy to the last follow‐up over the respective observation periods. The initial attack was excluded from ARR calculations consistent with prior reports [24].
To estimate annual T2 lesion rates and CEL rates, MRI from three time points was used: (1) MRI at MS diagnosis; (2) MRI before RTX start; and (3) MRI at the last follow‐up. The number of new lesions since the previous MRI was modelled over the interval between MRIs before and during RTX therapy. Annual rates were compared, again using negative binomial regression with log‐link. Models included the data of both observation periods and were adjusted by a binary variable indicating the observation periods (before or during RTX).
Rates of new CELs were compared descriptively [27]. EDSS before and during RTX therapy were compared using a Wilcoxon signed‐rank test for paired data. Statistical analysis was performed using R version 4.1.3 (R Core Team, 2022, Vienna, Austria). The R packages ggplot2 (Wickham, 2016) and ggpubr (Kassambara, 2020) were used for graphical presentation of the data. Missing values in the outcome variables were handled by pairwise exclusion. Denominators of given frequency ratios represent patients with available data.
All patients and their caregivers gave informed consent to the study. The study was approved by the local institutional ethical review boards in Austria (Ethikkommission der Medizinischen Universität Wien, no. EK1123) and Germany (Ethics Committee of the Witten/Herdecke University, Germany, BIOMARKER‐Study number AN4059) and by the national ethical review board in Sweden (Etikprövningsmyndigheten, Dnr 2020‐02378).
RESULTS
Patient characteristics
All pediatric MS patients starting treatment with RTX before the age of 19 who could be identified by local databases and networks were included. One patient was excluded due to insufficient data. In summary, a total of 61 patients (43 females, 70%) from centers in Sweden, Austria and Germany were included in this study. Median age at first MS symptoms was 14.9 years (IQR 2.6). Median age at MS diagnosis was 15.2 years (IQR 2.6). All patients had a diagnosis of relapsing–remitting MS. Cerebrospinal‐fluid‐specific oligoclonal bands were found in 54 out of 60 patients (90%) (Table 1). In the remaining patient, lumbar puncture was performed, but oligoclonal band data were missing.
TABLE 1.
Baseline characteristics.
| All (n = 61) | |
|---|---|
| Female sex, n (%) | 43 (70) |
| Countries, n (%) | |
| Sweden | 44/61 (72) |
| Austria | 12/61 (20) |
| Germany | 5/61 (8) |
| Age at first symptoms, median (IQR, range) | 14.86 (2.6; 8.39–17.45) |
| Age at MS diagnosis, median (IQR, range) | 15.17 (2.58; 8.62–18.01) |
| EDSS at MS diagnosis, median (IQR, range; n = 11) | 3 (1.5; 2–6.5) |
| Relapsing–remitting MS, n (%) | 61 (100) |
| Oligoclonal bands, n (%) | 54/60 a (90) |
| First DMT, n (%) | |
| Rituximab | 42/61 (69) |
| Interferone | 7/61 (11) |
| Natalizumab | 7/61 (11) |
| Fingolimod | 3/61 (5) |
| Dimethyl fumarate | 1/61 (2) |
| Glatirameracetate | 1/61 (2) |
| Age at start of first DMT, n (%) | 15.38 (2.53; 9.73–17.62) |
| Days first symptoms to DMT start, median (IQR, range) | 150 (211; 7–2917) |
| Age at RTX start, median (IQR, range) | 16.25 (2.11; 9.73–18.31) |
| Days first symptoms to RTX start, median (IQR, range) | 240 (497; 17–2917) |
| EDSS at RTX start, median (IQR, range; n = 58) | 1 (2; 0–4) |
Abbreviations: DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; IQR, interquartile range; MS, multiple sclerosis; n, number; RTX, rituximab.
Oligoclonal band data were not available in one patient.
Disease‐modifying therapies
In 42 of 61 patients (69%), the first DMT used was RTX, interferon‐beta in seven of 61 (11%), natalizumab in seven of 61 (11%), fingolimod in three of 61 (5%) and dimethyl fumarate or glatiramer acetate in one of 61 each (2%). In 15 patients, RTX was used as the second DMT, in three patients as the third (after interferon‐beta and dimethyl fumarate in two and after interferon‐beta and natalizumab in one) and in one patient as the fourth DMT (after glatiramer acetate, dimethyl fumarate and fingolimod). Median age at the start of the first DMT was 15.4 years (IQR 2.5), whereas the median time from the first symptom to DMT start was 150 days (IQR 211). Median age at the start of RTX was 16.2 years (IQR 2.1). The median time from the first symptom to the start of RTX was 240 days (IQR 497). The median EDSS at the start of RTX treatment was 1.0 (IQR 2) (Table 1).
RTX infusion regimen
Rituximab (RTX) was administered according to local infusion regimens. A total of 330 doses were administered between February 2009 and February 2022. The median number of doses per patient was 5 (IQR 4). Treatment regimens varied with respect to (1) the number of initial doses (one [n = 32] versus two doses [n = 29]); (2) the time interval between the two doses if there were two initial doses (2 weeks [n = 20]; 3 weeks [n = 3] and 4 weeks [n = 6]); (3) the overall dose given, ranging from 375 mg/m2 body surface area (n = 17) and 500 mg/m2 body surface area (n = 6) to 500 mg (n = 19), 750 mg (n = 4), 950 mg (n = 1) and 1000 mg (n = 13) per infusion (in one patient, the dosing was not available); and (4) the redosing interval at fixed intervals of 6 months (n = 51) versus redosing according to B cell repopulation within 4−11 months (n = 10). The most common infusion regimen was a single initial dose of 500 mg (n = 13) or 1000 mg (n = 9) repeated every 6 months, as recommended by the Swedish MS Society [28]. Due to the highly variable regimens with small numbers within each group, adjusting for treatment regimens was not feasible.
Clinical disease activity
Six out of 61 patients experienced one relapse each. Relapses occurred within 4 weeks after the first infusion in three patients; and seven, 12 and 14 months after RTX start in the other three patients. The ARR decreased from 0.60 (95% confidence interval [CI] 0.38–0.92) before the start of RTX (70.53 person‐years at risk) to 0.03 (95% CI 0.01–0.07) during RTX treatment (181.42 person‐years at risk). The rate ratio between the two observation periods was 0.06 (95% CI 0.02–0.14, p < 0.001) (Figure 1a). 90% (55 of 61) of patients remained relapse‐free during RTX treatment.
FIGURE 1.
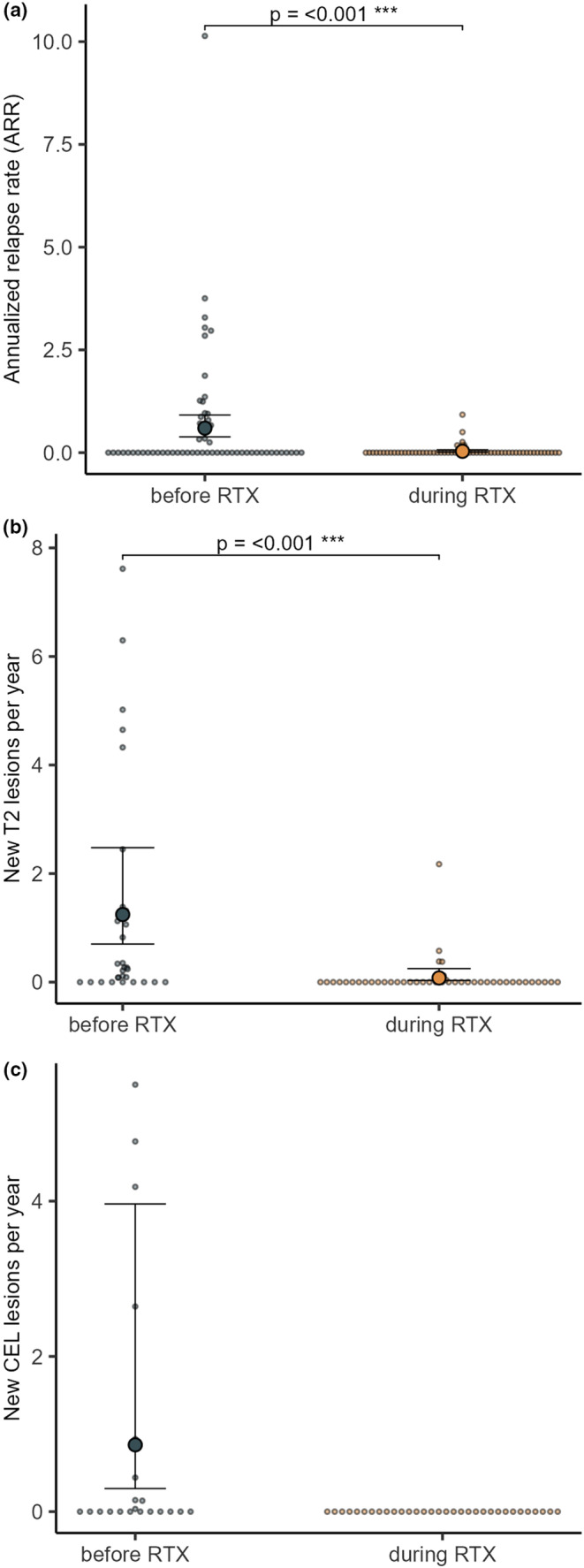
Annualized relapse rate, new T2 lesions and CELs before and during treatment with rituximab (RTX) (annual rate; 95% confidence interval). Small dots represent individual patients.
In a subgroup of 27 patients, a median of 9 (range 1–31) B cell counts was available during follow‐up. Amongst 20 of these 27 patients, some extent of repopulation was found at least once during follow‐up. Two relapses were documented in these 27 patients; one relapse occurred at the time of B cell return (7 months after RTX start), and one relapse was documented in a patient who was completely B cell depleted at the time of the relapse (6 months after the last RTX infusion and 12 months after RTX start). Relapses occurring within 4 weeks after treatment initiation were excluded from the analysis.
The median EDSS decreased from 1 (IQR 2) at RTX start to 0 (IQR 1.5) at last follow‐up (p = 0.027) (Figure 2).
FIGURE 2.
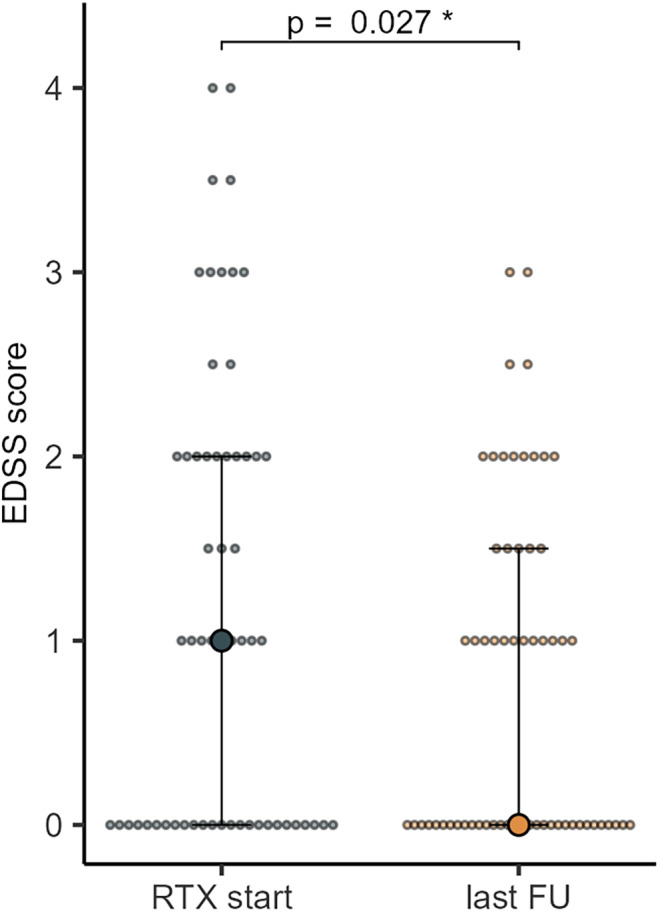
EDSS before rituximab (RTX) start and on last follow‐up (median; 95% confidence interval).
MRI activity
Magnetic resonance scans were performed at non‐standardized time points, with no predefined intervals between the scans before and after the first RTX infusion. Therefore MRI data from three time points were used: at MS diagnosis, before RTX start and at the last follow‐up. The median interval between MRI scans performed at the first two time points was 8.5 months (range 0.9−61.2 months) and the median interval between MRIs performed at the second two time points was 22.9 months (range 3.3−152.4 months).
The rate of new T2 lesions per year decreased from 1.25 (95% CI 0.70–2.48) to 0.08 (95% CI 0.03–0.25). The rate ratio between these observation periods was 0.06 (95% CI 0.02–0.17, p < 0.001) (Figure 1b). Annual rates of new CELs decreased from 0.860 (95% CI 0.30–3.96) before RTX to 0 during RTX (Figure 1c).
No evidence of disease activity (NEDA‐3) was seen in 70% (35 of 50) of patients during RTX.
Detailed data on statistical analyses are depicted in Table S1. Sensitivity analysis, excluding three of the major MS centers, is presented in Table S2.
Adverse events
Adverse events (AEs) were reported in 40 out of 60 patients (67%, data not available in n = 1 patient) and the majority (39 of 60 patients) were graded as mild to moderate. Infusion‐related AEs were the most frequently reported AEs in 29 of 60 patients (48%), followed by infectious complications in 12 of 60 patients (20%), hypogammaglobulinemia in nine of 52 patients (17%) and lymphopenia in four of 56 patients (7%). In four patients (7%), the treatment was permanently discontinued due to AEs, which was severe in one case (infusion‐related toxic reaction and dyspnea).
Last follow‐up and treatment persistence
Median time on RTX at the last follow‐up was 20.9 months (IQR 35.6, range 1.1–151.1). The overall treatment persistence of RTX was 90%.
Four patients discontinued RTX due to AEs and two patients due to disease activity. In total, 55/61 patients (90%) were followed until the end of the observation period. Individual clinical courses of the 61 patients are shown in Figure 3.
FIGURE 3.
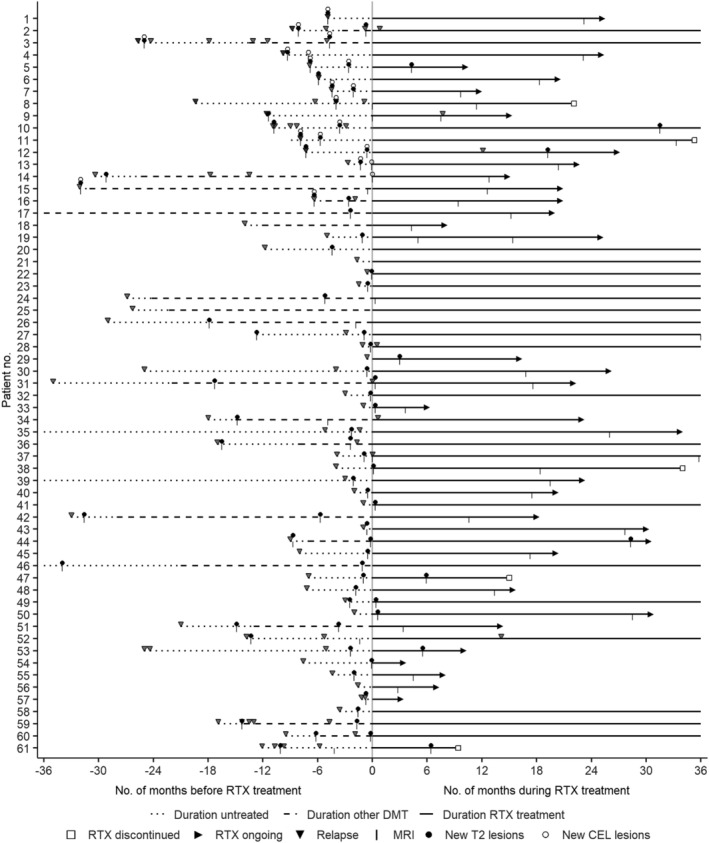
Individual clinical courses of pediatric‐onset MS patients. Lines indicate the duration of follow‐up (in months) before DMT (dotted), under DMT other than RTX (dashed) and under RTX therapy. Arrows at the end of the lines indicate continued RTX therapy after the last follow‐up, squares indicate discontinuation of RTX therapy before the end‐of‐study or patients lost to follow‐up. Triangles indicate relapses, vertical lines indicate MRIs, and points indicate detection of T2 lesions (brain and/or spinal) or CELs (brain and/or spinal). Zero on the x‐axis indicates the start of RTX therapy. CEL, contrast‐enhancing MRI lesion; DMT, disease‐modifying therapy; MRI, magnetic resonance imaging; RTX, rituximab.
DISCUSSION
Pediatric‐onset MS (POMS) is now recognized as a non‐benign inflammatory demyelinating disease associated with a high risk of early physical and cognitive impairment [3, 4], fatigue [6] and psychiatric comorbidities [29], leading to a significant impact on families and high healthcare resource utilization [6]. Therefore, there is increasing consensus on the need for early initiation of highly effective therapies [30, 31] in this young population. However, access to newer DMTs is limited in many countries, due to differing reimbursement rates of insurance providers.
In adult MS, recent phase 3 trials have shown the efficacy of the newer anti‐CD20‐therapies ocrelizumab and ofatumumab [12, 13, 14]. In pediatric MS, there are ongoing phase 3 trials evaluating the safety and efficacy of ocrelizumab compared with fingolimod (NCT05123703) and ofatumumab or siponimod versus fingolimod (NCT04926818).
Although RTX is only available for off‐label treatment of MS, it has become the most frequently prescribed DMT for treating relapsing–remitting MS in Sweden [21]. Data in the pediatric MS population are rare but consistently show significant reductions in relapse rates and MRI activity [22, 23, 24].
In this multicenter observational cohort study, effectiveness and safety from 61 pediatric MS patients treated with RTX over a median period of 20.9 months with a maximum treatment duration of 151 months are reported. Unique to our cohort is the use of RTX as a first‐line agent in 69% of patients.
Relapse rates, as well as the number of new T2 lesions or CELs, were significantly reduced after the onset of RTX and the median EDSS at last follow‐up was 0.0. 93% of patients remained free from relapses, 77% remained free from new T2 lesions and 100% were free from CELs. NEDA‐3, which is rarely reported in pediatric cohorts, was reached in 70% of patients.
Three out of the six documented relapses occurred within 4 weeks after the first RTX infusion, indicating overlap activity in this cohort of young and active MS patients. In contrast, it was not possible to determine the exact time point of the occurrence of new T2 lesions due to the highly variable MRI intervals before and after the start of RTX therapy and to the lack of a predefined re‐baselining MRI, which is a limitation of our study.
CD19+ counts were available in a subgroup of 27 patients. Twenty of these patients showed some extent of B cell return during follow‐up. Amongst the two relapses observed in these 27 patients, one occurred following B cell return, whilst the other occurred despite complete B cell depletion. Since MRI scans and serology were performed at non‐standardized intervals and variable frequencies, it was not possible to correlate MRI activity with B cell return. As shown in other cohorts [32], our data indicate that some extent of B cell repopulation does not lead to immediate return of relapse activity. However, further prospective data are needed in order to draw a conclusion on a correlation of B cell return and re‐occurrence of disease activity.
As one of the first studies, Salzer et al. reported the use of RTX in 14 pediatric MS patients who did not relapse after onset of RTX treatment [24]. Thirteen of these patients have also been included in our study, with longer follow‐up periods. Krysko et al. assessed the effectiveness of injectable DMTs versus newer DMTs and included 56 pediatric patients treated with RTX [22]. Whilst the study demonstrated that initial treatment with newer DMTs led to better disease control than treatment with injectable DMTs, no detailed data on the RTX subgroup were provided. A recently published Turkish case series provided data on 10 pediatric MS cases treated with ocrelizumab: none had relapses or new MRI lesions and the mean EDSS decreased from 1.75 to 1.20. One patient experienced anaphylaxis [33].
Only recently, Shukla et al. presented data on DMT use in 1092 patients from the US Network of Pediatric MS Centers including clinical and MRI data from 166 patients on RTX [23]. The relapse rate decreased from 1.01 to 0.12 and new T2 lesions were noted in only 10% of patients. NEDA‐2, including relapse and MRI, but not EDSS data, was achieved in 84% in the first 12 months. This is in contrast to our cohort in which new T2 lesions were found in 23% of patients. However, our patients were followed for a median observation period of 20.9 months and included EDSS scores in order to provide data on NEDA‐3.
In our cohort, AEs were reported in 67% of patients; most AEs. were infusion‐related and graded as mild to moderate. Hypogammaglobulinemia was found in nine out of 52 patients (17%). Although not common in our pediatric cohort, there is a known risk of hypogammaglobulinemia in particular on long‐term B cell depletion [12, 14] and therefore it has to be monitored also in the pediatric population. Whilst Krysko et al. reported a lower rate of AEs (26.3%) in POMS [22], in a randomized controlled study in adults AEs following RTX were detected in 98.6% [15]. Differences in study designs indicate that multicenter retrospective database studies may favor underreporting of minor AEs [22].
There are several limitations to our study. First, although data were collected prospectively, data analyses were performed retrospectively, and all patients were treated according to local protocols. Thus, there is high variability in treatment regimens and follow‐up management, which did not enable an adjustment for different treatment regimens. This also includes variable quality and sequences of MRI performed at each center according to local guidelines, including the lack of a re‐baselining MRI. To reduce potential local imaging bias, MR images were assessed by two experienced neuroradiologists specialized in imaging of POMS (patients from Sweden analyzed in Stockholm and patients from Austria and Germany analyzed in Vienna). Secondly, a selection bias has to be taken into account, as RTX is more often used in cases of higher disease activity. However, such selection bias will lead to underestimating the treatment effect. Thirdly, the limited sample size does not allow subgroup analyses. Fourthly, although significant, the EDSS decrease from 1.0 to 0.0 has no impact on activities of daily living. However, data on cognitive outcomes, a key problem in POMS [2, 4], accounting better for overall impairment, were not available in our cohort. Finally, the lack of a control group limits the interpretation of the data of this study.
In summary, our data suggest that RTX is an effective and safe treatment option in POMS when applied both as first‐line therapy and as a rescue therapeutic option. POMS is associated with higher inflammatory activity and irreversible disability at a younger age compared to adult‐onset MS [2, 3]. Therefore, as suggested recently [30, 31], the use of RTX as a first‐line agent, as seen in 69% of our patients, may prevent patients from the medical, social and emotional consequences of ongoing disease activity and reduce the risk of future disability in this particularly vulnerable population. In addition to the efforts on the development and evaluation of newer anti‐CD20 therapies in POMS our data underline that RTX is a treatment strategy with an excellent risk–benefit profile in pediatric MS patients at risk for unfavorable disease outcomes.
AUTHOR CONTRIBUTIONS
Barbara Kornek: Supervision; conceptualization; investigation; methodology; validation; writing – review and editing. Markus Breu: Conceptualization; methodology; writing – original draft; investigation; validation. Fredrik Sandesjö: Conceptualization; methodology; investigation; validation; writing – review and editing; writing – original draft. Ruxandra‐Iulia Milos: Investigation; methodology; software; validation; writing – review and editing. Jan Svoboda: Investigation; methodology; software; validation; writing – review and editing. Jonatan Salzer: Investigation; validation; writing – review and editing. Lisa Schneider: Methodology; validation; formal analysis; visualization; writing – review and editing. Julian Benedikt Reichelt: Investigation; methodology; project administration; writing – review and editing. Annikki Bertolini: Investigation; writing – review and editing. Astrid Blaschek: Investigation; writing – review and editing. Katharina Fink: Investigation; writing – review and editing. Romana Höftberger: Investigation; writing – review and editing. Jan Lycke: Investigation; writing – review and editing. Kevin Rostásy: Investigation; writing – review and editing. Rainer Seidl: Investigation; writing – review and editing. Sandy Siegert: Investigation; writing – review and editing. Ronny Wickström: Conceptualization; investigation; methodology; validation; writing – review and editing; supervision.
FUNDING INFORMATION
This study received support from StratNeuro, Karolinska Institutet, Stiftelsen Sunnerdahls Handikappfond, Sällskapet Barnavård and Hjärnfonden. RW was supported by Region Stockholm (clinical research appointment).
CONFLICT OF INTEREST STATEMENT
MB received speaker honoraria from Sanofi‐Genzyme. JoS received institutional consultancy fees from Mabion SA. KF received lecture honoraria from Merck; has served on scientific advisory boards for Biogen, Novartis, Merck, Roche and Celgene. JL received lecture honoraria from Biogen, Novartis, Merck, Roche, Axelion, Sanofi, Celgene and BMS; has served on scientific advisory boards for Almirall, Biogen, Novartis, Merck, Roche, Celgene, BMS and Sanofi; serves on the editorial board of the Acta Neurologica Scandinavica; has received unconditional research grants from Biogen and Novartis. KR received speaker honoraria from Merck and serves as a consultant for the Roche Operette2 trial. RW has served on scientific advisory boards for GW Pharma, Sobi and Octapharma, and has received speaker honoraria from Eisai and Sanofi. BK has received honoraria for speaking and participation in advisory boards from Biogen, BMS‐Celgene, Janssen, Merck, Novartis, Teva, Sanofi and Roche. FS, RM, JS, LS, JR, AB, AsB, RH, RS, SS report no conflicts of interest.
Supporting information
Table S1.
Table S2.
Breu M, Sandesjö F, Milos R‐I, et al. Rituximab treatment in pediatric‐onset multiple sclerosis. Eur J Neurol. 2024;31:e16228. doi: 10.1111/ene.16228
Markus Breu and Fredrik Sandesjö contributed equally to this work.
Ronny Wickström and Barbara Kornek contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chitnis T. Pediatric central nervous system demyelination. Continuum. 2019;25:793‐814. [DOI] [PubMed] [Google Scholar]
- 2. Gorman MP, Healy BC, Polgar‐Turcsanyi M, Chitnis T. Increased relapse rate in pediatric‐onset compared with adult‐onset multiple sclerosis. Arch Neurol. 2009;66:54‐59. [DOI] [PubMed] [Google Scholar]
- 3. Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356:2603‐2613. [DOI] [PubMed] [Google Scholar]
- 4. Ruano L, Branco M, Portaccio E, et al. Patients with paediatric‐onset multiple sclerosis are at higher risk of cognitive impairment in adulthood: an Italian collaborative study. Mult Scler. 2018;24:1234‐1242. [DOI] [PubMed] [Google Scholar]
- 5. Bartels F, Nobis K, Cooper G, et al. Childhood multiple sclerosis is associated with reduced brain volumes at first clinical presentation and brain growth failure. Mult Scler. 2019;25:927‐936. [DOI] [PubMed] [Google Scholar]
- 6. Greene N, Araujo L, Campos C, Dalglish H, Gibbs S, Yermilov I. The economic and humanistic burden of pediatric‐onset multiple sclerosis. JHEOR. 2022;9:103‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He A, Merkel B, Brown JWL, et al. Timing of high‐efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307‐316. [DOI] [PubMed] [Google Scholar]
- 8. Huppke P, Huppke B, Ellenberger D, et al. Therapy of highly active pediatric multiple sclerosis. Mult Scler. 2019;25:72‐80. [DOI] [PubMed] [Google Scholar]
- 9. Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta‐1a in pediatric multiple sclerosis. N Engl J Med. 2018;379:1017‐1027. [DOI] [PubMed] [Google Scholar]
- 10. Chitnis T, Banwell B, Kappos L, et al. Safety and efficacy of teriflunomide in paediatric multiple sclerosis (TERIKIDS): a multicentre, double‐blind, phase 3, randomised, placebo‐controlled trial. Lancet Neurol. 2021;20:1001‐1011. [DOI] [PubMed] [Google Scholar]
- 11. Vermersch P, Scaramozza M, Levin S, et al. Effect of dimethyl fumarate vs interferon β‐1a in patients with pediatric‐onset multiple sclerosis: the CONNECT randomized clinical trial. JAMA Netw Open. 2022;5:e2230439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hauser SL, Bar‐Or A, Comi G, et al. Ocrelizumab versus interferon beta‐1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221‐234. [DOI] [PubMed] [Google Scholar]
- 13. Hauser SL, Bar‐Or A, Cohen J, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546‐557. [DOI] [PubMed] [Google Scholar]
- 14. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209‐220. [DOI] [PubMed] [Google Scholar]
- 15. Hauser SL, Waubant E, Arnold DL, et al. B‐cell depletion with rituximab in relapsing−remitting multiple sclerosis. N Engl J Med. 2008;358:676‐688. [DOI] [PubMed] [Google Scholar]
- 16. Bar‐Or A, Calabresi PAJ, Arnold D, et al. Rituximab in relapsing−remitting multiple sclerosis: a 72‐week, open‐label, phase I trial. Ann Neurol. 2008;63:395‐400. [DOI] [PubMed] [Google Scholar]
- 17. Svenningsson A, Frisell T, Burman J, et al. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing−remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater‐blinded, phase 3, randomised controlled trial. Lancet Neurol. 2022;21:693‐703. [DOI] [PubMed] [Google Scholar]
- 18. Dale RC, Brilot F, Duffy LV, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology. 2014;83:142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghezzi A, Banwell B, Bar‐Or A, et al. Rituximab in patients with pediatric multiple sclerosis and other demyelinating disorders of the CNS: practical considerations. Mult Scler. 2021;27:1814‐1822. [DOI] [PubMed] [Google Scholar]
- 20. Beres SJ, Graves J, Waubant E. Rituximab use in pediatric central demyelinating disease. Pediatr Neurol. 2014;51:114‐118. [DOI] [PubMed] [Google Scholar]
- 21. Berntsson SG, Kristofferson A, Boström I, et al. Rapidly increasing off‐label use of rituximab in multiple sclerosis in Sweden—outlier or predecessor? Acta Neurol Scand. 2018;138:327‐331. [DOI] [PubMed] [Google Scholar]
- 22. Krysko KM, Graves J, Rensel M, et al. Real‐world effectiveness of initial disease‐modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020;88:42‐55. [DOI] [PubMed] [Google Scholar]
- 23. Shukla NM, Casper TC, Ness J, et al. Demographic features and clinical course of patients with pediatric‐onset multiple sclerosis on newer disease‐modifying therapies. Pediatr Neurol. 2023;145:125‐131. [DOI] [PubMed] [Google Scholar]
- 24. Salzer J, Lycke J, Wickström R, Naver H, Piehl F, Svenningsson A. Rituximab in paediatric onset multiple sclerosis: a case series. J Neurol. 2016;263:322‐326. [DOI] [PubMed] [Google Scholar]
- 25. Krupp LB, Tardieu M, Amatot MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune‐mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261‐1277. [DOI] [PubMed] [Google Scholar]
- 26. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017;17:162‐173. [DOI] [PubMed] [Google Scholar]
- 27. Akaishi T, Ishii T, Aoki M, Nakashima I. Calculating and comparing the annualized relapse rate and estimating the confidence interval in relapsing neurological diseases. Front Neurol. 2022;13:875456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sällskapet SM. Accessed 01 11, 2022. Available from: https://www.mssallskapet.se/wp‐content/uploads/2023/09/SMSS‐info‐om‐Rituximab‐230915.pdf
- 29. Portaccio E, De Meo E, Bellinvia A, Amato MP. Cognitive issues in pediatric multiple sclerosis. Brain Sci. 2021;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGinley M, Rossman IT. Bringing the HEET: the argument for high‐efficacy early treatment for pediatric‐onset multiple sclerosis. Neurotherapeutics. 2017;14:985‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandesjö F, Wassmer E, Deiva K, et al. Current international trends in the treatment of multiple sclerosis in children—impact of the COVID‐19 pandemic. Mult Scler Relat Disord. 2021;56:103277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID‐19. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1035. doi: 10.1212/NXI.0000000000001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bibinoğlu Amirov C, Saltik S, Yalçınkaya C, et al. Ocrelizumab in pediatric multiple sclerosis. Eur J Paediatr Neurol. 2023;43:1‐5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


