Abstract
Background and purpose
Because Becker muscular dystrophy (BMD) is a heterogeneous disease and only few studies have evaluated adult patients, it is currently still unclear which outcome measures should be used in future clinical trials.
Methods
Muscle magnetic resonance imaging, patient‐reported outcome measures and a wide range of clinical outcome measures, including motor function, muscle strength and timed‐function tests, were evaluated in 21 adults with BMD at baseline and at 9 and 18 months of follow‐up.
Results
Proton density fat fraction increased significantly in 10/17 thigh muscles after 9 months, and in all thigh and lower leg muscles after 18 months. The 32‐item Motor Function Measurement (MFM‐32) scale (−1.3%, p = 0.017), North Star Ambulatory Assessment (−1.3 points, p = 0.010) and patient‐reported activity limitations scale (−0.3 logits, p = 0.018) deteriorated significantly after 9 months. The 6‐min walk distance (−28.7 m, p = 0.042), 10‐m walking test (−0.1 m/s, p = 0.032), time to climb four stairs test (−0.03 m/s, p = 0.028) and Biodex peak torque measurements of quadriceps (−4.6 N m, p = 0.014) and hamstrings (−5.0 N m, p = 0.019) additionally deteriorated significantly after 18 months. At this timepoint, domain 1 of the MFM‐32 was the only clinical outcome measure with a large sensitivity to change (standardized response mean 1.15).
Discussion
It is concluded that proton density fat fraction imaging of entire thigh muscles is a sensitive outcome measure to track progressive muscle fat replacement in patients with BMD, already after 9 months of follow‐up. Finally, significant changes are reported in a wide range of clinical and patient‐reported outcome measures, of which the MFM‐32 appeared to be the most sensitive to change in adults with BMD.
Keywords: BMD, Dixon, magnetic resonance imaging, quantitative MRI, trial readiness
INTRODUCTION
Becker muscular dystrophy (BMD) is an X‐linked recessive muscular dystrophy caused by a deficient dystrophin protein due to a genetic defect in the Duchenne muscular dystrophy (DMD) gene [1]. BMD is a rare disease (prevalence 2.5/100,000) and typically leads to a progressive proximal weakness in adolescents, although a wide range in disease severity exists, including muscle cramps and serum creatine kinase elevation, isolated cardiomyopathy or severe weakness with wheelchair dependence [2]. Novel therapies are being developed for patients with BMD (e.g., the ARCH [NCT05160415] and GRAND CANYON [NCT05291091] trials by Edgewise Therapeutics), which make a thorough understanding of natural disease progression and the availability of valid outcome measures important [3, 4]. Whilst natural history studies in adults with BMD used to be scarce, much has been learned over the past few years. First, it was shown that muscle proton density fat fraction (PDFF) correlates strongly with a range of clinical outcome measures such as the 6‐min walking distance (6MWD), North Star Ambulatory Assessment (NSAA), 32‐item Motor Function Measurement scale (MFM‐32) and timed‐function tests in patients with BMD [5, 6, 7, 8]. More recently, longitudinal follow‐up studies have demonstrated that muscle PDFF measurements were more sensitive to change over 1–2 years than some clinical outcome measures (Biodex dynamometry, NSAA, 6MWD, 10‐m run velocity) in patients with BMD [9, 10, 11]. Furthermore, baseline thigh muscle PDFF was shown to be a predictor of disease progression on magnetic resonance imaging (MRI), which allows the possibility of selecting more rapidly progressing patients with BMD for future clinical trials [12]. Nevertheless, numerous clinical outcome measures and many muscles on MRI remain unstudied.
In this study, advanced 3D segmentation models were applied to comprehensively evaluate fat replacement in 27 entire muscles of the upper and lower leg and pelvis. Simultaneously some previously studied and novel clinical outcome measures in BMD were explored as well as patient‐reported outcome measures (PROMs) of activity limitations and quality of life. The objective was to assess which combination of muscle MRI and clinical outcome measures is most promising to measure the effect of a future therapy in a relatively short timeframe. Finally, the proximo‐distal profiles of fat replacement along the length of individual thigh muscles were explored to evaluate if this occurs non‐homogeneously and follows certain patterns of involvement.
METHODS
Patients and study design
In this prospective, longitudinal study, genetically confirmed adults with BMD who were still ambulatory at baseline (walking aids and part‐time use of a wheelchair were allowed) and had no contraindications for an MRI scan were included (Table 1). All included patients were symptomatic as asymptomatic serum creatine kinase elevation (hyperCKemia) was an exclusion criterion.
TABLE 1.
Demographic and clinical characteristics of the patients.
| Patient demographics | |
|---|---|
| Male sex | 21 (100%) |
| Current BMI (kg/m2) | 25.8 ± 6.0 |
| Age at baseline (years) | 40 (18–66) |
| Age at symptom onset (years) | 13 (4–50) |
| Disease duration (years) | 25 (1–59) |
| Ambulatory | 21 (100%) |
| Use of walking aids | 7 (33.3%) |
| Baseline 6MWD | |
| <300 m | 5 (23.8%) |
| 300–400 m | 3 (14.3%) |
| 400–500 m | 6 (28.6%) |
| >500 m | 7 (33.3%) |
| Calf hypertrophy | 17 (81.0%) |
Note: Data are shown as n (%), median (range) or as mean ± standard deviation.
Abbreviations: 6MWD, 6‐min walking distance; BMI, body mass index.
The following clinical outcome measures were acquired in patients at baseline and after 9 and 18 months: 6MWD, 10‐m walking test (10MWT), Medical Research Council (MRC) sum score (number of points), time to rise from floor test (TRF), time to climb four stairs test (TC4S), timed up and go test (TUG), NSAA, MFM‐32 (consisting of domains D1, D2 and D3) and Biodex® (System 4 Pro) dynamometry for isometric muscle strength measurement of the quadriceps and hamstrings muscle groups (as described in a previous study) [13, 14, 15, 16, 17, 18, 19, 20, 21]. The timed‐function tests (10MWT, TRF, TC4S, TUG) were analysed as a function of velocity (m/s) instead of time so a speed of 0 m/s corresponds to the patient being unable to perform the test [22]. The PROMs activity limitations scale (ActivLim) and Short Form 36‐item health survey (SF‐36) quality of life, which have been validated in a wide range of neuromuscular disorders, were also evaluated [23, 24]. Serum creatine kinase was measured in all patients. Finally, a muscle MRI scan with 6‐point Dixon and T1 images of the legs was performed at each study visit. The study was approved by the Ethics Committee Research UZ/KU Leuven and all participants provided written informed consent.
MRI data acquisition
Six‐point Dixon fast images in 3D (TR/TE1/ΔTE = 9.2/1.36/1.3 ms [TR repetition time, TE echo time], flip angle 12°, 125 slices, slice thickness 2 mm, field of view [FOV] 450 × 394 × 252 mm3, matrix 320 × 280 × 125, voxel size 1.2 × 1.2 2 mm3) and axial T1 turbo spin echo sequences (TR 412 ms, TE 4.0 ms, 30 slices with 8 mm slice thickness and 1 mm interslice gap, FOV 300 × 455 mm2, voxel size 1.4 × 1.4 mm2) were acquired with a 1.5 T Philips Ingenia MRI scanner (Philips Medical Systems, Best, The Netherlands). Three Dixon imaging stacks of the thighs (overlapping by 20 slices) were sufficient to capture all thigh muscles from origin to insertion, from the iliac crest to the tibial plateau. For technical reasons, only Dixon images of the right lower leg were acquired, and two overlapping stacks were always sufficient to capture the entire lower leg. The standard mDixon_QUANT protocol was used for Dixon imaging by Philips for an optimal signal‐to‐noise ratio and short acquisition time, followed by a correction for T1‐weighting in post‐processing as described by Liu et al. [25]. All participants were placed in a supine, feet‐first position on the MRI table and the legs were immobilized with sandbags.
MRI analysis
As described in previous studies, a custom‐made convolutional neural network was used to semi‐automatically obtain 3D segmentations of entire individual muscles and muscle groups of the thighs (n = 21) and right lower leg (n = 11) on the Dixon images (Figure S1) [21, 26, 27]. From the Dixon images, the PDFF image was calculated as fat/(fat + water) images and the 3D segmentations were used to calculate a mean PDFF per muscle. The volume‐weighted mean of muscles was used to calculate PDFF values for muscle groups and for the ‘total muscles’ parameters. For the thighs, the mean PDFF of the muscles in both legs is reported. The mean PDFF per muscle per slice (of the left and right leg combined) was used to evaluate the profile of muscle fat replacement along the proximo‐distal axis of the thigh muscles. All these calculations were performed with a custom‐made MATLAB script (MathWorks, Natick, MA, USA).
A subgroup analysis was performed to evaluate if baseline PDFF could explain a difference in the rate of muscle PDFF increase or clinical deterioration between patients. Previous studies in adults with BMD described a sigmoidal relation between the increase in thigh muscle PDFF over time and baseline PDFF, with slow increases in patients with a baseline PDFF of <20%, a gradually increasing rate in patients with a baseline PDFF of 45%–60% and finally a slight decrease in rate in patients with an even higher baseline PDFF [10, 12]. One study therefore divided patients into a ‘low baseline PDFF’ group of <35% and a ‘high baseline PDFF’ group with >35% [9]. In analogy with the existing literature, it was therefore decided to use the same, arbitrary cutoff values in this study.
Statistical analysis
RStudio® Desktop (Open‐Source License, version 4.1.2) was used for all statistical analyses. Significance level was determined at α = 0.05 and Holm's method was used to correct for multiple testing [28]. The Shapiro–Wilk test was used to determine normality of the data. For comparisons of clinical or radiological outcome measures between study visits and between subgroups, t tests and Wilcoxon ranked‐sum tests were used. To assess the correlation between PDFF and clinical outcome measures, Pearson and Spearman correlation coefficients were reported. The standardized response mean (SRM) was used to assess the effect size of changes in outcome measures over time and this was calculated as the mean change over time divided by the standard deviation of the change [10, 29]. For analysis of the homogeneity of fat replacement in each thigh muscle, a nonlinear mixed model with three‐knot b‐splines was compared with an intercept only mixed model, and an ANOVA likelihood ratio test was used to obtain a p value for comparison of these models based on the Akaike information criterion, as described in more detail in a previous study [26].
RESULTS
Patient characteristics
The demographic and clinical data of all participants are detailed in Table 1. None of the patients had been treated with corticosteroids. Two patients with BMD completed only the baseline study visit but not the visits at 9 and 18 months for personal reasons. One of these patients was severely affected and one very mildly affected (baseline total thigh PDFF and 6MWD of 73% and 151 m and 7% and 568 m, respectively). One other patient missed the visit at month 9 due to a hip fracture for which he was surgically treated. He participated again at month 18 but had lost ambulation.
Changes in PDFF of thigh and lower leg muscles after 9 and 18 months
After 9 months, PDFF increased significantly in 10/17 individual thigh muscles, in all three thigh muscle groups and in the total thigh muscles group (after Holm's correction; Table 2). After 18 months, the PDFF in all 17 individual thigh muscles had increased significantly by on average 3.1% (p < 0.001; Figure 1). The effect size of change (SRM) over 18 months was the largest for the vastus medialis, followed by the total thigh muscles and quadriceps muscle groups.
TABLE 2.
Evolution of PDFF in leg muscles in adults with BMD over 9 and 18 months of follow‐up.
| Muscles | Baseline | Month 9 | p value | SRM | Month 18 | p value | SRM |
|---|---|---|---|---|---|---|---|
| Biceps femoris long head | 45.4 ± 34.3 | 1.7 ± 2.1 | 0.001 | 0.82 | 2.7 ± 2.8 | <0.001 | 0.98 |
| Adductor magnus | 44.1 ± 32.6 | 1.6 ± 1.5 | <0.001 | 1.11 | 2.7 ± 2.6 | <0.001 | 1.04 |
| Semimembranosus | 42.9 ± 32.1 | 1.9 ± 2.3 | <0.001 | 0.83 | 2.7 ± 2.3 | <0.001 | 1.15 |
| Gluteus maximus | 41.8 ± 25.8 | 1.0 ± 1.4 | 0.002 | 0.70 | 3.1 ± 2.7 | <0.001 | 1.14 |
| Vastus lateralis | 39.1 ± 29.9 | 0.7 ± 2.6 | 0.212 | 0.28 | 2.5 ± 2.9 | 0.002 | 0.84 |
| Gluteus medius | 39.5 ± 25.8 | 1.7 ± 2.1 | 0.004 | 0.80 | 4.2 ± 4.4 | <0.001 | 0.95 |
| Vastus medialis | 38.0 ± 28.1 | 2.0 ± 3.0 | 0.002 | 0.66 | 3.1 ± 2.2 | <0.001 | 1.40 |
| Vastus intermedius | 35.6 ± 27.2 | 2.0 ± 2.1 | <0.001 | 0.92 | 3.0 ± 2.5 | <0.001 | 1.19 |
| Semitendinosus | 31.5 ± 29.5 | 1.2 ± 1.5 | 0.004 | 0.79 | 2.7 ± 3.4 | 0.001 | 0.78 |
| Gluteus minimus | 29.6 ± 22.6 | 0.8 ± 2.1 | 0.152 | 0.35 | 5.1 ± 6.6 | <0.001 | 0.78 |
| Adductor brevis | 27.9 ± 26.5 | 0.2 ± 2.9 | 0.610 | 0.07 | 2.1 ± 2.6 | 0.001 | 0.81 |
| Biceps femoris short head | 26.8 ± 25.2 | 1.5 ± 2.8 | 0.012* | 0.52 | 2.7 ± 3.5 | <0.001 | 0.78 |
| Rectus femoris | 23.0 ± 22.4 | 1.2 ± 2.3 | 0.046* | 0.51 | 2.2 ± 2.8 | 0.003 | 0.79 |
| Adductor longus | 23.1 ± 28.4 | 0.7 ± 1.0 | 0.014* | 0.64 | 2.4 ± 3.2 | 0.002 | 0.75 |
| Pectineus | 17.4 ± 24.1 | 0.7 ± 2.0 | 0.143 | 0.36 | 1.3 ± 2.0 | 0.006 | 0.67 |
| Gracilis | 17.1 ± 18.2 | 1.2 ± 1.6 | 0.002 | 0.75 | 2.7 ± 4.2 | 0.006 | 0.63 |
| Sartorius | 13.5 ± 12.6 | 0.7 ± 1.3 | 0.002 | 0.55 | 1.0 ± 2.0 | 0.020 | 0.48 |
| Hamstrings muscle group | 37.1 ± 30.0 | 1.3 ± 1.9 | 0.003 | 0.68 | 2.7 ± 2.7 | <0.001 | 1.00 |
| Adductors muscle group | 35.9 ± 29.1 | 1.4 ± 1.6 | 0.001 | 0.86 | 3.0 ± 2.9 | <0.001 | 1.02 |
| Quadriceps muscle group | 35.3 ± 26.7 | 1.5 ± 1.8 | <0.001 | 0.90 | 2.8 ± 2.3 | <0.001 | 1.23 |
| Total thigh muscles | 35.3 ± 25.8 | 1.3 ± 1.1 | <0.001 | 1.17 | 3.1 ± 2.4 | <0.001 | 1.29 |
| Gastrocnemius medialis | 33.6 ± 28.6 | 1.7 ± 2.3 | 0.007* | 0.74 | 5.5 ± 6.2 | <0.001 | 0.89 |
| Gastrocnemius lateralis | 23.7 ± 26.1 | 0.7 ± 1.6 | 0.080 | 0.44 | 4.0 ± 5.5 | <0.001 | 0.73 |
| Peroneus | 23.5 ± 19.2 | 0.4 ± 1.7 | 0.288 | 0.26 | 3.4 ± 3.3 | <0.001 | 1.01 |
| Soleus | 18.6 ± 15.7 | 0.6 ± 0.9 | 0.018* | 0.64 | 3.5 ± 4.0 | <0.001 | 0.87 |
| Tibialis anterior | 17.3 ± 16.2 | 0.6 ± 1.5 | 0.089 | 0.43 | 2.1 ± 2.6 | <0.001 | 0.82 |
| Extensor digitorum longus | 14.9 ± 13.3 | 0.5 ± 1.7 | 0.276 | 0.27 | 2.2 ± 2.0 | <0.001 | 1.13 |
| Flexor hallucis longus | 11.1 ± 8.8 | 0.9 ± 1.5 | 0.007* | 0.56 | 1.6 ± 1.5 | <0.001 | 1.08 |
| Popliteus | 9.3 ± 11.4 | 0.4 ± 1.7 | 0.358 | 0.22 | 1.7 ± 2.0 | <0.001 | 0.83 |
| Tibialis posterior | 8.5 ± 7.0 | 0.0 ± 0.9 | 1 | 0.01 | 1.3 ± 1.1 | <0.001 | 1.19 |
| Flexor digitorum longus | 6.6 ± 5.4 | 0.2 ± 1.4 | 0.099 | 0.13 | 1.5 ± 1.4 | <0.001 | 1.10 |
| Total lower leg muscles | 19.8 ± 15.6 | 0.6 ± 1.1 | 0.024* | 0.58 | 3.2 ± 3.2 | <0.001 | 0.99 |
Note: Muscles and muscle groups are ordered according to severity of muscle fat replacement at baseline. The baseline column shows the mean ± SD PDFF percentage for each muscle, and the 9‐ and 18‐month columns both show the absolute increase in PDFF percentage over this time compared to baseline. Significant p values are marked in bold, as well as SRMs with a large effect size (>0.80 following Cohen's rule). The asterisk (*) marks p values that were significant before Holm's correction for multiple testing.
Abbreviations: BMD, Becker muscular dystrophy; PDFF, proton density fat fraction; SD, standard deviation; SRM, standardized response mean.
FIGURE 1.
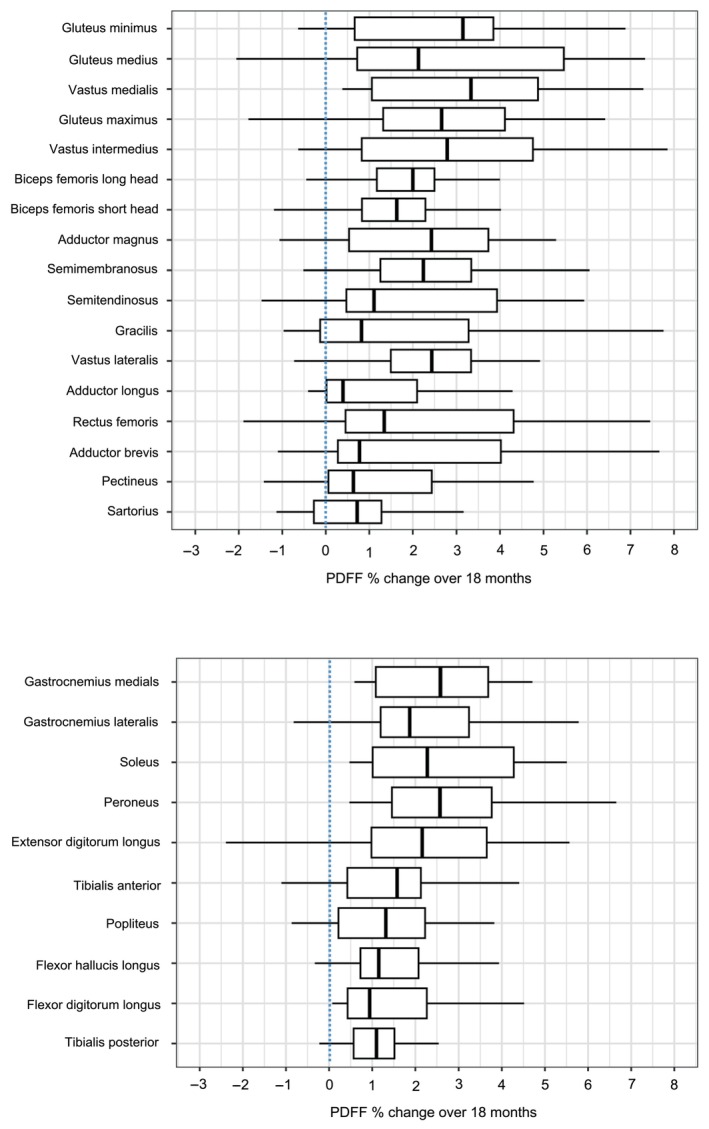
Boxplots of percentage change in PDFF of all thighs (left) and lower leg muscles (right) of adults with Becker muscular dystrophy over 18 months. PDFF, proton density fat fraction.
In the subgroup of patients with a baseline total thigh PDFF of <35% (n = 10) fat fraction increased by 2.4% ± 3.1% (p = 0.047, SRM 0.78) over 18 months, and in patients with a baseline total thigh PDFF of ≥35% (n = 11) fat fraction increased by 3.8% ± 1.5% (p < 0.001, SRM 2.44) over 18 months. The change in total thigh PDFF over 18 months did not differ significantly between these subgroups (p = 0.227).
There was a significant increase of PDFF in all lower leg muscles of patients after 18 months, but in none of these muscles after 9 months (after Holm's correction).
Changes in clinical and patient‐reported outcome measures over 9 and 18 months
The MFM‐32 total score, MFM‐32 D1, NSAA and ActivLim deteriorated significantly after 9 months, with the SRM showing a moderate sensitivity to change for these outcome measures (Table 3). After 18 months, these measures had further declined, and the 6MWD, 10MWT, TC4S and Biodex® dynamometry knee extension and flexion had now also deteriorated significantly in comparison to baseline. After Holm's correction only the deterioration in MFM‐32 D1 and MFM‐32 total scores remained significant at 18 months. The MFM‐32 D1 at 18 months was the only clinical outcome measure with a high sensitivity to change (SRM 1.15).
TABLE 3.
Clinical and patient‐reported outcome measures over 9 and 18 months of follow‐up.
| Outcome measures | Baseline | Month 9 | p value | SRM | Month 18 | p value | SRM |
|---|---|---|---|---|---|---|---|
| 6MWD (m) | 433.2 ± 178.8 | −15.1 ± 34.8 | 0.083 | 0.43 | −28.7 ± 57.3 | 0.042* | 0.50 |
| 10MWT (m/s) | 1.7 ± 0.8 | −0.1 ± 0.1 | 0.097 | 0.41 | −0.1 ± 0.3 | 0.032* | 0.40 |
| TRF (m/s) | 0.2 ± 0.2 | 0.0 ± 0.1 | 0.294 | 0.16 | 0.0 ± 0.1 | 0.351 | 0.25 |
| TC4S (m/s) | 0.3 ± 0.3 | 0.0 ± 0.1 | 0.870 | 0.04 | 0.0 ± 0.1 a | 0.028* | 0.45 |
| TUG (m/s) | 0.5 ± 0.1 | 0.0 ± 0.1 | 0.627 | 0.12 | 0.0 ± 0.2 | 0.793 | 0.06 |
| NSAA (points/34) | 22.1 ± 10.5 | −1.3 ± 2.0 | 0.010* | 0.65 | −2.5 ± 3.2 | 0.006* | 0.78 |
| MFM‐32 D1 (%) | 62.1 ± 31.5 | −3.7 ± 4.8 | 0.016* | 0.76 | −6.1 ± 5.3 | <0.001 | 1.15 |
| MFM‐32 D2 (%) | 95.2 ± 7.4 | +0.6 ± 3.5 | 0.784 | 0.17 | −4.2 ± 12.2 | 0.076 | 0.35 |
| MFM‐32 D3 (%) | 99.8 ± 1.04 | −0.3 ± 1.1 | 1 | 0.24 | −1.5 ± 3.2 | 0.095 | 0.47 |
| MFM‐32 (%) | 82.8 ± 15.0 | −1.3 ± 2.1 | 0.017* | 0.62 | −4.4 ± 6.4 | <0.001 | 0.69 |
| MRC sum score (points/60) | 50.6 ± 8.4 | +0.8 ± 2.7 | 0.241 | 0.26 | −0.6 ± 6.4 | 0.682 | 0.08 |
| MRC sum score (points/150) | 132.7 ± 17.7 | +1.0 ± 3.8 | 0.284 | 0.28 | +0.3 ± 3.3 | 0.727 | 0.09 |
| Peak torque quadriceps (N m) | 48.3 ± 52.2 | −1.5 ± 12.0 | 0.286 | 0.13 | −4.6 ± 7.4 | 0.014* | 0.62 |
| Peak torque hamstrings (N m) | 54.9 ± 30.8 | +1.5 ± 6.7 | 0.368 | 0.22 | −5.0 ± 8.4 | 0.019* | 0.59 |
| CK (U/L) | 1419 ± 2153 | −189 ± 2059 | 0.616 | 0.09 | −231 ± 1976 | 0.671 | 0.12 |
| ActivLim (logits) | 3.0 ± 3.0 | −0.3 ± 0.6 | 0.018* | 0.58 | −0.6 ± 1.1 | 0.021* | 0.48 |
| SF‐36 (%) | |||||||
| Physical functioning | 50.0 ± 35.2 | −1.4 ± 12.0 | 0.629 | 0.11 | +0.8 ± 14.8 | 0.938 | 0.05 |
| Role limitations, physical | 67.9 ± 36.4 | 0.0 ± 34.3 | 1 | 0.00 | −14.5 ± 37.6 | 0.109 | 0.39 |
| Body pain | 75.4 ± 22.0 | −0.4 ± 10.4 | 1 | 0.04 | −6.7 ± 15.0 | 0.067 | 0.45 |
| General health perception | 54.8 ± 21.7 | −3.1 ± 14.5 | 0.383 | 0.21 | −1.6 ± 18.6 | 0.715 | 0.09 |
| Vitality | 57.4 ± 20.0 | +6.9 ± 17.8 | 0.075 | 0.39 | −2.6 ± 12.8 | 0.726 | 0.20 |
| Social functioning | 68.5 ± 27.8 | +6.3 ± 22.0 | 0.244 | 0.28 | +7.9 ± 20.9 | 0.117 | 0.38 |
| Role limitations, emotional | 76.2 ± 35.2 | +1.9 ± 21.3 | 0.713 | 0.09 | −5.3 ± 33.8 | 0.586 | 0.16 |
| Mental health | 72.4 ± 18.5 | +0.7 ± 11.1 | 0.888 | 0.06 | −3.6 ± 8.4 | 0.080 | 0.42 |
Note: The baseline column shows the mean ± SD for each outcome measure, and the 9‐ and 18‐month columns both show the changes over this time compared to baseline. The first four listed domains of the SF‐36 pertain to physical well‐being and the last four to emotional well‐being, with a higher percentage indicating better health. Significant p values are marked in bold, as well as SRMs with a large effect size (>0.80 following Cohen's rule). The asterisk (*) marks p values that were significant before Holm's correction for multiple testing.
Abbreviations: 6MWD, 6‐min walking distance; 10MWT, 10‐m walking test; ActivLim, Activity Limitations scale; CK, creatine kinase; MFM‐32, 32‐item Motor Function Measurement scale (domains D1, D2 and D3); MRC, Medical Research Council; NSAA, North Star Ambulatory Assessment; SD, standard deviation; SF‐36, Short Form 36‐item health survey; SRM, standardized response mean; TC4S, time to climb four stairs test; TRF, time to rise from floor test; TUG, timed up and go test.
TC4S speed decreased by 0.03 m/s.
Post hoc comparison of subgroups of patients with a baseline total thigh PDFF of <35% and ≥35% showed that the NSAA decreased significantly (p = 0.048) more in the group with a higher baseline PDFF over 18 months, but no significant differences were found for any of the other clinical outcome measures (despite a general trend towards faster deterioration in the high baseline PDFF group).
All clinical outcome measures correlated strongly with total thigh PDFF at baseline, except for MFM‐32 D3 (Figure 2). Quadriceps and hamstrings dynamometry muscle strength also correlated with the respective PDFF of these muscle groups. The evolution in clinical outcome measures over 9 or 18 months did not correlate with total thigh PDFF changes during the study. Also no significant correlation between total thigh PDFF and body mass index (ρ = 0.14, p = 0.554) or creatine kinase values (ρ = −0.08, p = 0.737) at baseline was found.
FIGURE 2.
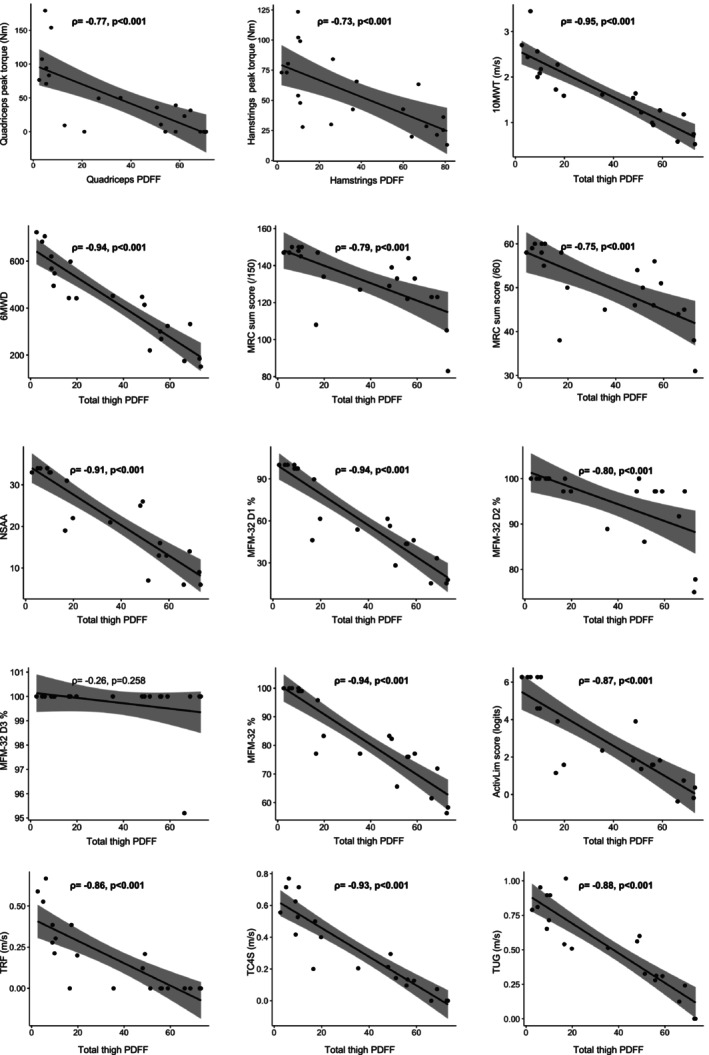
Correlations between PDFF and clinical outcome measures at baseline. The grey areas around the regression lines are 95% confidence intervals. Quantitative strength measurements with dynamometry of the quadriceps and hamstrings muscle groups are correlated with the PDFF of the quadriceps and hamstrings muscle groups, respectively. All other clinical outcome measures are correlated with the total thigh muscles PDFF. Only domain 3 of MFM‐32 did not correlate significantly with PDFF. MFM‐32, Motor Function Measurement scale; PDFF, proton density fat fraction.
Finally, SF‐36 quality of life measurements did not change significantly over 9 or 18 months of follow‐up.
Patterns of non‐uniform fat replacement in thigh muscles of patients with BMD
Fat replacement was not homogeneously distributed along the length of any of the thigh muscles (p < 0.001 for all). Figure 3 shows the patterns of fat replacement within thigh muscles of patients with BMD at baseline. Muscles are roughly grouped together based on similarities in the fat replacement patterns on a group level (despite some individual variation). Overall, muscles with patterns can be discerned following a cubic function with fat replacement occurring first either more distally in the muscle (Figure 3a) or more proximally (Figure 3b). Next, fat replacement can also occur more in the centre than at the origin/insertion (Figure 3c), resembling a downward facing parabola, and in some muscles there is little coherence between patients and they are thus ‘undefined’ (Figure 3d). In several muscles the predominant pattern also changes depending on the stage of fat replacement. For example, the cubic function pattern in muscles with a more proximal fat replacement (vastus lateralis/intermedius and gluteus medius/minimus) appears to change to an upward facing (U) parabola pattern in more severely affected patients.
FIGURE 3.
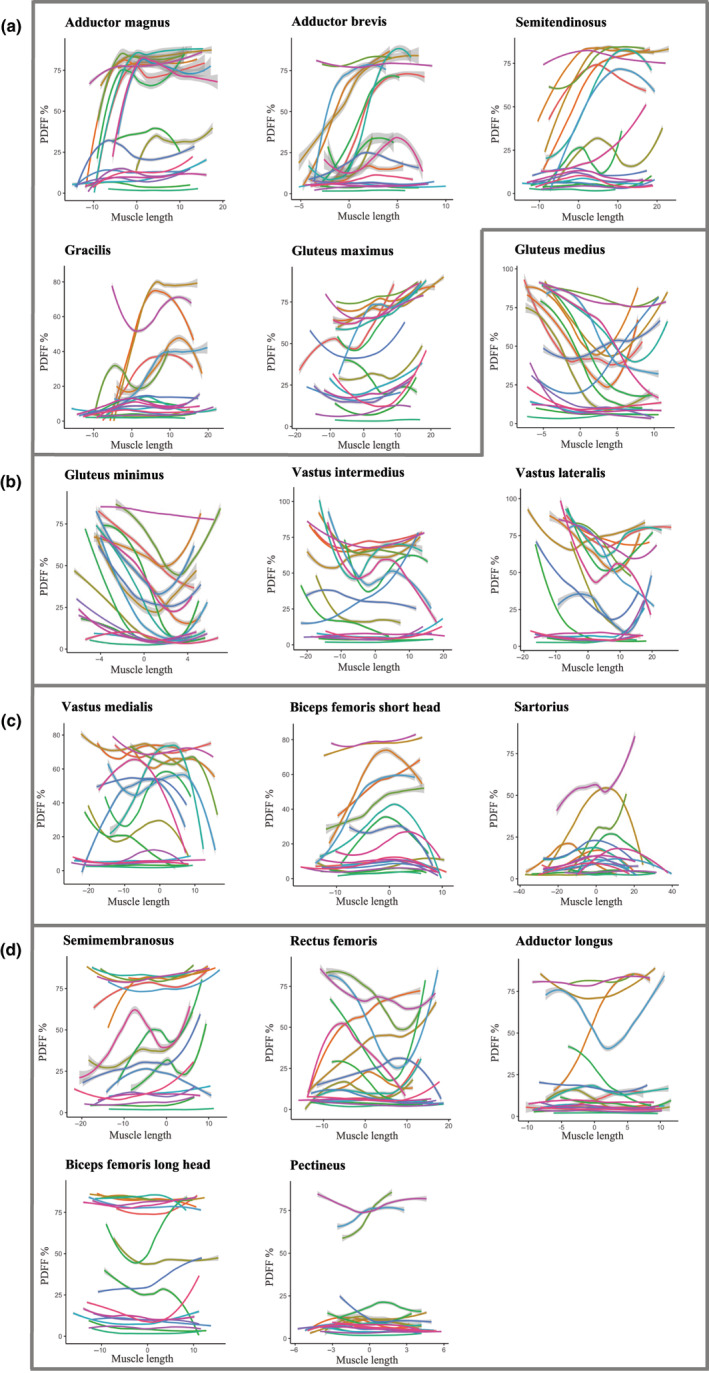
Patterns of fat replacement within thigh muscles of patients with Becker muscular dystrophy at baseline. The PDFF percentage is shown for each of the 17 thigh muscles on the y‐axis, with the x‐axis representing the length of the muscle in centimetres from proximal (left, negative) to distal (right, positive). The most voluminous slice of the muscle is centred at muscle length ‘0’ on the x‐axis to enable easy visual comparison of the same parts of muscles from patients with varying thigh lengths in one graph. Each coloured line represents an individual patient, consisting of about 50–250 individual PDFF measurements from all (2 mm thick) Dixon MRI slices covering that muscle. These lines were smoothed with a generalized additive model function with confidence intervals shown as grey bars around the lines, to aid in visual representation. Thigh muscles were grouped together according to similarities in the predominant observable fat replacement patterns: (a) muscles with more pronounced fat replacement distally, (b) muscles with more pronounced fat replacement proximally, (c) muscles with more fat replacement in their centre than at the origin/insertion (resembling a downward facing parabola) and (d) muscles with little coherence between patients and thus an ‘undefined’ pattern.
DISCUSSION
It was shown that, even over a relatively short follow‐up period of 9 months, PDFF measurements of thigh muscles were sensitive to change in adults with BMD, whereas the PDFF of lower leg muscles increased significantly after 18 months. It was also shown that especially the MFM‐32, but potentially also the NSAA, ActivLim, isometric thigh muscle strength testing with Biodex dynamometry and 6MWD could be useful in future studies in adults with BMD. Finally, the patterns of fat replacement within thigh muscles in BMD were described.
Proton density fat fraction changes in thigh and lower leg muscles of adults with BMD
In this study, PDFF was assessed in 17 individual thigh muscles and 10 muscles of the lower leg in adults with BMD. This enabled us to report on previously unstudied muscles such as the glutei, of which the medius and maximus were shown to be highly sensitive to change. A short 9‐month interval was chosen for the evaluation of outcome measures, as opposed to a similar study which reported change over 2 years, because the ability to assess disease progression in a shorter time‐window is important for future clinical trials [10]. That study by van de Velde et al. reported a high sensitivity to change (SRM >0.80) of PDFF over 2 years in the quadriceps muscle group, individual quadriceps muscles and the average of all thigh muscles. A high SRM was additionally reported in the adductors muscle group, adductor magnus, biceps femoris long head, semimembranosus and gluteus medius muscles over only 9 months. After 18 months, the hamstrings muscle group, adductor brevis and gluteus maximus could also be added to this list, as well as all the muscles of the lower leg except for the gastrocnemius lateralis. The observed increase of 1.3% in all thigh muscles over 9 months and 3.1% over 18 months is very similar to that reported in two recent studies in adults with BMD (1%–2% per year) [10, 11]. Similar to previous studies in adults with BMD, it was shown that PDFF increased faster in thighs of patients with a higher baseline fat fraction (≥35%) than those with a lower baseline fat fraction (<35%). Although the difference between these groups was not significant, this may be the case in a larger study population [9, 12].
After 9 months, only muscles of the thighs (and not lower legs) had shown a significant increase in PDFF, which confirms that these are more sensitive to change over short periods of time and thus ideally suited as an outcome measure of disease progression in clinical trials.
Clinical and patient‐reported outcome measures in adults with BMD
It was found that both the NSAA and MFM‐32 already showed a significant decline after 9 months in patients with BMD, with specifically D1 of the MFM‐32 showing the highest sensitivity to change (SRM 0.76). The NSAA had previously been reported to change significantly in patients with BMD over 1–2 years [10, 16]. Of course, it is not necessary to include both the MFM‐32 D1 and the NSAA as outcome measures in a clinical trial as both focus on ambulatory functions and they overlap considerably. Our study suggests a slight superiority of the MFM‐32 (D1) to detect clinical deterioration, as it was the only clinical outcome measure that had a high SRM (1.15) after 18 months and remained significant after Holm's correction. Domains 2 and 3 of the MFM‐32 were not sensitive to change in this population of ambulatory patients but might potentially be of more use in later stages of the disease.
Although it had previously been shown that quantitative strength assessment of the thigh muscles can change significantly over 2 years of follow‐up, this was confirmed after 18 months of follow‐up [10].
In this study, it was hypothesized that patients with a higher thigh total muscle PDFF could also experience a more rapid decline in muscle strength and function. It was shown that the NSAA decreased significantly more over 18 months in patients with a baseline total thigh PDFF of ≥35%, which suggests that patients with a higher PDFF at baseline experience not just a faster rate of fat replacement on MRI but also of clinical deterioration.
The significant decrease of 28.7 m in 6MWD in patients after 18 months is approximately the estimated minimal clinically important difference for this outcome measure [22]. This further supports the use of the 6MWD in adults with BMD, even though it appears to be less sensitive over shorter follow‐up periods than MFM‐32, NSAA and Biodex measurements. The significant changes in 10MWT and TC4S after 18 months were very small, with small SRMs, and therefore appear less suited as an outcome measure of disease progression.
A novel aspect of this study in adults with BMD was the inclusion of PROMs. It was demonstrated that the ActivLim score could detect a significant functional decline in adults with BMD even after only 9 months. This advocates for its use both in clinical trials and in clinical practice, especially given that more frequently used outcome measures showed no difference (e.g., MRC sum score) over this short timeframe. The other PROM, the SF‐36 health status, did not detect changes in health status over 9 or 18 months of follow‐up, but it could have merits over a longer timeframe.
Patterns of non‐uniform fat replacement in thigh muscles of patients with BMD
This is the first study to analyse entire leg muscles from origin to insertion in BMD. This also ensured optimal accuracy of repeated PDFF measurements as it eliminated selection and repositioning biases caused by inhomogeneous muscle fat replacement, as discussed previously [10, 21, 26].
Whilst the distribution of fat replacement in the vastus lateralis and semitendinosus muscles in adults with BMD had already been explored, similar fat replacement patterns were confirmed for these two muscles and additionally patterns were reported for 15 other thigh muscles in this study, thereby demonstrating that fat replacement did not occur homogeneously along the length of any of the thigh muscles in adults with BMD [10].
Limitations
First, as BMD is a rare disease, our study population was relatively small. However, sample size was comparable to similar studies in the literature, and our study comprised a more comprehensive analysis of the natural history in adults with BMD, with PDFF measurements in as many as 27 individual entire leg muscles and evaluation of 11 clinical outcome measures and PROMs [9, 10]. This allowed for a comparison of the relative sensitivity of all these outcome measures to change in a single study. However, correction for multiple statistical analyses was consequently severe, and some results therefore became insignificant, although this should not be interpreted as irrelevant. One of the patients lost ambulation during the study after a traumatic hip fracture but was still included for analysis because this development is an accurate representation of the natural history of late‐stage ambulatory patients with BMD.
Second, it would be interesting to evaluate the change in fat replacement patterns in individual muscles over time, but a longer follow‐up period would be needed to make such observations.
Third, although the ActivLim scale is not specifically validated in adults with BMD, it was chosen to include as an outcome measure because it has been validated in a wide range of neuromuscular disorders (including Duchenne muscular dystrophy) and is generally applied to assess functional limitations in neuromuscular disorders [23]. Nevertheless, given its potential as an outcome measure in future clinical trials, an additional validation would first be preferable.
Finally, the focus was on a subsection of patients who were still ambulatory as these patients are most likely to be targeted for clinical trials. However, as most studies adopt this approach, little is known about outcome measures in more severely affected patients with BMD and further study needs to be done in non‐ambulatory patients to define sensitive outcome measures in this group of patients as well. Most of the outcome measures used in the current study specifically targeted ambulatory patients (e.g., 6MWD…), but the PROMs and MFM (specifically domains 2 and 3) could potentially still be useful in non‐ambulatory patients, as could PDFF analyses of the upper limb muscles.
CONCLUSIONS
It is concluded that PDFF imaging of entire individual thigh muscles and muscle groups is a sensitive outcome measure to track progressive muscle fat replacement in patients with BMD, already after 9 months of follow‐up. PDFF measurements of lower leg muscles increased significantly after 18 months. In addition to PDFF imaging of entire individual thigh muscles it is suggested that future clinical trials include the MFM‐32, and potentially also the Biodex, 6MWD and ActivLim scales because of their moderately strong sensitivity to change. It was also shown that fat replacement did not occur homogeneously in any of the 17 investigated thigh muscles and the associated patterns of fat replacement within these muscles are described. The results of this study, which combined a quantitative MRI analysis of all muscles of the upper and lower leg with an evaluation of an extensive range of clinical outcome measures and PROMs, can be used to optimize clinical trial design in future therapeutic studies in adults with BMD.
AUTHOR CONTRIBUTIONS
Bram De Wel: Conceptualization; investigation; writing – original draft; writing – review and editing; visualization; methodology; formal analysis; project administration; data curation. Louise Iterbeke: Writing – review and editing; formal analysis. Lotte Huysmans: Writing – review and editing; formal analysis. Ronald Peeters: Writing – review and editing; methodology. Veerle Goosens: Writing – review and editing. Nicolas Dubuisson: Writing – review and editing. Peter van den Bergh: Writing – review and editing. Vinciane Van Parijs: Writing – review and editing. Gauthier Remiche: Writing – review and editing. Liesbeth De Waele: Writing – review and editing. Frederik Maes: Writing – review and editing; methodology. Patrick Dupont: Methodology; writing – review and editing; formal analysis. Kristl G. Claeys: Conceptualization; investigation; funding acquisition; writing – original draft; writing – review and editing; methodology; project administration; resources; supervision; data curation.
FUNDING INFORMATION
This study received research funding from the Klinische Onderzoeks en OpleidingsRaad (KOOR) of UZ Leuven and the Kan‐GO! Fund of KU Leuven. BDW is supported by the Research Foundation – Flanders (FWO, PhD fellowship fundamental research grant number 1159121N). The work of FM and LH is supported in part by the Internal Funds KU Leuven under Grant C24/18/047 and by the Flemish Government under the ‘Onderzoeksprogramma Artificiële Intelligentie (AI) Vlaanderen’ programme.
CONFLICT OF INTEREST STATEMENT
KGC is Chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. Several authors are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD) and the European Reference Network for Rare Neurological Diseases (ERN‐RND). ND has received compensation for lectures and/or advisory boards from Alnylam, Sanofi, Jansen and ArgenX. The authors report no disclosures relevant to the paper.
Supporting information
Figure S1.
ACKNOWLEDGEMENTS
The patients are thanked for their participation in the study. The authors are also grateful to Stefan Ghysels, Kris Byloos and Guido Putzeys of the MRI study team at UZ Leuven for their help with scanning the study participants.
De Wel B, Iterbeke L, Huysmans L, et al. Lessons for future clinical trials in adults with Becker muscular dystrophy: Disease progression detected by muscle magnetic resonance imaging, clinical and patient‐reported outcome measures. Eur J Neurol. 2024;31:e16282. doi: 10.1111/ene.16282
DATA AVAILABILITY STATEMENT
The anonymized supporting datasets analysed during the current study are available to qualified investigators from the corresponding author on reasonable request.
REFERENCES
- 1. Waldrop MA, Flanigan KM. Update in Duchenne and Becker muscular dystrophy. Curr Opin Neurol. 2019;32:722‐727. [DOI] [PubMed] [Google Scholar]
- 2. Bushby KMD, Thambyayah M, Gardner‐Medwin D. Prevalence and incidence of Becker muscular dystrophy. Lancet. 1991;337:1022‐1024. [DOI] [PubMed] [Google Scholar]
- 3. Shieh PB. Emerging strategies in the treatment of Duchenne muscular dystrophy. Neurotherapeutics. 2018;15:840‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fortunato F, Farnè M, Ferlini A. The DMD gene and therapeutic approaches to restore dystrophin. Neuromuscul Disord. 2021;31:1013‐1020. [DOI] [PubMed] [Google Scholar]
- 5. Maggi L, Moscatelli M, Frangiamore R, et al. Quantitative muscle MRI protocol as possible biomarker in Becker muscular dystrophy. Clin Neuroradiol. 2021;31:257‐266. [DOI] [PubMed] [Google Scholar]
- 6. Fischer D, Hafner P, Rubino D, et al. The 6‐minute walk test, motor function measure and quantitative thigh muscle MRI in Becker muscular dystrophy: a cross‐sectional study. Neuromuscul Disord. 2016;26:414‐422. [DOI] [PubMed] [Google Scholar]
- 7. Comi GP, Niks EH, Cinnante CM, et al. Characterization of patients with Becker muscular dystrophy by histology, magnetic resonance imaging, function, and strength assessments. Muscle Nerve. 2022;65:326‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheikh AM, Rudolf K, de Stricker BJ, Witting N, Vissing J. Patients with Becker muscular dystrophy have severe paraspinal muscle involvement. Front Neurol. 2021;12:613483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheikh AM, Rudolf K, Witting N, Vissing J. Quantitative muscle MRI as outcome measure in patients with Becker muscular dystrophy—a 1‐year follow‐up study. Front Neurol. 2021;11:613489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Velde NM, Hooijmans MT, Sardjoe Mishre AS, et al. Selection approach to identify the optimal biomarker using quantitative muscle MRI and functional assessments in Becker muscular dystrophy. Neurology. 2021;97:e513‐e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comi GP, Niks EH, Vandenborne K, et al. Givinostat for Becker muscular dystrophy: a randomized, placebo‐controlled, double‐blind study. Front Neurol. 2023;14:1095121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veeger TTJ, van de Velde NM, Keene KR, et al. Baseline fat fraction is a strong predictor of disease progression in Becker muscular dystrophy. NMR Biomed. 2022;35:e4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleyweg RP, van der Meché FGA, Schmitz PIM. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve. 1991;14:1103‐1109. [DOI] [PubMed] [Google Scholar]
- 14. Crapo RO, Casaburi R, Coates AL, et al. ATS statement guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111‐117. [DOI] [PubMed] [Google Scholar]
- 15. Pizzato TM, Baptista CR, Martinez EZ, Sobreira CF, Mattiello Sverzut AC. Prediction of loss of gait in Duchenne muscular dystrophy using the ten meter walking test rates. JGSGT. 2016;7:1‐6. [Google Scholar]
- 16. Bello L, Campadello P, Barp A, et al. Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies. Sci Rep. 2016;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Podsiadlo D, Richardson S. The timed ‘up & go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142‐148. [DOI] [PubMed] [Google Scholar]
- 18. Barp A, Bello L, Caumo L, et al. Muscle MRI and functional outcome measures in Becker muscular dystrophy. Sci Rep. 2017;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bérard C, Payan C, Hodgkinson I, Fermanian J, The MFM Collaborative Study Group . A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005;15:463‐470. [DOI] [PubMed] [Google Scholar]
- 20. Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112(1):267‐275. [DOI] [PubMed] [Google Scholar]
- 21. de Wel B, Huysmans L, Peeters R, et al. Prospective natural history study in 24 adult patients with LGMDR12 over 2 years' follow‐up: quantitative MRI and clinical outcome measures. Neurology. 2022;99(6):e638‐e649. [DOI] [PubMed] [Google Scholar]
- 22. Mcdonald CM, Henricson EK, Abresch RT, et al. The 6‐minute walk test and other clinical endpoints in Duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013;48:357‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Batcho CS, van den Bergh P, van Damme P, et al. How robust is ACTIVLIM for the follow‐up of activity limitations in patients with neuromuscular diseases? Neuromuscul Disord. 2016;26:211‐220. [DOI] [PubMed] [Google Scholar]
- 24. Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Med Care. 1992;30:473‐483. [PubMed] [Google Scholar]
- 25. Liu CY, McKenzie CA, Yu H, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T1 and noise. Magn Reson Med. 2007;58(2):354‐364. [DOI] [PubMed] [Google Scholar]
- 26. de Wel B, Huysmans L, Depuydt CE, Maes F. Histopathological correlations and fat replacement imaging patterns in recessive limb‐girdle muscular dystrophy type 12. J Cachexia Sarcopenia Muscle. 2023;14(3):1468‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huysmans L, de Wel B, Claeys KG, Maes F. Automated MRI quantification of volumetric per‐muscle fat fractions in the proximal leg of patients with muscular dystrophies. Front Neurol. 2023;14:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65‐70. [Google Scholar]
- 29. Nuñez‐Peralta C, Alonso‐Pérez J, Llauger J, et al. Follow‐up of late‐onset Pompe disease patients with muscle magnetic resonance imaging reveals increase in fat replacement in skeletal muscles. J Cachexia Sarcopenia Muscle. 2020;11:1032‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Data Availability Statement
The anonymized supporting datasets analysed during the current study are available to qualified investigators from the corresponding author on reasonable request.


