Abstract
Background and purpose
In amyotrophic lateral sclerosis (ALS), there is an unmet need for more precise patient characterization through quantitative, ideally operator‐independent, assessments of disease extent and severity. Radially sampled averaged magnetization inversion recovery acquisitions (rAMIRA) magnetic resonance imaging enables gray matter (GM) and white matter (WM) area quantitation in the cervical and thoracic spinal cord (SC) with optimized contrast. We aimed to investigate rAMIRA‐derived SC GM and SC WM areas and their association with clinical phenotype and disability in ALS.
Methods
A total of 36 patients with ALS (mean [SD] age 61.7 [12.6] years, 14 women) and 36 healthy, age‐ and sex‐matched controls (HCs; mean [SD] age 63.1 [12.1] years, 14 women) underwent two‐dimensional axial rAMIRA imaging at the inter‐vertebral disc levels C2/3–C5/C6 and the lumbar enlargement level T max. ALS Functional Rating Scale–revised (ALSFRS‐R) score, muscle strength, and sniff nasal inspiratory pressure (SNIP) were assessed.
Results
Compared to HCs, GM and WM areas were reduced in patients at all cervical levels (p < 0.0001). GM area (p = 0.0001), but not WM area, was reduced at T max. Patients with King's Stage 3 showed significant GM atrophy at all levels, while patients with King's Stage 1 showed significant GM atrophy selectively at T max. SC GM area was significantly associated with muscle force at corresponding myotomes. GM area at C3/C4 was associated with ALSFRS‐R (p < 0.001) and SNIP (p = 0.0016).
Conclusion
Patients with ALS assessed by rAMIRA imaging show significant cervical and thoracic SC GM and SC WM atrophy. SC GM area correlates with muscle strength and clinical disability. GM area reduction at T max may be an early disease sign. Longitudinal studies are warranted.
Keywords: amyotrophic lateral sclerosis, imaging markers, magnetic resonance imaging, motor neuron diseases, spinal cord imaging
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease leading to damage of both upper (UMNs) and lower motor neurons (LMNs). It is a heterogeneous disease with substantial differences in clinical presentation at onset as well as speed and pattern of disease progression [1, 2]. Precise patient characterization through objective, ideally operator‐independent, strategies to define the extent and severity of the disease is therefore warranted, not only to improve our understanding of the disease, but also to potentially help in the evaluation of upcoming treatment approaches, both in clinical and research settings.
Neuroimaging markers have increasingly been investigated in ALS [3, 4, 5]. The spinal cord (SC), containing the LMNs and axonal processes of UMNs, has been the target of several studies assessing SC metrics as potential imaging markers in ALS. Most of these studies focused on SC total cross‐sectional area (TCA) assessments in the cervical SC, reporting positive associations between cervical TCA and disease severity and disability [4, 6, 7, 8, 9]. One study investigating cervical SC gray matter (GM) atrophy in a cohort of spinal disease onset patients with relatively slow disease courses showed that cervical SC GM atrophy was more sensitive than TCA atrophy in distinguishing patients from controls [10]. One further study observed selective cervical GM atrophy in patients early in the disease course [11]. Microstructural alterations have also been investigated in the SC white matter (WM) using diffusion tensor imaging, however, associations with disability are equivocal [12, 13, 14].
The radially sampled averaged magnetization inversion recovery acquisitions (rAMIRA) magnetic resonance imaging (MRI) method enables high‐resolution imaging of the SC GM and WM in clinically feasible short acquisition times, with an optimized contrast behavior in both the cervical and thoracic cord [15, 16].
Using rAMIRA we recently demonstrated significant cervical and thoracic SC GM atrophy in patients with a presumed pure LMN disorder, the post‐polio syndrome (PPS), finding strong segment‐wise associations between SC GM area and muscle force in the corresponding muscles, indicating the clinical relevance of the observed atrophy [17].
In this study, we aimed to investigate cervical and thoracic SC GM and SC WM areas using rAMIRA imaging in patients with ALS compared to age‐ and sex‐matched healthy controls (HCs), and the associations of these metrics with segmental and respiratory muscle strength, and established markers of disability (ALS Functional Rating Scale–revised [ALSFRS‐R] [18]) to evaluate the potential of SC GM and WM areas as clinically feasible imaging metrics for LMN and SC corticospinal tract degeneration in ALS.
METHODS
Recruitment and inclusion
We recruited patients with possible, probable, or definite ALS according to the revised El Escorial (rEE) criteria [19] from the ALS Clinic of the University Hospital Basel and the ALS Clinic Sankt Gallen, Switzerland. Detailed inclusion and exclusion criteria are listed in the Appendix (Table A1). Age and sex‐matched HCs were recruited via flyers in the hospital and local sports clubs.
Spinal cord GM and WM areas have not previously been investigated based on rAMIRA imaging in ALS patients; thus, a conventional sample size calculation could not be performed. We chose the sample size of 36 ALS patients and 36 HCs based on significant differences between cervical and lower thoracic SC GM areas observed in a group of 20 patients with a pure LMN disorder (PPS) compared to HCs [17], considering the expected larger phenotypic heterogeneity of ALS as a combined LMN and UMN disorder. This sample size is in line with previous studies investigating the SC GM in ALS [10, 11].
Protocol approval, registration, and consent
This study was approved by the local ethics committee (EKNZ 2018–00032) and registered at ClinicalTrials.gov (identifier NCT05764434). We obtained written informed consent from each participant.
Clinical assessment
Clinical assessments were performed within 2 weeks of MRI. King's stage [20], total ALSFRS‐R [18] scores and bulbar, upper limb, lower limb, and respiratory subscores were obtained. Muscle strength was assessed according to the Medical Research Council definitions [21] and segmental muscle strength was assessed using handheld dynamometry (MicroFet 2; Hoggan Scientific, USA) according to the ENCALS protocol for muscle strength assessment [22]. Sniff nasal inspiratory pressure (SNIP) was assessed using a MicroRPM handheld device (SmartMedical, UK).
MRI scan acquisition and analysis
All participants were investigated with the same 3‐Tesla whole‐body scanner (Magnetom Prisma; Siemens Healthineers, Germany) at the University Hospital Basel using a 64‐channel head and neck coil additionally to the built‐in spine coil. Sagittal T2‐weighted images of the SC were acquired to enable precise acquisition of the two‐dimensional axial rAMRIA images perpendicular to the SC. Axial two‐dimensional rAMIRA [15, 16] images were acquired at the inter‐vertebral disc levels C2/3, C3/4, C4/5, C5/6, and T max. T max—the lumbar enlargement level—was visually determined at the widest sagittal diameter of the lower thoracic SC. The rAMIRA acquisition protocol used in this study was based on the optimized rAMIRA protocol developed for thoracic SC imaging [16]. In brief, the rAMIRA sequence consisted of an initial inversion recovery preparation followed by a time‐limited, cine‐like balanced steady‐state free precession (bSSFP) readout module. The readout acquired radially sampled, segmented k‐space data for five simultaneous inversion images of different tissue contrast in the SC. The mean inversion times were TIeff = [174, 239, 304, 368, 433] ms. The inversion images were averaged to yield a signal‐to‐noise and contrast‐to‐noise improved morphological SC image [17]. Further rAMIRA acquisition parameters were: field of view = 128 × 128 mm2, 512 readout samples (included 2 × oversampling), 260 radial projections, 10 segments, isotropic in‐plane resolution 0.50 × 0.50 mm2, slice thickness 8 mm, TRbSSFP = 6.49 ms (“echo spacing”), TEbSSFP = 3.23 ms, excitation flip angle 50°, receiver bandwidth = 310 Hz/px, averages = 2. We used cardiac triggering to mitigate potential pulsation artifacts using a standard infrared finger clip. Thus, the overall TR of the rAMIRA sequence depended on the pulse rate and was kept around 3 s. For a heartbeat of 60 beats per minute, the resulting acquisition time (TA) corresponded to TA = 2 min 39 s per slice.
In general, SC image quality was very good. Seven images (from three patients) out of 359 images could not be analyzed due to insufficient image quality, either due to movement artifacts (at C4/C5 n = 1, at C5/C6 n = 1, and at T max n = 2) or reduced signal due to an intolerance of the head coil (at C3/C4, C4/C5, and C5/C6 n = 1). One image at T max could not be obtained due to operation material in the vicinity of the thoracic SC in one person in the HC group.
Spinal cord metrics were segmented according to an established approach [17, 23] using the software JIM 7 (www.xinapse.com). Given the high interrater reliability of this approach with intraclass coefficients >0.874 [17], the segmentation process was performed by one experienced rater blinded to participant status.
Statistical analysis
We analyzed data using SPSS 28 and JMP Pro 16.
-
I
(a) Comparison of rAMIRA‐derived SC metrics between patients and HC.
Using Shapiro–Wilk testing, we found SC metrics to be normally distributed, hence, in the primary analysis we performed comparisons of SC GM and WM areas between patients and HCs using independent t‐tests. To compensate for multiple testing in the primary analysis, we performed a Bonferroni correction with a correction factor of 15 for three outcomes (TCA, GM area, and WM area) and five inter‐vertebral disc levels, resulting in a significance level of α <0.0033.
As secondary analyses, we calculated linear regression models with SC GM area at each level as the outcome variable, respectively, to estimate differences between HCs and patients stratified by (b) onset region (bulbar, cervical, and lumbar); (c) level of diagnostic certainty (possible, probable, probable laboratory‐supported and definite ALS according to the rEE criteria), and (d) King's stage, using age and sex as fixed predictor variables.
Analogous analyses were performed with WM areas as outcome, respectively, and are reported in the Appendix. To adjust for multiple testing, we performed a Bonferroni correction for each secondary analysis with a correction factor of 10 (for GM and WM area each at five levels), resulting in a significance level of α < 0.005.
-
II
Associations between limb and respiratory muscle strength and GM areas at corresponding cord segments.
To assess associations between limb and respiratory muscle strength and SC GM area at the corresponding cord segments, respectively, we performed linear regression analyses with (a) muscle strength as outcome and the SC GM area at the corresponding cord segment as respective predictor variables (following the results of a post mortem study [24] and an in vivo MRI study in healthy individuals [25], the inter‐vertebral disc level C3/C4, C4/C5, C5/C6, and T max correspond to the cord segments C4/5, C5/C6, C7/C8, and L5, respectively), and (b) SNIP (a marker of diaphragm strength) as the outcome and the SC GM area at the inter‐vertebral disc level C3/C4 (corresponding to the cord segment C4/C5) as the predictor, adjusting for age and sex.
-
III
Associations with disability.
Finally, the association between our proposed imaging markers and overall disability was assessed using multivariable regression analyses, with ALSFRS‐R score as the outcome variable and SC GM area and SC WM area at the level C3/C4 as predictor variables, respectively, adjusted for age and sex. We chose this level as it represents cervical enlargement.
RESULTS
Clinical and demographic characteristics
Between May 2018 and October 2020, we screened 69 patients; 36 patients with ALS according to the rEE criteria and 36 age‐ and sex‐matched HCs were included. The inclusion and exclusion process is summarized in Figure A1. Demographic data are summarized in Table 1. Overall, patients and HCs tolerated the MRI and clinical examinations very well. No adverse events were documented.
-
I
Comparison of rAMIRA‐derived SC metrics between patients and HCs.
TABLE 1.
Clinical data and demographics of patients with amyotrophic lateral sclerosis and healthy control subjects.
| Patients with ALS | HC group | |
|---|---|---|
| Sex: male/female | 22/14 | 22/14 |
| Mean age (SD), min–max, years | 61.7 (12.6) 30.2–92.9 | 63.1 (12.1) 29.5–87.4 |
| Mean disease duration (SD), min–max, months | 32.87 (38.9) 6.0–223.2 | NA |
| Diagnosis according to rEE, n (%) | ||
| Possible | 5 (13.9) | NA |
| Probable | 11 (30.6) | |
| Probable laboratory‐supported | 12 (33.3) | |
| Definite | 6 (16.7) | |
| Familial ALS (mutation detected) | 2 (0.6) | |
| Disease onset type, n (%) | ||
| Bulbar | 6 (16.6) | NA |
| Spinal | 30 (83.3) | |
| Upper limb | 15 | |
| Lower limb | 14 | |
| Thoracic | 1 | |
| Mean ALSFRS‐R | 37.7 | NA |
| Min–max ALSFRS‐R | 22–48 | |
| King's Stage, n | ||
| 1 | 9 | NA |
| 2 | 8 | |
| 3 | 19 | |
| Progression type (according to Labra et al., 2016 [34]), n | ||
| Fast | 2 | NA |
| Intermediate | 8 | |
| Slow | 26 | |
| BMI (SD), min–max, kg/m2 | 24.7 (3.8) 18.1–35.1 | 24.7 (3.2) 19.1–34.6 |
| Riluzole medication, n | 32 | NA |
| Edaravone medication (additional to Riluzole), n | 3 | NA |
| Familial ALS with confirmed genetic mutation a , n | 2 (both C9orf72) | NA |
| Segmental muscle strength | ||
| Deltoid, mean (SD), N | 77.0 (54.17) | 140.3 (44.4) |
| Brachioradialis, mean (SD), N | 130.7 (74.7) | 207.2 (49.0) |
| FDI, mean (SD), N | 15.8 (12.3) | 35.2 (8.5) |
| Hand grip, mean (SD), kg | 19.9 (15.7) | 36.6 (11.) |
| Foot dorsiflexors, mean (SD), N | 123.4 (83.6) | 213.0 (55.0) |
| SNIP, mean (SD), cmH2O | 68.1 (30.10) | 92.3 (26.2) |
Note: Segmental muscle strength values were calculated using a mean of left and right muscle strength in each participant.
Abbreviations: ALS, amyotrophic lateral sclerosis; FDI, first dorsal interosseous; HC, healthy control; kg, kilogram; N, Newton; SNIP, sniff nasal inspiratory pressure.
Patients without a positive family history for ALS were not genetically investigated in this study.
(a) SC total cord and GM areas were significantly reduced at inter‐vertebral disc levels C2/C3, C3/C4, C4/C5, C5/C6 and T max in patients with ALS compared to HCs. WM areas were significantly reduced at all cervical levels (Figure 1 and Table 2). At the levels of the cervical and lumbar enlargements SC GM area reductions were most pronounced, with relative reductions (rr) at the levels C3/C4 and T max of 15.1% and 20.2%, respectively (Table 2).
FIGURE 1.
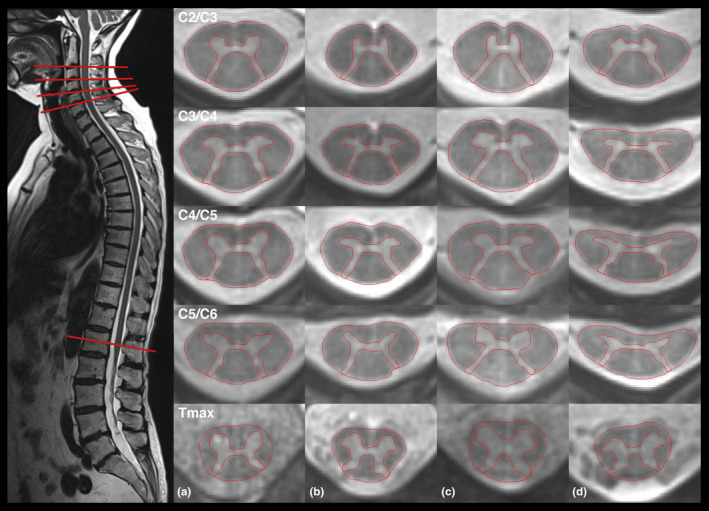
Radially sampled averaged magnetization inversion recovery acquisitions (rAMIRA) images of the spinal cord at inter‐vertebral disc levels C2/C3, C3/C4, C4/C5, C5/C6, and T max of two matched pairs: Left: Acquisition of the rAMIRA images perpendicular to the spinal cord (healthy control person). Right: Column A: healthy control person, female, 63.9 years; Column B: patient with amyotrophic lateral sclerosis (ALS), female, 63.3 years; Column C: healthy control person, male, 60.7 years; Column D: patient with ALS, male, 61.5 years.
TABLE 2.
Comparison of total cord area (TCA), and gray (GM) and white matter (WM) areas of patients with amyotrophic lateral sclerosis and healthy control subjects (n = 36, respectively) via independent t‐tests, and relative reductions of TCA, GM area, and WM area.
| TCA level | Group | Mean TCA /SD, mm2 | SE | Difference | SE of the difference | p | 95% CI | 95% CI |
|---|---|---|---|---|---|---|---|---|
| C2C3 |
ALS HC |
75.93/8.76 84.21/6.56 |
1.46 1.09 |
−8.27 | 1.82 | <0.001 | −11.92 | −4.63 |
| C3C4 |
ALS HC |
77.26/7.77 88.13/7.85 |
1.31 1.31 |
−10.87 | 1.85 | <0.001 | −14.57 | −7.18 |
| C4C5 |
ALS HC |
80.24/9.11 88.83/8.62 |
1.56 1.44 |
−8.59 | 2.12 | <0.001 | −12.82 | −4.36 |
| C5C6 |
ALS HC |
75.98/9.66 85.44/7.29 |
1.66 1.22 |
−9.45 | 2.04 | <0.001 | −13.52 | −5.39 |
| T max |
ALS HC |
55.31/8.30 62.54/6.93 |
1.42 1.17 |
−7.23 | 1.84 | <0.001 | −10.90 | −3.56 |
| GM area level | ||||||||
| C2C3 |
ALS HC |
14.21/1.72 15.67/1.61 |
0.29 0.27 |
−1.46 | 0.39 | <0.001 | −2.24 | −0.68 |
| C3C4 |
ALS HC |
16.68/2.23 19.67/2.70 |
0.38 0.45 |
−2.99 | 0.59 | <0.001 | −4.17 | −1.82 |
| C4C5 |
ALS HC |
18.14/2.13 20.52/2.44 |
0.36 0.41 |
−2.38 | 0.55 | <0.001 | −3.48 | −1.29 |
| C5C6 |
ALS HC |
17.04/2.27 19.91/1.95 |
0.39 0.32 |
−2.86 | 0.50 | <0.001 | −3.87 | −1.86 |
| T max |
ALS HC |
20.25/3.32 25.28/3.33 |
0.57 0.56 |
−5.03 | 0.80 | <0.001 | −6.63 | −3.43 |
| WM area level | ||||||||
| C2C3 |
ALS HC |
61.72/7.80 68.53/5.61 |
1.30 0.94 |
−6.81 | 1.60 | <0.001 | −10.00 | −3.62 |
| C3C4 |
ALS HC |
60.58/6.03 68.46/6.46 |
1.02 1.08 |
−7.88 | 1.48 | <0.001 | −10.84 | −4.92 |
| C4C5 |
ALS HC |
62.10/7.87 68.31/6.93 |
1.35 1.15 |
−6.20 | 1.77 | <0.001 | −9.74 | −2.67 |
| C5C6 |
ALS HC |
58.94/8.20 65.53/6.05 |
1.41 1.01 |
−6.59 | 1.71 | <0.001 | −10.01 | −3.17 |
| T max |
ALS HC |
35.06/5.88 37.26/4.60 |
1.01 0.78 |
−2.20 | 1.27 | 0.088 | −4.73 | 0.34 |
| Relative reductions | |||
|---|---|---|---|
| Level | rr TCA, % | rr GM area, % | rr WM area, % |
| C2C3 | 9.8 | 9.3 | 9.9 |
| C3C4 | 12.2 | 15.1 | 11.4 |
| C4C5 | 10.0 | 11.8 | 9.5 |
| C5C6 | 11.4 | 14.7 | 10.5 |
| T max | 11.8 | 20.2 | 6.1 |
Note: p values after Bonferroni correction with a significance level of <0.0033 are marked bold.
Abbreviations: ALS, amyotrophic lateral sclerosis; CI, confidence interval of the difference between TCA means; GM, gray matter; HC, healthy control; rr, relative reduction; SE, standard error; TCA, total cord area; WM, white matter.
Secondary analyses
(b) Comparing subgroups with different disease‐onset regions versus HCs, patients with a spinal disease onset (either cervical or lumbar) had significantly reduced cervical and thoracic SC GM areas, while patients with bulbar onset showed significant SC GM area reductions only at the T max level (Table 3). In patients with a lumbar onset, SC WM areas were significantly reduced at all cervical levels, in patients with a cervical onset only at upper cervical SC levels (Table A2).
TABLE 3.
Comparison of spinal cord gray matter area means of patients with amyotrophic lateral sclerosis and a bulbar, cervical, or lumbar disease onset and healthy control subjects as reference, adjusted for age and sex.
| Inter‐vertebral disc level | Group | Adjusted mean GM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | Bulbar onset | 14.98/0.70 | −0.76/0.76 | −2.27 | 0.76 | 0.3236 |
| Cervical onset | 14.10/0.45 | −1.64/0.52 | −2.67 | −0.60 | 0.0024 | |
| Lumbar onset a | 14.06/0.43 | −1.68/0.51 | −2.69 | −0.67 | 0.0015 | |
| Controls | 15.74/0.28 | |||||
| C3/C4 | Bulbar onset | 18.44/1.18 | −1.25/1.27 | −3.78 | 1.28 | 0.3280 |
| Cervical onset | 15.99/0.67 | −3.70/0.78 | −5.24 | −2.15 | <0.0001 | |
| Lumbar onset a | 16.69/0.64 | −3.00/0.76 | −4.52 | −1.49 | 0.0002 | |
| Controls | 19.69/0.42 | |||||
| C4/C5 | Bulbar onset | 19.67/1.10 | −0.85/1.18 | −3.22 | 1.51 | 0.4727 |
| Cervical onset | 17.64/0.65 | −2.89/0.74 | −4.37 | −1.40 | 0.0002 | |
| Lumbar onset a | 18.00/0.60 | −2.52/0.71 | −3.94 | −1.11 | 0.0007 | |
| Controls | 20.53/0.40 | |||||
| C5/C6 | Bulbar onset | 18.39/1.01 | −1.56/1.08 | −3.71 | 0.59 | 0.1522 |
| Cervical onset | 17.00/0.59 | −2.94/0.68 | −4.30 | −1.59 | <0.0001 | |
| Lumbar onset a | 16.59/0.54 | −3.36/0.64 | −4.65 | −2.07 | <0.0001 | |
| Controls | 19.95/0.35 | |||||
| T max | Bulbar onset | 20.49/1.44 | −4.85/1.56 | −7.96 | −1.73 | 0.0028 |
| Cervical onset | 21.21/0.94 | −4.13/1.08 | −6.28 | −1.97 | 0.0003 | |
| Lumbar onset a | 19.17/0.90 | −6.17/1.06 | −8.29 | −4.05 | <0.0001 | |
| Controls | 25.34/0.58 |
Note: p values with a significance level of <0.005 (after Bonferroni correction) are marked bold.
Abbreviations: CI, confidence interval of the difference between means; GM, gray matter; SE, standard error.
One patient with thoracic onset was classified as lumbar onset since this was the second involved region.
(c) Comparing patients with different levels of diagnostic certainty according to the rEE criteria versus HCs, we found significantly reduced SC GM areas in patients with probable, probable laboratory‐supported and definite ALS (Table 4). There were no significant differences between patients with probable and probable laboratory‐supported ALS, and the results for these patients are reported collectively as “probable ALS”. Patients with a possible ALS diagnosis showed significant SC GM atrophy only at T max. WM area reductions were most pronounced at cervical SC levels, but were not significant at T max (Table A3). Adding the results of SC imaging to judge regional involvement in ALS (using the normal mean –2 SD as a cut‐off for pathological atrophy at each level), it would be possible to reclassify one out of five patients with a diagnosis of possible ALS, according to the rEE criteria, as probable ALS.
TABLE 4.
Comparison of spinal cord gray matter area means of patients with amyotrophic lateral sclerosis (ALS) with different diagnostic certainty levels based on the revised El Escorial (rEE) criteria and healthy control subjects as reference, adjusted for age and sex. The ‘probable’ category refers to both probable and probable laboratory supported ALS based on the rEE criteria.
| Inter‐vertebral disc level | ALS diagnosis acc. to rEE | Adjusted mean GM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | Possible | 14.63/0.64 | −1.12/0.69 | −2.49 | 0.26 | 0.1108 |
| Probable | 14.57/0.32 | −1.17/0.41 | −1.99 | −0.34 | 0.0062 | |
| Definite | 12.52/0.64 | −3.22/0.69 | −4.60 | −1.84 | <0.0001 | |
| Controls | 15.74/0.26 | |||||
| C3/C4 | Possible | 17.69/0.96 | −2.02/1.04 | −4.10 | 0.05 | 0.0559 |
| Probable | 17.11/0.49 | −2.60/0.63 | −3.86 | −1.35 | 0.0001 | |
| Definite | 14.00/0.96 | −5.71/1.04 | −7.79 | −3.64 | <0.0001 | |
| Controls | 19.71/0.40 | |||||
| C4/C5 | Possible | 19.48/0.92 | −1.07/0.99 | −3.05 | 0.91 | 0.2853 |
| Probable | 18.17/0.47 | −2.38/0.60 | −3.58 | −1.18 | 0.0002 | |
| Definite | 16.29/1.01 | −4.26/1.07 | −6.41 | −2.12 | 0.0002 | |
| Controls | 20.55/0.38 | |||||
| C5/C6 | Possible | 18.64/0.82 | −1.32/0.89 | −3.10 | 0.46 | 0.1432 |
| Probable | 17.00/0.42 | −2.96/0.54 | −4.04 | −1.88 | <0.0001 | |
| Definite | 15.31/0.90 | −4.66/0.96 | −6.58 | −2.73 | <0.0001 | |
| Controls | 19.96/0.34 | |||||
| T max | Possible | 20.25/1.38 | −5.08/1.49 | −8.06 | −2.10 | 0.0012 |
| Probable | 20.53/0.70 | −4.80/0.89 | −6.59 | −3.01 | <0.0001 | |
| Definite | 18.36/1.71 | −6.98/1.80 | −10.57 | −3.38 | 0.0003 | |
| Controls | 25.33/0.58 |
Note: p values with a significance level of <0.005 (after Bonferroni correction) are marked bold.
Abbreviations: CI, confidence interval of the difference between means; rEE, revised El Escorial; SE, standard error.
(d) At the time of scanning, patients were at King's Stages 1–3 (Table 1). While patients with King's Stage 3 showed significant SC GM area reductions at all cervical and thoracic levels compared to HCs, patients with King's Stage 1 in this cohort showed significant GM area reduction only at T max (Table 5). In general, SC WM area reductions were most pronounced at upper cervical cord levels, with reductions being significant in patients with King's Stages 2 and 3 compared to HCs (Table A4).
-
II
Associations between limb and respiratory muscle strength and GM areas at corresponding cord segments.
TABLE 5.
Comparison of gray matter area means of patients with amyotrophic lateral sclerosis with different King's Stages (1–3) and healthy control subjects as reference, adjusted for age and sex.
| Inter‐vertebral disc level | King's Stage | Adjusted mean GM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | 1 | 14.92/0.56 | −0.81/0.63 | −2.06 | 0.44 | 0.2010 |
| 2 | 14.09/0.58 | −1.64/0.65 | −2.94 | −0.35 | 0.0135 | |
| 3 | 13.98/0.39 | −1.76/0.47 | −2.69 | −0.82 | 0.0004 | |
| Controls | 15.73/0.28 | |||||
| C3/C4 | 1 | 18.20/0.82 | −1.48/0.92 | −3.32 | 0.35 | 0.1120 |
| 2 | 17.19/0.85 | −2.49/0.95 | −4.39 | −0.60 | 0.0108 | |
| 3 | 15.66/0.59 | −4.02/0.70 | −5.41 | −2.62 | <0.0001 | |
| Controls | 19.68/0.41 | |||||
| C4/C5 | 1 | 18.81/0.78 | −1.72/0.88 | −3.48 | 0.04 | 0.0553 |
| 2 | 18.71/0.82 | −1.82/0.91 | −3.63 | −0.001 | 0.0499 | |
| 3 | 17.48/0.57 | −3.05/0.68 | −4.41 | −1.69 | <0.0001 | |
| Controls | 20.52/0.39 | |||||
| C5/C6 | 1 | 18.61/0.63 | −1.29/0.71 | −2.70 | 0.12 | 0.0717 |
| 2 | 18.48/0.65 | −1.42/0.73 | −2.88 | 0.03 | 0.0549 | |
| 3 | 15.49/0.46 | −4.41/0.54 | −5.50 | −3.33 | <0.0001 | |
| Controls | 19.90/0.31 | |||||
| T max | 1 | 21.47/1.13 | −3.81/1.28 | −6.36 | −1.26 | 0.0040 |
| 2 | 20.94/1.18 | −4.34/1.32 | −6.98 | −1.70 | 0.0017 | |
| 3 | 19.19/0.84 | −6.09/0.99 | −8.07 | −4.11 | <0.0001 | |
| Controls | 25.28/0.58 |
Note: p values with a significance level of <0.005 (after Bonferroni correction) are marked bold.
Abbreviations: CI, confidence interval of the difference between means; GM, gray matter; SE, standard error.
(a) Results are summarized in Table 6. In multivariate regression analyses adjusting for age and sex, SC GM area at the inter‐vertebral disc level C3/C4 (corresponding to the spinal segment C4‐C5 [24, 25]) explained 37.1% of shoulder anteversion strength variance (p = 0.0006). Likewise, SC GM area at the level C4/C5 (corresponding to the spinal segment C6) explained 16.3% of brachioradialis strength variance (p = 0.0396); GM area at level C5/C6 (corresponding to spinal segments C7‐C8) explained 30.8% of hand grip strength variance (p = 0.0027) and 26.2% of first dorsal interosseous strength variance (p = 0.0068). Furthermore, GM area at T max (the lumbar enlargement level) accounted for 18.4% of foot dorsal extension strength variance (p = 0.0279).
TABLE 6.
Association between clinical outcomes —muscle strength, Amyotrophic Lateral Sclerosis (ALS) Functional Rating Scale revised total score and upper limb subscore—and gray matter area at corresponding inter‐vertebral disc levels in patients with ALS, covarying for age and sex.
| Model | Outcome | Factor | Standardized estimate | SE | t value | p |
|---|---|---|---|---|---|---|
| Model 1: adj. r 2 = 37.1%; p = 0.0006 | Muscle strength (N); Deltoid | Intercept | 0.004 | 0.14 | 0.03 | 0.9769 |
| GM area at C3/C4 | 0.63 | 0.14 | 4.37 | 0.0001 | ||
| Age | −0.06 | 0.15 | −0.42 | 0.6778 | ||
| Sex | 0.09 | 0.14 | 0.63 | 0.5310 | ||
| Model 2: adj. r 2 = 16.3%; p = 0.0396 | Muscle strength (N); Brachioradialis | Intercept | 0.024 | 0.16 | 0.15 | 0.8801 |
| GM area at C4/C5 | 0.41 | 0.16 | 2.49 | 0.0186 | ||
| Age | −0.24 | 0.16 | −1.47 | 0.1509 | ||
| Sex | 0.06 | 0.17 | 0.35 | 0.7260 | ||
| Model 3: adj. r 2 = 30.8%; p = 0.0027 | Muscle strength (kg); Hand Grip | Intercept | −0.003 | 0.15 | −0.02 | 0.9840 |
| GM area at C5/C6 | 0.51 | 0.15 | 3.38 | 0.0020 | ||
| Age | −0.19 | 0.15 | −1.26 | 0.2174 | ||
| Sex | 0.21 | 0.16 | 1.37 | 0.1794 | ||
| Model 4: adj. r 2 = 26.2%; p = 0.0068 | Muscle strength (N); First dorsal interosseus | Intercept | 0.003 | 0.15 | 0.02 | 0.9865 |
| GM area at C5/C6 | 0.56 | 0.16 | 3.58 | 0.0012 | ||
| Age | −0.06 | 0.16 | −0.39 | 0.6958 | ||
| Sex | 0.15 | 0.16 | 0.94 | 0.3572 | ||
| Model 5: adj. r 2 = 18.4%; p = 0.0279 | Muscle strength (N); Tibialis anterior | Intercept | 0.020 | 0.16 | 0.12 | 0.9034 |
| GM area at T max | 0.47 | 0.17 | 2.81 | 0.0087 | ||
| Age | −0.07 | 0.18 | −0.39 | 0.6959 | ||
| Sex | 0.14 | 0.17 | 0.83 | 0.4142 | ||
| Model 6: adj. r 2 = 32.5%; p = 0.0016 | SNIP (cmH2O) | Intercept | −0.005 | 0.14 | −0.03 | 0.9724 |
| GM area at C3/C4 | 0.36 | 0.15 | 2.45 | 0.0200 | ||
| Age | −0.35 | 0.15 | −2.29 | 0.0287 | ||
| Sex | 0.16 | 0.15 | 1.07 | 0.2911 | ||
| Model 7: adj. r 2 = 40.5%; p = 0.0002 | ALSFRS‐R total score | Intercept | 38.210 | 0.97 | 39.37 | <0.0001 |
| GM area at C3/C4 | 4.83 | 1.01 | 4.77 | <0.0001 | ||
| Age | 0.39 | 1.04 | 0.37 | 0.7103 | ||
| Sex | −1.21 | 1.01 | −1.19 | 0.2419 |
p values with a significance level <0.05 are marked in bold.
Abbreviations: ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Rating Scale–revised; GM, gray matter; kg, kilograms; N, Newton; SE, standard error; SNIP, sniff nasal inspiratory pressure.
(b) Spinal cord GM area at the inter‐vertebral disc level C3/C4 (corresponding to the spinal segment C4‐C5 supplying the diaphragm [24, 25]), explained 32.5% of SNIP variance (p = 0.0016).
-
III
Associations with disability.
In multivariate regression analyses covarying for age and sex, the SC GM area closest to the cervical enlargement (C3/C4) explained 40.5% of ALSFRS‐R total score variance (p = 0.0002; Table 6). In contrast, SC WM area at C3/C4 was not a significant predictor of ALSFRS‐R, neither in univariate analysis, nor when added to the models above.
DISCUSSION
In this study, we investigated SC GM and SC WM in patients with ALS in both the cervical and thoracic SC, the latter of which is notoriously challenging to image due to its increased susceptibility to artifacts. Using axial two‐dimensional rAMIRA imaging we found significant SC GM and SC WM atrophy. SC GM atrophy was most pronounced at the cervical and lumbar enlargement levels, exceeding the relative SC WM reductions. While SC WM atrophy—as anatomically expected—was relatively homogeneously distributed across SC levels, the SC GM atrophy appeared to be patchier, suggesting compartmentalized cord GM atrophy in ALS, in line with earlier electrophysiological studies [26].
The SC GM area correlated with muscle strength in the corresponding myotome, and at C3/C4 with diaphragm strength, indicating that this atrophy metric conveys regionally relevant information.
The SC GM area at the inter‐vertebral disc level with the largest relative area reduction, C3/C4, also reflects the overall disease burden in ALS: The SC GM area at C3/C4 explained 40.5% of ALSFRS‐R variance, while the SC WM area at C3/C4 did not correlate with disability.
While the main histopathological correlate of SC WM atrophy is axonal loss, the loss and/or size reduction of alpha‐, gamma‐, and interneurons in the anterior horn [27] and changes in the neuropil may contribute to the SC GM atrophy observed in patients with ALS in vivo [28]. Histopathological data demonstrated an average reduction in LMN of 55% (with inter‐individual variations of 8%–90%) in ALS post mortem, with largest reductions observed in the reported region of onset in the majority of patients [28].
One of the key challenges in imaging marker development in ALS is posed by the phenotypic heterogeneity in terms of onset regions and spatial progression patterns due to different and potentially coexisting (regional vs. remote; contiguous vs. noncontiguous) disease‐spreading mechanisms [29]. We observed significant GM atrophy at T max in patients with nonspinal, that is, bulbar onset in the absence of significant cervical GM atrophy, suggesting a multifocal, noncontiguous progression pattern in these patients, in line with reports by Sekiguchi et al. [26]. In the patients in our cohort with bulbar disease onset, the percentage relative reduction at the lumbar enlargement level was three‐ to fourfold higher than that observed at cervical levels. This comparatively large reduction makes it unlikely that this observation can be solely explained by differences in measurement precision due to the relatively larger GM size at T max.
Interestingly, this non‐uniform (focal or multifocal) SC GM atrophy was not accompanied by a similarly heterogeneous SC WM atrophy in these patients. The percentage relative reductions of SC WM area along the cervical and thoracic cord in bulbar onset patients were rather homogeneously distributed among levels, ranging from 7.7% to 10%.
Using the King's staging method as an established tool to categorize disease spread based on clinical milestones, we found significant cervical and thoracic GM atrophy in patients classified as King's Stage 3, and selective significant SC GM atrophy at T max in patients in King's Stages 1 and 2 (with only four out of nine patients with King's Stage 1 presenting symptoms attributable to the lumbar region). Again, this indicates the sensitivity of GM imaging in this region to early (partially still clinically silent) alterations. While WM area was reduced at the upper cervical levels in King's Stages 2 and 3, WM reductions at T max were not significant. These results complement a finding reported recently by Nigri et al. [11], who observed selective GM area reductions in the absence of WM reductions at the cervical enlargement in a study focused on the cervical cord including 25 patients with King' Stage 1.
Categorizing patients in terms of diagnostic certainty at the time of imaging using the rEE, we found significant SC GM atrophy at all SC levels in patients with probable or definite ALS, while patients with a diagnosis of possible ALS showed significant SC GM atrophy only at T max. Taking SC GM atrophy into account as an additional sign of regional LMN involvement in ALS, led to reclassification of one out of five patients with a diagnosis of “possible” ALS (based on the rEE criteria) into the category of “probable” ALS. Given the relatively small number of patients in this diagnostic category in our study, this result requires confirmation in larger studies.
Not surprisingly, SC WM area reductions were slightly pronounced at the upper cervical levels. At these levels, alterations of the UMN axons (that further caudally synapse with LMNs to upper limb, thoracic and lower limb muscles) can be captured simultaneously, enhancing the sensitivity of detecting changes in a presumed multifocal disease. At the levels C2/C3 and C3/C4, significant SC WM area reductions were detected in patients at all levels of diagnostic certainty, even in the group with possible ALS.
The significant positive association observed between SC GM areas (as presumed metrics of LMN damage) and regional clinical metrics, that is, muscle force, as well as with general disability assessed by the ALSFRS‐R, is in line with what has been reported in other LMN disorders such as PPS [17].
In contrast to LMN degeneration‐related atrophic paralyses, impairment due to UMN damage primarily results in movement slowness—at least stage‐dependent—instead of weakness [30], which might partly explain the lack of an association between SC WM atrophy (due to UMN damage) and disability parameters in this cohort. The SC WM alterations observed could still be clinically compensated at the time of scan but might become relevant in the future course of the disease [10].
One recent study did not find atrophy of the TC area at the lumbar enlargement in a cohort of ALS patients with predominant upper limb dysfunction [31], however, these results may be partially attributed to sex imbalances between the patient and control group with a higher proportion of men in the ALS group masking disease‐related differences. By contrast, we found SC GM atrophy to be consistently present and, more importantly, in patients with earlier diseases/lesser disease burden, making the SC GM area at T max an appealing target for further studies.
The anatomical inter‐subject variability of SC area measurements in HCs can be substantial. In recent years, efforts have been made to develop normalization strategies to reduce this anatomical variability [32, 33] to increase sensitivity and specificity in detecting pathology‐associated changes. One promising strategy used the product of the spinal canal anterior–posterior and lateral diameter [33]. However, after using this normalization approach in our study, the standard deviation was not consistently reduced, and even resulted in a relative increase of the standard deviation at T max. Therefore, we present the non‐normalized values in this study.
We must acknowledge some limitations to our study. Firstly, our sample size was relatively small, although it was similar to that in previous studies in this field [10, 11]. As we deployed rAMIRA, an MRI approach recently developed at the University of Basel on Siemens scanners, we primarily chose a single‐center design to ensure the highest image quality and consistency in image acquisitions. Multicentric evaluations to investigate larger cohorts and to also confirm the results of the subgroup analyses are the planned next steps.
Secondly, our patient group was heterogeneous in terms of disease onset type, UMN/LMN predominance and symptom progression. However, heterogeneity, in terms of first symptoms, progression spreading pattern, bulbar and respiratory involvement and clinical disability, is one of the main hallmarks of ALS. Our quantitative imaging markers show promise even in this heterogeneous group, highlighting their robustness and broad applicability. Moreover, the majority of patients was not investigated at disease onset, but 1–2 or more years after symptom onset, thus not allowing firm conclusions about the diagnostic value of SC GM atrophy.
Finally, our study included only two patients with familial ALS and a confirmed genetic mutation, and systematic genetic testing in patients with sporadic ALS was not performed. Thus, we cannot estimate the value of these novel imaging metrics in genetic ALS forms. The evaluation of patients with mutations, and presymptomatic mutation carriers is needed.
In conclusion, in this study, we show significant SC GM and WM atrophy in the cervical and thoracic SC in ALS patients as compared to age‐ and sex‐matched HCs using rAMIRA imaging. Longitudinal studies are necessary and underway to explore the sensitivity to change and possible prognostic value of SC GM and WM atrophy in ALS. The methodology presented here might be helpful to better understand the respective contributions of LMN and SC corticospinal tract degeneration in ALS.
AUTHOR CONTRIBUTIONS
Maria Janina Wendebourg: Investigation; writing – original draft; data curation; project administration; formal analysis; visualization. Matthias Weigel: Conceptualization; writing – review and editing; methodology. Claudia Weidensteiner: Writing – review and editing. Laura Sander: Writing – review and editing. Eva Kesenheimer: Writing – review and editing; investigation. Nicole Naumann: Investigation; writing – review and editing. Tanja Haas: Investigation. Philipp Madoerin: Investigation; visualization. Nathalie Braun: Writing – review and editing. Christoph Neuwirth: Writing – review and editing. Markus Weber: Writing – review and editing. Kathleen Jahn: Investigation; writing – review and editing. Ludwig Kappos: Writing – review and editing; supervision. Cristina Granziera: Writing – review and editing; supervision. Kathi Schweikert: Writing – review and editing; investigation. Michael Sinnreich: Writing – review and editing. Oliver Bieri: Writing – review and editing; methodology; supervision. Regina Schlaeger: Supervision; conceptualization; investigation; funding acquisition; writing – review and editing; methodology; formal analysis; project administration; resources.
FUNDING INFORMATION
This study was funded by the Gottfried and Julia Bangerter Rhyner Foundation, by the Freiwillige Akademische Gesellschaft Basel, by the Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung, as well as by the Neuromuscular Research Association Basel (NeRAB).
APPENDIX 1.
TABLE A1.
Inclusion and exclusion criteria for patients with amyotrophic lateral sclerosis and healthy control subjects.
| Patients with ALS | Age‐ and sex‐matched healthy control subjects |
|---|---|
| Inclusion criteria | |
| >18 years of age | >18 years of age |
| Neurologist‐confirmed diagnosis of a possible, probable or definite ALS according to rEE criteria | |
| Ability to lie in an MRI scanner for 1 h | Able to lie in MRI scanner for 1 h |
| Exclusion criteria | |
| Active other neurological or neuromuscular condition explaining the symptoms or a significant part of it | Neurological or neuromuscular conditions |
| Other neurological or neuromuscular conditions interfering with the examinations | |
| Severe cervical spinal stenosis or other relevant disease of the spine or spinal cord | Severe cervical spinal stenosis or other relevant disease of the spine or spinal cord |
| Other severe chronic disease | Other severe chronic disease |
| Pregnancy | Pregnancy |
| General contraindications against MRI scanning (e.g. metal implants, pacemakers) | General contraindications against MRI |
| Not able to read the patient information due to language barriers | Not able to read the patient information due to language barriers |
| Major cognitive deficit impacting the ability to read and understand the patient information and/or to follow the instructions of the study personnel | Major cognitive deficit impacting the ability to read and understand the patient information and/or to follow the instructions of the study personnel |
Abbreviation: ALS, amyotrophic lateral sclerosis; MRI, magnetic resonance imaging; rEE, revised El Escorial.
TABLE A2.
Comparison of spinal cord white matter area means of patients with a bulbar, cervical, or lumbar amyotrophic lateral sclerosis disease onset and healthy control subjects as reference adjusted for age and sex.
| Inter‐vertebral disc level | Group | Adjusted mean WM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | Bulbar onset | 62.14/2.71 | −6.25/2.94 | −12.12 | −0.38 | 0.0372 |
| Cervical onset | 61.00/1.73 | −7.39/2.00 | −11.39 | −3.40 | 0.0004 | |
| Lumbar onset a | 61.34/1.65 | −7.05/1.96 | −10.96 | −3.14 | 0.0006 | |
| Controls | 68.39/1.08 | |||||
| C3/C4 | Bulbar onset | 63.20/2.92 | −5.29/3.13 | −11.53 | 0.96 | 0.0958 |
| Cervical onset | 60.18/1.66 | −8.31/1.92 | −12.13 | −4.48 | <0.0001 | |
| Lumbar onset a | 59.72/1.58 | −8.77/1.87 | −12.51 | −5.03 | <0.0001 | |
| Controls | 68.49/1.03 | |||||
| C4/C5 | Bulbar onset | 63.12/3.42 | −5.25/3.66 | −12.56 | 2.06 | 0.1559 |
| Cervical onset | 63.95/2.00 | −4.43/2.29 | −9.02 | 0.17 | 0.0586 | |
| Lumbar onset a | 59.68/1.85 | −8.70/2.19 | −13.08 | −4.32 | 0.0002 | |
| Controls | 68.37/1.21 | |||||
| C5/C6 | Bulbar onset | 59.60/3.25 | −5.99/3.48 | −12.93 | 0.95 | 0.0896 |
| Cervical onset | 61.32/1.90 | −4.27/2.18 | −8.63 | 0.10 | 0.0551 | |
| Lumbar onset a | 56.12/1.75 | −9.48/2.08 | −13.63 | −5.32 | <0.0001 | |
| Controls | 65.59/1.15 | |||||
| T max | Bulbar onset | 34.51/2.07 | −2.67/2.25 | −7.17 | 1.82 | 0.2394 |
| Cervical onset | 35.00/1.35 | −2.18/1.56 | −5.29 | 0.94 | 0.1671 | |
| Lumbar onset a | 34.50/1.30 | −2.68/1.53 | −5.73 | 0.38 | 0.0850 | |
| Controls | 37.18/0.83 |
Note: p values after Bonferroni correction with a significance level of <0.0083 are marked bold.
Abbreviations: CI, confidence interval of the difference between means; WM, white matter.
One patient with a thoracic onset was classified as lumbar onset since this was the following second involved region.
TABLE A3.
Comparison of spinal cord white matter area means of patients with amyotrophic lateral sclerosis (ALS) with different diagnostic certainty levels according to the revised El Escorial (rEE) criteria and healthy control subjects adjusted for age and sex. The ‘probable’ category refers to both probable and probable laboratory supported ALS according to the rEE criteria.
| Inter‐vertebral disc level | ALS diagnosis based on rEE criteria | Adjusted mean WM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | Possible | 59.50/2.52 | −8.90/2.73 | −14.35 | −3.46 | 0.0017 |
| Probable | 62.79/1.27 | −5.62/1.63 | −8.87 | −2.36 | 0.0010 | |
| Definite | 57.48/2.53 | −10.92/2.72 | −16.36 | −5.48 | 0.0002 | |
| Controls | 68.40/1.05 | |||||
| C3/C4 | Possible | 60.73/2.43 | −7.78/2.63 | −13.04 | −2.52 | 0.0043 |
| Probable | 61.51/1.25 | −7.01/1.59 | −10.19 | −3.82 | <0.0001 | |
| Definite | 56.16/2.44 | −12.35/2.63 | −17.60 | −7.10 | <0.0001 | |
| Controls | 68.51/1.01 | |||||
| C4/C5 | Possible | 62.62/2.93 | −5.75/3.17 | −12.09 | 0.59 | 0.0746 |
| Probable | 62.55/1.51 | −5.82/1.92 | −9.66 | −1.98 | 0.0035 | |
| Definite | 58.27/3.22 | −10.10/3.43 | −16.96 | −3.25 | 0.0045 | |
| Controls | 68.37/1.22 | |||||
| C5/C6 | Possible | 57.98/2.83 | −7.60/3.06 | −13.71 | −1.48 | 0.0157 |
| Probable definite | 59.52/1.46 | −6.06/1.85 | −9.76 | −2.35 | 0.0017 | |
| Controls | 56.15/3.10 | −9.43/3.31 | −16.04 | −2.82 | 0.0059 | |
| 65.58/1.17 | ||||||
| T max | Possible | 33.69/1.96 | −3.49/2.13 | −7.74 | 0.76 | 0.1060 |
| Probable definite | 35.06/0.99 | −2.11/1.27 | −4.66 | 0.43 | 0.1020 | |
| Controls | 34.06/2.44 | −3.12/2.56 | −8.24 | 2.01 | 0.2286 | |
| 37.18/0.83 |
Note: p values after Bonferroni correction with a significance level of <0.0083 are marked bold.
Abbreviations: CI, confidence interval of the difference between means; rEE, revised El Escorial Criteria; WM, white matter; SE, standard error.
TABLE A4.
Comparison of white matter area means of patients with amyotrophic lateral sclerosis at different King's Stages (1–3), with healthy control subjects as reference, with adjustment for age and sex.
| Inter‐vertebral disc level | King's Stage | Adjusted mean WM area/SE, mm2 | Difference between means/SE | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|
| C2/C3 | 1 | 62.55/2.16 | −5.86/2.43 | −10.71 | −1.01 | 0.0185 |
| 2 | 60.07/2.25 | −8.34/2.51 | −13.34 | −3.33 | 0.0014 | |
| 3 | 61.35/1.52 | −7.06/1.81 | −10.68 | −3.44 | 0.0002 | |
| Controls | 68.41/1.07 | |||||
| C3/C4 | 1 | 62.44/2.07 | −6.04/2.32 | −10.68 | −1.40 | 0.0115 |
| 2 | 60.70/2.15 | −7.78/2.40 | −12.57 | −2.99 | 0.0019 | |
| 3 | 59.34/1.48 | −9.14/1.76 | −12.66 | −5.62 | <0.0001 | |
| Controls | 68.48/1.03 | |||||
| C4/C5 | 1 | 66.12/2.41 | −2.20/2.70 | −7.59 | 3.20 | 0.4191 |
| 2 | 60.23/2.50 | −8.09/2.79 | −13.65 | −2.52 | 0.0051 | |
| 3 | 60.52/1.76 | −7.79/2.08 | −11.95 | −3.63 | 0.0004 | |
| Controls | 68.31/1.20 | |||||
| C5/C6 | 1 | 62.66/2.31 | −2.84/2.59 | −8.02 | 2.34 | 0.2780 |
| 2 | 58.35/2.40 | −7.15/2.68 | −12.50 | −1.80 | 0.0096 | |
| 3 | 56.84/1.69 | −8.66/2.00 | −12.66 | −4.66 | <0.0001 | |
| Controls | 65.50/1.15 | |||||
| T max | 1 | 34.90/1.64 | −2.27/1.85 | −5.97 | 1.42 | 0.2236 |
| 2 | 34.68/1.71 | −2.49/1.91 | −6.32 | 1.33 | 0.1973 | |
| 3 | 34.60/1.22 | −2.56/1.43 | −5.43 | 0.30 | 0.0786 | |
| Controls | 37.17/0.83 |
Note: p values after Bonferroni correction with a significance level of <0.0083 are marked in bold.
Abbreviations: CI, confidence interval of the difference between means; WM, white matter; SE, standard error.
FIGURE A1.
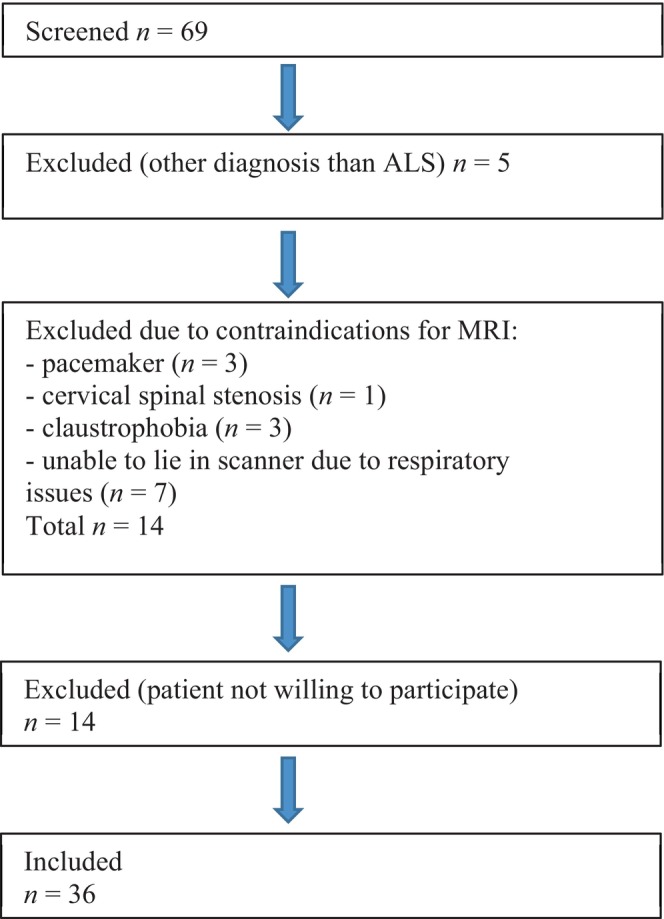
Flowchart overview of inclusion of patients with amyotrophic lateral sclerosis (ALS). MRI, magnetic resonance imaging.
Wendebourg MJ, Weigel M, Weidensteiner C, et al. Cervical and thoracic spinal cord gray matter atrophy is associated with disability in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2024;31:e16268. doi: 10.1111/ene.16268
DATA AVAILABILITY STATEMENT
Upon reasonable request we will render the detailed results derived from the reported analyses available.
REFERENCES
- 1. Tard C, Defebvre L, Moreau C, Devos D, Danel‐Brunaud V. Clinical features of amyotrophic lateral sclerosis and their prognostic value. Rev Neurol (Paris). 2017;173(5):263‐272. doi: 10.1016/j.neurol.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 2. Goyal NA, Berry JD, Windebank A, et al. Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS. Muscle Nerve. 2020;62(2):156‐166. doi: 10.1002/mus.26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menke RA, Agosta F, Grosskreutz J, Filippi M, Turner MR. Neuroimaging endpoints in amyotrophic lateral sclerosis. Neurotherapeutics. 2017;14(1):11‐23. doi: 10.1007/s13311-016-0484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Mendili MM, Cohen‐Adad J, Pelegrini‐Issac M, et al. Multi‐parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PLoS One. 2014;9(4):e95516. doi: 10.1371/journal.pone.0095516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verber NS, Shepheard SR, Sassani M, et al. Biomarkers in motor neuron disease: a state of the art review. Front Neurol. 2019;3(10):291. doi: 10.3389/fneur.2019.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen‐Adad J, El Mendili MM, Morizot‐Koutlidis R, et al. Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(1):30‐38. doi: 10.3109/17482968.2012.701308 [DOI] [PubMed] [Google Scholar]
- 7. Branco LM, De Albuquerque M, De Andrade HM, et al. Spinal cord atrophy correlates with disease duration and severity in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):93‐97. doi: 10.3109/21678421.2013.852589 [DOI] [PubMed] [Google Scholar]
- 8. Querin G, El Mendili MM, Lenglet T, et al. Spinal cord multi‐parametric magnetic resonance imaging for survival prediction in amyotrophic lateral sclerosis. Eur J Neurol. 2017;24(8):1040‐1046. doi: 10.1111/ene.13329 [DOI] [PubMed] [Google Scholar]
- 9. van der Burgh HK, Westeneng HJ, Meier JM, et al. Cross‐sectional and longitudinal assessment of the upper cervical spinal cord in motor neuron disease. Neuroimage Clin. 2019;24:101984. doi: 10.1016/j.nicl.2019.101984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paquin MÊ, El Mendili MM, Gros C, Dupont SM, Cohen‐Adad J, Pradat PF. Spinal cord gray matter atrophy in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2018;39(1):184‐192. doi: 10.3174/ajnr.A5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nigri A, Dalla Bella E, Ferraro S, et al. Cervical spinal cord atrophy in amyotrophic lateral sclerosis across disease stages. Ann Clin Transl Neurol. 2023;10(2):213‐224. doi: 10.1002/acn3.51712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valsasina P, Agosta F, Benedetti B, et al. Diffusion anisotropy of the cervical cord is strictly associated with disability in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2007;78(5):480‐484. doi: 10.1136/jnnp.2006.100032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nair G, Carew JD, Usher S, Lu D, Hu XP, Benatar M. Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. NeuroImage. 2010;53:576‐583. doi: 10.1016/j.neuroimage.2010.06.060 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Liu L, Ma L, et al. Preliminary study on cervical spinal cord in patients with amyotrophic lateral sclerosis using MR diffusion tensor imaging. Acad Radiol. 2014;21(5):590‐596. [DOI] [PubMed] [Google Scholar]
- 15. Weigel M, Bieri O. Spinal cord imaging using averaged magnetization inversion recovery acquisitions. Magn Reson Med. 2018;79:1870‐1881. doi: 10.1002/mrm.26833 [DOI] [PubMed] [Google Scholar]
- 16. Weigel M, Haas T, Wendebourg MJ, Schlaeger R, Bieri O. Imaging of the thoracic spinal cord using radially sampled averaged magnetization inversion recovery acquisitions. J Neurosci Methods. 2020;1(343):108825. doi: 10.1016/j.jneumeth.2020.108825 [DOI] [PubMed] [Google Scholar]
- 17. Wendebourg MJ, Weigel M, Richter L, et al. Spinal cord gray matter atrophy is associated with functional decline in post‐polio syndrome. Eur J Neurol. 2022;29(5):1435‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci. 1999;169(1–2):13‐21. doi: 10.1016/s0022-510x(99)00210-5 [DOI] [PubMed] [Google Scholar]
- 19. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on motor neuron diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293‐299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 20. Balendra R, Al Khleifat A, Fang T, Al‐Chalabi A. A standard operating procedure for King's ALS clinical staging. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3–4):159‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: nerve injuries research committee. His Majesty's stationery office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 figures. Brain. 2010;133(10):2838‐2844. [DOI] [PubMed] [Google Scholar]
- 22. Shefner JM, Liu D, Leitner ML, et al. Quantitative strength testing in ALS clinical trials. Neurology. 2016;87(6):617‐624. doi: 10.1212/WNL.0000000000002941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlaeger R, Papinutto N, Panara V, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol. 2014;76(4):568‐580. doi: 10.1002/ana.24241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kameyama T, Hashizume Y, Sobue G. Morphologic features of the normal human cadaveric spinal cord. Spine. 1996;21:1285‐1290. doi: 10.1097/00007632-199606010-00001 [DOI] [PubMed] [Google Scholar]
- 25. Papinutto N, Schlaeger R, Panara V, et al. 2D phase‐sensitive inversion recovery imaging to measure in vivo spinal cord gray and white matter areas in clinically feasible acquisition times. J Magn Reson Imaging. 2015;42:698‐708. doi: 10.1002/jmri.24819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sekiguchi T, Kanouchi T, Shibuya K, et al. Spreading of amyotrophic lateral sclerosis lesions—multifocal hits and local propagation? J Neurol Neurosurg Psychiatry. 2014;85(1):85‐91. doi: 10.1136/jnnp-2013-305617 [DOI] [PubMed] [Google Scholar]
- 27. Kiernan JA, Hudson AJ. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain. 1991;114:843‐853. doi: 10.1093/brain/114.2.843 [DOI] [PubMed] [Google Scholar]
- 28. Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral‐caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68(19):1576‐1582. doi: 10.1212/01.wnl.0000261045.57095.56 [DOI] [PubMed] [Google Scholar]
- 29. Kanouchi T, Ohkubo T, Yokota T. Can regional spreading of amyotrophic lateral sclerosis motor symptoms be explained by prion‐like propagation? J Neurol Neurosurg Psychiatry. 2012;83(7):739‐745. doi: 10.1136/jnnp-2011-301826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kent‐Braun JA, Walker CH, Weiner MW, Miller RG. Functional significance of upper and lower motor neuron impairment in amyotrophic lateral sclerosis. Muscle Nerve. 1998;21(6):762‐768. doi: [DOI] [PubMed] [Google Scholar]
- 31. Barry RL, Torrado‐Carvajal A, Kirsch JE, et al. Selective atrophy of the cervical enlargement in whole spinal cord MRI of amyotrophic lateral sclerosis. Neuroimage Clin. 2022;36:103199. doi: 10.1016/j.nicl.2022.103199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kesenheimer EM, Wendebourg MJ, Weigel M, et al. Normalization of spinal cord Total cross‐sectional and gray matter areas as quantified with radially sampled averaged magnetization inversion recovery acquisitions. Front Neurol. 2021;12:637198. doi: 10.3389/fneur.2021.63719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papinutto N, Asteggiano C, Bischof A, et al. Intersubject variability and normalization strategies for spinal cord Total cross‐sectional and gray matter areas. J Neuroimaging. 2020;30(1):110‐118. doi: 10.1111/jon.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Labra J, Menon P, Byth K, Morrison S, Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87(6):628‐632. doi: 10.1136/jnnp-2015-310998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request we will render the detailed results derived from the reported analyses available.


